Abstract
OBJECTIVE
To evaluate the extent of pancreatic β-cell function in a large number of insulin-dependent diabetic patients with a disease duration of 50 years or longer (Medalists).
RESEARCH DESIGN AND METHODS
Characterization of clinical and biochemical parameters and β-cell function of 411 Medalists with correlation with postmortem morphologic findings of 9 Medalists.
RESULTS
The Medalist cohort, with a mean ± SD disease duration and age of 56.2 ± 5.8 and 67.2 ± 7.5 years, respectively, has a clinical phenotype similar to type 1 diabetes (type 1 diabetes): mean ± SD onset at 11.0 ± 6.4 years, BMI at 26.0 ± 5.1 kg/m2, insulin dose of 0.46 ± 0.2 u/kg, ∼94% positive for DR3 and/or DR4, and 29.5% positive for either IA2 or glutamic acid decarboxylase (GAD) autoantibodies. Random serum C-peptide levels showed that more than 67.4% of the participants had levels in the minimal (0.03–0.2 nmol/l) or sustained range (≥0.2 nmol/l). Parameters associated with higher random C-peptide were lower hemoglobin A1C, older age of onset, higher frequency of HLA DR3 genotype, and responsiveness to a mixed-meal tolerance test (MMTT). Over half of the Medalists with fasting C-peptide >0.17 nmol/l responded in MMTT by a twofold or greater rise over the course of the test compared to fasting. Postmortem examination of pancreases from nine Medalists showed that all had insulin+ β-cells with some positive for TUNEL staining, indicating apoptosis.
CONCLUSIONS
Demonstration of persistence and function of insulin-producing pancreatic cells suggests the possibility of a steady state of turnover in which stimuli to enhance endogenous β cells could be a viable therapeutic approach in a significant number of patients with type 1 diabetes, even for those with chronic duration.
The incidence of type 1 diabetes is increasing around the world and age at onset is becoming younger (1–4). More than 90% of diabetic patients will develop significant vascular complications resulting in loss of visual acuity, kidney failure, and increased risk of cardiovascular diseases (5–7). Enhancing endogenous insulin production in diabetic patients can substantially improve glycemic control and decrease complications. However, the comparative analysis of residual pancreatic function and morphology in long-term diabetic patients has not been studied. The Joslin 50-Year Medalist Study has been characterizing as a large cohort of patients who have been insulin dependent for 50 years or longer (8,9). Surprisingly, preliminary screening by random serum C-peptide levels suggests that a majority of Medalists may still be producing insulin.
Studies on individuals with extreme duration of type 1 diabetes are rare, but they can be uniquely useful in identifying protective factors against the development of complications and for the preservation of endogenous insulin-producing β-cells (10). Bain et al. (8) characterized the golden years cohort, a population of people with 50 or more years of type 1 diabetes from the U.K., for complication status and other clinical parameters, but not for residual insulin production. Lohr and Kloppel (9) reported residual β-cells in 46% of their 16 autopsied cases of individuals with more than 21 years duration of diabetes. Pipeleers and Ling (10) reported residual β-cells in 40% of 43 patients with 10–30 years duration of diabetes and onset after 7 years of age. More recently, Meier et al. (11) reported the presence of insulin-containing β-cells in 88% of the pancreases from 42 type 1 diabetic patients with duration of diabetes ranging from 4–67 years of whom 14 had diabetes for 32 or more years. Gianani et al. (12) reported that 3 of 13 pancreases of people with childhood-onset diabetes for 10 years or longer were positive for insulin, but only 1 of these had either DR3 or DR4 allele. However, no clinical premortem studies on β-cell function were reported in any of these studies.
In the present study, we are reporting the clinical and physiologic characterization of 411 patients with insulin-dependent diabetes of 50 years or longer duration, particularly with regard to their pancreatic β-cell function. In addition, uniquely, multiple samples of pancreases from 9 Medalists were analyzed morphologically and correlated with premortem data to determine whether the clinical evidence of residual insulin production correlates with the pancreatic histologic findings.
RESEARCH DESIGN AND METHODS
U.S. residents who received Joslin 50-Year Medals were recruited for participation. The Joslin 50-Year Medal is available to any individual who provides a medical record or three other forms of documentation of 50 or more years of insulin-dependent diabetes. Information regarding the program is advertised in publications by the American Diabetes Association and the Juvenile Diabetes Research Foundation, as well as by individual physicians, Medalists, and the general media. By September 30, 2008, a total of 476 medals had been awarded to U.S. residents since the beginning of this study, 431 (90.5%) appointments had been made, and 411 Medalists had completed a study visit. The reasons for nonparticipation were death in 2 cases, inability to travel/poor health (8), family member illness (5), and/or work (4). Informed consent was obtained from all subjects prior to study participation. The Joslin Committee on Human Subjects approved the study.
Most Medalists (88%) received routine endocrine care outside the Joslin Diabetes Center. For the data presented here, all study subjects were evaluated at the Joslin Diabetes Center by medical history, clinical exams, and blood and urine analysis. Hemoglobin A1C (A1C) was determined by high-performance liquid chromatography (Tosoh G7 and 2.2, Tokyo, Japan). Lipid profiles were determined by standard enzymatic methods (using kits from Roche Diagnostics, Indianapolis, IN; Denka Seiken, Tokyo, Japan; and AsahiKasei, Tokyo, Japan). Serum C-peptide was determined by radioimmunoassay (Beckman Coulter, Fullerton, CA) and validated at the Northwest Lipid Research Laboratory at the University of Washington as previously described (13). The methods of Yu et al. (14) were used at both the Joslin Diabetes Center and the Barbara Davis Center for Childhood Diseases to assay IA2 and GAD 65 autoantibodies.
Mixed-meal tolerance test.
Any individual with a random C-peptide greater than or equal to 0.1 nmol/l was invited back for a mixed-meal tolerance test (MMTT). Participants were instructed to fast overnight. At presentation, a blood glucose level was taken via glucometer, and if the individual's blood glucose level was between 5.5 and 8.25 mmol/l, the test was performed. Otherwise, individuals were given insulin or a snack to reach the appropriate range before the test was begun (15). Serum samples were assayed for C-peptide and glucose levels, as detailed above, at time points 0, 30, 60, 90, and 120 min.
Genotyping.
DRB1 and DQB1 genotyping was done using linear arrays of immobilized sequence-specific oligonucleotides similar to previously described methodology, with direct sequencing of DRB1 exon 2 to differentiate DRB1*04 subtypes (16).
All Medalists as of January 1, 2008, with a random C-peptide in excess of 0.1 nmol/l (n = 50) were genotyped for HNF4A, GCK, TCF1, IPF1, and TCF2 (maturity onset of diabetes in the young [MODY] 1–5, respectively) by Athena Diagnostics (Worcester, MA).
Histologic studies.
Pancreases were obtained through the Juvenile Diabetes Research Foundation-sponsored Network for Pancreatic Organ Donors with Diabetes (nPOD) program from 50-year Medalists who consented to postmortem organ donation. The pancreases were procured by the National Disease Research Interchange and shipped to the nPOD Pathology Core (University of Florida, Gainesville, FL) where they were breadloafed into up to 20 blocks, fixed in 10% buffered formalin, and processed routinely for paraffin embedding. Sections from blocks spanning the whole pancreas (6–20 blocks per pancreas, mean 15) were available for eight of the nine; only 2 blocks were recovered for the remaining pancreases. At the Joslin Diabetes Center, paraffin sections were microwaved for antigen retrieval and immunostained with guinea pig anti-bovine insulin (1:200, Linco), rabbit anti-bovine glucagon (1:2000 M. Appel, University of Massachusetts), mouse anti-human Ki67 (1:200, Becton, Dickinson and Company), and rabbit anti-human CD3 (1:50, Dako) (17). CD3-positive cells in islets were assessed only in regions of the pancreas without many CD3+ cells in the parenchyma. TUNEL (Roche POD TUNEL kit) was performed on microwaved sections of those pancreases without extensive autolysis. Amyloid was detected using thioflavin S (16). Images were taken on an Olympus BH2 microscope or in confocal mode with a Zeiss LSM 410 microscope.
Statistical analysis.
All variables were visually inspected and analyzed for distribution to determine appropriate statistical methods for analysis. A Wilcoxon rank sum analysis was used for two-way comparisons involving independent continuous variables, and the Fisher exact test was used to analyze the relationship of categorical variables. ANOVA and tests for linear trend were performed using generalized linear models designating group as categorical or ordinal as appropriate. A paired t test was used to determine if the differences between fasting and peak values during MMTT were statistically significant. P values ≤ 0.05 was considered statistically significant. Stata version 11 (College Station, TX) and SAS version 9.2 (Cary, NC) were used to perform analyses.
RESULTS
As of September 30, 2008, a total of 411 Medalists had participated in this study; 47% were male (Table 1). The average age was 67.2 ± 7.4 years, mean age at diagnosis of diabetes was 11.0 ± 6.5 years, and mean duration of diabetes was 56.2 ± 5.8 years. Medalists had a favorable lipid profile (calculated HDL 1.6 ± 0.58 mmol/l, LDL 2.2 ± 0.6 mmol/l, total cholesterol 4.2 ± 0·9 mmol/l, triglycerides 0.9 ± 0.5 mmol/l). Average insulin dose per kilogram was 0.46 ± 0.2 u/kg. The frequency of HLA risk alleles DR3 and DR4 (0602 excluded) was greater than 93% (Table 1). The prevalence of participants who were autoantibody positive was 29.7% (111) for either antigen; 14.9% (55) and 18.4% (69) for IA2 only or GAD only, respectively (Table 1).
TABLE 1.
Characteristics of Medalist study participants
| Male (%) | 47.0 (192) |
| A1C (%) | 7.3 ± 1.1 |
| Age (years) | 67.2 ± 7.4 |
| Age at diagnosis (years) | 11.0 ± 6.5 |
| Duration (years) | 56.2 ± 5.8 |
| BMI (kg/m2) | 26.0 ± 5.1 |
| C-peptide (nmol/l) | 0.07 ± 0.12 |
| Cholesterol (mmol/l) | 4.2 ± 0.9 |
| Calculated HDL (mmol/l) | 1.6 ± 0.5 |
| LDL (mmol/l) | 2.2 ± 0.6 |
| Triglycerides (mmol/l) | 0.9 ± 0.5 |
| Insulin dose (u/kg) | 0.46 ± 0.2 |
| Family history of diabetes | |
| Any diabetes | 29.7 (122) |
| Type 1 diabetes | 12.9 (53) |
| DR3* | 38.8 (116) |
| DR4* | 52.0 (156) |
| DR3 or DR4* | 93.7 (295) |
| DR3/4* | 39.1 (118) |
| IA2 or GAD* | 29.7 (111) |
| IA2* | 14.9 (56) |
| GAD* | 18.4 (69) |
| Proliferative diabetic retinopathy* | 55 (163) |
| Microalbuminuria (ACR <7.91)* | 13.1 (45) |
| Neuropathy (MNSI >2)* | 60.6 (183) |
| Cardiovascular disease* | 48.3 (160) |
Data are means ± SD or % (n).
*Percentages reflect calculations done on data available. ACR, albumin-to-creatinine ratio; MNSI, Michigan Neuropathy Screening Instrument.
In addition to characterizing basic clinical traits, we examined family history and the presence of MODY polymorphisms. The family histories of both type 1 diabetes and type 2 diabetes of all patients were reviewed. We determined that 12.9% of Medalists had at least one first-degree relative with type 1 diabetes, and 29.7% had a first-degree relative with any type of diabetes (Table 1). No significant differences were observed in the frequency of first degree relatives with type 1 diabetes or any type of diabetes mellitus when random C-peptide levels were compared (Table 2). Additionally, 50 individuals with random C-peptide levels in excess of 0.1 nmol/l were genotyped for risk polymorphisms in MODY genes 1–5 ([1] HNF4A, [2] GCK, [3] TCF1, [4] IPF1, [5] TCF2). Of those typed, only 4 were found to have risk polymorphisms (HNF4A, IPF1 and 2 had TCF1). Only 1 of the 4 identified with MODY polymorphisms had a C-peptide level greater than 0.2 nmol/l. Analyses were done excluding these individuals and there was no difference in statistical results.
TABLE 2.
Characteristics of Medalist study participants by DCCT categories of residual insulin production
| Undetectable <0.03 nmol/l | Minimal 0.03–0.2 nmol/l | Sustained ≥0.2 nmol/l | P* | |
|---|---|---|---|---|
| N (%) | 33.0 (126) | 64.4 (246) | 2.6 (10) | |
| Male | 42.6 (55) | 50.2 (123) | 52.9 (7) | 0.4 |
| A1C (%) | 7.5 ± 1.0 | 7.1 ± 1.1 | 7.32 ± 0.7 | 0.005 |
| Age (years) | 67.5 ± 8.1 | 67.0 ± 7.2 | 71.7 ± 8.3 | 0.09 |
| Age at diagnosis (years) | 10.9 ± 6.8 | 10.9 ± 6.1 | 16.2 ± 8.6 | 0.02 |
| Duration (years) | 56.4 ± 6.0 | 56.1 ± 5.7 | 55.5 ± 4.1 | 0.7 |
| BMI (kg/m2) | 26.7 ± 2.8 | 26.0 ± 4.3 | 23.8 ± 3.6 | 0.5 |
| Insulin dose (u/kg) | 0.47 ± 0.2 | 0.5 ± 0.2 | 0.4 ± 0.2 | 0.5 |
| Cholesterol (mmol/l) | 4.2 ± 0.9 | 4.2 ± 0.9 | 4.2 ± 1.1 | 0.8 |
| Calculated HDL (mmol/l) | 1.6 ± 0.9 | 1.6 ± 0.5 | 1.7 ± 0.6 | 0.7 |
| LDL (mmol/l) | 2.2 ± 0.6 | 2.2 ± 0.6 | 2.2 ± 0.9 | 0.5 |
| Triglycerides (mmol/l) | 0.92 ± 0.5 | 0.89 ± 0.51 | 0.98 ± 0.65 | 0.9 |
| Family history of diabetes† | ||||
| Any diabetes | 27.8 (35) | 31.7 (78) | 20.0 (2) | 0.6 |
| Type 1 diabetes | 11.9 (15) | 14.2 (35) | 10.0 (1) | 0.8 |
| DR3† | 33.6 (39) | 43.8 (84) | 57.1 (4) | 0.03 |
| DR4† | 42.2 (49) | 57.7 (112) | 42.9 (3) | 0.03 |
| DR3/4† | 44.8 (52) | 36.6 (71) | 14.3 (1) | 0.14 |
| DR3 or DR4 | 95.7 (116) | 92.2 (177) | 100.0 (7) | 0.4 |
| IA2 or GAD† | 32.8 (40) | 27.2 (64) | 40.0 (4) | 0.7 |
| IA2† | 16.3 (20) | 13.9 (33) | 0 | 0.3 |
| GAD† | 21.1 (26) | 16.0 (38) | 36.4 (4) | 0.1 |
| Proliferative DR† | 53.9 (56) | 52.7 (97) | 36.4 (4) | 0.5 |
| Microalbuminuria (ACR <7.91)† | 15.1 (18) | 10.8 (19) | 23.1 (3) | 0.3 |
| Neuropathy (MNSI >2)† | 60.4 (67) | 60.7 (99) | 60.0 (6) | 0.9 |
| Cardiovascular disease† | 46.4 (52) | 50.0 (96) | 50.0 (5) | 0.8 |
| MMTT response | 0 (0/3) | 14.2 (3/21) | 57.1 (4/7) | <0.0001 |
Data are means ± SD or % (n).
†Percentages reflect calculations done on data available.
*P values resulted from ANOVA.
To further examine the role of residual C-peptide, Medalists were categorized based on their serum random C-peptide measures. These categories were derived from the Diabetes Control and Complications Trial (DCCT) criteria for examining residual C-peptide production based on response to MMTT. These categories were as follows: undetectable, ≤0.03 nmol/l; minimal, 0.03–0.2 nmol/l; and sustained, ≥0.2 nmol/l (17). Our categorization was done using the patient's random C-peptide measure. We hypothesize that our means of categorization may underestimate the degree of insulin production, as random C-peptide levels are not necessarily at their maximum since production was likely not stimulated in most cases. There were 33.0% in the undetectable category, 64.4% with minimal, and 2.6% with sustained random serum C-peptide levels, resulting in 67.4% (n = 256) with at least detectable C-peptide levels (Table 2). Analyses indicated a significant difference in glycemic control as measured by glycated hemoglobin across the DCCT-defined groups of random serum C-peptide levels, however, did not increase linearly with C-peptide levels (7.5 ± 1.0%, 7.1 ± 1.1%, and 7.3 ± 0.7%, respectively, P = 0.005). The group with sustained levels of random C-peptide had a much higher mean of age at diagnosis (16.2 ± 8.6 years) compared with each of the other groups (mean of 10.9 years in each of the other groups, ANOVA P = 0.02). MHC HLA risk alleles were also differentially distributed across these three groups of random C-peptide (Table 2). The sustained group had the highest frequency of DR3 risk alleles, whereas the minimal group (0.03–0.2 nmol/l) had the highest frequency of DR4 risk alleles. Of interest is the higher frequency of the DR3 risk allele among those with sustained random C-peptide production compared with those with undetectable random levels (57.1% vs. 33.6% DR3). The presence of islet cell antibodies, either IA2 or GAD, was not different across the three groups; however, the frequency of IA2 autoantibodies was lower than that of GAD in all groups, with none present in the sustained group (Table 2). As shown in Table 2, there was no difference across the three groups in terms of sex, age, duration of disease, A1C, family history of diabetes, BMI, insulin dose, lipid profile, or prevalence of microvascular or macrovascular complications.
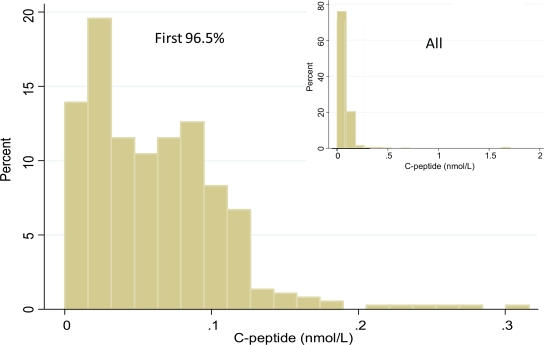
There were 14 individuals who had significantly higher random C-peptide levels than the majority (in excess of 0.17 nmol/l, representing the top 3.5%) (Fig. 1). On average these individuals had an older age at diagnosis versus the rest of the cohort (17.4 ± 7.4 vs. 10.8 ± 6.3 years, P = 0.0008, respectively). Trends that did not reach statistical significance comparing this group with the rest of the cohort were higher frequency of DR3 or DR4 risk alleles (100% vs. 93.2%) and lower prevalence of autoantibodies (20.0% vs. 28.7%).
FIG. 1.
Distribution of the first 97% of C-peptide levels among 50-year Medalists. Inset shows C-peptide values from all values. These pictures demonstrate the outlying 3% in excess of 0.17 nmol/l. (A high-quality color representation of this figure is available in the online issue.)
MMTT.
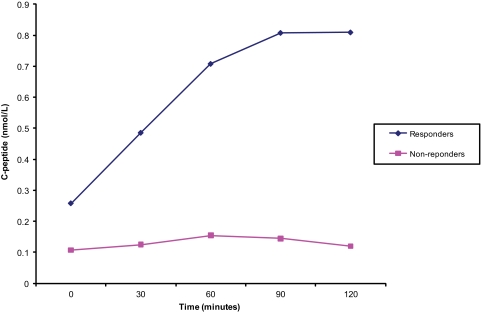
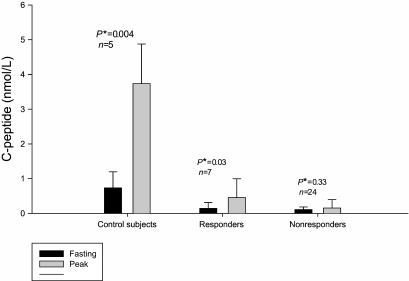
The physiologic characterization of the C-peptide production in the Medalists was studied through MMTT. A total of 31 individuals were invited to the Joslin Diabetes Center for MMTT based on their random C-peptide levels being greater than 0.1 nmol/l. In addition, 6 nondiabetic age-matched controls were also studied. Thirteen of the 31 Medalist participants who returned for the MMTT responded with doubling of C-peptide levels over their level at time 0 min (Fig. 2). As shown in Fig. 3, the nondiabetic age-matched controls had fasting levels (mean ± SD) of 0.73 ± 0.5 nmol/l and stimulated levels at 60 min of 3.74 ± 1.1 nmol/l, (P < 0.004). The 13 Medalist responders had fasting C-peptide levels of 0.14 ± 0.2 nmol/l and reached a maximum of 0.45 ± 0.54 nmol/l at 90 min (P = 0.03). In contrast, the 18 nonresponders had fasting C-peptide level of 0.11 ± 0.1 nmol/l and a maximum level of 0.15 ± 0.2 nmol/l at 90 min (P = 0.33) (Fig. 3). Analysis demonstrated a higher proportion of responders were in the sustained C-peptide group. It is clear that those diabetic patients with sustained random levels of C-peptides (57.1%) were significantly more responsive to MMTT, defined as at least a doubling of baseline fasting measure of C-peptide, than the minimal group (14.2%). Additionally, the sustained C-peptide group showed a significantly greater response to MMTT at 36.4% compared with the minimal group as established by random C-peptide level 15.0%, (P < 0.001).
FIG. 2.
MMTT average response curves for responders and nonresponders. (A high-quality color representation of this figure is available in the online issue.)
FIG. 3.
Mean C-peptide levels from MMTT at baseline and peak value of control subjects, responders, and nonresponders. *P value is from a paired t-test. Error bars represent standard deviation.
Histology.
Pancreases from 9 Medalists representing all three DCCT categories of C-peptide production were recovered after death for pathologic analysis (Table 3). All were DR3, DR4, or DR3/DR4 positive; and 3 (33%) were antibody positive to GAD or IA2 autoantibodies. None of the pancreases studied came from patients who had a MODY risk polymorphism, and none of these have been reported before.
TABLE 3.
Summary of findings in nine Medalist's pancreases, including insulin, Ki67, and TUNEL staining in cells
| ID | Sex | Age (years) | Age dx (years) | HbA1C (%) | AB | HLA | C-peptide (nmol/l) | Insulin+ | Ki67+ | TUNEL+ | CD3+ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | F | 60 | 1 | 8.8 | GAD+ | DR3/DR4 | 0.01 | Very few, scattered singlets | ND | Insulin + clusters | In a few islets |
| M2 | M | 59 | 7 | 5.6 | Neg | DR3 | 0.02 | Scattered in separate lobes, + in ducts | Neg | Autolysis | In a few islets |
| M3 | M | 89 | 5 | 9 | Neg | DR4 | 0.04 | Scattered in separate lobes, + in ducts | In ducts | Autolysis | ND |
| M4 | M | 78 | 4 | 6.6 | IA2+ | DR3 | 0.06 | Scattered, clusters up to 8 cells | In islets; no insulin+ cells | +Cells, but not insulin+ | ND |
| M5 | F | 71 | 8 | 5.7 | Neg | DR3/DR4 | 0.06 | Scattered, clusters and some within islets | ND | Autolysis | ND |
| M6 | F | 73 | 7 | 7.3 | Neg | DR3/DR4 | 0.09 | Scattered, within islets, rare cluster | ND | Neg | ND |
| M7 | F | 72 | 5 | 9.8 | GAD+ | DR3/DR4 | 0.1 | Few scattered in small islets, rare glucagon+ in ducts | Neg | Autolysis | ND |
| M8 | F | 79 | 23 | 6.7 | Neg | DR3/DR4 | 0.16 | 50% islets none; 25% normal; 25% with amyloid | ND | Couple insulin+ | In a few islets |
| M9 | M | 88 | 30 | 7.1 | Neg | DR3 | 1.66 | All islets insulin+, Some small islets all insulin+; + in ducts | Insulin+ | ND | In a few islets |
dx, duration; ND, not determined; Neg, negative; Age dx, age of diagnosis; AB, antibody.
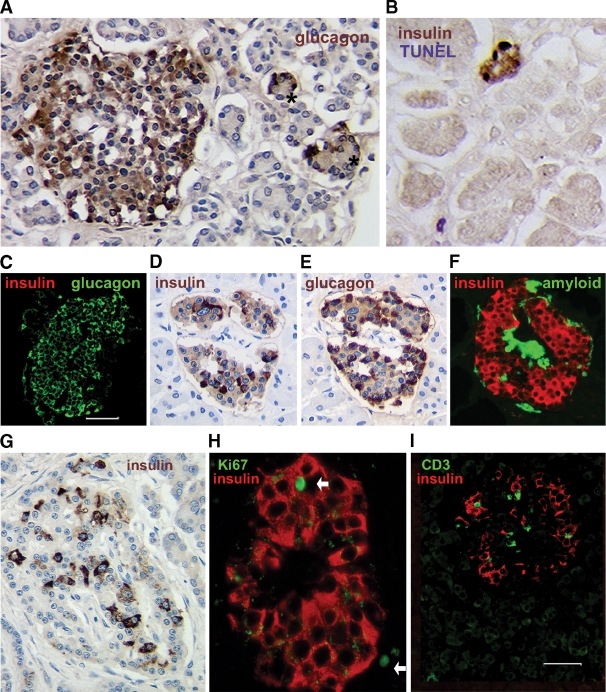
Insulin+ cells were observed in all the pancreases as scattered single extrainsular cells or small clusters in some lobes (Table 3). In the seven Medalists who had onset of diabetes at age 8 or younger, most islets were atrophic with no insulin staining (Fig. 4A and C), although there were often also small islets with a few central cells that did not stain for glucagon. Although these unstained central cells are hypothesized to be degranulated β-cells, as yet no specific β-cell markers have stained positive. However, in two of these Medalists, insulin-positive cells were found within occasional islets.
FIG. 4.
Histologic findings in pancreases from nine Medalists. In seven of nine pancreases, there were mainly atrophic islets (A) in which all or almost all cells were immunostained for glucagon, with occasional small islets that had peripheral glucagon+ cells with unstained central cells (asterisks), and rare small clusters or scattered single insulin+ cells (B); all nine pancreases had these scattered insulin+ cells. In one antibody-positive Medalist, some insulin+ cells were TUNEL-positive (B). In another pancreas (from Medalist 8) (C–F), about half of the pancreas had only atrophic islets (C), although there were lobes within the body and tail of the pancreas, with most islets with significant proportion of insulin+ cells (D and E, adjacent sections) and some with amyloid deposits (F) (thioflavin S+). In another late-onset diabetes pancreas (Medalist 9; age 30 at onset) (G–I), every islet had at least one insulin+ cell, and most had 10–20% of normal (G). In this last Medalist pancreas, rare Ki67+insulin+ cells (arrows indicate Ki67+ nuclei, both within the islet and nearby) (H) were found. I: In three pancreases, CD3 cells were rarely found in insulin+ islets, whereas no other CD3 cells were found in the microscopic field. (A high-quality digital representation of this figure is available in the online issue.)
In the pancreases from the two Medalists (Medalists 8 and 9, Table 3) who had later onset diabetes (23 and 30 years) and responded by doubling their C-peptide during MMTT, considerably more insulin+ cells were found, and these were clearly within islets (Fig. 4D–I). In both, there were islets depleted of insulin+ cells, as well as islets with considerable proportion of insulin+ cells. Intriguingly, in Medalist 8 (onset at 23 years), half of the pancreases had only atrophic islets (Fig. 4C), but in a lobular pattern there were islets with significant insulin+ cells (Fig. 4D–F) and even some with amyloid deposits (Fig. 4F). Clinical evaluations of Medalist 8 showed her to be positive for DR3 and DR4 and well controlled with an A1C of 6.7%. Two MMTTs in Medalist 8 confirmed a 360% rise in C-peptide at 90 min.
Turnover of β-cells was supported by a few TUNEL+ insulin+ cells in islets or clusters in two of four (50%) TUNEL-stained pancreases (Fig. 4B) and by Ki67+ islet cells in two other pancreases; only one of which had insulin+ Ki67+ cells (Fig. 4H).
In three (33%) antibody-negative Medalists, a few CD3+ cells were found in insulin+ islets (Fig. 4I), and in one, antibody-positive without noticeable insulin+ cells within islets, a few islets had a CD3+ cell. There were no other CD3 cells in the low magnification fields of these pancreases.
DISCUSSION
This study represents the first comprehensive analysis of the clinical characteristics affecting β-cell function in a large cohort of insulin-dependent patients of chronic duration (18). We have correlated these parameters with postmortem pancreatic pathology of some of the same patients. The results showed that a majority have the ability to produce insulin endogenously, as suggested by random serum C-peptide levels and response to MMTT, and confirmed by the presence of insulin+ cells in all nine available Medalist pancreases, even in those with undetectable random C-peptide values (19). This prevalence of elevated C-peptide levels (67.4% >0.03 nmol/l) in the Medalist cohort is the highest among published studies of type 1 diabetic patients (19,20). The DCCT reported only 11% of patients screened by MMTT, mean duration of 2.3 ± 0.3 years, had this level of C-peptide (17). Although there can be differences between random C-peptide levels, which could be caused by fasting and those resulting from a MMTT, it is likely that the random serum C-peptides would underestimate residual C-peptide levels, biasing our results to the null. However, this has not been studied in individuals with extreme duration of diabetes. Even with this high percentage of retained insulin production, the clinical and biochemical characteristics of this population are consistent with type 1 diabetes: low BMI (26 ± 5.1 kg/m2), onset of disease at 11 ± 6.5 years, lipid profile of high HDL (1.6 ± 0.5 mmol/l) and normal LDL (2.2 ± 0.6 mmol/l) and lack of insulin resistance per daily insulin dose (0.46 ± 0.2 u/kg). Furthermore, this group, genetically, had a high frequency of HLA diabetes risk alleles DR3 and/or DR4 (93%) (21,22). Also consistent with type 1 diabetes, 29.5% of Medalists were positive for IA2 or GAD autoantibodies (23,24). Thus, it is likely that most of the Medalists studied have autoimmune type 1 diabetes.
The random serum C-peptide levels in the Medalists spanned a wide range, from undetectable to >1 nmol/l. Detailed analysis of Medalists' clinical parameters according to their C-peptide levels provided very few clues regarding the preservation of endogenous insulin production, suggesting that multiple factors contribute. Dividing the cohort into three groups according to C-peptide levels provided interesting findings, such as the finding that better glycemic control and later age of onset were associated with higher C-peptide levels.
A small subset of Medalists with high C-peptide levels was associated with older age at onset and more β-cells. The pancreases from two of this subgroup (Table 3) had nearly 10% of normal β-cell mass, whereas neither Lohr and Kloppel (20) nor Meier et al. (11) found a correlation of later age of onset with residual β-cells, although Pipeleers and Ling did (10). It is possible that the differential preservation of insulin producing cells in the Medalists could have led to their long-term survival.
The MMTT responsiveness of the cohort indicates that the higher random or fasting C-peptide, the greater propensity to respond, suggesting some retained β-cells have preserved function. It is interesting to note that in the MMTT, the fasting and maximum levels of C-peptide response of the sustained C-peptide group were ∼10–20% of those of age-matched nondiabetic controls. For the nonresponders, the C-peptide levels at fasting and maximum response were less than 2–3% of the nondiabetic control. The relative differences in the C-peptide levels among these groups corresponded to the number of β-cells detected by postmortem morphologic analysis of the pancreases. Our histologic studies showed that nine of nine Medalist pancreases, even those (Medalist 1 and 2) who did not have detectable random C-peptide, had residual insulin+ β-cells as singlets and small clusters; several (Table 3) had insulin-positive cells within islets. All of these would have been classified as pattern A by the criteria recently reported (12) since they all were DR3, DR4, or DR3/4, Caucasian, and had insulin-deficient islets.
The studies of the Medalists provide strong evidence indicating that residual β-cells in type 1 diabetic patients of chronic duration are in a steady state of cellular apoptosis and proliferation, even in individuals with diabetes of over 50 years duration. Meier et al. (11) reported both increased apoptosis and evidence of chronic inflammation in pancreases from people who had type 1 diabetes of various durations ranging from 4 to 67 years. Interestingly, insulin+ β-cells were co-labeled with apoptotic and proliferative markers. In addition, positive CD3 cells were detected in a few islets in all pancreases studied. Clearly more data derived from these pancreases and of other Medalist patients will be important to solidify these conclusions.
Because these are human tissues, there is no way to determine whether the residual β-cells survived the initial autoimmune destruction or formed at some later time. Although rare β-cell replication was seen, in most of the pancreases the majority of insulin-positive cells were single cells, often within the ducts. A recent report by Thorel et al. (25) demonstrated in a murine model that in β-cell–depleted mice, α-cells could differentiate into β-cells in mice after prolonged duration of diabetes (i.e., 10 months). This raises the possibility that the residual C-peptide observed in the Medalists represents a slow increase of β-cell mass/function in this group of patients with diabetes of extreme duration. It should be noted that in 5 of the pancreases, the only insulin-positive cells were singlets or small clusters, and even in the four with insulin-positive cells within islets, there was no colocalization of insulin and glucagon. Thus, the source of residual β-cells in the Medalists is unclear, but it is likely that they may be derived from multiple sources.
In summary, the study of a large group of insulin-dependent diabetic patients of extreme duration characterized clinically, biochemically, and histologically has provided the surprising finding that residual functional β-cells remain even after 50 years in a majority of these type 1 diabetic patients. The data presented in this study clearly support that even under prolonged autoimmune and metabolic stress, pancreatic β-cells can be replenished. Thus, the amelioration of autoimmune stress, together with stimulus for regeneration of endogenous β-cells, could be a feasible approach to improve endogenous insulin production in a substantial number of patients with type 1 diabetes.
ACKNOWLEDGMENTS
This study is supported by grants from the Juvenile Diabetes Research Foundation 8-2005-358, 8-2008-363, 25-2008-383; National Institutes of Health K12-16335, T32-DK-007260, R24-DK-083957 01, P30-DK-036836-23, Brehm Foundation, Beatson Foundation; and Eli Lilly (F3Z US X024).
No other potential conflicts of interest relevant to this article were reported.
H.A.K. and J.K.S. researched, collected, and analyzed data and wrote and reviewed the manuscript. J.L. collected data. A.D., L.P.A., and G.E. reviewed and edited the manuscript. S.B.-W. and G.L.K. reviewed data and reviewed and edited the manuscript.
The authors would like to acknowledge the tireless work of our research team, Leah Whelan and Joshua Geltman of the Joslin Diabetes Center, and the participants of the 50-Year Medalist Study.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of type I diabetes—the analysis of the data on published incidence trends. Diabetologia 1999;42:1395–1403 [DOI] [PubMed] [Google Scholar]
- 2.Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 2000;23:1516–1526 [DOI] [PubMed] [Google Scholar]
- 3.Harjutsalo V, Sjoberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 2008;371:1777–1782 [DOI] [PubMed] [Google Scholar]
- 4.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 5.Gale EA. Glucose control in the UKPDS: what did we learn? Diabet Med 2008;25Suppl. 2:9–12 [DOI] [PubMed] [Google Scholar]
- 6.Walsh MG, Zgibor J, Borch-Johnsen K, Orchard TJ. A multinational assessment of complications in type 1 diabetes: the DiaMond substudy of complications (DiaComp) level 1. Diab Vasc Dis Res 2006;3:84–92 [DOI] [PubMed] [Google Scholar]
- 7.Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care 2008;31:1360–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bain SC, Gill GV, Dyer PH, Jones AF, Murphy M, Jones KE, Smyth C, Barnett AH. Characteristics of type 1 diabetes of over 50 years duration (the Golden Years Cohort). Diabet Med 2003;20:808–811 [DOI] [PubMed] [Google Scholar]
- 9.Keenan HA, Costacou T, Sun JK, Doria A, Cavellerano J, Coney J, Orchard TJ, Aiello LP, King GL. Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50-year Medalist Study. Diabetes Care 2007;30:1995–1997 [DOI] [PubMed] [Google Scholar]
- 10.Pipeleers D, In't Veld P, Pipeleers-Marichal M, Gorus F. The β cell population in type 1 diabetes. Novartis Found Symp 2008;292:19–24; discussion 24–31, 122–129, 202–203 [DOI] [PubMed] [Google Scholar]
- 11.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained β cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 2005;48:2221–2228 [DOI] [PubMed] [Google Scholar]
- 12.Gianani R, Campbell-Thompson M, Sarkar SA, Wasserfall C, Pugliese A, Solis JM, Kent SC, Hering BJ, West E, Steck A, Bonner-Weir S, Atkinson MA, Coppieters K, von Herrath M, Eisenbarth GS. Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia 2010;53:690–698 [DOI] [PubMed] [Google Scholar]
- 13.Mandrup-Poulsen T. β cell death and protection. Ann N Y Acad Sci 2003;1005:32–42 Erratum published online 5 June 2010 [DOI] [PubMed] [Google Scholar]
- 14.Yu L, Rewers M, Gianani R, Kawasaki E, Zhang Y, Verge C, Chase P, Klingensmith G, Erlich H, Norris J, Eisenbarth GS. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab 1996;81:4264–4267 [DOI] [PubMed] [Google Scholar]
- 15.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, Battelino T, Haastert B, Ludvigsson J, Pozzilli P, Lachin JM, Kolb H; Type 1 Diabetes Trial Net Research Group; European C-Peptide Trial Study Group Mixed-meal tolerance test versus glucagon stimulation test for the assessment of β-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008;31:1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hull RL, Watts MR, Kodama K, Shen ZP, Utzschneider KM, Carr DB, Vidal J, Kahn SE. Genetic background determines the extent of islet amyloid formation in human islet amyloid polypeptide transgenic mice. Am J Physiol Endocrinol Metab 2005;289:E703–E709 [DOI] [PubMed] [Google Scholar]
- 17.Steffes MW, Sibley S, Jackson M, Thomas W. β-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003;26:832–836 [DOI] [PubMed] [Google Scholar]
- 18.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 1965;14:619–633 [DOI] [PubMed] [Google Scholar]
- 19.Henquin JC, Cerasi E, Efendic S, Steiner DF, Boitard C. Pancreatic β-cell mass or β-cell function? That is the question! Diabetes Obes Metab 2008;10Suppl. 4:1–4 [DOI] [PubMed] [Google Scholar]
- 20.Lohr M, Kloppel G. Residual insulin positivity and pancreatic atrophy in relation to duration of chronic type 1 (insulin-dependent) diabetes mellitus and microangiopathy. Diabetologia 1987;30:757–762 [DOI] [PubMed] [Google Scholar]
- 21.Sanjeevi CB, Sedimbi SK, Landin-Olsson M, Kockum I, Lernmark A. Risk conferred by HLA-DR and DQ for type 1 diabetes in 0–35-year age group in Sweden. Ann N Y Acad Sci 2008;1150:106–111 [DOI] [PubMed] [Google Scholar]
- 22.Davies JL, Kawaguchi Y, Bennett ST, Copeman JB, Cordell HJ, Pritchard LE, Reed PW, Gough SCL, Jenkins SC, Palmer SM, Balfour KM, Rowe BT, Farrall M, Barnett AH, Bain SC, Todd JA. A genome-wide search for human type 1 diabetes susceptibility genes. Nature 1994;371:130–136 [DOI] [PubMed] [Google Scholar]
- 23.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Chase HP, Eisenbarth GS. Number of autoantibodies (against insulin, GAD or ICA512/IA2) rather than particular autoantibody specificities determines risk of type I diabetes. J Autoimmun 1996;9:379–383 [DOI] [PubMed] [Google Scholar]
- 24.Orban T, Sosenko JM, Cuthbertson D, Krischer JP, Skyler JS, Jackson R, Yu L, Palmer JP, Schatz D, Eisenbarth G; Diabetes Prevention Trial-Type 1 Study Group Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 2010;464:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]