Abstract
OBJECTIVE
Cerebral microvascular disease associated with type 2 diabetes may exacerbate the effects of aging on cognitive function. A considerable homology exists between the retinal and cerebral microcirculations; a hypothesized association between diabetic retinopathy (DR) and cognitive decline was examined in older people with type 2 diabetes.
RESEARCH DESIGN AND METHODS
In the population-based Edinburgh Type 2 Diabetes Study, 1,046 men and women aged 60–75 years with type 2 diabetes underwent standard seven-field binocular digital retinal photography and a battery of seven cognitive function tests. A general cognitive ability score (g) was generated by principal components analysis. The Mill-Hill Vocabulary Scale was used to estimate premorbid cognitive ability. DR was graded using a modification of the Early Treatment of Diabetic Retinopathy Scale.
RESULTS
After age and sex adjustment, a significant relationship was observed with increasing severity of DR (none, mild, and moderate to severe) for most cognitive measures. Participants with moderate-to-severe retinopathy had the worst g and the worst performances on the individual tests. There was a significant interaction between sex and retinopathy for g. In male subjects, the associations of retinopathy with g (and with tests of verbal fluency, mental flexibility, and processing speed but not memory and nonverbal reasoning) persisted (P < 0.05) when further adjusted for vocabulary (to estimate lifetime cognitive decline), depression, sociodemographic characteristics, cardiovascular risk factors, and macrovascular disease.
CONCLUSIONS
DR was independently associated with estimated lifetime cognitive decline in older men with type 2 diabetes, supporting the hypothesis that cerebral microvascular disease may contribute to their observed accelerated age-related cognitive decline. A sex interaction with stronger findings in men requires further confirmation.
Type 2 diabetes is associated with an increased risk of age-related cognitive impairment and decline in addition to higher incidences of stroke and dementia (1–3). Relatively little is known about the risk factors associated with the deleterious effects of type 2 diabetes on accelerated cognitive aging, although cerebral microvascular disease may be important (4–6). However, direct in vivo evaluation of the cerebral microcirculation is difficult and the vessels themselves are too small to permit detailed visualization with current neuroimaging methods.
Retinal and cerebral small vessels share similar embryological origin, size, structure, and physiological characteristics (including the blood-brain and blood-retinal barrier) (7,8). The retinal vascular bed can be directly visualized noninvasively with retinal photography. Typical retinopathic changes associated with diabetes are associated with white matter lesions in the brain, magnetic resonance imaging (MRI)-defined cerebral infarcts, and incident stroke (9–11). We hypothesized that increasing severity of diabetic retinopathy (DR) would be associated with poorer cognitive ability and with greater cognitive decline, thus providing evidence for an effect of cerebral microvascular disease on accelerated age-related cognitive decline in people with type 2 diabetes. Previous studies have found a significant association between the presence of microaneurysms and reduced performance on measures of fluid intelligence, processing speed, and attention ability in young adults with type 1 diabetes (12) and between retinopathy and risk of cognitive impairment and decline in the general population (13–15), but to our knowledge the relationship between DR and cognition in older people with type 2 diabetes has not been examined.
The aim of the present investigation was to determine the association of DR with both cognitive ability and estimated lifetime cognitive decline in a large, well-characterized sample of men and women aged 60–75 years with type 2 diabetes (the Edinburgh Type 2 Diabetes Study [ET2DS]). Cognitive function was measured using a battery of neuropsychological tests assessing major cognitive domains, and cognitive decline was estimated by using a test of vocabulary to adjust for premorbid cognitive function.
RESEARCH DESIGN AND METHODS
The ET2DS is a population-based cohort study designed to investigate potentially modifiable risk factors for cognitive decrements in type 2 diabetes. The study commenced in 2006/2007 as a cross-sectional survey of 547 men and 519 women aged 60–75 with type 2 diabetes. Participants were recruited at random, in 5-year age bands, from the Lothian Diabetes Register, which is a computerized database containing clinical details on over 20,000 patients with known type 2 diabetes living in Lothian, Scotland. The Lothian Research Ethics Committee approved the study, and fully informed written consent was obtained from each participant. Details of the study recruitment and examination procedures have previously been described in detail (16). Study participants have been shown to be representative of the target population of older men and women with type 2 diabetes living in the general population (17).
Cognitive assessment.
Specially trained research assistants administered a detailed battery of cognitive tests in a standard order during a single session in a quiet and well-lit room following tests for adequate visual acuity and capillary blood glucose levels (>4.0 mmol/l). Cognitive ability was assessed using tests of immediate and delayed nonverbal memory and verbal declarative memory (Faces and Family Pictures Subtest [FACES] and Logical Memory I (LM) from the Wechsler Memory Scale-IIIU.K. [18]); nonverbal reasoning, working memory, information processing speed (Matrix Reasoning [MR], Letter-Number Sequencing [LNS], and Digit Symbol Test [DST] from the Wechsler Adult Intelligence Scale 3rd Edition [19]); executive function (Borkowski Verbal Fluency Test [VFT] [20]) and mental flexibility (Trail Making Test-Part B [TMTB] [21]). Vocabulary (“crystallized” intelligence) was measured using the combined version of the Junior and Senior Form A synonyms of the Mill Hill Vocabulary Scale [MHVS] (22). As results on vocabulary-based tests vary little with aging, they can be used to estimate peak prior cognitive ability (23,24). Late-life cognition adjusted for vocabulary correlates highly with actual cognitive change (25). The Mini-Mental State Examination (MMSE) is often used as a screening for dementia and was included as a general mental assessment for possible cognitive pathology (26). Depressed mood was assessed using the Hospital Anxiety and Depression Scale (27).
Physical examination.
A self-administered questionnaire was used to collect data on age, sex, educational attainment, diabetes history and treatment modality, smoking history, alcohol consumption, medication use, and history of cardiovascular disease, including the World Health Organization (WHO) Chest Pain (28) and Edinburgh Claudication (29) questionnaires. Clinical measurements included plasma A1C and fasting total and HDL serum cholesterol, height, weight, waist and hip circumferences, a resting 12-lead electrocardiogram, and brachial blood pressures as previously described (16). Additional information on macrovascular disease was obtained from the Information and Services Division of NHS Scotland on all medical and surgical discharges from Scottish hospitals since 1981 (SMR01 scheme), and any K09 or K010 codes indicating cardiovascular or cerebrovascular disease were extracted.
Retinal photography and grading.
Retinal photography was performed approximately 2–3 weeks after each subject's initial visit to the research clinic for cognitive and physiological testing. Of the 1,046 participants who underwent both cognitive and retinal examinations, two were excluded who did not have gradable photographs for DR severity in either eye, leaving 1,044 who provided data for this analysis. After pupillary dilatation, standard seven-field nonstereoscopic color photographs were taken of both eyes at 35° using a high-resolution digital retinal camera. All photographs were graded by two trained optometrists, working independently and according to the scale described by the Early Treatment Diabetic Retinopathy Study (ETDRS) research group (30). Inter- and intraobserver variations and the validity of this grading system have previously been evaluated (31). For each eye, the maximum grade in any of the seven photographic fields was determined for each of the characteristic lesions of DR and was used in defining the final retinopathy levels, varying from level 10 (no retinopathy) to level 81 (advanced proliferative retinopathy). For the purpose of this study, a score of 81 was added for panretinal photocoagulation scars only if the laser treatment was for DR. The retinopathy level for a participant was assigned on the basis of the severity scores of the worse eye. If the photographs from an eye could not be graded, the scores for the other eye were used. Discrepancies in the final level score at subject level between the graders were resolved in the first instance by discussion between them. Unresolved discrepancies were further reviewed and arbitrated by an ophthalmologist.
Data analyses.
Smoking status was defined as current, former, or never. The average weekly alcohol intake was defined as standard drinks per week (32). Duration of diabetes was estimated from the age at diagnosis. Diabetes treatment was classified into three groups: 1) diet control only, 2) oral antidiabetes agents without insulin, and 3) insulin injection with or without oral agents. Waist-to-hip ratio (WHR) was calculated as the waist circumference divided by the hip circumference in centimeters. BMI was defined as weight in kilograms divided by the square of height in meters. Hypercholesterolemia was defined as serum total cholesterol ≥5 mmol/l or use of medication prescribed by a doctor to lower blood lipids level. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥85 mmHg or use of medication prescribed by a doctor to lower blood pressure. Coronary heart disease (myocardial infarction and/or angina) was defined if two of the first three of the following criteria were met or if both the first and last criteria were met: 1) subject recall of a doctor's diagnosis of myocardial infarction or angina, 2) positive WHO chest pain questionnaire, 3) electrocardiogram evidence of ischemia, and 4) prior hospital discharge (ICD) code for ischemic heart disease. Cerebrovascular disease (stroke and/or transient ischemic attack [TIA]) was defined if two of three of the following criteria were met: 1) subject recall of a doctor's diagnosis of stroke or TIA, 2) prior hospital discharge code consistent with stroke or TIA, and 3) confirmation by clinical notes review. Intermittent claudication was based on a positive response to the Edinburgh Claudication Questionnaire. Any macrovascular disease was defined as a history of myocardial infarction, angina, stroke, TIA, or peripheral arterial disease. DR was defined as an ETDRS level of ≥20, i.e., the presence of microaneurysms alone or with any of the following lesions: hemorrhages, cotton wool spots, intraretinal microvascular abnormalities, hard exudates, venous beading, venous loops and/or reduplication, fibrous proliferations, preretinal hemorrhage, vitreous hemorrhage, and new vessels. This was further divided into mild nonproliferative DR (NPDR) (levels 20–35), moderate-to-severe NPDR (levels 43–53), and proliferative DR (levels 61–81). Due to small numbers in the proliferative retinopathy group, severity of DR was collapsed into three categories (none, mild, and moderate–severe); the two most severe categories were combined into moderate–severe DR and used in all subsequent analyses. A measure of general cognitive ability, representing the variance common to all the cognitive tests except MHVS, was generated. This was done by subjecting the seven cognitive tests (FACES, LM, MR, LNS, DST, VFT, and TMTB) to a principal-components analysis. Scree slope analysis and the eigenvalues-greater-than-1 rule both indicated a single component (which accounted for 44% of the total variance, on which all tests had high loadings), thus validating the use of a general factor. Scores on this first unrotated principal component were saved as standardized scores (mean ± SD 0 ± 1).
All continuous variables were normally distributed except for the TMTB for which a natural logarithmic transformation of scores was used, as well as depression scores (normalized through a n + 1 logarithmic transformation), duration of diabetes, and alcohol intake (square root transformations).
Three categories of DR (none, mild, and moderate–severe) were compared on all demographic and medical variables using tests of linearity included in ANOVA for continuous data and χ2 analyses for categorical data. ANCOVA was used for comparing cognitive test scores in subjects according to severity of DR and in assessing the effects of retinopathy severity on the imputed cognitive change from estimated peak prior cognitive function (MHVS scores) at the same time as adjusting for possible confounders. Adjustment variables were introduced into the models in three cumulative steps. In the first step, age and sex were entered into all models. Then MHVS was entered to obtain an estimate of cognitive change. Finally, education level, vascular risk factors (alcohol intake, smoking status, WHR, systolic blood pressure, and total cholesterol), the presence of macrovascular disease, and depression symptoms were additionally adjusted. These variables were selected as possible confounders if they showed a significant association with DR on univariate analysis or if, in previous literature, a variable was reported to be associated with both DR and cognitive test performance. We did not include duration of diabetes or A1C in the main multivariate model because it is likely that prolonged exposure to hyperglycemia underlies the development of DR and their inclusion in the model could result in overadjustment. Interactions between DR and other covariates were also assessed. All tests were two tailed, and a two-sided P value ≤0.05 was taken to indicate statistical significance. η2 values were used to convey effect sizes. All analyses were performed using SPSS, version 14.0, for Windows (33).
RESULTS
Characteristics of the total ET2DS population (n = 1,066) and comparisons with nonparticipants from the target population have previously been presented (17). A total of 1,044 subjects had retinal photographs available for grading. These subjects did not differ significantly from subjects without photographs either available or suitable for grading (n = 22) with respect to diabetes duration or treatment, mean A1C, cardiovascular risk factors, or prevalence of macrovascular disease (data not shown). However, they did perform significantly better on tests of nonverbal memory (mean ± SD FACES scores 66.0 ± 7.8 vs. 58.3 ± 7.0; P < 0.001), verbal declarative memory (mean LM scores 25.4 ± 8.2 vs. 20.0 ± 5.9; P = 0.003), and general cognitive ability (mean g scores 0.00 ± 1.00 vs. −0.53 ± 0.77; P = 0.014).
Of the 1,044 type 2 diabetic participants, 339 (32.5%) subjects had any DR. Of these, 292 (86.1%) had mild NPDR, 32 (9.4%) had moderate-to-severe NPDR, and 15 (4.4%) had proliferative retinopathy (n = 19 and n = 8 for men in the latter two categories, respectively). A comparison of characteristics according to DR severity is given in Table 1. Subjects with DR were more likely to be receiving insulin treatment and had higher mean A1C, diabetes duration, and WHR. They also had a higher prevalence of previous stroke/TIA.
TABLE 1.
Characteristics of study participants according to severity of DR
| No DR | Mild DR | Moderate-to-severe DR | P for trend | |
|---|---|---|---|---|
| n | 705 | 292 | 47 | |
| Mean age (years) | 67.3 ± 4.2 | 67.4 ± 4.2 | 67.1 ± 4.2 | 0.962 |
| Male sex | 49.2 (347) | 55.1 (161) | 57.4 (27) | 0.061 |
| Education level | ||||
| University | 16 (113) | 16.1 (47) | 19.1 (9) | 0.312 |
| Other profession/technical qualification | 27.2 (192) | 32.9 (96) | 25.5 (12) | |
| Secondary/primary school | 56.7 (400) | 51.0 (149) | 55.3 (26) | |
| Diabetes-related variables | ||||
| Median diabetes duration (years) | 5.5 (3.4–9.4) | 9.3 (5.1–14.4) | 17.1 (12.1–22.9) | <0.001 |
| Treatment | ||||
| Diets | 23.8 (168) | 9.2 (27) | 2.1 (1) | <0.001 |
| Oral antidiabetes agents only | 65.7 (463) | 63.4 (185) | 38.3 (18) | |
| Insulin with or without oral antidiabetes agents | 10.5 (74) | 27.4 (80) | 59.6 (28) | |
| A1C (%) | 7.3 ± 1.1 | 7.5 ± 1.1 | 8.4 ± 1.4 | <0.001 |
| Vascular risk factors | ||||
| BMI (kg/m2) | 31.4 ± 5.5 | 31.3 ± 6.1 | 32.3 ± 5.3 | 0.521 |
| WHR | 0.96 ± 0.07 | 0.97 ± 0.08 | 0.99 ± 0.09 | 0.001 |
| Smoking status | ||||
| Never | 38.0 (268) | 39.4 (115) | 53.2 (25) | 0.112 |
| Former | 47.4 (334) | 47.6 (139) | 36.2 (17) | |
| Current | 14.6 (103) | 13.0 (38) | 10.6 (5) | |
| Median average alcohol intake (drinks/week) | 1 (0–7) | 0.6 (0–6.8) | 1 (0–3.8) | 0.291 |
| Systolic blood pressure (mmHg) | 133.3 ± 15.8 | 133.41 ± 7.4 | 132.8 ± 19.9 | 0.972 |
| Hypertension | 84.3 (594) | 84.6 (247) | 95.7 (45) | 0.152 |
| Total cholesterol (mmol/l) | 4.3 ± 0.9 | 4.2 ± 0.9 | 4.3 ± 1.0 | 0.201 |
| HDL cholesterol (mmol/l) | 1.30 ± 0.4 | 1.29 ± 0.4 | 1.17 ± 0.4 | 0.083 |
| Hypercholesterolemia | 89.9 (634) | 88.4 (258) | 100.0 (47) | 0.382 |
| Cardiovascular disease variables | ||||
| Myocardial infarction and/or angina | 30.2 (213) | 31.5 (92) | 44.7 (21) | 0.111 |
| Stroke and/or TIA | 6.2 (44) | 13.4 (39) | 14.9 (7) | <0.001 |
| Intermittent claudication | 5.5 (39) | 6.5 (19) | 8.5 (4) | 0.353 |
| Any macrovascular disease (myocardial infarction, angina, stroke, TIA, or IC) | 35.3 (249) | 38.4 (112) | 51.1 (24) | 0.046 |
Data are means ± SD, % (n), or median (interquartile range) unless otherwise indicated. TIA, transient ischemic attack. Bold values are statistically significant values (P ≤ 0.05). IC, intermittent claudication.
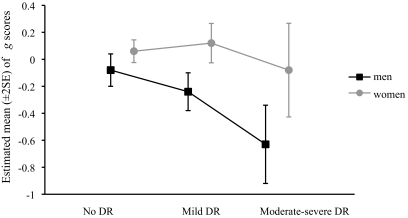
In the total study population, age- and sex-adjusted general cognitive ability (g) was significantly lower in the group with moderate-to-severe DR (mean −0.44 [95% CI −0.73 to −0.16]) compared with the group without DR (0.05 [−0.03 to 0.12]; P = 0.003), with an intermediate score for the mild DR group (−0.04 [−0.17 to 0.07]) (P for trend = 0.003). No significant associations were found between DR and estimated premobid cognitive ability (MHVS score). The association between DR and current general cognitive ability (g) remained statistically significant after adjustment for MHVS, but in this model there was a significant interaction between sex and retinopathy for g (P = 0.030). Men appeared to be more affected by severity of retinopathy than women (Fig. 1). The total study population was stratified by sex, and the effects of retinopathy on g and individual cognitive tests were examined separately for men and women (Table 2). Following age adjustment, significant associations were only found in men between DR and g, VFT and TMTB, and in both sexes with DST. When adjusted for MHVS to estimate lifetime change in cognitive ability, the retinopathy-DST association lost statistical significance in women but remained significant in men. When further adjusted for a wide range of potentially confounding variables, including macrovascular disease, associations in men persisted between DR and g (P for trend <0.001; η2 = 0.020), VFT (P for trend = 0.001; η2 = 0.020), TMTB (P for trend = 0.009; η2 = 0.012), and DST (P for trend = 0.001; η2 = 0.032) tests (assessing executive function, mental flexibility, and processing speed, respectively). No statistically significant differences across any of the retinopathy categories were found for the LM, MR, or LNS tests for either sex. There was not any significant interaction of retinopathy and any of the variables other than sex.
FIG. 1.
Estimated mean (±2 SE) of general factor (g) scores of men and women according to severity of DR.
TABLE 2.
Multivariable-adjusted mean (SE) of cognitive scores by severity of DR in men and women
| Men |
Women |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No DR | Mild DR | Moderate-to-severe DR | P for trend | Effect of DR* | No DR | Mild DR | Moderate-to-severe DR | P for trend | Effect of DR* | |
| n | 347 | 161 | 27 | — | 358 | 131 | 20 | — | ||
| Age adjusted | ||||||||||
| VFT | 38.0 (0.7) | 36.0 (1.0) | 31.5 (2.5) | 0.006 | 36.8 (0.7) | 36.6 (1.1) | 36.9 (2.9) | 0.965 | ||
| FACES | 65.0 (0.4) | 64.1 (0.6) | 62.4 (1.4) | 0.052 | 67.6 (0.4) | 67.5 (0.7) | 64.8 (1.8) | 0.277 | ||
| Ln (TMTB) | 4.68 (0.02) | 4.70 (0.03) | 4.94 (0.08) | 0.013 | 4.65 (0.02) | 4.68 (0.04) | 4.82 (0.09) | 0.126 | ||
| DST | 48.2 (0.7) | 45.8 (1.1) | 36.8 (2.7) | <0.001 | 52.4 (0.8) | 51.1 (1.3) | 44.3 (3.3) | 0.037 | ||
| LM | 24.8 (0.4) | 24.2 (0.6) | 23.8 (1.5) | 0.400 | 26.1 (0.4) | 26.8 (0.7) | 23.2 (1.9) | 0.711 | ||
| MR | 13.4 (0.3) | 14.0 (0.4) | 12.2 (1.0) | 0.971 | 12.0 (0.3) | 12.3 (0.5) | 14.3 (1.2) | 0.106 | ||
| LNS | 9.8 (0.2) | 9.8 (0.2) | 9.0 (0.6) | 0.473 | 9.6 (0.1) | 9.6 (0.2) | 9.2 (0.6) | 0.599 | ||
| g | 0.01 (0.05) | −0.10 (0.08) | −0.63 (0.19) | 0.003 | 0.08 (0.05) | 0.03 (0.09) | −0.24 (0.23) | 0.222 | ||
| MHVS | 31.1 (0.3) | 32.0 (0.4) | 31.2 (1.0) | 0.245 | 30.7 (0.3) | 30.3 (0.4) | 29.0 (1.1) | 0.138 | ||
| Age and MHVS adjusted | ||||||||||
| VFT | 38.4 (0.6) | 35.5 (0.9) | 31.7 (2.3) | <0.001 | 36.6 (0.6) | 36.9 (1.0) | 38.7 (2.5) | 0.533 | ||
| FACES | 65.1 (0.4) | 63.9 (0.6) | 62.4 (1.4) | 0.016 | 67.5 (0.4) | 67.6 (0.7) | 65.6 (1.7) | 0.512 | ||
| Ln (TMTB) | 4.67 (0.02) | 4.71 (0.03) | 4.93 (0.07) | 0.002 | 4.65 (0.02) | 4.67 (0.03) | 4.77 (0.08) | 0.268 | ||
| DST | 48.5 (0.7) | 45.4 (1.0 | 37.0 (2.4) | <0.001 | 52.4 (0.7) | 51.3 (1.2) | 46.1 (3.0) | 0.072 | ||
| LM | 24.9 (0.4) | 23.8 (0.6) | 23.9 (1.4) | 0.148 | 26.0 (0.4) | 27.0 (0.7) | 24.4 (1.7) | 0.723 | ||
| MR | 13.5 (0.2) | 13.7 (0.4) | 12.3 (0.9) | 0.584 | 12.0 (0.3) | 12.3 (0.4) | 14.9 (1.0) | 0.052 | ||
| LNS | 9.9 (0.1) | 9.7 (0.2) | 9.1 (0.5) | 0.185 | 9.6 (0.1) | 9.7 (0.2) | 9.5 (0.5) | 0.934 | ||
| g | 0.04 (0.04) | −0.15 (0.06) | −0.61 (0.15) | <0.001 | 0.05 (0.04) | 0.07 (0.07) | −0.05 (0.18) | 0.828 | ||
| Fully adjusted† | ||||||||||
| VFT | 38.4 (0.7) | 35.5 (1.0) | 32.0 (2.4) | 0.001 | 0.020 | 37.0 (0.7) | 37.3 (1.0) | 39.8 (2.6) | 0.417 | 0.001 |
| FACES | 65.1 (0.4) | 64.1 (0.6) | 63.2 (1.5) | 0.062 | 0.006 | 67.6 (0.4) | 67.8 (0.7) | 66.2 (1.7) | 0.811 | 0.000 |
| Ln (TMTB) | 4.67 (0.02) | 4.71 (0.03) | 4.91 (0.08) | 0.009 | 0.012 | 4.66 (0.02) | 4.67 (0.04) | 4.74 (0.09) | 0.364 | 0.002 |
| DST | 49.0 (0.7) | 45.6 (1.0) | 38.8 (2.6) | 0.001 | 0.032 | 52.4 (0.8) | 50.8 (1.2) | 46.6 (3.1) | 0.060 | 0.006 |
| LM | 24.7 (0.5) | 23.8 (0.6) | 24.0 (1.5) | 0.236 | 0.002 | 26.1 (0.5) | 26.9 (0.7) | 24.5 (1.7) | 0.857 | 0.000 |
| MR | 13.5 (0.3) | 13.8 (0.4) | 12.6 (0.9) | 0.857 | 0.000 | 12.1 (0.3) | 12.5 (0.5) | 14.7 (1.1) | 0.056 | 0.006 |
| LNS | 9.8 (0.2) | 9.7 (0.2) | 9.5 (0.5) | 0.481 | 0.001 | 9.6 (0.1) | 9.7 (0.3) | 9.6 (0.6) | 0.907 | 0.000 |
| g | 0.04 (0.05) | −0.14 (0.07) | −0.49 (0.15) | <0.001 | 0.020 | 0.07 (0.05) | 0.08 (0.07) | 0.02 (0.18) | 0.919 | 0.000 |
†Further adjusted for education, alcohol intake, smoking status, WHR, systolic blood pressure, total cholesterol, major macrovascular disease, and depression symptoms.
*Effect size of DR as indexed by η2 (the proportion of variance) in the fully adjusted model. Bold values are statistically significant values (P ≤ 0.05).
Analyses were repeated after excluding individuals with prevalent stroke or history of TIA and those with MMSE scores <24. None of the associations reported were essentially altered by these exclusions (data not shown). In a final analysis, associations were also adjusted for A1C and duration of diabetes. Adjustment for both attenuated the association between DR and TMTB to borderline significance (P = 0.059) in men but did not change other results.
DISCUSSION
In this representative population of older people with type 2 diabetes, increasing severity of DR was associated with poorer general cognitive ability. When analyzed separately by sex, a significant dose-response relationship was found only in men and for individual tests of verbal fluency, information processing speed, and mental flexibility (but not memory and nonverbal reasoning). These associations persisted after adjustment for estimated premorbid cognitive ability (vocabulary scores), suggesting that in men, DR was not only associated with cognitive ability in later life but also with increased estimated lifetime cognitive decline. To estimate cognitive decline, it was not possible to adjust for the same cognitive test applied at an earlier age, but all the nonvocabulary tests were significantly correlated and vocabulary scores were strongly loaded on general cognitive ability (coefficient 0.57; P < 0.001). After additional control for education, vascular risk factors, macrovascular disease, and mood, associations between DR and estimated cognitive decline remained significant, suggesting that they were not simply due to confounding by other vascular mechanisms.
To our knowledge, no previous studies have specifically examined the relationship between DR and cognitive functioning in a large group of older people with type 2 diabetes from the general population using comprehensive and detailed assessment of retinal signs. Two previous studies did not show an association between DR and cognitive function in older diabetic patients (34,35), but these were based on either a direct ophthalmoscopic examination of the retina or a single-field retinal photograph. These methods image only a small portion of the retina and with considerably less rigor compared with multiple-field photographs and as a result, the prevalence of DR signs may have been underestimated. Our findings are consistent with those from studies of predominantly nondiabetic older subjects from the general population. In the Blue Mountains Eye Study (14), subjects with hypertension aged 49 years and older who had evidence of retinopathy signs were significantly more likely to score poorly (<23) on the MMSE, and in the Cardiovascular Health Study there was a significant association between retinopathy and worse performance on information-processing speed in subjects aged >69 years (36). The Atherosclerosis Risk in Communities Study (ARIC) also reported that retinopathy was independently associated with poorer cognitive function in middle-aged people free of stroke (37), both in those with and in those without diabetes and hypertension. Longitudinal findings from the 14-year follow-up of the same cohort showed that people with retinopathy had a greater 10-year decline in verbal fluency and information-processing speed (but not in delayed verbal memory) compared with those without retinal microvascular abnormalities (15).
Due to the considerable homology between retinal and cerebral microvasculature, retinal vascular changes are likely to provide an indirect marker of concomitant changes in the brain microvasculature. It is possible that accelerated cognitive aging associated with type 2 diabetes may arise, at least in part, from the cumulative impact of a disruption of blood-brain barrier and/or the ischemic injuries leading to diverse changes in brain parenchyma. As our findings persisted after subjects with recognized clinical stroke were excluded, perivascular brain damage and/or ischemia from asymptomatic cerebral small vessel disease (SVD) could explain the cognitive associations. For example, microinfarcts and MRI signs of cerebral SVD (e.g., lacunar infarcts and white matter lesions) have been shown to contribute importantly to cognitive impairment and vascular dementia (38–41). Furthermore, increased blood-brain barrier permeability has been demonstrated in people with type 2 diabetes (4), and alteration in the blood-brain barrier secondary to microvascular endothelial dysfunction may be an important pathophysiological mechanism in the initiation or worsening of cerebral SVD (42). Cerebral SVD predominantly affects the subcortical associative areas of deep gray matter (the basal ganglia and thalamus) and white matter structures, with disruption of integrity of frontal subcortical circuits. This may lead to deficits in executive functioning and information processing. Our results in type 2 diabetes are consistent with a cognitive profile that is associated with cerebral SVD.
A striking finding of the present study was a significant interaction of DR with sex such that the negative associations of DR with several cognitive measures were statistically significant only in men. This difference was not anticipated a priori. To our knowledge, no previous study has examined whether sex modifies the relation of retinopathy with cognition. The sex-specific effect of DR on cognitive functioning could be influenced by lower prevalence of DR (29.7 vs. 35.1%; P = 0.058), coronary heart disease (24.4 vs. 37.8%; P < 0.001); and stroke and/or TIA (5.5 vs. 11.6%; P < 0.001) in women compared with men, but adjusting for macrovascular disease did not alter the sex-specific effects. It is also possible that other unknown risk factors that were not examined in this analysis (e.g., subclinical atherosclerosis, estrogen use, or physical activity) could play a mediating role. Although several hypotheses have been proposed in an attempt to explain sex difference observed in some studies, including male-female differences in brain reserve and differential susceptibility to normal or pathological age-associated changes, there is no clear empirical evidence on the effect of sex on cognition in the general population or in people with diabetes.
Despite the representativeness of our study participants to the target population in terms of demographic and clinical characteristics, the possibility of participation and/or survival bias cannot be completely ruled out. Indeed, it has been hypothesized that diabetes may be a more potent risk factor for cognitive dysfunction in women than in men due to a higher risk of diabetes-related macrovascular disease and early loss of possible protective effects of estrogen (due to earlier menopause) on cognitive functioning (43). It is possible that women with more severe diabetes (those more likely to have retinopathy and cognitive decline) may have died of macrovascular disease before they could be recruited into the study, thereby leading to conservative estimates of associations between retinopathy and cognitive decline. However, the degree of variation of g scores in men and women in the study population was similar and, as shown in Table 1, people with any retinopathy were only slightly more likely to be male. It therefore seems unlikely that survival bias could be the full explanation for the sex difference. Given that we had no specific a priori reason for assuming that our association of DR with cognition would be different between males and females, and given that other studies have not suggested the existence of such a sex difference, interpretation of the interaction between sex and DR must be treated with caution until it has been replicated in other large studies.
One of the main strengths of this study was the use of high-quality retinal photographs and detailed grading of DR using full seven-field photography and the low amount of missing retinal grading data. Other strengths included the application of a detailed cognitive test battery covering the major cognitive domains, although use of a single test to assess each particular cognitive domain could mean that only limited aspects of what may be considered complex mental functions were actually determined (20). The study population had a verified clinical diagnosis of type 2 diabetes and has been shown to be representative of the target population of elderly men and women with the full range of severity of type 2 diabetes living in the general population.
The use of a general cognitive factor, g, helped avoid potential problems caused by multiple testing and residual confounding by peak prior mental ability was minimized by controlling for performance on a test of vocabulary rather than relying solely on level of education as in many previous studies. Extensive phenotyping for potential confounding factors also enabled a comprehensive multivariate analysis. However, the presence of microvascular complications other than retinopathy in the no DR group (e.g., neuropathy and nephropathy), leading to shared microvascular risk burden between the retinopathy and retinopathy-free subjects, may have tended to reduce differences toward the null.
The effect size for retinopathy in these older men with type 2 diabetes was small after multivariate adjustment. Such a small effect size for risk factors associated with cognitive decline is not uncommon, including the effects of other potentially important factors (e.g., apolipoprotein E genotype and smoking) (44,45). It is possible that even very modest effects of a risk factor may lead to larger cognitive deficits and possibly dementia with further aging. Furthermore, reduction of such a risk factor could have a significant impact on cognition in the diabetic population as a whole by producing a positive shift in the overall population distribution of cognitive ability. However, while our findings support the hypothesis that reduction of microvascular disease might reduce the rate of cognitive decline, the clinical significance of our findings remains to be established. The major limitation of the study is that analyses were cross-sectional, with measures of retinal and cognitive function made almost simultaneously, limiting our ability to determine whether DR preceded or occurred in parallel with cognitive decline. Prospective studies are required to clarify the temporal sequence of these associations. Such a follow-up project involving the present study population for the actual cognitive change is underway.
In conclusion, DR was independently associated with estimated lifetime cognitive decline in older men with type 2 diabetes, supporting the hypothesis that cerebral microvascular disease may contribute to the accelerated age-related cognitive decline observed in diabetic populations. If the above findings are substantiated, diabetes-associated cognitive dysfunction may be amenable to therapeutic and preventive strategies that are targeted specifically at protecting the cerebral microvasculature and reducing the risk of developing even mild microvascular disease in an aging diabetic population.
Acknowledgments
This study was supported by a grant from the UK Medical Research Council. J.F.P., I.J.D., and B.M.F. are members of the University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative, which is funded by the Biotechnology of Biological Sciences Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, and Medical Research Council.
No potential conflicts of interest relevant to this article were reported.
J.D. researched data, contributed to discussion, and wrote the manuscript. M.W.J.S. researched data, contributed to discussion, and reviewed and edited the manuscript. R.M.R. contributed to discussion and reviewed and edited the manuscript. B.M.F. contributed to discussion and reviewed and edited the manuscript. I.J.D. researched data, contributed to discussion, and reviewed and edited the manuscript. F.G.R.F. contributed to discussion and reviewed and edited the manuscript. A.J.L. contributed to discussion and reviewed and edited the manuscript. J.M. researched data, contributed to discussion, and reviewed and edited the manuscript. P.H. researched data, contributed to discussion, and reviewed and edited the manuscript. K.S. researched data, contributed to discussion, and reviewed and edited the manuscript. J.F.P. researched data, contributed to discussion, and reviewed and edited the manuscript.
Parts of this study were presented in abstract form at the Diabetes U.K. Annual Professional Conference, Liverpool, U.K., 4–6 March 2010.
We thank staff and participants of the ET2DS and staff at the Wellcome Trust Clinical Research Facility and the Princess Alexandra Eye Pavillion in Edinburgh, where the clinical examinations were performed.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Cukierman T, Gerstein HAC, Williamson JD. Cognitive decline and dementia in diabetes-systematic overview of prospective observational studies. Diabetologia 2005;48:2460–2469 [DOI] [PubMed] [Google Scholar]
- 2.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64–74 [DOI] [PubMed] [Google Scholar]
- 3.Sander D, Sander K, Poppert H. Stroke in type 2 diabetes. Br J Diabetes Vasc Dis 2008;8:222–229 [Google Scholar]
- 4.Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood-brain barrier permeability in type 2 diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry 2003;74:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manschot SM, Brands AM, van der Grond J, Kessels RP, Algra A, Kappelle LJ, Biessels GJ. Brian magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes 2006;55:1106–1113 [DOI] [PubMed] [Google Scholar]
- 6.van Harten B, Oosterman J, Muslimovic D, van Loon BJ, Scheltens P, Weinstein HC. Cognitive impairment and MRI correlates in the elderly patients with type 2 diabetes mellitus. Age Ageing 2007;36:164–170 [DOI] [PubMed] [Google Scholar]
- 7.Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease : a rationale based on homology between cerebral and retinal microvasculatures. J Anat 2005;206:319–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwa VI, van der Sande JJ, Stam J, Tijmes N, Vrooland JL. Retinal arterial changes correlate with cerebral small-vessel disease. Neurology 2002;59:1536–1540 [DOI] [PubMed] [Google Scholar]
- 9.Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, Liao DP, Hubbard LD, Mosley TH. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA 2002;288:67–74 [DOI] [PubMed] [Google Scholar]
- 10.Cooper LS, Wong TY, Klein R, Sharrett AR, Bryan RN, Hubbard LD, Couper DJ, Heiss G, Sorlie PD. Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: the Atherosclerosis Risk in Communities Study. Stroke 2006;37:82–86 [DOI] [PubMed] [Google Scholar]
- 11.Cheung N, Wong TY. Diabetic retinopathy and systemic vascular complications. Prog Retin Eye Res 2008;27:161–176 [DOI] [PubMed] [Google Scholar]
- 12.Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJ, Wardlaw J, Deary IJ, Frier BM. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes 2003;52:149–156 [DOI] [PubMed] [Google Scholar]
- 13.Ding J, Patton N, Deary IJ, Strachan MW, Fowkes FG, Mitchell RJ, Price JF. Retinal microvascular abnormalities and cognitive dysfunction: a systematic review. Br J Ophthalmol 2008;92:1017–1025 [DOI] [PubMed] [Google Scholar]
- 14.Liew G, Mitchell P, Wong TY, Lindley RI, Cheung N, Kaushik S, Wang JJ. Retinal microvascular signs and cognitive impairment. J Am Geriatr Soc 2009;57:1892–1896 [DOI] [PubMed] [Google Scholar]
- 15.Lesage SR, Mosley TH, Wong TY, Szklo M, Knopman D, Catellier DJ, Cole SR, Klein R, Coresh J, Coker LH, Sharrett AR. Retinal microvascular abnormalities and cognitive decline: the ARIC 14-year follow-up study. Neurology 2009;73:862–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price JF, Reynolds RM, Mitchell RJ, Williamson RM, Fowkes FG, Deary IJ, Lee AJ, Frier BM, Hayes PC, Strachan MW. The Edinburgh Type 2 Diabetes Study: study protocol. BMC Endocr Disord 2008;8:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marioni RE, Strachan MW, Reynolds RM, Lowe GD, Mitchell RJ, Fowkes FG, Frier BM, Lee AJ, Butcher I, Rumley A, Murray GD, Deary IJ, Price JF. Association between raised inflammatory markers and cognitive decline in elderly people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes 2010;59:710–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wechsler D. Manual of the Wechsler Memory Scale—Revised. New York, Psychological Corporation, 1987 [Google Scholar]
- 19.Wechsler D. Wechsler Adult Intelligence Scale (UK). 3rd ed London, Psychological Corporation, 1998 [Google Scholar]
- 20.Lezak M. Neuropsychological Assessment. 3rd ed Oxford, Oxford University Press, 1995 [Google Scholar]
- 21.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York, Oxford University Press, 1991 [Google Scholar]
- 22.Raven J, Raven JC, Court JH. Manual for Raven's Progressive Matrices and Vocabulary Scales. Oxford, U.K., Oxford Psychologists Press, 1998 [Google Scholar]
- 23.Salthouse TA. Localizing age-related individual differences in a hierarchical structure. Intelligence 2004;32:541–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deary IJ, Whalley LJ, Crawford JR. An ‘instantaneous’ estimate of a lifetime's cognitive change. Intelligence 2004;32:113–119 [Google Scholar]
- 25.Crawford JR, Deary IJ, Starr J, Whalley LJ. The NART as an index of prior intellectual functioning: a retrospective validity study covering a 66-year interval. Psychol Med 2001;31:451–458 [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE. Mini Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiat Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 27.Zigmond AS, Snaith RP. The hospital anxiety and depression scales. Acta Psychiatr Scand 1983;67:361–370 [DOI] [PubMed] [Google Scholar]
- 28.Rose G, McCartney P, Reid DD. Self-administration of a questionnaire on chest pain and intermittent claudication. Br J Prev Soc Med 1977;31:42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leng GC, Fowkes FG: The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol 1992;45:1101–1109 [DOI] [PubMed] [Google Scholar]
- 30.Early Treatment Diabetic Retinopathy Study Research Group: Grading diabetic retinopathy fromstereoscopic color fundus photographs: an extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology 1991;98:786–806 [PubMed] [Google Scholar]
- 31.Early Treatment Diabetic Retinopathy Study Research Group: Fundus photographic risk factors for progression of diabetic retinopathy: ETDRS report number 12. Ophthalmology 1991;98:823–833 [PubMed] [Google Scholar]
- 32.Dawson D. Methodological issues in measuring alcohol use. Alcohol Res Health 2003;27:18–29 [PMC free article] [PubMed] [Google Scholar]
- 33.SPSS for Windows. Chicago, IL, SPSS Inc., 2004 [Google Scholar]
- 34.Manschot SM, Biessels GJ, de Valk H, Algra A, Rutten GE, van der Grond J, Kappelle LJ. Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia 2007;50:2388–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umegaki H, Iimuro S, Kaneko T, Araki A, Sakurai T, Ohashi Y, Iguchi A, Ito H. Factors associated with lower Mini Mental State Examination scores in elderly Japanese diabetes mellitus patients. Neurobiol Aging 2008;29:1022–1026 [DOI] [PubMed] [Google Scholar]
- 36.Baker ML, Marino Larsen EK, Kuller LH, Klein R, Klein BE, Siscovick DS, Bernick C, Manolio TA, Wong TY. Retinal microvascular signs, cognitive function, and dementia in older persons. Stroke 2007;38:2041–2047 [DOI] [PubMed] [Google Scholar]
- 37.Wong TY, Klein R, Sharrett AR, Nieto FJ, Boland LL, Couper DJ, Mosley TH, Klein BE, Hubbard LD, Szklo M. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons: the Atherosclerosis Risk in Communities Study. Stroke 2002;33:1487–1492 [DOI] [PubMed] [Google Scholar]
- 38.White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, Masaki K, Launer L, Markesbery WR. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci 2002;977:9–23 [DOI] [PubMed] [Google Scholar]
- 39.Vermeer SE, Prins ND, Den HT, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–1222 [DOI] [PubMed] [Google Scholar]
- 40.Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, Koudstaal PJ, Hofman A, Breteler MM. Cerebral small vessel disease and decline in information processing speed, executive function and memory. Brain 2005;128:2034–2041 [DOI] [PubMed] [Google Scholar]
- 41.van der Flier WM, van Straaten EC, Barkhof F, Verdelho A, Madureira S, Pantoni L, Inzitari D, Erkinjuntti T, Crisby M, Waldemar G, Schmidt R, Fazekas F, Scheltens P. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke 2005;36:2116–2120 [DOI] [PubMed] [Google Scholar]
- 42.Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease-systematic review and meta-analysis. Neurobiol Aging 2009;30:337–352 [DOI] [PubMed] [Google Scholar]
- 43.Coker LH, Shumaker SA. Type 2 diabetes mellitus and cognition: an understudied issue in women's health. Psychosom Res 2003;54:129–139 [DOI] [PubMed] [Google Scholar]
- 44.Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, Carothers A, Whalley LJ: Cognitive change and the APOE epsilon 4 allele. Nature 2002;418:932. [DOI] [PubMed] [Google Scholar]
- 45.Stewart MC, Deary IJ, Fowkes FG, Price JF. Relationship between lifetime smoking, smoking status at older age and human cognitive function. Neuroepidemiology 2006;26:83–92 [DOI] [PubMed] [Google Scholar]