Abstract
OBJECTIVE
Chemerin is a recently discovered hepatoadipokine that regulates adipocyte differentiation as well as chemotaxis and activation of dendritic cells and macrophages. Chemerin was reported to modulate insulin sensitivity in adipocytes and skeletal muscle cells in vitro and to exacerbate glucose intolerance in several mouse models in vivo. In humans, chemerin was shown to be associated with multiple components of the metabolic syndrome including BMI, triglycerides, HDL cholesterol, and hypertension. This study aimed to examine the effect of chemerin on weight, glucose and lipid metabolism, as well as atherosclerosis in vivo.
RESEARCH DESIGN AND METHODS
We used recombinant adeno-associated virus to express human chemerin in LDL receptor knockout mice on high-fat diet.
RESULTS
Expression of chemerin did not significantly alter weight, lipid levels, and extent of atherosclerosis. Chemerin, however, significantly increased glucose levels during the intraperitoneal glucose tolerance test without affecting endogenous insulin levels and the insulin tolerance test. Chemerin reduced insulin-stimulated Akt1 phosphorylation and activation of 5′AMP-activated protein kinase (AMPK) in the skeletal muscle, but had no effect on Akt phosphorylation and insulin-stimulated AMPK activation in the liver and gonadal adipose tissue.
CONCLUSIONS
Chemerin induces insulin resistance in the skeletal muscle in vivo. Chemerin is involved in the cross talk between liver, adipose tissue, and skeletal muscle.
Chemerin was initially described in 2003 as a novel chemoattractant protein (1) that, upon enzymatic proteolysis, modulates chemotaxis and activation of dendritic cells and macrophages through distinct G protein-coupled receptors such as CMKLR1, GPR1, and CCRL2 (2–4). More recently, chemerin was reported as a hepatoadipokine that regulates adipocyte differentiation in an autocrine/paracrine manner via CMKLR1 and modulates the expression of adipocyte genes involved in glucose and lipid homeostasis (5,6). Conflicting data exist regarding the effect of chemerin on insulin signaling in adipocytes in vitro. Kralisch et al. (7) demonstrated that chemerin downregulated insulin-stimulated glucose uptake in 3T3-L1 adipocytes, while Takahasi et al. (8) reported the opposite. In skeletal muscle cells, chemerin was shown to impair insulin signaling and glucose uptake, a process partially mediated by activation of the extracellular signal–regulated kinases-1/2 (9). In humans, chemerin correlated with systemic markers of inflammation such as high-sensitivity C-reactive protein, interleukin-6, and tumor necrosis factor-α and was associated with components of the metabolic syndrome including BMI, triglycerides, HDL cholesterol, and hypertension in different populations (5,10–13). In morbidly obese patients undergoing bariatric surgery, weight loss was associated with improvement of various metabolic parameters and a sustained reduction of chemerin levels (14,15). Taken together, available data suggest that chemerin may play a role in glucose and lipid metabolism as well as inflammation. Surprisingly little data, however, are currently available about the potential effects of chemerin on the regulation of these parameters in vivo.
Recently, Ernst et al. (16) reported that acute administration of recombinant human chemerin exacerbated glucose intolerance, lowered serum insulin levels, and decreased tissue glucose uptake in ob/ob, db/db, and diet-induced obese (DIO) but not in normoglycemic wild-type mice. As acute injection of chemerin may not reflect the effect of chronically elevated chemerin levels seen in obesity, we designed the present study to investigate the impact of long-term chemerin overexpression on body weight, glucose and lipid metabolism, as well as atherosclerosis in LDL receptor knockout (LDLRKO) mice on a high-fat diet (HFD). As human chemerin fully binds to and activates murine CMKLR1 (17), we expressed human chemerin using a recombinant adeno-associated virus (AAV)-based vector in this well-established mouse model of diet-induced insulin resistance, dyslipidemia, and atherosclerosis.
RESEARCH DESIGN AND METHODS
Generation of recombinant adeno-associated viral constructs.
All constructs were expressed under control of the thyroxine-binding globulin promoter (18,19). Transgenes were full-length human chemerin (preprochemerin, aa 1–163) and β-galactosidase (LacZ) as control. To produce AAV vectors encapsidated in an AAV8 capsid (AAV2.8), a pseudotyping strategy was performed. Vectors (AAV.chemerin, AAV.lacZ) were purified using a standard cesium sedimentation method, and titers were determined via TaqMan analysis using probes and primers targeting the bovine GH poly(A+) region of the vectors (18,19).
Animal studies.
Male LDLRKO (B6.12957-LDLrtm1Her/J) mice were obtained from Charles River Laboratories (Sulzfeld, Germany). Mice (5 weeks of age) were injected intravenously via the tail vein with 1 × 1013 particles of AAV.chemerin or AAV.lacZ. LDLRKO mice (n = 13 per group) were kept on a HFD (0.21% cholesterol, 21% butterfat; ssniff, Soest, Germany) for 16 weeks. After 12 h of overnight fasting, blood was drawn before and several time points after virus injection. The study was approved by the District Government of Upper Bavaria.
The intraperitoneal glucose tolerance test (ipGTT) and the insulin tolerance test (ITT) were performed 12 and 14 weeks after AAV injection, respectively (detailed description in the supplement available online at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0362/DC1).
At the end of the study (16 weeks after injection), mice (n = 12 per group; one death per group during the study) were injected intraperitoneally with insulin (n = 6 per group) (0.02 units/g body weight) or saline (n = 6) to determine protein levels and phosphorylation of Akt1 and 5′AMP-activated protein kinase (AMPK)-α. Mice were killed 15 min after injection and organs were harvested. Liver, skeletal muscle, and gonadal adipose tissue were freeze-clamped in liquid nitrogen in situ and stored at −80°C for further analysis. Aortas of LDLRKO mice were harvested for en face quantification of atherosclerotic lesions.
Blood sample assays.
Human and murine serum chemerin levels were determined using commercially available enzyme-linked immunosorbent assays (ELISAs) (R&D Systems, Wiesbaden, Germany). Plasma insulin levels were measured with an ultrasensitive insulin immunoassay (Alpco, Salem, MA). Serum total cholesterol, triglyceride, and HDL cholesterol levels were measured enzymatically on an Alcyon 300 analyzer (Abbott GmbH, Wiesbaden, Germany) using commercial reagents (Diasys, Holzheim, Germany).
Analysis of mRNA expression.
Total RNA was extracted as previously described (19). Real-time PCR was performed using the Realplex4 Mastercycler (Eppendorf, Hamburg, Germany). Expression levels of genes of interest were determined using the Assays-On-Demand TaqMan primers and probes (Applied Biosystems, Carlsbad, CA) according to the manufacturer's instructions (supplemental data in the online appendix).
Chemerin expression.
One μl of murine and human serum samples were subjected to 10% BisTris gel and transferred to a polyvinylidene fluoride (PVDF) membrane (Immobilon-P, Zefa-Laborservice, Germany). Human chemerin was detected with goat anti-human chemerin antibody (concentration 1:1,000; R&D Systems, Wiesbaden, Germany) and peroxidase-conjugated AffiniPureRabbit anti-goat IgG (H+L) (concentration 1:10.000; Jackson ImmunoResearch, Baltimore, MD) as the secondary antibody.
Insulin signaling and activation of AMPK-α.
Liver, skeletal muscle, and adipose tissue samples were homogenized in radioimmunoprecipitation assay-buffer (50 mmol/l Tris-HCl, pH 7.4; 1% NP-40; 0,25% Na-deoxycholate; 150 mmol/l NaCl; 1 mmol/l EDTA; 1 mmol/l phenylmethylsulfonyl fluoride; 1 μg/ml of each aprotinin, leupeptin, pepstatin; 1 mmol/l Na3VO4; 1 mmol/l NaF), and lysates were subjected to 10% BisTris gel and transferred to PVDF membrane (Immobilon-P; Zefa-Laborservice, Germany). Anti Akt1 (C73H10) (concentration 1:1,000), phospho-Akt (Thr308) (C31E5) (concentration 1:1,000), AMPK-α (23A3) (concentration 1:1,000), phospho-AMPK-α (Thr172) (D79.5E) (concentration 1:1,000), and anti-rabbit IgG horseradish peroxidase-linked antibodies (concentration 1:10.000) were obtained from Cell Signaling (Danvers, MA). Anti-actin antibody was purchased from Sigma (Sigma, St. Louis, MO).
Histological analysis.
For the quantification of the aortic plaques, the mouse aorta was carefully harvested and stained with Sudan IV (Sigma, Germany) as previously described (18,19). The extent of the lesion area was determined using Nikon Nis Elements D3.0 (Nikon, Düsseldorf, Germany). Data are reported as the percentage of the aortic surface covered by lesions (total surface area of the atherosclerotic lesions divided by the total surface area of the aorta).
Statistical analysis.
Data are expressed as the mean ± SEM. Analysis of the data was performed using the unpaired Student t test. Statistical significance for all comparisons was assigned at P < 0.05.
RESULTS
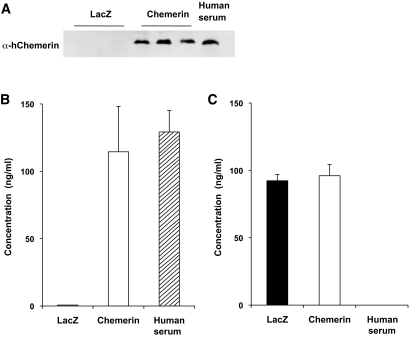
To examine the effect of chemerin expression on body weight, glucose and lipid metabolism, as well as atherosclerosis in vivo, LDLRKO mice on HFD were injected with 1 × 1013 particles of AAV.chemerin or control (AAV.lacZ). Injection of AAV.chemerin resulted in specific and sustained chemerin expression over the course of the study with serum levels comparable with those seen in healthy human volunteers (Fig. 1A and B). Expression of human chemerin did not alter murine chemerin serum levels (Fig. 1C).
FIG. 1.
A: Western blot analysis of chemerin expression in serum samples of LDLRKO mice 16 weeks after injection with 1 × 1013 particles of AAV.lacZ or AAV.chemerin and a healthy human volunteer. B: Expression of chemerin in LDLRKO mice 16 weeks after injection with 1 × 1013 particles of AAV.lacZ or AAV.chemerin (n = 12 per group) and in 3 healthy human volunteers as assessed by ELISA. C: Murine chemerin levels in LDLRKO mice 16 weeks after injection with 1 × 1013 particles of AAV.lacZ or AAV.chemerin (n = 12 per group) as assessed by ELISA.
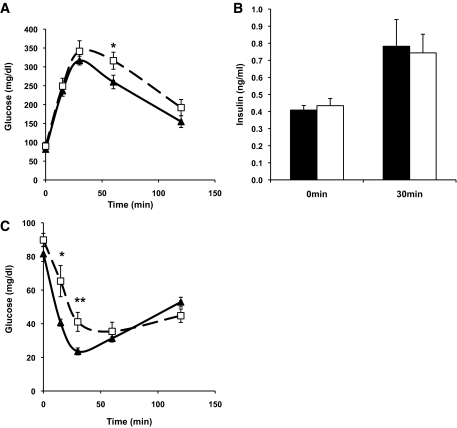
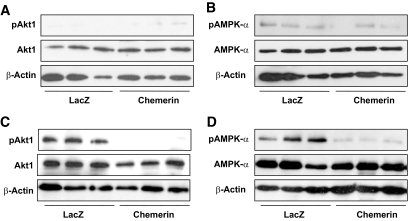
Mice injected with AAV.chemerin or AAV.lacZ had a similar change in body weight over the 16-week study period (supplemental Fig. 1, found in the online appendix). Compared with control mice, chemerin-expressing mice showed significantly increased glucose levels during both the ipGTT (without affecting endogenous insulin levels) and the ITT (Fig. 2), indicating that overexpression of human chemerin induces insulin resistance in LDLRKO mice. To determine whether expression of chemerin resulted in a tissue-specific pattern of insulin resistance, insulin signaling was examined in liver, adipose tissue, and skeletal muscle. Expression of chemerin substantially reduced insulin-stimulated Akt1 phosphorylation and AMPK-α activation in the skeletal muscle (Fig. 3). In contrast, mice injected with AAV.chemerin showed no effect on basal and insulin-stimulated hepatic Akt1 phosphorylation, AMPK-α activation, and the mRNA expression of the gluconeogenic enzymes G6P and PEPCK (supplemental Fig. 2, found in the online appendix). In gonadal adipose tissue, expression of chemerin slightly decreased phospho-AMPK-α levels in the absence of insulin but did not alter insulin-stimulated AMPK-α activation and Akt1 signaling (supplemental Fig. 3, found in the online appendix). In conclusion, expression of chemerin induced glucose intolerance via induction of insulin resistance in the skeletal muscle.
FIG. 2.
Effect of chemerin on glucose (A) and insulin (B) levels during the ipGTT in LDLRKO mice 12 weeks after injection with AAV.lacZ (black triangle) or AAV.chemerin (white square) (n = 10 per group). Effect of chemerin on glucose levels during the ITT (C) in LDLRKO mice 14 weeks after injection with AAV.lacZ (black triangle) or AAV.chemerin (white square) (n = 10 per group). *P < 0.05, **P = 0.01.
FIG. 3.
Effect of chemerin on basal and insulin-stimulated Akt1 phosphorylation (pAkt1) (A, C) and AMPK-α phosphorylation (pAMPK-α) (B, D) in the skeletal muscle of LDLRKO mice injected with AAV.lacZ or AAV.chemerin (data are representative for n = 6 per group).
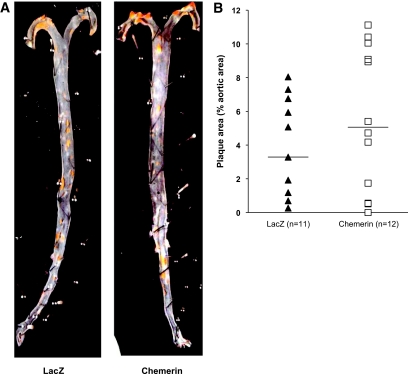
Mice-expressing chemerin did not show significantly altered total cholesterol, triglyceride, HDL cholesterol, and non-HDL cholesterol levels compared with controls (supplemental Fig. 4, found in the online appendix). Likewise, expression of chemerin did not change the mean atherosclerotic lesion area as determined by en face analysis of the entire aorta (Fig. 4).
FIG. 4.
Effect of chemerin on mean atherosclerotic lesion area as determined by en face analysis of the entire aorta in LDLRKO mice injected with AAV.lacZ (n = 11) or AAV.chemerin (n = 12). (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
Our study aimed to determine the effect of human chemerin on weight, glucose and lipid metabolism, as well as atherosclerosis in vivo in LDLRKO mice on HFD. We demonstrated that expression of chemerin with serum levels comparable with those seen in healthy human volunteers induced insulin resistance in the skeletal muscle with increased glucose levels during both the ipGTT and the ITT. Expression of chemerin, however, did not significantly alter weight, lipid levels, and extent of atherosclerotic lesion area.
Ernst et al. (16) reported that acute administration of recombinant human chemerin exacerbated glucose intolerance and lowered serum insulin levels in ob/ob and db/db mice but had no effect on glucose tolerance in wild-type mice on chow diet. Chemerin injection of DIO mice also resulted in impaired glucose tolerance but (except for the 2-h time point) did not reduce insulin levels during GTT. Chemerin significantly decreased liver but not skeletal muscle glucose uptake in db/db mice. Consistent with Ernst et al. (16), long-term expression of human chemerin in our diet-induced mouse model of insulin resistance and atherosclerosis resulted in glucose intolerance without altering insulin levels. We did not assess the effect of chemerin on glucose uptake in different tissues. However, given the results of the present study, we would have expected decreased glucose uptake in the skeletal muscle consistent with data from Sell et al. (9) demonstrating that chemerin impaired insulin signaling and glucose uptake in skeletal muscle cells in vitro. Unfortunately, Ernst et al. (16) did not report the effect of acute chemerin administration on glucose uptake in DIO mice. Further studies, including clamp techniques, are necessary to better characterize the effect of chemerin on glucose metabolism in vivo and to address the mechanism by which chemerin reduced insulin-stimulated Akt1 phosphorylation and AMPK-α activation in the skeletal muscle. In vitro, Sell et al. (9) identified activation of p38 MAP kinase, extracellular signal–regulated kinases-1/2, and p56 as potential mechanisms that might mediate the effect of chemerin on insulin resistance in skeletal muscle cells.
In contrast to epidemiological data (5,12,13), chemerin had no significant effect on triglyceride and HDL cholesterol levels in our study. Studies in mice with a more human-like lipoprotein metabolism (e.g., APOE*3-Leiden.CETP transgenic mice) may be required to reproduce the lipid phenotype seen in humans.
Lack of chemerin on the atherosclerotic lesion area does not contradict previous studies demonstrating that chemerin modulates activation of immune cells in inflamed tissues of patients with skin disorders (20,21) or mouse models of peritonitis and lung disease (3,17). One could speculate that chemerin may affect early atherosclerotic plaque development and/or plaque morphology rather than extent of atherosclerotic lesion area. In patients with stable chest pain, however, chemerin was not associated with coronary atherosclerotic plaque morphology as determined by computed tomography-angiography (12).
Chemerin is synthesized as a secreted precursor, prochemerin, which is poorly active on the chemerin receptor CMKLR1 (1). Prochemerin can be rapidly converted into inactive, proinflammatory, or anti-inflammatory chemerin variants by proteolytic removal of carboxy-terminal peptides mediated by distinct proteases of the coagulation and inflammation cascades (3,22–24). Unfortunately, the antibodies used in our study for Western blot analysis, human and murine chemerin ELISAs, detect prochemerin, chemerin, and other proteolytically processed forms but do not allow us to distinguish between these peptides. Since distinct chemerin forms differ substantially in their bioactivity, the significance for total chemerin assessment is limited thus complicating the interpretation of our results. It is reasonable to speculate that since both human and murine chemerin activate the murine chemerin receptor CMKLR1 (17), overall increased chemerin levels resulted in glucose intolerance and insulin resistance in the skeletal muscle of LDLRKO mice on HFD. However, it is also conceivable that expression of chemerin altered its enzymatic proteolysis thus modulating both murine and human chemerin bioactivity.
In summary, we determined in this present study the effect of human chemerin on weight, glucose and lipid metabolism, as well as atherosclerosis in vivo. We demonstrated that expression of chemerin induced insulin resistance in the skeletal muscle of LDLRKO mice on HFD. Expression of chemerin, however, did not significantly alter weight, lipid levels, and extent of atherosclerotic lesion area.
Supplementary Material
ACKNOWLEDGMENTS
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector. No potential conflicts of interest relevant to this article were reported.
M.B. wrote the manuscript, researched the data, and revised the manuscript. K.R. researched the data and reviewed/edited the manuscript. C.L. researched the data, contributed to the discussion, and reviewed/edited the manuscript. J.Z. researched the data and reviewed/edited the manuscript. B.G. contributed to the discussion and reviewed/edited the manuscript. K.G.P contributed to the discussion and reviewed/edited the manuscript. M.L. designed the study, contributed to the discussion, and reviewed/edited the manuscript. U.C.B designed the study, wrote the manuscript, and revised the manuscript.
Parts of this study were presented in poster form at the 46th annual meeting of the European Association for the Study of Diabetes, Stockholm, Sweden, 20–24 September 2010.
We are indebted to Elisabeth Fleischer-Brielmaier, Inge Biller-Friedmann, Daniela Bureik, and Kerstin Henze from the Department of Internal Medicine II, University of Munich, Munich, Germany, for their expert technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brézillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med 2003;198:977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zabel BA, Nakae S, Zúñiga L, Kim JY, Ohyama T, Alt C, Pan J, Suto H, Soler D, Allen SJ, Handel TM, Song CH, Galli SJ, Butcher EC. Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J Exp Med 2008;205:2207–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med 2008;205:767–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnea G, Strapps W, Herrada G, Berman Y, Ong J, Kloss B, Axel R, Lee KJ. The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci U S A 2008;105:64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007;148:4687–4694 [DOI] [PubMed] [Google Scholar]
- 6.Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem 2007;282:28175–28188 [DOI] [PubMed] [Google Scholar]
- 7.Kralisch S, Weise S, Sommer G, Lipfert J, Lossner U, Bluher M, Stumvoll M, Fasshauer M. Interleukin-1beta induces the novel adipokine chemerin in adipocytes in vitro. Regul Pept 2009;154:102–106 [DOI] [PubMed] [Google Scholar]
- 8.Takahashi M, Takahashi Y, Takahashi K, Zolotaryov FN, Hong KS, Kitazawa R, Iida K, Okimura Y, Kaji H, Kitazawa S, Kasuga M, Chihara K. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3–L1 adipocytes. FEBS Lett 2008;582:573–578 [DOI] [PubMed] [Google Scholar]
- 9.Sell H, Laurencikiene J, Taube A, Eckardt K, Cramer A, Horrighs A, Arner P, Eckel J. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes 2009;58:2731–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weigert J, Neumeier M, Wanninger J, Filarsky M, Bauer S, Wiest R, Farkas S, Scherer MN, Schäffler A, Aslanidis C, Schölmerich J, Buechler C. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrinol (Oxf) 2010;72:342–348 [DOI] [PubMed] [Google Scholar]
- 11.Tan BK, Chen J, Farhatullah S, Adya R, Kaur J, Heutling D, Lewandowski KC, O'Hare JP, Lehnert H, Randeva HS. Insulin and metformin regulate circulating and adipose tissue chemerin. Diabetes 2009;58:1971–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehrke M, Becker A, Greif M, Stark R, Laubender RP, von Ziegler F, Lebherz C, Tittus J, Reiser M, Becker C, Göke B, Leber AW, Parhofer KG, Broedl UC. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol 2009;161:339–344 [DOI] [PubMed] [Google Scholar]
- 13.Bozaoglu K, Segal D, Shields KA, Cummings N, Curran JE, Comuzzie AG, Mahaney MC, Rainwater DL, VandeBerg JL, MacCluer JW, Collier G, Blangero J, Walder K, Jowett JB. Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population. J Clin Endocrinol Metab 2009;94:3085–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sell H, Divoux A, Poitou C, Basdevant A, Bouillot JL, Bedossa P, Tordjman J, Eckel J, Clément K. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 2010;95:2892–2896 [DOI] [PubMed] [Google Scholar]
- 15.Ress C, Tschoner A, Engl J, Klaus A, Tilg H, Ebenbichler CF, Patsch JR, Kaser S. Effect of bariatric surgery on circulating chemerin levels. Eur J Clin Invest 2010;40:277–280 [DOI] [PubMed] [Google Scholar]
- 16.Ernst MC, Issa M, Goralski KB, Sinal CJ. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology 2010;151:1998–2007 [DOI] [PubMed] [Google Scholar]
- 17.Luangsay S, Wittamer V, Bondue B, De Henau O, Rouger L, Brait M, Franssen JD, de Nadai P, Huaux F, Parmentier M. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J Immunol 2009;183:6489–6499 [DOI] [PubMed] [Google Scholar]
- 18.Lebherz C, Sanmiguel J, Wilson JM, Rader DJ. Gene transfer of wild-type apoA-I and apoA-I Milano reduce atherosclerosis to a similar extent. Cardiovasc Diabetol 2007;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehrke M, Lebherz C, Millington SC, Guan HP, Millar J, Rader DJ, Wilson JM, Lazar MA. Diet-dependent cardiovascular lipid metabolism controlled by hepatic LXRalpha. Cell Metab 2005;1:297–308 [DOI] [PubMed] [Google Scholar]
- 20.Skrzeczyńska-Moncznik J, Wawro K, Stefańska A, Oleszycka E, Kulig P, Zabel BA, Sułkowski M, Kapińska-Mrowiecka M, Czubak-Macugowska M, Butcher EC, Cichy J. Potential role of chemerin in recruitment of plasmacytoid dendritic cells to diseased skin. Biochem Biophys Res Commun 2009;380:323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skrzeczyńska-Moncznik J, Stefańska A, Zabel BA, Kapińska-Mrowiecka M, Butcher EC, Cichy J. Chemerin and the recruitment of NK cells to diseased skin. Acta Biochim Pol 2009;56:355–360 [PMC free article] [PubMed] [Google Scholar]
- 22.Zabel BA, Zuniga L, Ohyama T, Allen SJ, Cichy J, Handel TM, Butcher EC. Chemoattractants, extracellular proteases, and the integrated host defense response. Exp Hematol 2006;34:1021–1032 [DOI] [PubMed] [Google Scholar]
- 23.Zabel BA, Allen SJ, Kulig P, Allen JA, Cichy J, Handel TM, Butcher EC. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J Biol Chem 2005;280:34661–34666 [DOI] [PubMed] [Google Scholar]
- 24.Guillabert A, Wittamer V, Bondue B, Godot V, Imbault V, Parmentier M, Communi D. Role of neutrophil proteinase 3 and mast cell chymase in chemerin proteolytic regulation. J Leukoc Biol 2008;84:1530–1538 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.