Abstract
OBJECTIVE
We tested the hypothesis that an increase in insulin per se, i.e., in the absence of zinc, suppresses glucagon secretion during euglycemia and that a decrease in insulin per se stimulates glucagon secretion during hypoglycemia in humans.
RESEARCH DESIGN AND METHODS
We measured plasma glucagon concentrations in patients with type 1 diabetes infused with the zinc-free insulin glulisine on three occasions. Glulisine was infused with clamped euglycemia (∼95 mg/dl [5.3 mmol/l]) from 0 to 60 min on all three occasions. Then, glulisine was discontinued with clamped euglycemia or with clamped hypoglycemia (∼55 mg/dl [3.0 mmol/l]) or continued with clamped hypoglycemia from 60 to 180 min.
RESULTS
Plasma glucagon concentrations were suppressed by −13 ± 3, −9 ± 3, and −12 ± 2 pg/ml (−3.7 ± 0.9, −2.6 ± 0.9, and −3.4 ± 0.6 pmol/l), respectively, (all P < 0.01) during zinc-free hyperinsulinemic euglycemia over the first 60 min. Glucagon levels remained suppressed following a decrease in zinc-free insulin with euglycemia (−14 ± 3 pg/ml [−4.0 ± 0.9 pmol/l]) and during sustained hyperinsulinemia with hypoglycemia (−14 ± 2 pg/ml [−4.0 ± 0.6 pmol/l]) but increased to −3 ± 3 pg/ml (−0.9 ± 0.9 pmol/l) (P < 0.01) following a decrease in zinc-free insulin with hypoglycemia over the next 120 min.
CONCLUSIONS
These data indicate that an increase in insulin per se suppresses glucagon secretion and a decrease in insulin per se, in concert with a low glucose concentration, stimulates glucagon secretion. Thus, they document that insulin is a β-cell secretory product that, in concert with glucose and among other signals, reciprocally regulates α-cell glucagon secretion in humans.
The regulation of pancreatic islet α-cell glucagon secretion by nutrients, hormones, neurotransmitters, and drugs is complex and incompletely understood (1–8). It involves direct signaling of α-cells (1) and indirect signaling of α-cells by β-cell (2–4) and δ-cell (5) secretory products, the autonomic nervous system (6,7), and gut incretins (8). Among the intraislet mechanisms, there is evidence that indirect reciprocal β-cell–mediated signaling of α-cells normally predominates over direct α-cell signaling in the regulation of glucagon secretion in humans (9–13). The physiological concept is as follows: 1) A β-cell secretory product, or products, tonically restrains α-cell glucagon secretion during postabsorptive euglycemia. 2) A decrease in β-cell secretion, in concert with a low α-cell glucose concentration, signals an increase in α-cell glucagon secretion during hypoglycemia (9–12). 3) An increase in β-cell secretion negates direct α-cell stimulation and thus results in no change or even suppression of α-cell glucagon secretion following a mixed meal (13).
Among the various candidate signaling molecules—insulin, zinc, γ-aminobutyric acid, and amylin among others (4)—there is evidence that insulin is a β-cell secretory product that normally restrains basal α-cell glucagon secretion (14). First, administered insulin suppresses glucagon secretion in several species (4,14,15) including humans (16). However, most available insulin preparations contain zinc and zinc is cosecreted with insulin from β-cells and it has been reported that a decrease in pancreatic arterial zinc, but not in insulin depleted of zinc, increases glucagon secretion during hypoglycemia in streptozotocin diabetic rats, leading the authors to conclude that the inhibitory β-cell secretory product is zinc—not insulin (17). Second, perfusion of the rat (3) and the human (18) pancreas (and incubation of rat islets [15]) with an antibody to insulin increases glucagon release. Because insulin circulates as a zinc-free monomer (19), that finding seemingly implicates insulin directly as an α-cell inhibitory factor and suggests that zinc is not the only β-cell product that reciprocally regulates α-cell glucagon secretion. However, it could be reasoned that the antibody binds insulin-zinc complexes before they dissociate at physiological pH and with dilution. Third, findings—that siRNA-mediated knockdown of insulin receptors prevented the effect of low glucose concentrations to increase glucagon release from isolated mouse islets (20) and that blockade of insulin signaling with the phosphatidylinositol 3-kinase inhibitor wortmannin prevented the effect of high glucose concentrations to decrease glucagon release from isolated rat and human islets (21)—also appear to implicate insulin as the relevant β-cell secretory product.
We tested the hypothesis that an increase in insulin per se, i.e., in the absence of an increase in zinc, suppresses glucagon secretion during euglycemia and that a decrease in insulin per se, in concert with low glucose concentrations, stimulates glucagon secretion in humans. To do so, we studied patients with type 1 diabetes, individuals with essentially no endogenous insulin secretion and no α-cell glucagon secretory response to hyperinsulinemic hypoglycemia (22–29) but viable α-cells as evidenced by a glucagon secretory response to administered amino acids (22,30–32), on three separate occasions. Plasma glucagon concentrations were measured during infusion of the zinc-free insulin glulisine with clamped euglycemia over 60 min on all three occasions. Then, glucagon concentrations were measured following discontinuation of glulisine, to produce a sharp decrease in systemic and therefore α-cell zinc-free insulin levels, over 120 min with clamped euglycemia on one occasion or clamped hypoglycemia on another occasion. On a third occasion, glucagon levels were measured during sustained glulisine infusion with clamped hypoglycemia. The data document 1) a decrease in plasma glucagon during zinc-free hyperinsulinemic euglycemia and 2) an increase in plasma glucagon following a decrease in zinc-free insulin during hypoglycemia but not following a decrease in insulin without hypoglycemia or during hypoglycemia without a decrease in insulin.
RESEARCH DESIGN AND METHODS
Seventeen patients with type 1 diabetes gave written consent to participate in this study, which was approved by the Washington University Human Research Protection Office and conducted at the Washington University Clinical Research Unit. Eight were women, and nine were men. The mean ± SD age was 30 ± 10 years, BMI 26.1 ± 3.9 kg/m2, duration of type 1 diabetes 16 ± 9 years, and A1C 7.5 ± 0.8%. With unmeasurably low values assigned the assay detection limit of 0.1 ng/ml (0.03 nmol/l), their mean fasting C-peptide concentration was 0.2 ± 0.1 ng/ml (0.07 ± 0.03 nmol/l). Exclusion criteria included a serum creatinine concentration >1.5 mg/dl, untreated proliferative retinopathy, or clinical evidence of autonomic neuropathy; known macrovascular or central nervous system disease, anemia, or pregnancy; or potentially interfering medications. Eight were using a multiple daily injection insulin regimen with a long-acting insulin analog as the basal insulin and a rapid-acting insulin analog as the prandial insulin, and nine were using continuous subcutaneous insulin infusion with a rapid-acting insulin analog.
The patients took their last dose of long-acting insulin, if used, the morning of the day prior to each study and managed their diabetes with a rapid-acting insulin until they reported to the Clinical Research Unit that evening. They then fasted overnight and their plasma glucose concentrations were maintained at ∼100 mg/dl (5.6 mmol/l) with variable doses of intravenous regular insulin (Novolin R; Novo Nordisk Inc, Princeton, NJ). On the morning of each study, lines were inserted into an antecubital vein for infusions and into a hand vein with that hand subsequently kept in a ∼55°C plexiglas box for arterialized venous sampling. The patients remained supine through the remainder of the study. Blood samples were obtained, and heart rates and blood pressures (Propaq Encore; Protocol Systems, Beaverton, OR) were recorded, at 15-min intervals from −15 to 210 min. Then 5.0 g arginine was injected intravenously and additional blood samples were obtained at 213, 215, and 217 min (3, 5, and 7 min after arginine injection). From 0 to 60 min, insulin glulisine (Apidra; sanofi-aventis U.S., Bridgewater, NJ) was infused intravenously in a dose of 12.0 mU · kg−1 · min−1 and, based on bedside plasma glucose measurements every 5 min, glucose was infused intravenously to clamp plasma glucose concentrations at ∼95 mg/dl (5.3 mmol/l). Then, glulisine infusions were discontinued and, in random sequence, plasma glucose concentrations were either held at ∼95 mg/dl (5.3 mmol/l) or clamped at ∼55 mg/dl (3.0 mmol/l) from 60 to 180 min. On a subsequent occasion, glulisine infusion was continued to 180 min and plasma glucose concentrations were clamped at ∼55 mg/dl (3.0 mmol/l) in 12 of the patients.
Analytical methods.
Plasma glucose concentrations were measured with a glucose oxidase method (YSI Glucose Analyzer, Yellow Springs Instruments, Yellow Springs, OH). Plasma C-peptide, growth hormone, and cortisol concentrations were measured with two-site chemiluminescent assays (Immulite 1000; Siemens, Los Angeles, CA). Because insulin glulisine does not cross-react in the Immulite insulin assay, an older insulin radioimmunoassay (33) was used to estimate plasma glulisine concentrations. Preliminary data indicated that the latter radioimmunoassay detects ∼75–80% of diluted glulisine based on the concentration stated on the label. Plasma glucagon and pancreatic polypeptide concentrations were measured with Linco radioimmunoassays (Millipore, Temecula, CA). Plasma epinephrine and norepinephrine were measured with a single isotope derivative (radioenzymatic) method (34). Blood lactate (35) and serum nonesterified fatty acids (36) were measured with enzymatic methods. Symptoms were quantitated by asking the patients to score (from 0, none, to 6, severe) each of 12 symptoms classified on the basis of our published data (37): six neurogenic symptoms (adrenergic: heart pounding, shaky, tremulous, and nervous/anxious; cholinergic: sweaty, hungry, and tingling) and six neuroglycopenic symptoms (difficulty thinking/confused, tired/drowsy, weak, warm, faint, and dizzy).
Statistical methods.
Except where specified otherwise, data are reported as means ± SE. Time and condition-related variables were analyzed by repeated-measures mixed-model analysis of variance. P values <0.05 were considered to indicate significant differences.
RESULTS
Plasma insulin glulisine, glucose, and glucagon.
Infusion of insulin glulisine raised mean plasma insulin concentrations to ∼500 μU/ml (3,000 pmol/l) by 60 min. Following discontinuation of glulisine infusions at 60 min, mean insulin levels fell sharply, reaching baseline levels before 180 min, in both studies; insulin levels continued to rise when glulisine infusions were continued from 60 to 180 min in the third study (Fig. 1).
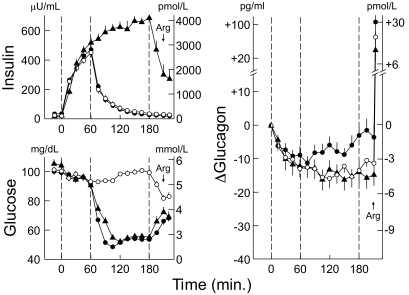
FIG. 1.
Mean ± SE plasma insulin and glucose concentrations and change (Δ) in plasma glucagon concentrations in patients with type 1 diabetes during infusions of zinc-free insulin glulisine with clamped euglycemia from 0 to 60 min on three occasions (decrements in plasma glucagon all P < 0.01) and following 1) discontinuation of insulin glulisine with clamped euglycemia (○), 2) discontinuation of insulin glulisine with clamped hypoglycemia (●) (increments in plasma glucagon P < 0.01), and 3) continuation of insulin glulisine with clamped hypoglycemia (▴), each from 60 to 180 min. Arg, arginine.
When insulin glulisine was infused from 0 to 60 min, plasma glucose concentrations were held, by glucose infusion, at ∼95 mg/dl (5.3 mmol/l) on all three occasions. When insulin glulisine infusions were discontinued after 60 min, glucose levels were held at ∼95 mg/dl (5.3 mmol/l) on one occasion and were lowered and then held at ∼55 mg/dl (3.0 mmol/l) on the other occasion. When insulin glulisine infusion was continued from 60 to 180 min, glucose levels were lowered and then held at ∼55 mg/dl (3.0 mmol/l).
Mean ± SE plasma glucagon concentrations were 67 ± 4, 64 ± 4, and 46 ± 3 pg/ml (19.2 ± 1.1, 18.4 ± 1.1, and 13.2 ± 1.1 pmol/l), respectively, at 0 min and decreased to 54 ± 3, 55 ± 4, and 34 ± 3 pg/ml (15.5 ± 0.9, 15.8 ± 1.1, and 9.8 ± 0.9 pmol/l) during glulisine hyperinsulinemia at 60 min. Thus, plasma glucagon levels were suppressed by −13 ± 3, −9 ± 3, and −12 ± 2 pg/ml (−3.7 ± 0.9, −2.6 ± 0.9, and −3.4 ± 0.6 pmol/l) during zinc-free hyperinsulinemic euglycemia from 0 to 60 min (all P < 0.01). Glucagon levels remained suppressed following a decrease in zinc-free insulin after 60 min with euglycemia to 180 min (−14 ± 3 pg/ml [−4.0 ± 0.9 pmol/l]) and during sustained zinc-free hyperinsulinemia with hypoglycemia from 60 to 180 min (−14 ± 2 pg/ml [4.0 ± 0.6 pmol/l]) but increased following a decrease in zinc-free insulin after 60 min with hypoglycemia from 60 to 180 min to −3 ± 3 pg/ml (−0.9 ± 0.09 pmol/l) (P < 0.01).
Other neuroendocrine and metabolism measurements and symptoms, heart rate, and blood pressures.
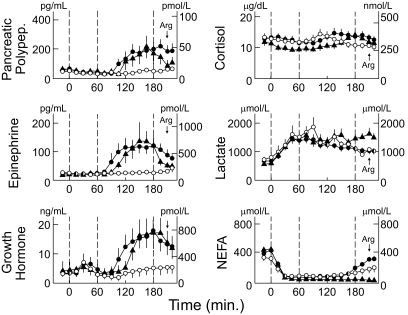
None of the other neuroendocrine levels displayed the pattern of glucagon. Plasma pancreatic polypeptide concentrations tended to decline during zinc-free hyperinsulinemic euglycemia but increased similarly during hypoglycemia, with falling insulin levels and sustained hyperinsulinemia. The latter was also true for plasma epinephrine, growth hormone, and cortisol concentrations. Blood lactate concentrations rose similarly during hyperinsulinemia on all three occasions. Serum nonesterified fatty acid concentrations were suppressed (Fig. 2). Mean symptom scores, heart rates, and systolic and diastolic blood pressures were similar on all three occasions (data not shown).
FIG. 2.
Mean ± SE plasma pancreatic polypeptide (polypep), epinephrine, growth hormone, cortisol, blood lactate, and serum nonesterified fatty acid (NEFA) concentrations in patients with type 1 diabetes during infusions of zinc-free insulin glulisine with clamped euglycemic from 0 to 60 min on three occasions and 1) following discontinuation of insulin glulisine with clamped euglycemia (○), 2) following discontinuation of insulin glulisine with clamped hypoglycemia (●), and 3) continuation of insulin glulisine with clamped hypoglycemia (▴), each from 60 to 180 min. Arg, arginine.
DISCUSSION
Despite a body of evidence indicating that insulin is a β-cell secretory product that reciprocally regulates α-cell glucagon secretion (rev. in 4,14,16), it has been concluded that zinc, not insulin, is the relevant β-cell secretory product (17). Therefore, we tested the hypothesis that an increase in insulin per se, i.e., in the absence of a change in zinc, suppresses glucagon secretion during euglycemia and that a decrease in insulin per se stimulates glucagon secretion during hypoglycemia in humans. To do so, we measured plasma glucagon concentrations in demonstrably endogenous insulin-deficient patients with type 1 diabetes during controlled differences in zinc-free insulin levels—produced by infusion of the zinc-free commercial insulin glulisine (38)—combined with controlled euglycemia and hypoglycemia. Glulisine was first infused for 60 min with clamped euglycemia on three occasions. Then glulisine was 1) discontinued with euglycemia maintained over 2 h, 2) discontinued with hypoglycemia maintained over 2 h, and 3) continued with hypoglycemia maintained over 2 h.
Our hypothesis was confirmed. First, a sharp increase in zinc-free insulin suppressed plasma glucagon concentrations during clamped euglycemia on all three occasions. Second, plasma glucagon concentrations remained suppressed following a sharp decrease in systemic, and thus α-cell, zinc-free insulin with clamped euglycemia and during continued zinc-free hyperinsulinemia with clamped hypoglycemia, but plasma glucagon concentrations increased following a sharp decrease in zinc-free insulin with clamped hypoglycemia. These data also illustrate that a decrease in insulin, as demonstrated earlier for a decrease in β-cell secretion (12), signals an increase in glucagon secretion only in the setting of hypoglycemia.
The decrease in zinc-free insulin did not restore a normal glucagon response to hypoglycemia in these patients with type 1 diabetes. However, that would not be expected even if signaling of α-cells were normalized. Patients with type 1 diabetes have glucagon secretory responses to direct α-cell stimuli such as oral alanine (30), intravenous amino acids (31), and oral amino acids (32). However, their maximum stimulated plasma glucagon concentrations are only ∼20–25% those of nondiabetic individuals (30–32). That finding was also documented in the present study. The peak glucagon response to intravenous arginine was ∼25% of that in our earlier study of nondiabetic individuals (12). The extent to which that represents a decreased glucagon response per α-cell, a decrease in α-cell mass (39), or both in type 1 diabetes is not known.
Normally, insulin and zinc ions create a hexameric crystalline structure stored in pancreatic β-cell secretory granules at pH 5.5. Upon secretion, the hexameric insulin structure dissociates into the biologically active insulin monomer and zinc ions—probably because of a combination of a rapid decrease in zinc ion pressure and the change in pH from 5.5 to 7.4 (19). In our patients with type 1 diabetes and little if any endogenous insulin secretion, the circulating insulin was almost exclusively glulisine. Glulisine contains no exogenous zinc (38) and, because insulin circulates as a zinc-free monomer at physiological pH (19), it would not have bound endogenous zinc. Thus, by changing the rate of glulisine infusion we were able to change insulin action independent of zinc action.
There are limitations to this study. First, the changes in plasma glucagon concentrations were rather small in relation to the total measured values. The radioimmunoassay used detects species in addition to 3,500 Da glucagon, although changes in measured values are thought to represent changes in biologically active glucagon (40,41). Second, while the first two studies (decrements in zinc-free insulin with euglycemia and with hypoglycemia) were performed in random sequence, the second control (no decrement in insulin with hypoglycemia) was performed later in a subset of the patients. The latter may explain the lower mean glucagon levels in that limb. Third, we used a high dose of insulin glulisine that was intended to mimic normal α-cell insulin concentrations. That may not have been necessary. Fourth, because an effect of a decrease in zinc to stimulate glucagon secretion during hypoglycemia in an insulin-deficient model has already been demonstrated (17,42), we did not document that a decrease in a zinc-containing insulin also produces a hypoglycemia-associated increment in glucagon secretion in patients with type 1 diabetes.
We have no clear explanation for the difference between our finding and that of Zhou et al. (17), who found no increase in glucagon secretion when insulin from which zinc had been removed was discontinued during hypoglycemia in diabetic rats. They studied rats, and we studied humans. Their sample size was about one-third of our sample size. While they provided evidence that very high concentrations of their zinc-depleted insulin preparation (i.e., 1.0 μmol/l) was biologically active in vitro, dose-response data indicating that biological activity was not reduced by the zinc removal process were not presented.
Again, the regulation of glucagon secretion is complex and not limited to effects of insulin or of other β-cell secretory products (1–8,43). For example, the decrease in nonesterified fatty acids may have been involved in the suppression of glucagon secretion during hyperinsulinemic euglycemia and the increase in epinephrine may have been involved in the stimulation of glucagon secretion during hypoinsulinemic hypoglycemia in our studies.
The finding that β-cell secretion (9–13), including that by insulin (present data), reciprocally regulates α-cell glucagon secretion in humans is potentially clinically relevant (13). Loss of the glucagon response to hypoglycemia in type 1 diabetes and advanced type 2 diabetes and less suppression of glucagon following a meal in diabetes may both be the result, at least in part, of β-cell failure (13). The former is thought to be relevant to the pathogenesis of iatrogenic hypoglycemia and the latter to that of hyperglycemia in diabetes (12,13,40).
In conclusion, these data indicate that insulin per se suppresses glucagon secretion during euglycemia and that a decrease in insulin per se, in concert with low glucose levels, signals an increase in glucagon secretion during hypoglycemia. Thus, they document that insulin is a β-cell secretory product that, in concert with glucose and among other signals, reciprocally regulates α-cell glucagon secretion in humans.
ACKNOWLEDGMENTS
This study was supported, in part, by National Institutes of Health grants R37-DK27085, UL1-RR24992, and by a fellowship award from the American Diabetes Association.
P.E.C. has served as a consultant to Marcadia Biotech and Merck in the past year. No other potential conflicts of interest relevant to this article were reported.
B.A.C. planned and performed the study and wrote the manuscript. P.E.C. planned the study and wrote the manuscript.
The authors acknowledge the assistance of the staff of the Washington University Clinical Research Unit and the technical assistance of Krishan Jethi, Licia Rowe, Laura Karsteter, Nalima Parikh, Tanya Eden, Shirley Frei, Janice Bathon, Paula Blood, and David Gibson. Janet Dedeke prepared this manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 2735.
REFERENCES
- 1.Rorsman P, Salehi SA, Abdulkader F, Braun M, MacDonald PE. KATP-channels and glucose-regulated glucagon secretion. Trends Endocrinol Metab 2008;19:277–284 [DOI] [PubMed] [Google Scholar]
- 2.Samols E, Tyler J, Marks V. Glucagon-insulin interrelationships. In Glucagon: Molecular Physiology, Clinical and Therapeutic Implications. Lefebvre P, Unger RH. Eds. Elmsford, NY, Pergamon Press, 1972, p. 151–174 [Google Scholar]
- 3.Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 1984;74:2296–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocrine Reviews 2007;28:84–116 [DOI] [PubMed] [Google Scholar]
- 5.Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson IC, Low MJ, Christie MR, Persaud SJ, Jones PM. Somatostatin secreted by islet δ-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 2009;58:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taborsky GJ, Jr, Ahrén B, Havel PJ. Autonomic mediation of glucagon secretion during hypoglycemia. Diabetes 1998;47:995–1005 [DOI] [PubMed] [Google Scholar]
- 7.Marty N, Dallaporta M, Thorens B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology 2007;22:241–251 [DOI] [PubMed] [Google Scholar]
- 8.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–1439 [DOI] [PubMed] [Google Scholar]
- 9.Banarer S, McGregor VP, Cryer PE. Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes 2002;51:958–965 [DOI] [PubMed] [Google Scholar]
- 10.Gosmanov NR, Szoke E, Israelian Z, Smith T, Cryer PE, Gerich JE, Meyer C. Role of the decrement in intraislet insulin for the glucagon response to hypoglycemia in humans. Diabetes Care 2005;28:1124–1131 [DOI] [PubMed] [Google Scholar]
- 11.Israelian Z, Gosmanov NR, Szoke E, Schorr M, Bokhari S, Cryer PE, Gerich JE, Meyer C. Increasing the decrement in insulin secretion improves glucagon responses to hypoglycemia in advanced type 2 diabetes. Diabetes Care 2005;28:2691–2696 [DOI] [PubMed] [Google Scholar]
- 12.Raju B, Cryer PE. Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes. Diabetes 2005;54:757–764 [DOI] [PubMed] [Google Scholar]
- 13.Cooperberg BA, Cryer PE. β-Cell–mediated signaling predominates over direct α-cell signaling in the regulation of glucagon secretion in humans. Diabetes Care 2009;32:2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bansal P, Wang Q. Insulin as a physiological modulator of glucagon secretion. Am J Physiol Endocrinol Metab 2008;295:E751–E761 [DOI] [PubMed] [Google Scholar]
- 15.Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 2005;54:1808–1815 [DOI] [PubMed] [Google Scholar]
- 16.Galassetti P, Davis SN. Effects of insulin per se on neuroendocrine and metabolic counter-regulatory responses to hypoglycaemia. Clin Sci 2000;99:351–362 [PubMed] [Google Scholar]
- 17.Zhou H, Zhang T, Harmon JS, Bryan J, Robertson RP. Zinc, not insulin, regulates the rat alpha-cell response to hypoglycemia in vivo. Diabetes 2007;56:1107–1112 [DOI] [PubMed] [Google Scholar]
- 18.Brunicardi FC, Kleinman R, Moldovan S, Nguyen TH, Watt PC, Walsh J, Gingerich R. Immunoneutralization of somatostatin, insulin and glucagon causes alterations in islet cell secretion in the isolated perfused human pancreas. Pancreas 2001;23:302–308 [DOI] [PubMed] [Google Scholar]
- 19.Søndergaard LG, Stoltenberg M, Flyvbjerg A, Brock B, Schmitz O, Danscher G, Rungby J. Zinc ions in beta-cells of obese, insulin resistant, and type 2 diabetic rats traced by autometallography. APMIS 2003;111:1147–1154 [DOI] [PubMed] [Google Scholar]
- 20.Diao J, Asghar Z, Chan CB, Wheeler MB. Glucose-regulated glucagon secretion requires insulin receptor expression in pancreatic alpha-cells. J Biol Chem 2005;280:33487–33496 [DOI] [PubMed] [Google Scholar]
- 21.Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y, Wheeler MB, Braun M, Wang Q. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab 2006;3:47–58 [DOI] [PubMed] [Google Scholar]
- 22.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha-cell defect. Science 1973;182:171–173 [DOI] [PubMed] [Google Scholar]
- 23.Polonsky K, Bergenstal R, Pons G, Schneider M, Jaspan J, Rubenstein A. Relation of counterregulatory responses to hypoglycemia in type I diabetics. N Engl J Med 1982;307:1106–1112 [DOI] [PubMed] [Google Scholar]
- 24.White NH, Skor DA, Cryer PE, Levandoski LA, Bier DM, Santiago JV. Identification of type I diabetic patients at increased risk for hypoglycemia during intensive therapy. N Engl J Med 1983;308:485–491 [DOI] [PubMed] [Google Scholar]
- 25.Bolli GB, De Feo P, De Cosmo S, Perriello G, Ventura MM, Massi Benedetti M, Santeusanio F, Gerich JE, Brunetti P. A reliable and reproducible test for adequate glucose counterregulation in type I diabetes mellitus. Diabetes 1984;33:732–737 [DOI] [PubMed] [Google Scholar]
- 26.Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. J Clin Invest 1993;91:819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bischof MG, Bernroider E, Ludwig C, Kurzemann S, Kletter K, Waldhäusl W, Roden M. Effect of near physiologic insulin therapy on hypoglycemia counterregulation in type-1 diabetes. Horm Res 2001;56:151–158 [DOI] [PubMed] [Google Scholar]
- 28.Banarer S, Cryer PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes. Diabetes 2003;52:1195–1203 [DOI] [PubMed] [Google Scholar]
- 29.Kishore P, Gabriely I, Cui MH, Di Vito J, Gajavelli S, Hwang JH, Shamoon H. Role of hepatic glycogen breakdown in defective counterregulation of hypoglycemia in intensively treated type 1 diabetes. Diabetes 2006;55:659–666 [DOI] [PubMed] [Google Scholar]
- 30.Wiethop BV, Cryer PE. Glycemic actions of alanine and terbutaline in IDDM. Diabetes Care 1993;16:1124–1130 [DOI] [PubMed] [Google Scholar]
- 31.Caprio S, Tamborlane WV, Zych K, Gerow K, Sherwin RS. Loss of potentiating effect of hypoglycemia on the glucagon response to hyperaminoacidemia in IDDM. Diabetes 1993;42:550–555 [DOI] [PubMed] [Google Scholar]
- 32.Rossetti P, Porcellati F, Busciantella Ricci N, Candeloro P, Cioli P, Nair KS, Santeusanio F, Bolli GB, Fanelli CG. Effect of oral amino acids on counterregulatory responses and cognitive function during insulin-induced hypoglycemia in nondiabetic and type 1 diabetic people. Diabetes 2008;57:1905–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuzuya H, Blix PM, Horwitz DL, Steiner DF, Rubenstein AH. Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes 1977;26:22–29 [DOI] [PubMed] [Google Scholar]
- 34.Shah SD, Clutter WE, Cryer PE. External and internal standards in the single isotope derivative (radioenzymatic) measurement of plasma norepinephrine and epinephrine. J Lab Clin Med 1985;106:624–629 [PubMed] [Google Scholar]
- 35.Lowry OH, Passoneau JV, Hasselberger FX, Schultz DW. Effect of ischemia on known substrates and cofactors of the glycolytic pathway in brain. J Biol Chem 1964;239:18–30 [PubMed] [Google Scholar]
- 36.Hosaka K, Kikuchi T, Mitsuhida N, Kawaguchi A. A new colorimetric method for the determination of free fatty acids with acyl-CoA synthase and acyl-CoA oxidase. J Biochem 1981;89:1799–1803 [DOI] [PubMed] [Google Scholar]
- 37.Towler DA, Havlin CE, Craft S, Cryer P. Mechanism of awareness of hypoglycemia. Perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes 1993;42:1791–1798 [DOI] [PubMed] [Google Scholar]
- 38.Becker RHA. Insulin glulisine complementing basal insulins: a review of structure and activity. Diabetes Technol Ther 2007;9:109–121 [DOI] [PubMed] [Google Scholar]
- 39.Pechhold K, Zhu X, Harrison VS, Lee J, Chakrabarty S, Koczwara K, Gavrilova O, Harlan DM. Dynamic changes in pancreatic endocrine cell abundance, distribution, and function in antigen-induced and spontaneous autoimmune diabetes. Diabetes 2009;58:1175–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007;28:253–283 [DOI] [PubMed] [Google Scholar]
- 41.Cherrington AD. Control of glucose production in vivo by insulin and glucagon. In Handbook of Physiology: The Endocrine Pancreas and Regulation of Metabolism. Sect. 7, vol. II Cherrington AD, Jefferson LS. Eds. New York, Oxford University Press, 2001, pp 759–785 [Google Scholar]
- 42.Slucca M, Harmon JS, Oseid EA, Bryan J, Robertson RP. ATP-sensitive K+ channel mediates the zinc switch-off signal for glucagon response during glucose deprivation. Diabetes 2010;59:128–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quesada I, Tudurí E, Ripoll C, Nadal A. Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol 2008;199:5–19 [DOI] [PubMed] [Google Scholar]