Abstract
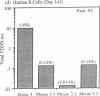
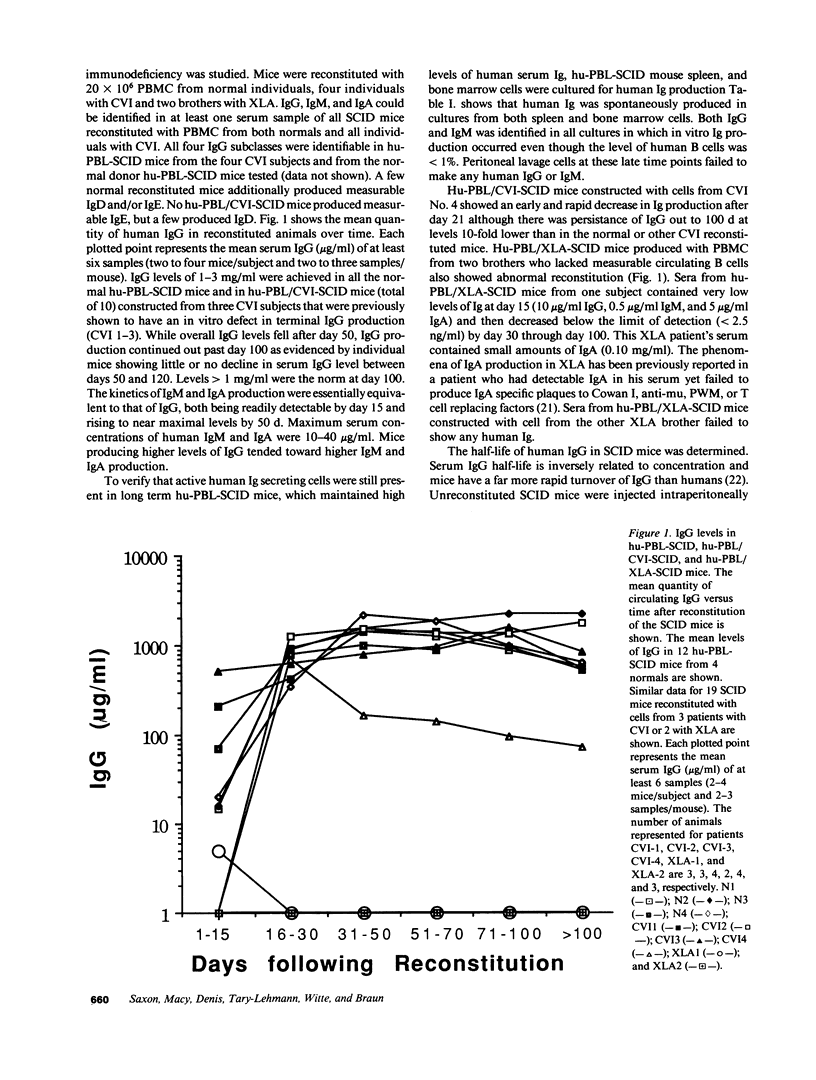
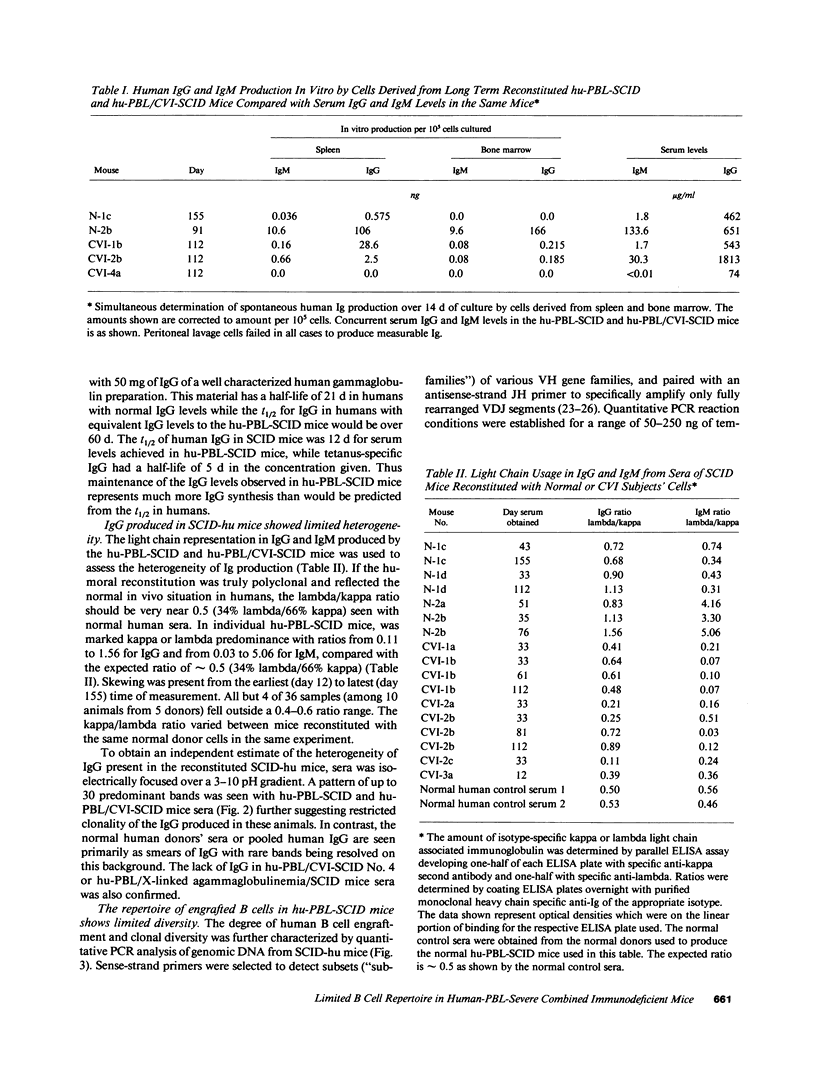
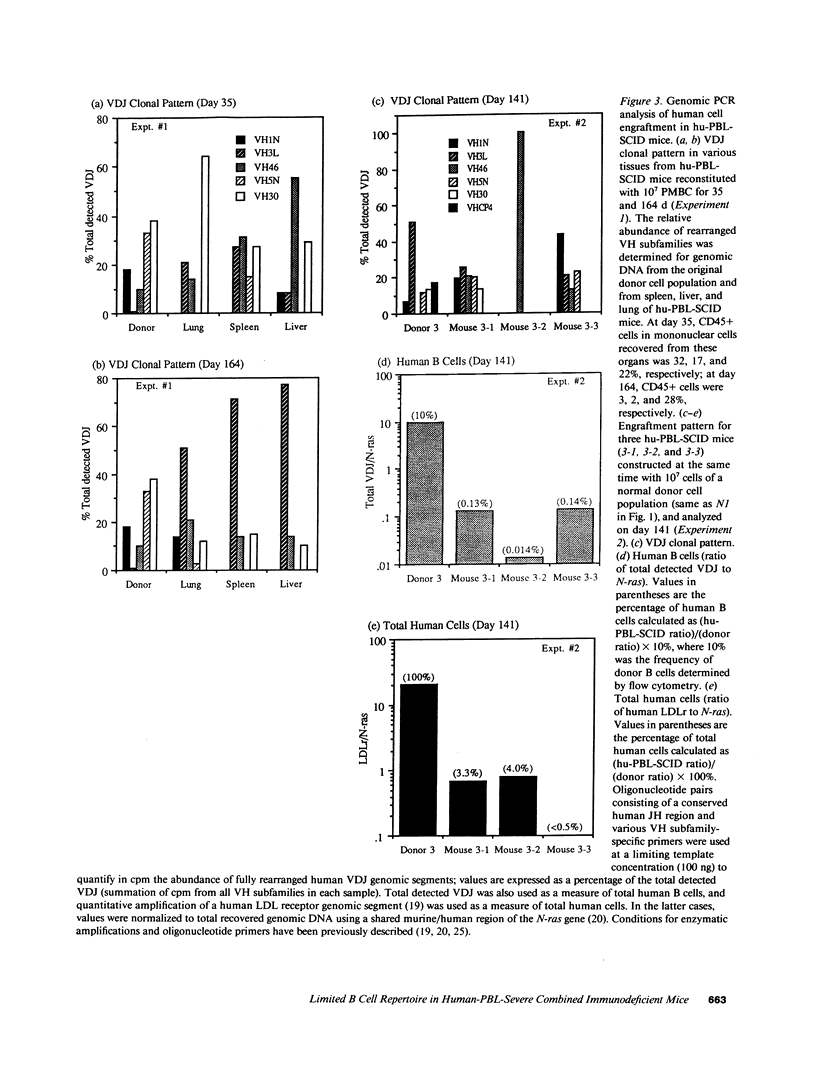
The ability to engraft human PBMC or fetal tissue immune cells in the severe combined immunodeficient (SCID) mouse has created a need for characterization of these systems and their application to disease models. We demonstrate that SCID mice reconstituted with PBMC support the growth and differentiation of a restricted set of B cells. Human IgG levels of 1-2 mg/ml (10-20% of normal human serum levels) were routinely achieved in spite of a serum half life of only 12 d. Ig levels peaked around 50 d and Ig production was maintained for greater than 100 d. The Ig was greater than 85% IgG though some IgM, IgA, IgD, and even IgE could be detected. However, the human IgG produced in hu-PBL-SCID mice was pauci-clonal when analyzed by isoelectric focusing and by kappa/lambda light chain usage. Using a new polymerase chain reaction based analysis capable of monitoring individual VH family utilization, we found that the engrafted B cells showed skewed and restricted human VH subfamily utilization. These parameters were markedly variable among hu-PBL-SCID mice reconstituted from the same donor cell population at both early (21-50 d) and late stages (greater than 100 d). Hu-PBL/CVI-SCID mice constructed with cells from patients with common variable immunodeficiency with an in vitro block in terminal B cell differentiation produced human Ig responses that were quantitatively the same as those produced by hu-PBL-SCID mice from normal donors. The hu-PBL-SCID system using PBMC appears to lead to growth and Ig production by a small number of B cells and results in a restricted B cell repertoire.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold A., Cossman J., Bakhshi A., Jaffe E. S., Waldmann T. A., Korsmeyer S. J. Immunoglobulin-gene rearrangements as unique clonal markers in human lymphoid neoplasms. N Engl J Med. 1983 Dec 29;309(26):1593–1599. doi: 10.1056/NEJM198312293092601. [DOI] [PubMed] [Google Scholar]
- Ashman R. F., Saxon A., Stevens R. H. Profile of multiple lymphocyte functional defects in acquired hypogrammaglobulinemia, derived from in vitro cell recombination analysis. J Allergy Clin Immunol. 1980 Apr;65(4):242–256. doi: 10.1016/0091-6749(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Becker-André M., Hahlbrock K. Absolute mRNA quantification using the polymerase chain reaction (PCR). A novel approach by a PCR aided transcript titration assay (PATTY). Nucleic Acids Res. 1989 Nov 25;17(22):9437–9446. doi: 10.1093/nar/17.22.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. E., Mellis S. J., Pollock R., Smith C. L., Suh H., Heinke B., Kowal C., Surti U., Chess L., Cantor C. R. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988 Mar;7(3):727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., King L. Unique V gene usage by B-Ly1 cell lines, and a discordance between isotype switch commitment and variable region hypermutation. J Mol Cell Immunol. 1989;4(3):121–128. [PubMed] [Google Scholar]
- Buckley R. H. Immunodeficiency diseases. JAMA. 1987 Nov 27;258(20):2841–2850. [PubMed] [Google Scholar]
- Crescenzi M., Seto M., Herzig G. P., Weiss P. D., Griffith R. C., Korsmeyer S. J. Thermostable DNA polymerase chain amplification of t(14;18) chromosome breakpoints and detection of minimal residual disease. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4869–4873. doi: 10.1073/pnas.85.13.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchosal M. A., McConahey P. J., Robinson C. A., Dixon F. J. Transfer of human systemic lupus erythematosus in severe combined immunodeficient (SCID) mice. J Exp Med. 1990 Sep 1;172(3):985–988. doi: 10.1084/jem.172.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye R. A., Benz C. C., Liu E. Detection of amplified oncogenes by differential polymerase chain reaction. Oncogene. 1989 Sep;4(9):1153–1157. [PubMed] [Google Scholar]
- Govan H. L., 3rd, Valles-Ayoub Y., Braun J. Fine-mapping of DNA damage and repair in specific genomic segments. Nucleic Acids Res. 1990 Jul 11;18(13):3823–3830. doi: 10.1093/nar/18.13.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero I., Villasante A., Corces V., Pellicer A. Loss of the normal N-ras allele in a mouse thymic lymphoma induced by a chemical carcinogen. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7810–7814. doi: 10.1073/pnas.82.23.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krams S. M., Dorshkind K., Gershwin M. E. Generation of biliary lesions after transfer of human lymphocytes into severe combined immunodeficient (SCID) mice. J Exp Med. 1989 Dec 1;170(6):1919–1930. doi: 10.1084/jem.170.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. H., Gyllensten U. B., Cui X. F., Saiki R. K., Erlich H. A., Arnheim N. Amplification and analysis of DNA sequences in single human sperm and diploid cells. Nature. 1988 Sep 29;335(6189):414–417. doi: 10.1038/335414a0. [DOI] [PubMed] [Google Scholar]
- Macy E., Kemeny M., Saxon A. Enhanced ELISA: how to measure less than 10 picograms of a specific protein (immunoglobulin) in less than 8 hours. FASEB J. 1988 Nov;2(14):3003–3009. doi: 10.1096/fasebj.2.14.3263291. [DOI] [PubMed] [Google Scholar]
- Mayer L., Fu S. M., Cunningham-Rundles C., Kunkel H. G. Polyclonal immunoglobulin secretion in patients with common variable immunodeficiency using monoclonal B cell differentiation factors. J Clin Invest. 1984 Dec;74(6):2115–2120. doi: 10.1172/JCI111636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Kaneshima H., Shultz L. D., Lieberman M., Weissman I. L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988 Sep 23;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988 Sep 15;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Reddy S., Piccione D., Takita H., Bankert R. B. Human lung tumor growth established in the lung and subcutaneous tissue of mice with severe combined immunodeficiency. Cancer Res. 1987 May 1;47(9):2456–2460. [PubMed] [Google Scholar]
- Saiki O., Ralph P., Cunningham-Rundles C., Good R. A. Three distinct stages of B-cell defects in common varied immunodeficiency. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6008–6012. doi: 10.1073/pnas.79.19.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki O., Ralph P., Cunningham-Rundles C., Good R. A. Three distinct stages of B-cell defects in common varied immunodeficiency. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6008–6012. doi: 10.1073/pnas.79.19.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanz I., Kelly P., Williams C., Scholl S., Tucker P., Capra J. D. The smaller human VH gene families display remarkably little polymorphism. EMBO J. 1989 Dec 1;8(12):3741–3748. doi: 10.1002/j.1460-2075.1989.tb08550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon A., Giorgi J. V., Sherr E. H., Kagan J. M. Failure of B cells in common variable immunodeficiency to transit from proliferation to differentiation is associated with altered B cell surface-molecule display. J Allergy Clin Immunol. 1989 Jul;84(1):44–55. doi: 10.1016/0091-6749(89)90177-2. [DOI] [PubMed] [Google Scholar]
- Saxon A., Mitsuyasu R., Stevens R., Champlin R. E., Kimata H., Gale R. P. Designed transfer of specific immune responses with bone marrow transplantation. J Clin Invest. 1986 Oct;78(4):959–967. doi: 10.1172/JCI112686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Schowalter D. B., Sommer S. S. The generation of radiolabeled DNA and RNA probes with polymerase chain reaction. Anal Biochem. 1989 Feb 15;177(1):90–94. doi: 10.1016/0003-2697(89)90019-5. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Macy E., Morrow C., Saxon A. Characterization of a circulating subpopulation of spontaneous antitetanus toxoid antibody producing B cells following in vivo booster immunization. J Immunol. 1979 Jun;122(6):2498–2504. [PubMed] [Google Scholar]
- Stevens R. H., Tamaroff M., Saxon A. Inability of patients with common variable hypogammaglobulinemia to generate lymphjoblastoid B cells following booster immunization. Clin Immunol Immunopathol. 1980 Jul;16(3):336–343. doi: 10.1016/0090-1229(80)90139-7. [DOI] [PubMed] [Google Scholar]
- Syvänen A. C., Bengtström M., Tenhunen J., Söderlund H. Quantification of polymerase chain reaction products by affinity-based hybrid collection. Nucleic Acids Res. 1988 Dec 9;16(23):11327–11338. doi: 10.1093/nar/16.23.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
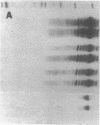
- Valles-Ayoub Y., Govan H. L., 3rd, Braun J. Evolving abundance and clonal pattern of human germinal center B cells during childhood. Blood. 1990 Jul 1;76(1):17–23. [PubMed] [Google Scholar]
- Waldmann T. A., Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- Ward E. S., Güssow D., Griffiths A. D., Jones P. T., Winter G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature. 1989 Oct 12;341(6242):544–546. doi: 10.1038/341544a0. [DOI] [PubMed] [Google Scholar]
- Ware C. F., Donato N. J., Dorshkind K. Human, rat or mouse hybridomas secrete high levels of monoclonal antibodies following transplantation into mice with severe combined immunodeficiency disease (SCID). J Immunol Methods. 1985 Dec 27;85(2):353–361. doi: 10.1016/0022-1759(85)90144-9. [DOI] [PubMed] [Google Scholar]