Summary
Recently reports have indicated that both cimetidine and ranitidine delay cell proliferation in rats following 70% partial hepatectomy and result in an increased mortality following this procedure. The present study was designed to determine whether three H2 blocking agents (cimetidine, ranitidine, famotidine) and a new, powerful antisecretory drug (omeprazole) specifically influence hepatocyte proliferation in primary culture. Hepatocytes were isolated from livers of normal male rats by the standard collagenase perfusion technique. Hepatic DNA synthesis and percent of labelled nuclei were determined after 48 h incubation. Hepatocytes in culture were incubated with the H2 blocking agents and omeprazole or with different concentrations of serum obtained from sham-operated or 70% hepatectomized rats treated or not with the same agents. Rats were injected intraperitoneally at 8:00 a.m. on two consecutive days. In hepatectomized rats, the first dose was injected at 8:00 a.m. immediately after surgery, the second, 24 h later. The serum of sham-operated or 70% hepatectomized rats that did not receive drugs served as control. No changes in DNA synthesis, percentage of labelled nuclei and transaminase were detected when the agents were added to the hepatocytes in culture at concentrations within the effective pharmacological dosage and 30 times higher. Similarly, no changes in these parameters were obtained when different concentrations of serum obtained from sham-operated rats treated with H2 blocking agents or omeprazole were added to the basal culture medium. However, a significant inhibition of DNA synthesis and of percentage of labelled nuclei was observed when hepatocytes were incubated in the presence of serum from 70% hepatectomized rats that had been treated with cimetidine or with ranitidine. The serum of 70% hepatectomized rats treated with famotidine and omeprazole had no effect on hepatocyte proliferation in vitro. No effect on transaminase was found in these conditions.
Introduction
It has recently been reported that the administration of cimetidine and ranitidine to rats following 70% partial hepatectomy (PH) significantly delays and inhibits hepatic DNA synthesis and the percentage of mitosis [1,2]. No direct evidence has been presented to explain the mechanism of this inhibition. Both these drugs continue to be widely used after major hepatic resection for the prophylaxis of gastric complications (‘stress ulcer syndrome’) [3–6] and are also widely prescribed to patients with chronic hepatic disorders and esophageal varices [7–9]. Thus, a more specific evaluation of the effect of these drugs on liver regeneration is warranted.
The experiments reported here were designed to determine the effects of these compounds on hepatocyte proliferation in vitro. The two widely used agents, cimetidine and ranitidine, as well as a new, potent H2 blocking agent, famotidine [10], and a new inhibitor of acid secretion, omeprazole [11], were evaluated. Either the antisecretory agents or the serum taken from rats undergoing PH or sham operation and treated with these agents were tested. The H2 blocking agents and omeprazole were added directly to hepatocyte cultures in serum-free medium in the presence or absence of epidermal growth factor (EGF).
Materials and Methods
Animals
Male Fischer F(344) rats, weighing 180–200 g, were obtained from Hilltop Lab Animals, Inc., Scott-dale, PA. The animals were housed in a temperature (20 ± 1 °C) and light (6 a.m.–6 p.m.) controlled room and received food and water ad libitum. Seventy percent PH was performed according to the method described by Higgins and Anderson [12]. Sham operation consisted of a laparotomy and gentle manual manipulation of the liver. All surgical procedures were performed between 7:30 and 9:00 a.m.
Materials
Collagenase type I (125–250 U/mg) was purchased from Worthington Diagnostic Systems, Freehold, NJ. MEM (minimal essential medium) and fetal calf serum (FCS) were purchased from GIBCO Laboratories, Grand Island, NY. Insulin, EGF, HEPES, pyruvic acid and proline were purchased from Sigma Chemical Company, St. Louis, MO. [methyl-3H] Thymidine (50–80 Ci/mmol) was purchased from New England Nuclear, Boston, MA. Cimetidine was obtained from Smith, Kline and French Lab Co., Caroline, PR, ranitidine from Glaxo, Inc., Research Triangle Park, NC, famotidine from Sigma Tau Co., Rome, Italy, omeprazole from Bracco, Milan, Italy. Aqueous Counting Scintillant (ACS) was purchased from Amersham, Arlington Heights, IL.
Isolation of hepatocytes
Hepatocytes were isolated from 7-week-old male rats weighing 180–200 g, by a modification of the in situ two-step collagenase perfusion technique of Seglen [13] described by Jirtle et al. [14]. The liver was perfused via a cannula in the inferior vena cava with 250 ml buffer (142 mM NaCl, 6.7 mM KC1, 10 mM HEPES, pH 7.4) followed by 250 ml of the same buffer containing in addition 5.7 mM CaCl2 and 0.5 mg/ml collagenase. The hepatocytes were dispersed and washed twice with cold Ca2+-free perfusion buffer and resuspended in MEM supplemented with proline (26 mM), pyruvate (1 mM), aspartate (0.2 mM), serine (0.2 mM), gentamycin (40μg/ml). For attachment, insulin (10−7 M) and FCS (5%) were added. Viability was determined by trypan blue exclusion [15] and only preparations having greater than 90% viability were used. Cell number was determined with a hemocytometer.
The cells were plated at a cell density of 2 × 105 in 35-mm Falcon ‘Primaria’ tissue culture dishes in 1.5 ml medium and maintained at 37 °C and at 5% C02 atmosphere. Following a 3-h attachment period, MEM was added with the specified additions as reported. EGF and insulin, when added, were at a concentration of 10 ng/ml and 10−7 M, respectively.
Determination of [3H] thymidine incorporation and DNA synthesis
After a 3-h period, necessary for the hepatocyte attachment, the medium was changed and specific additions were made. [3H] Thymidine, 7.5 μCi/dish, was present from 24–48 h or from 48–72 h. Each experimental group comprised four dishes. When the cells were harvested, two dishes were used to determine DNA content using the microfluorometrie method of Setaro and Morley [16], and two dishes of each group were treated as described by Michalopoulos et al. [17] to measure [3H] thymidine incorporation.
Serum studies
Forty normal rats were sham-operated, divided into five groups and injected as follows: group 1, cimetidine (2.4 mg/200 g body wt.); group II, ranitidine (0.8 mg/200 g body wt.); group III, famotidine (0.11 mg/200 g body wt.); group IV, omeprazole (0.06 mg/200 g body wt.); group V, saline. Another 40 rats underwent PH, were divided into five groups and injected in the same manner as the sham group. All animals were operated on before 8 a.m. and all were injected i.p. at 8 a.m. on the day of operation and at 8 a.m. the following day with the drugs dissolved in saline (0.5 ml) with the exception of omeprazole which was dissolved in PEG-500. Under ether anesthesia, blood was taken from the animals 3 h after the final injection. Blood samples of these animals, as well as blood taken from a group of eight normal unhepatectomized rats, were taken for hormonal study.
Autoradiography
For autoradiographic studies, hepatocytes plated on ‘Primaria’ dishes were fixed and stained for glucose-6-phosphate dehydrogenase directly on the dishes, as reported by us [18]. [3H] Thymidine, 7.5 μCi/dish, was present for 24 h prior to fixation. The dishes were covered with emulsion (Kodak NTB3) and developed after 10 days. The labelling index was determined by counting a total of 1000 cells per dish. The glucose-6-phosphate dehydrogenase positivity clearly showed that the cultured cells were parenchymal hepatic cells [18].
Hormone studies
The plasma levels of immunoreactive insulin (IRI) were determined by radioimmunoassay using an insulin kit obtained from Serono Diagnostics (Brain-tree, MO). The detection limit of the assay was 5 μU/ml.
The plasma levels of immunoreactive glucagon (IRG) were determined with a glucagon kit obtained from Serono Diagnostics. This kit was specifically chosen for its high degree of accuracy, precision and specificity as has been reported [19,20]. Samples for the glucagon assay were collected in chilled tubes containing 500 units of a trypsin inhibitor (aprotinin) and 1.2 mg of sodium EDTA/ml of whole blood collected for assay. The detection limit for the assay was 15 pg/ml. The results were also expressed as insulin/glucagon molar ratio which was calculated as suggested by Muller [21] using the formula [IRI (μU/ml)/IRG (pg/ml)] × 23.33 = ratio. This formula assumes that insulin has a molecular weight of 6000 Da and a biologic activity of 25 units/mg. It also assumes that glucagon has a molecular weight of 3500 Da. Serum testosterone and estradiol were determined by specific radioimmunoassays as described previously [22].
Cimetidine determination
The blood level of cimetidine was determined with HPLC using the method of Mihaley et al. [23]. Blood was taken under ether anesthesia from three untreated sham and PH rats (control), nine sham-operated and nine PH rats treated with cimetidine as reported in Materials and Methods. In the untreated rats, the blood was taken at 8 a.m. and used as a control. In the sham-operated and the PH operated rats (three from each group), blood was taken 1, 2 and 3 h after the last injection of cimetidine on the second day of treatment.
Determination of aminotransferases in the medium
Hepatocytes were seeded as described above, and test compounds were added 3 h later (first medium change) at the concentrations indicated in Table 1. The medium, after 48 h of incubation, was centrifuged at 2000 × g for 15 min, and an aliquot of the supernatant was diluted with an equal volume of phosphate-buffered saline. Activity of alanine aminotransferase (ALT) (EC 2.6.1.2) was determined with a spectrophotometer (Perkin-Elmer Coleman 124D, Maywood, IL) by the lactate dehydrogenase coupling method as originally described by Karmen [24,25]. The activities of transaminase in the medium are expressed in U/ml. One unit of activity is defined as the amount of enzyme, which produces 1 μmol of NAD per min under the conditions of the assay procedure.
TABLE 1. Effect of Cimetidine, Ranitidine, Famotidine and Omeprazole on Viability, DNA Concentration and Transaminase Levels of Hepatocytes in ‘primary cultures’.
Hepatocyte isolation has been described under ‘Materials and Methods’. Cells were plated in MEM supplemented with 5% FCS and insulin (10−7 M). After 3 h attachment, the medium and floating cells were removed and serum-free MEM plus insulin (10−7 M) was added. Antisecretory agents were added as indicated. Cells were cultured for 72 h and processed every 24 h for viability and DNA determinations. ALT was determined and expressed as U/ml only for the dishes stopped after 72 h of incubation. For each time point, triplicate culture dishes were assayed. The results are expressed as the average from 9 dishes and 3 different animals (mean ± S.D.).
| 24 h | 48 h | 72 h | ALT (U/ml) | ||||
|---|---|---|---|---|---|---|---|
| Viability (%) | DNA (μg/dish) | Viability (%) | DNA (μg/dish) | Viability (%) | DNA (μg/dish) | ||
| Control | 95 ± 3 | 3.2 ± 0.2 | 97 ± 3 | 3.3 ± 0.2 | 98 ± 2 | 3.28 ± 0.3 | 9± 3 |
| Cimetidine 27 × 1C−6 g/ml | 97 ± 2 | 3.1 ± 0.2 | 98 ± 2 | 3.2 ± 0.3 | 98 ± 1 | 3.28 ± 0.2 | 11 ± 5 |
| Ranitidine 6.0 × l0−6g/ml | 98 ± 1 | 3.1 ± 0.3 | 97 ± 3 | 3.4 ± 0.35 | 96 ± 4 | 3.05 ± 0.4 | 10 ± 9 |
| Famotidine 2.1 × l0−6g/ml | 95 ± 2 | 3.2 ± 0.1 | 96 ± 1 | 2.95 ± 1.2 | 97 ± 1 | 3.08 ± 0.7 | 12 ± 3 |
| Omeprazole 1.8 × l0−6g/ml | 95 ± 1 | 3.2 ± 0.1 | 97 ± 2 | 2.9 ± 1.1 | 97 ± 2 | 3.3 ± 0.9 | 8± 2 |
Statistical study
The unpaired Student' s t-test was used for statistical analysis of the data using the program available on the Hewlett-Packard 9815S (Hewlett-Packard Co., Palo Alto, CA).
Results
Table 1 demonstrates the results of cimetidine, ranitidine, famotidine, omeprazole and saline treatment on the viability, DNA, and ALT of hepatocytes in primary cultures. As is reported in our previous paper [18], 98% of cells attached to the dish were hepatocytes. The H2 blocking agents were added to hepatocytes cultured in serum-free medium in the presence of insulin alone (10−7 M). This medium is optimal for preserving all the metabolic properties of hepatocytes without promoting proliferation. Moreover, it is ideal for metabolic as well as toxicological studies. The drug concentrations listed in Table 1 are 30 times higher than the maximum concentration found in the blood of patients and animals treated with a single therapeutic dose of H2 blockers [26–29]. There were no significant differences in the viability or DNA concentration of hepatocytes and transaminase in the medium in the presence or absence of the H2 blocking agents and omeprazole, suggesting that these drugs have no direct toxic effect on hepatocytes in culture.
Fig. 1 depicts the effect of various concentrations of cimetidine, ranitidine, famotidine and omeprazole on the proliferation of hepatocytes cultured for 48 h in serum-free medium. Conditions were the same as in the previous experiment, with the exception of an addition of EGF to the medium which promoted hepatocyte proliferation. None of these drugs affected the hepatocyte proliferation stimulated by EGF. Further confirmation of these results is reported in Table 2, which lists the concentration of DNA and the percent labelled nuclei index of hepatocyte proliferation. This stimulation of hepatocyte proliferation over that observed in the culture incubated in the presence of insulin alone was not affected by the presence of drugs. The same results were obtained when, under the same conditions, serum of PH or non-PH rats was added. Fig. 2 outlines the effect of various serum concentrations (10–50% final concentration) on the DNA synthesis of hepatocytes in culture. Serum was obtained from sham-operated animals which had received i.p. cimetidine, ranitidine, famotidine, omeprazole and saline. No statistical difference was found in DNA synthesis between any of these groups. The effect of serum, obtained from rats that underwent PH and received H2 blocking agents and omeprazole, on DNA synthesis and percentage of labelled nuclei is depicted in Fig. 3. The DNA synthetic activity of hepatocytes cultured in the presence of different concentrations of serum from hepatectomized rats treated with the drugs was compared with the values obtained using the serum from hepatectomized rats treated with saline. The serum of PH rats treated with cimetidine inhibited DNA synthesis and percent of labelled nuclei at all the concentrations of serum used. Although the inhibitory effect of cimetidine was partially covered by the different serum concentrations, the percent of the effect increased in relation to the amount of serum used (10% serum = 29% inhibition; 40% serum = 38% inhibition). The serum of PH rats injected with ranitidine inhibited hepatocyte proliferation only at the higher serum concentrations, i.e., 40–50%. No inhibitory effects were observed using the serum of PH animals treated with famotidine or omeprazole. The figure also shows the value of ALT in these conditions. No significant variation of ALT was observed in the presence of 40% serum concentrations.
Fig. 1.
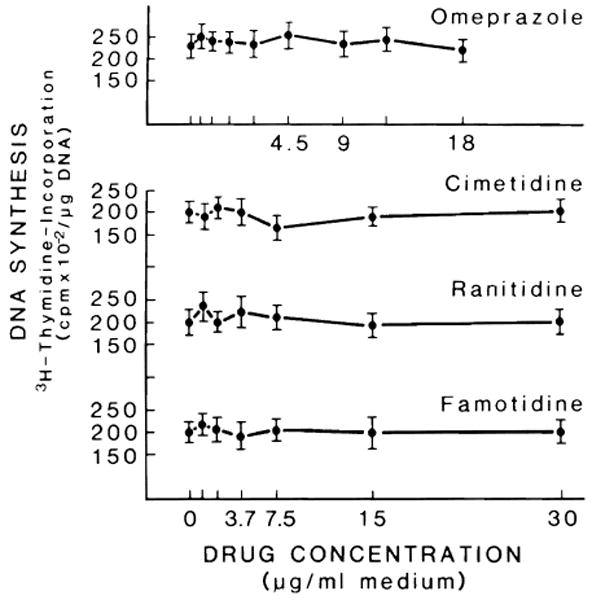
Effect of cimetidine, ranitidine, famotidine and omeprazole on DNA synthesis of hepatocytes in primary culture. Hepatocyte isolation has been described under ‘Materials and Methods’. Cells were plated in MEM supplemented with 5% FCS and insulin (10−7 M). After 3 h, the medium and floating cells were discarded and serum-free medium plus insulin (10−7 M), EGF (10 ng/ml) and proline (0.26 mM) were added. H2 blocking agents were added at different concentrations on the basis of their blood level after administration of a therapeutic dose; cimetidine, ranitidine, famotidine (0.62–30 μg/ml) and omeprazole (0.06–1.8 μg/ml). Cultures were exposed to 7.5 μCi [3H] thymidine for the last 24 h and processed after 48 h for determination of DNA synthesis. For each medium, triplicate culture dishes from 5 different animals were used. Results (mean ± S.D.) are expressed as cpm × 10−2/μg DNA.
TABLE 2. DNA Concentration and Labelling Index of Hepatocyte Cultures Grown in the Presence of Cimetidine, Ranitidine, Famotidine, and Omeprazole.
Culture conditions were as for Fig. 1. After 48 h of incubation, dishes were processed for DNA concentration and % labelled nuclei. Concentrations of H2 blocking agents were 30 times higher than the blood value found in patients injected with one pharmacological dose of the drugs. Cultures without H2 blocking agents were used as control. DNA concentration was expressed as μg/dish. The results are expressed as the average from 9 dishes and 3 different animals (mean ± S.D.). DNA in the labelled nuclei of hepatocytes incubated in the presence of insulin alone was 2.8 ± 0.33 μg/dish; percent of labelled nuclei in the same conditions was 4.5 ± 7.
| DNA (μg/dish) | Labelled nuclei (%) | |
|---|---|---|
| Control (saline) | 6.2 ± 1.2 | 10 ± 1 |
| Cimetidine 27 × 10−6g/ml | 5.2 ± 1.8 | 9± 2 |
| Ranitidine 6.0 × 10-6g/ml | 5.8 ± 1.2 | 10 ± 2 |
| Famotidine 2.1 × 10-6g/ml | 5.5 ± 2.0 | 12 ± 2 |
| Omeprazole 1.8 × 10-6g/ml | 5.9 ± 1.8 | 11 ± 3 |
Fig. 2.
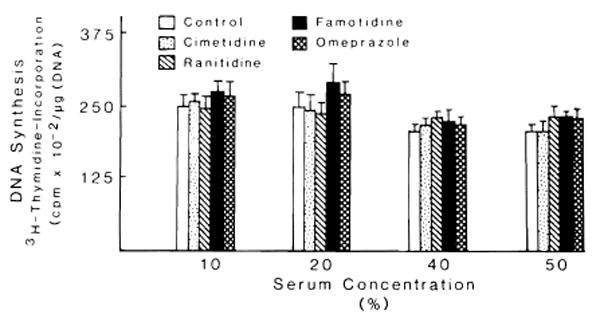
Effect of serum obtained from rats sham-operated and treated with H2 blocking agents and omeprazole on DNA synthesis of hepatocytes in primary culture. The experimental conditions are described in ‘Materials and Methods’. Culture conditions were as in Fig. 1, with the exception that serum from rats treated with or without (as control) H2 blocking agents were added instead of EGF. Four concentrations (10–50%) of serum were used. Results (mean ± S.D.) are expressed as cpm × 10−2/μg DNA. For each medium, triplicate culture dishes from 5 different experiments were used.
Fig. 3.
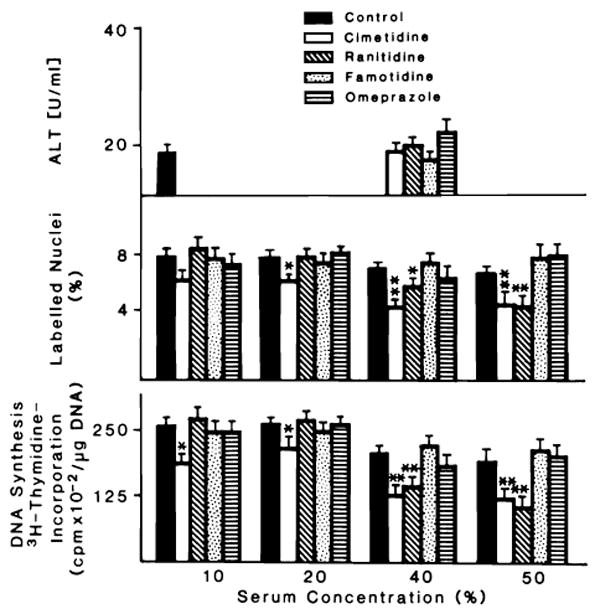
Effect of different concentrations of serum obtained from 70% hepatectomized rats treated or not with cimetidine, ranitidine, famotidine and omeprazole on DNA synthesis, % labelling index and ALT of hepatocytes in primary culture. The experimental conditions are described in ‘Materials and Methods’. Culture conditions were as reported in Fig. 1, with the exception that serum from treated and not treated (as control) 70% hepatectomized rats was added instead of EGF. Four final concentrations (10, 20, 40 and 50%) of serum were used. Exposure to [3H] thymidine was for 24 h. Individual culture dishes were processed for autoradiography and DNA synthesis. A total of 1000 cells randomly selected were counted to obtain the percent of labelled cells. Results are presented as mean ± S.D. (DNA synthesis = cpm × 10−2/μg DNA; labelled nuclei = % of labelled nuclei; aminotransferase = U/ml). For each medium, triplicate culture dishes from 5 different experiments were used. Significantly different from the control value observed in each group: *P < 0.05; **P < 0.01.
Table 3 reports the values of glucagon, insulin, insulin/glucagon ratio, estrogen and testosterone in sham-operated rats and in rats who underwent a 70% PH 24 h before, treated and not treated with antisecretory substances. The significant variation due to the surgical procedures (P < 0.05) for insulin, glucagon and testosterone was not affected by the administration of H2 blocking agents or omeprazole. The hormonal determinations were done in five animals of each group.
TABLE 3. Determination of Insulin, Glucagon, IRI/IRG, Estrogen and Testosterone in Sham-Operated Rats and in PH Rats Treated in our Study with H2 Blocking Agents or Omeprazole.
| Glucagon (pg/ml) | Insulin (μU/ml) | IRI/IRG | Estrogen (pg/ml) | Testosterone (ng/ml) | |
|---|---|---|---|---|---|
| Sham rats | |||||
| control | 180 ± 56 | 27 ± 2 | 3.50 | 8.3 ± 3 | 1.7 ± 0.33 |
| cimetidine | 173 ± 68 | 24 ± 3 | 3.23 | 7.3 ± 2 | 1.3 ± 0.35 |
| ranitidine | 183 ± 22 | 18 ± 7 | 2.29 | 3.5 ± 5 | 1.4 ± 0.15 |
| famotidine | 198 ± 64 | 28 ± 2 | 3.29 | 10.3 ± 4 | 1.5 ± 0.40 |
| omeprazole | 193 ± 85 | 25 ± 3 | 4.10 | 6.4 ± 3 | 2.0 ± 0.50 |
| 70% PH rats | |||||
| control | 410 ± 110 | 20 ± 2.0 | 1.14 | 7.9 ± 2.1 | 0.30 ± 0.03 |
| cimetidine | 460 ± 150 | 24 ± 2.4 | 1.20 | 7.8 ± 3.0 | 0.95 ± 0.01 |
| ranitidine | 540 ± 100 | 29 ± 3.0 | 1.25 | 6.9 ± 4.0 | 0.18 ± 0.02 |
| famotidine | 480 ± 90 | 26 ± 1.5 | 1.26 | 9.3 ± 3.0 | 0.32 ± 0.03 |
| omeprazole | 420 ± 70 | 27 ± 7.2 | 1.50 | 8.1 ± 2.0 | 0.48 ± 0.04 |
Control, saline; cimetidine, 2.4 mg/rat; ranitidine, 0.8 mg/rat; famotidine, 0.11 mg/rat; omeprazole, 0.06 mg/rat.
Fig. 4 reports the level of cimetidine in sham-operated and PH rats treated with cimetidine as is reported in Materials and Methods. Cimetidine was not found in the blood of untreated sham and PH rats. Sham-operated treated rats have high blood levels of cimetidine 1 h after its administration, which decreases over a period of 3 h. In contrast, the blood level of cimetidine in PH-treated rats remained constant over the same period. We only studied the levels of cimetidine in these experiments since it demonstrated the most significant effect on regeneration. We did not measure cimetidine level immediately after surgery to avoid any influence of surgical stress and/or anesthesia in drug kinetics.
Fig. 4.
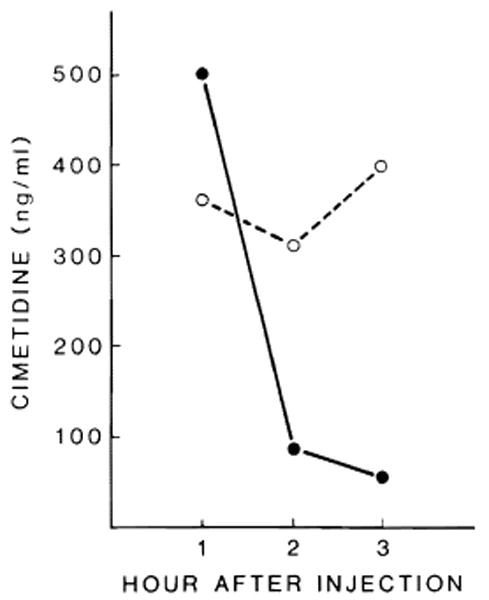
Cimetidine levels in sham-operated (●–––●) and PH (○– – –○) rats.
Discussion
Two commonly used H2 blocking agents, cimetidine and ranitidine, have been demonstrated recently to interfere significantly with DNA synthesis in a model of 70% partially hepatectomized rats [1,2]. Although the change (hemodynamics) produced by these agents was ruled out as a possible mechanism of action [2], no further attempts to define the basis of this observation have been reported. It is now well established that hepatoeytes in culture with serum-supplemented or serum-free medium can be maintained for several days and can be induced to synthesize DNA by providing certain hormones or growth factors [15,18,30–33]. This represents a model in vitro system to evaluate candidates which are proposed to interfere with hepatocyte proliferation. This system has been used in the present studies as the basis for defining the effect of three H2 blocking agents (cimetidine, ranitidine, famotidine) and a gastric secretion inhibitory agent (omeprazole).
The results reported here clearly demonstrate that first- (cimetidine and ranitidine) and second (famotidine)-generation H2 blocking agents and omeprazole do not exert any measurable direct control on hepatocyte proliferation in vitro. The addition of these agents, also when used at very high concentrations (30 times the active pharmacological dose found in the blood of patients treated with a single therapeutic dosage of the drugs), does not affect the viability, the DNA content or the proliferating activity of hepatocytes maintained in primary culture for a period of 72 h.
To further define their possible influence on hepatocyte proliferation, the effect of serum obtained from rats treated with H2 blocking agents or omeprazole was studied. Two sets of experiments were compared, one using the serum of sham-operated animals, the other using serum of 70% hepatectomized rats. The most significant results were observed when the serum of 70% hepatectomized rats treated with the drugs was added to the culture medium (Fig. 3). Serum from hepatectomized rats treated with cimetidine inhibited hepatic proliferation in a manner that was somewhat related to the concentration of serum used. Serum of PH animals treated with ranitidine resulted in inhibition of DNA synthesis only at very high concentrations, while the serum of PH animals treated with famotidine and omeprazole did not affect hepatic proliferation at any of the concentrations used. It is evident from these studies, therefore, that the significant inhibition of DNA synthesis and percent labelled nuclei that is modulated by cimetidine and ranitidine is not due to the actual drug but is manifested by their ability to induce some variation(s) in the composition of serum during the course of hepatic regeneration.
Many recent investigations and reviews have focused on the permissive effect, or possibly the initiating role, of hormones in hepatic regeneration [34–36]. Insulin and glucagon were the first two hormones whose so-called hepatotrophic action was associated with liver regeneration [37,38]. Since then, many studies have shown that other hormonal changes, especially those related to sex hormones, occur during the first 48 h of liver regeneration [39–49].
On the other hand, the ability of H2 blocking agents to interfere with the hormonal state has been well known since the beginning of the clinical use of cimetidine [50]. Variations of the hypothalamic-pituitary-gonadal axis have been reported during treatment with cimetidine which have been associated with increased serum prolactin levels, impotence and gynecomastia [51,52].
Only a few reports regarding these effects are described for ranitidine [51–54] while none have been reported for famotidine or omeprazole [55]. The data reported here demonstrate that insulin and glucagon as well as estrogen and testosterone blood levels do not change in the PH animal despite the treatment regimen imposed on the rats. Therefore, the inhibitory effect on hepatic proliferation is not due to the hormonal changes measured in these studies in the serum of PH rats. The influence of other hormones and growth factors (e.g., pituitary, thyroid, etc.) on the results reported here cannot be ruled out.
The only difference found by us was the higher level of cimetidine in the serum of PH rats compared with that observed in the serum of sham-operated rats. This difference suggests that either the liver has an important role in the catabolism of cimetidine or that increased blood cimetidine levels following PH are due to some degree of renal dysfunction.
Such mechanisms could be acting under the conditions described here; in fact, the effect found by us using serum from PH rats treated with cimetidine could be due to the metabolites of this substance.
These results are not in contrast to the demonstration that the H2 blocking agents, added directly to the hepatocytes cultured in vitro, have no effect in spite of the high concentration used. It is known that when hepatocyte proliferation is studied in vitro, one of the optimal conditions is low cellular density in the culture in order to prevent the phenomenon of the contact inhibition of proliferation. The amount of metabolite produced by such a low cellular population could not reach an effective concentration in the medium, capable of inhibiting hepatocyte proliferation. This same phenomenon could be true for ranitidine, which has a similar inhibitory activity of hepatocyte proliferation at a much higher concentration.
Based on these results, further experiments are in progress to assess hepatocyte proliferation in culture in the presence of cimetidine and ranitidine metabolites to completely clarify this phenomenon. In the interim, we emphasize that our results, confirming those found by Kanashima in vivo, focus attention on the need for caution and careful evaluation when this drug is administered to the patient immediately following hepatectomy.
Acknowledgments
Supported by Research Grants from the Veterans Administration and Project Grant No. AM-29961 from the National Institutes of Health, Bethesda, Maryland and by Grant No. 87/01291-44 from the Consiglio Nazionale dell Ricerche, Italy.
References
- 1.Kanashima R, Nagasue N, Furusawa M, et al. Inhibitory effect of cimetidine on liver regeneration after two-thirds hepatectomy in rats. Am J Surg. 1983;146:293–298. doi: 10.1016/0002-9610(83)90400-2. [DOI] [PubMed] [Google Scholar]
- 2.Kanashima R, Nagasue N, Sakato K. Ranitidine as an inhibitor of liver regeneration. Am J Surg. 1985;149:223–227. doi: 10.1016/s0002-9610(85)80074-x. [DOI] [PubMed] [Google Scholar]
- 3.Foster JH, Lawler MR, Welborn MB, et al. Recent experience with major hepatic resection. Ann Surg. 1968;167:651–668. doi: 10.1097/00000658-196805000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinkerton JA, Sawyers JL, Foster JH. A study of the postoperative course after hepatic lobectomy. Ann Surg. 1971;173:800–811. doi: 10.1097/00000658-197105000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartier F, Gauthier-Lafaye P, Lareng L, et al. Cimetidine in patients at risk of stress ulcers: a multicentre controlled trial. Intensive Care Med. 1980;6:54–62. [Google Scholar]
- 6.David E, Kelly KA. Acute postoperative peptic ulceration. Surg Clin North Am. 1969;49:1111–1120. doi: 10.1016/s0039-6109(16)38944-7. [DOI] [PubMed] [Google Scholar]
- 7.Speranza V, Basso N, Baragani M, et al. Prophylaxis of acute gastroduodenal mucosal lesions: H2 receptor antagonist in peptic ulcer disease and progress in histamine research. Eur Symp Italy. 1979:155–158. [Google Scholar]
- 8.MacDougall BRD, Bailey RJ, Williams R. H2 receptor antagonist and antacids in the prevention of acute gastrointestinal hemorrhage in fulminant hepatic failure. Lancet. 1977;i:617–619. doi: 10.1016/s0140-6736(77)92055-4. [DOI] [PubMed] [Google Scholar]
- 9.Eden K, Kern F., Jr Current status of cimetidine in upper gastrointestinal bleeding. Gastroenterology. 1978;74:466–467. [PubMed] [Google Scholar]
- 10.Paoluzi P, Torsoli A, Bianchi-Porro G, Lazzaroni M, Barbara L, Francavilla A. Famotidine (MK-208) in the treatment of gastric ulcer. Digestion. 1985;32:38–44. doi: 10.1159/000199260. [DOI] [PubMed] [Google Scholar]
- 11.Rune SJ, Bonnevie O, Nielsen AM, et al. Gastric acid secretion and duodenal ulcer healing during treatment with omeprazole. Scand J Gastroenterol. 1984;19:882–884. [PubMed] [Google Scholar]
- 12.Higgins GM, Anderson RM. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 13.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 14.Jirtle RL, Michalopoulos G, McLaine JR, et al. Transplantation system for determining the clonogenic survival of parenchymal hepatocytes exposed to ionizing radiation. Cancer Res. 1981;41:3512–3518. [PubMed] [Google Scholar]
- 15.McGowan JA, Bucher NRL. Pyruvate promotion of DNA synthesis in serum-free primary cultures of adult rat hepatocytes. In Vitro (Rockville) 1983;19:159–166. doi: 10.1007/BF02618054. [DOI] [PubMed] [Google Scholar]
- 16.Setaro F, Morley CGD. A modified fluorometrie method for the determination of microgram quantities of DNA from cell tissue cultures. Anal Biochem. 1976;71:313–317. doi: 10.1016/0003-2697(76)90043-9. [DOI] [PubMed] [Google Scholar]
- 17.Michalopoulos G, Houck KA, Dolan ML, et al. Control of hepatocyte replication by two serum factors. Cancer Res. 1984;44:4414–4419. [PubMed] [Google Scholar]
- 18.Francavilla A, Ove P, Polimeno L, et al. Epidermal growth factor and proliferation in rat hepatocytes in primary cultures isolated at different times after partial hepatectomy. Cancer Res. 1986;46:1318–1323. [PMC free article] [PubMed] [Google Scholar]
- 19.Kenny J, Say RR. Glucagon like activity extractable from the gastrointestinal tract of man and other animals. J Endocrinol. 1962;25:1–7. doi: 10.1677/joe.0.0250001. [DOI] [PubMed] [Google Scholar]
- 20.Chisholm DJ, Alford FP, Harewood MS, et al. Nature and biologic activity of ‘extrapancreatic glucagon’ studies in pancreatectomized cats. Metabolism. 1978;27:262–273. doi: 10.1016/0026-0495(78)90106-3. [DOI] [PubMed] [Google Scholar]
- 21.Muller WA, Faloona GR, Unger RH. The influence of the antecedent diet upon glucagon and insulin secretion. N Engl J Med. 1971;285:1450–1454. doi: 10.1056/NEJM197112232852603. [DOI] [PubMed] [Google Scholar]
- 22.Van Thiel DH, Gavaler JS, Cobb CF, Sherins RJ, Lester R. Alcohol induced testicular atrophy in the adult male rat. Endocrinology. 1979;105:888–894. doi: 10.1210/endo-105-4-888. [DOI] [PubMed] [Google Scholar]
- 23.Mihaley GH, Ockbain S, Jones DB, Hanson RC, Small-wood RA. High pressure liquid chromatographic determination of cimetidine in plasma and urine. J Pharm Sci. 1982;71:5909–5912. doi: 10.1002/jps.2600710528. [DOI] [PubMed] [Google Scholar]
- 24.Rej R, Horder M. Aspartate aminotransferase. In: Bergmeyer J, Grassl M, editors. Methods of Enzymatic Analysis. 3rd. III. Weinheim: Verlag Chemie; 1983. pp. 416–433. [Google Scholar]
- 25.Horder M, Rej R. Alanine aminotransferase. In: Bergmeyer J, Grassl M, editors. Methods of Enzymatic Analysis. 3rd. III. Weinheim: Verlag Chemie; 1983. pp. 444–456. [Google Scholar]
- 26.Rowlny-Jones D, Burland WL, Griffiths R. Pharmacokinetic and pharmacological properties of cimetidine. Proceedings of an International Symposium on Histamine H2 Receptor Antagonist; 10-11 November 1977; Göttingen, F.R.G. Amsterdam. [Google Scholar]
- 27.Woodings EP. Ranitidine H2 receptor antagonist. Gut. 1980;21:187–191. doi: 10.1136/gut.21.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan R. Clinical pharmacology of famotidine. Summary of data from the United States. Ital J Gastroenterol. 1984;16:171–174. [Google Scholar]
- 29.Regordh CG, Gabrielsonn M, Hoffman KY, Lofberg I, Skonteng I. Pharmacokinetics and metabolism of omeprazole in animal and man. An overview. Scand J Gastroenterol. 1985;20(suppl 108):79–94. doi: 10.3109/00365528509095821. [DOI] [PubMed] [Google Scholar]
- 30.Richman RA, Claus TH, Pilkis SJ, et al. Hormonal stimulation of DNA synthesis in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci USA. 1976;73:3589–3593. doi: 10.1073/pnas.73.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman DL, Claus TH, Pilkis SJ. Hormonal regulation of DNA synthesis in primary cultures of adult rat hepatocytes. Exp Cell Res. 1981;135:283–290. doi: 10.1016/0014-4827(81)90164-6. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa K, Watanabe K, Koga M. Induction of mitosis in primary cultures of adult rat hepatocytes under serum-free conditions. Biochem Biophys Res Commun. 1982;104:259–265. doi: 10.1016/0006-291x(82)91968-4. [DOI] [PubMed] [Google Scholar]
- 33.Michalopoulos G, Cianciulli HD, Novotny R, et al. Liver regeneration studies with rat hepatocytes in primary culture. Cancer Res. 1982;42:4673–4680. [PubMed] [Google Scholar]
- 34.Bucher NLR. Insulin, glucagon and the liver. In: Weber G, editor. Advances in Enzyme Regulation. Vol. 15. Oxford: Pergamon Press; 1976. [DOI] [PubMed] [Google Scholar]
- 35.Cahill GF., Jr Physiology of insulin in man. Diabetes. 1971;20:7857–7899. doi: 10.2337/diab.20.12.785. [DOI] [PubMed] [Google Scholar]
- 36.Francavilla A, Porter KA, Benichou J, et al. Liver regeneration in dogs. Morphologic and chemical changes. J Surg Res. 1978;25:409–419. doi: 10.1016/s0022-4804(78)80005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starzl TE, Porter KA, Francavilla A, et al. 100 years of the hepatotrophic controversy. In: Porter R, Whelan J, editors. Hepatotrophic Factors Ciba Foundation Symposium No 55. Amsterdam: Elsevier; 1978. [DOI] [PubMed] [Google Scholar]
- 38.Starzl TE, Francavilla A, Halgrimson CG, et al. The origin, hormonal nature, and action of hepatotrophic substances in portal venous blood. Surg Gynecol Obstet. 1973;137:179–199. [PMC free article] [PubMed] [Google Scholar]
- 39.Starzl TE, Francavilla A, Porter KA, et al. The effect upon the liver of evisceration with or without hormone replacement. Surg Gynecol Obstet. 1978;146:524–532. [PMC free article] [PubMed] [Google Scholar]
- 40.Leffert H, Alexander NM, Faloona G, et al. Specific endocrine and hormonal receptor changes associated with regeneration in adult rats. Proc Natl Acad Sci USA. 1975;72:4033–4036. doi: 10.1073/pnas.72.10.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leffert NL, Koch KS. Proliferation of hepatocytes. In: Porter R, Whelan J, editors. Hepatotrophic Factors Ciba Foundation Symposium No 55. Amsterdam: Excerpta Medica; 1977. [DOI] [PubMed] [Google Scholar]
- 42.Leffert HL, Koch KS, Moran T, et al. Hormonal control of rat liver regeneration. Gastroenterology. 1979;76:1470–1482. [PubMed] [Google Scholar]
- 43.Rixon RH, Whitfield JF. The control of liver regeneration by parathyroid hormone and calcium. J Cell Physiol. 1976;87:147–156. doi: 10.1002/jcp.1040870203. [DOI] [PubMed] [Google Scholar]
- 44.Canzanelli AD, Rapport D, Guild R. Control of liver regeneration and nucleic acid content by thyroid: with observations of the effects of pyrimidines. Am J Physiol. 1949;157:225–233. doi: 10.1152/ajplegacy.1949.157.2.225. [DOI] [PubMed] [Google Scholar]
- 45.Castellano TJ, Schiffman RL, Jacob MC, et al. Suppression of liver cell proliferation by glucocorticoid hormone: a comparison of normally growing and regenerating tissue in the immature rat. Endocrinology. 1978;102:1107–1112. doi: 10.1210/endo-102-4-1107. [DOI] [PubMed] [Google Scholar]
- 46.Russell WE, Bucher NLR. Vasopressin modulates liver regeneration in the Brattleboro rat. Am J Physiol. 1983;245:321–324. doi: 10.1152/ajpgi.1983.245.2.G321. [DOI] [PubMed] [Google Scholar]
- 47.Francavilla A, Di Leo A, Eagon PK, et al. Regenerating rat liver: correlation between estrogen receptor localization and deoxyribonucleic acid synthesis. Gastroenterology. 1984;86:562–567. [PMC free article] [PubMed] [Google Scholar]
- 48.Eagon PK, Porter LE, Francavilla A, et al. Estrogen and androgen receptors in liver: their role in liver disease and regeneration. Semin Liver Dis. 1985;5:59–69. doi: 10.1055/s-2008-1041758. [DOI] [PubMed] [Google Scholar]
- 49.Francavilla A, Eagon PK, Di Leo A, et al. Sex hormone related functions in regenerating male rat liver. Gastroenterology. 1986;91:1263–1270. doi: 10.1016/s0016-5085(86)80026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Funder JW, Mercer JE. Cimetidine, a histamine H2 receptor antagonist, occupies androgen receptors. J Clin Endocrinol Metab. 1979;48:189–191. doi: 10.1210/jcem-48-2-189. [DOI] [PubMed] [Google Scholar]
- 51.Pearce P, Funder JW. Histamine H2-receptor antagonist; radio-receptor assay for antiandrogenic side effects [Abstract] Clin Exp Pharmacol Physiol. 1980;7:442. [Google Scholar]
- 52.Penden NR, Boyd EJS, Browning MCK, et al. Effects of two histamine H2-receptor blocking drugs on basal levels of gonadotrophins, prolactin, testosterone and oestradiol 17β during treatment of duodenal ulcer in male patients. Acta Endocrinol. 1981;96:564–568. doi: 10.1530/acta.0.0960564. [DOI] [PubMed] [Google Scholar]
- 53.Lombardo L. Reversible amenorrhoea after ranitidine treatment. Lancer. 1982;i:224. doi: 10.1016/s0140-6736(82)90789-9. [DOI] [PubMed] [Google Scholar]
- 54.Delitala G, Devilla L, Pende A, et al. Stimulation of prolactin induced by ranitidine, and antagonist of H2-receptors in man. J Endocrinol Invest. 1980;3:12–16. [Google Scholar]
- 55.Walan A. Summary of the worldwide clinical experience with omeprazole; Abstracts from the Second International Symposium on Omeprazole: the 8th Congress of Gastroenterology World Congresses; Sao Paulo, Brazil. 1986. p. 14. [Google Scholar]


