Abstract
The 14th EGFL-repeat (Ten14) of human tenascin cytotactin activates the epidermal growth factor receptor (EGFR) with micromolar affinity; however, unlike EGF, Ten14-mediated activation of EGFR does not lead to receptor internalization. As the divergent signaling pathways downstream of EGFR have been shown to be triggered from plasma membrane and cytosolic locales, we investigated whether Ten14-mediated surface restriction of EGFR resulted in altered biochemical and cellular responses as compared to EGF. Molecules associated with migratory cascades were activated to a relatively greater extent in response to Ten14, with very weak activation of proliferation-associated cascades. Activation of phospholipase C γ (PLCγ) and m-calpain, associated with lamellipod protrusion and tail retraction, respectively, were noted at even at sub-saturating doses of Ten14. However, activation of ERK/MAPK, p90RSK, and Elk1, factors affecting proliferation, remained low even at high Ten14 concentrations. Similar activation profiles were observed for EGF-treated cells at 4°C, a maneuver that limits receptor internalization. We demonstrate a concurrent effect of such altered signaling on biophysical responses—sustained migration was observed at levels of Ten14 that activated PLCγ, but did not stimulate proliferation significantly. Here, we present a novel class of EGFR ligands that can potentially signal as a part of the extracellular matrix, triggering specific intracellular signaling cascades leading to a directed cellular response from an otherwise pleiotropic receptor. This work extends the signaling paradigm of EGFL repeat being presented in a restricted fashion as part of the extracellular matrix.
Many cell surface receptors elicit pleiotropic cellular responses when activated, although some of these responses might be mutually exclusive in any given or at a given time point. One prime example involves the epidermal growth factor receptor (EGFR), which upon ligandation, triggers cell migration and proliferation, two responses that do not occur simultaneously (Wells, 1999). How a cell distinguishes between these two outcomes likely involves differential activation of the myriad of intracellular signaling pathways that are activated by this receptor (Bhalla and Iyengar, 1999).
We have demonstrated previously that EGFR-mediated migration and proliferation are distinct cell responses that negatively impact each other; that is, when cells are driven to migrate, the fraction of the cell population undergoing proliferating diminishes (Chen et al., 1994a, 1996b). EGFR-induced motility requires the activation of phospholipase C-γ (PLCγ) (Chen et al., 1996a,b; Polk, 1998), whose activation negatively impacts EGFR-mediated cell proliferation. Interestingly, both proliferation and migration are downstream of extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK); however, motility requires ERK to be activated at the plasma membrane (Glading et al., 2001), while proliferation can be triggered by ERK at either the plasma membrane or from intracellular sites (Chen et al., 1994a; Wang et al., 2002). Additionally, cell proliferation requires ERK translocation into the nucleus along with other transcription factors such as Ets-related protein ELK1 and the 90-kDa ribosome S6 protein kinase (p90RSK) that are activated by intracellular ERK (Ebisuya et al., 2005; Rocks et al., 2006). Thus, cellular responses mediated by EGFR signaling appear to be triggered independently by the spatial separation of these key molecules. This mode of signal control adds an important dimension to controlling biophysical responses downstream of EGFR (Burke et al., 2001; Di Fiore and De Camilli, 2001; Ebisuya et al., 2005; Rocks et al., 2006).
EGFR is activated by soluble growth factors such as EGF, TGFα, amphiregulin, heparin-binding EGF, betacellulin and a few virally encoded factors (Citri and Yarden, 2006). These peptides bind with very high affinity and lead to internalization of EGFR, activating both motogenic and mitogenic cascades in the process (Wells, 2000). Ligand binding is key to internalization of EGFR—unliganded EGFR fail to internalize and activate downstream molecules from such locales (Haugh et al., 1999b).
This led us to speculate that a ligand that limits EGFR activity in a manner so as to trigger signaling selectively from the cell surface might elicit a different spectrum of responses than the classical soluble EGFR ligands such as EGF and TGFα. We and others recently demonstrated that a new class of ligands for EGFR—EGF-like (EGFL) repeats—can activate EGFR with binding modes qualitatively distinct from the classical soluble peptide ligands for EGFR (Swindle et al., 2001; Schenk et al., 2003). Select EGFL repeats of tenascin cytotactin, an extracellular matrix protein, bind with ultra-low affinity and a fast off-rate, leading to compartmentalization of active receptors at the cell surface without internalization of either receptor or ligand (Iyer et al., 2007). This direct activation of EGFR is distinct from the indirect activation that occurs secondary to integrin-mediated signaling elicited by other domains of these proteins ( Jones et al., 1997). This may be characteristic of binding of EGFL repeats in general (Schenk and Quaranta, 2003; Tran et al., 2004, 2005), allowing for a novel signaling mechanism distinct from classical growth factors.
We hypothesized that compartmentalization of liganded EGFR at the cell surface would lead to selective activation of intracellular cascades and that this would influence the overall cell response. Specifically, we postulated that in response to the transient nature of binding of EGFL repeats, EGFR would activate PLCγ and m-calpain at the cell surface, leading to enhanced migration but lacking the tonic intracellular activation of ERK that drives the cells toward proliferation. Our results indicate relatively robust activation of molecules associated with the migratory cascade downstream of EGFR in response to Ten14, leading to preferential activation of cell migration at concentrations of Ten14 that failed to stimulate proliferation. This work presents a novel mechanism by which ECM proteins containing EGFL repeats signal EGFR, leading to a more selective and directed cell response from a potentially pleiotropic receptor.
Materials and Methods
Expression and purification of EGF-like repeat proteins
Mid-log phase cultures of Escherichia coli strain BL21/DE3/pLys-S (Stratagene, La Jolla, CA) transformed with the individual expression plasmids were induced for recombinant protein expression (Ten14 or mEGF) with 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) for 3 h at 37°C. Bacteria were harvested by centrifugation for 10 min at 5,000g at 4°C, and bacterial lysates were prepared by extraction with 0.02 culture volumes of B-PER detergent (Pierce, Rockford, IL). Recombinant proteins were purified from bacterial lysates by nickel-agarose chromatography with imidazole elution as stated previously (Swindle et al., 2001). Purified protein was dialyzed against PBS, 0.25 mM β-mercaptoethanol for 24 h at room temperature.
Mitogenesis assay
Cells were quiesced for 24 h in quiescence medium (serum-free growth medium supplemented with 0.5% dialyzed fetal calf serum) (Swindle et al., 2001). The ligand-induced 3[H]-thymidine incorporation assay has been described previously (Chen et al., 1994a). In brief, after quiescence cells were exposed to EGF or various concentrations of EGF-like repeat proteins for 24 h. 3[H]-thymidine was added to the cells for the last 10 h to determine stimulation of proliferation. Mitogenesis assays were performed in tandem with in vitro wound healing assays with common stocks of ligand. Proliferation was assessed in the presence of both pharmacologic and molecular inhibitors for ERK/MAPK and PLCγ1, respectively.
Pharmacological abrogation of ERK/MAPK signaling was accomplished by preincubation for 30 min in the presence of 20 μM PD98059 (MEK inhibitor, Biosource, Camarillo, CA) following which media containing both ligand and inhibitor was added to the cells.
For molecular interventions, NR6WT cells were transfected with 20 μM of the Smartpool siRNA duplex against PLCγ1 (Dharmacon, Chicago, IL). Scrambled siRNA and control oligonucleotide sequences were used for PLCγ1 and ERK/MAPK, respectively, as controls. Knockdown of the respective substrate molecules was verified using immunoblotting using the appropriate antibodies.
In vitro wound healing assay
Basal and EGF-induced migration was assessed by the ability of the cells to move into an acellular area (Chen et al., 1994a). Cells were plated on a 24-well plastic dish and grown to confluence in DME with 7.5% FBS. After quiescence for 24 h in medium with 0.5% dialyzed FBS, an area was denuded by a rubber policeman. The cells were then treated with or without ligand at 37°C. Photographs were taken at 0 h and 24 h, and the distance traveled by the cells at the acellular front was determined. Motility assays were performed in tandem with mitogenesis assays with common stocks of ligand, and also tested for inhibitors of PLCγ1 and ERK/MAPK in a similar fashion as described above.
Cell-based calpain assay
NR6WT fibroblasts were plated with equal density and quiesced at 50% confluence in a Labtek II glass chamber (Nunc, Rochester, NY) and loaded for 20 min at 37°C with 50 μM Boc-LM-CMAC (Invitrogen, Carlsbad, CA), a synthetic calpain substrate (Glading et al., 2001). After loading, the cells were treated with growth factor for 10 min and then mounted on glass slides, and images of the Boc-LM-CMAC fluorescence were obtained using prefixed exposures to enable comparisons between specimens. Equal density of cells per field was obtained via inspection under light microscopy. The BOC substrate is designed so that calpain cleavage results in fluorescence. Following imaging, representative images were quantified for blue fluorescence (with at least 15 cells per field) and graphed using Microsoft Excel. For cells that were incubated at 4°C, cells were loaded with BOC-LM-CMAC, then preincubated at 4°C, and ligand added at 4°C for 5 min.
In order to specifically assess the role of m-calpain, similar experiments were performed in the presence of 10 μM CI-IV (Calbiochem, San Diego, CA), at this concentration a selective inhibitor of m-calpain. In addition, the experiment was performed separately in the presence of either 10 μM PD98059 (MEK inhibitor), 50 μM BAPTA, or both to assess the contributions of m-calpain and mu-calpain to the overall calpain activation. For all cases, quiesced cells were preincubated with inhibitors for 30 min before the addition of Boc-LM-CMAC and then treated with different concentration of EGF and Ten14 for 10 min.
Immunofluorescence assays
To assess localization of p90RSK and active (tyrosyl-phosphorylated) versus total EGFR, 10,000 NR6WT cells quiesced on glass coverslips and treated with increasing concentrations of EGF or Ten14. In order to assay for p90RSK activation, treatment was performed for 30 min whereas EGFR localization was assessed for ligand treatment for 20 min. For experiments performed at 4°C to observe active EGFR localization when endocytosis is blocked, the coverslips were preincubated on ice for 30 min prior to treatment, following which ligands were added on ice. After washing with cold PBS, cells were fixed in 4% paraformaldehyde for 30 min and lysed for 30 min with buffer containing 1% triton X-100, 1 mM PMSF, and 1 μg/ml aprotonin (all at final concentrations), followed by blocking in 5% BSA. In order to assess the localization of total versus phosphorylated EGFR, cells were incubated overnight at 4°C in a mixture of rabbit polyclonal anti-EGFR antibody (Santa Cruz Biotechnology, Santa Crux, CA) and mouse monoclonal phospho-EGFR antibody (Upstate, Chicago, IL), both at a final concentration of 2 μg/ml. After a brief wash in PBS containing 0.5% BSA, coverslips were incubated in a mixture of Alexa Fluor 647 anti-mouse secondary antibody, Alexa Fluor 488 anti-rabbit secondary antibody (both at 1 μg/ml) and 25 μg/ml propidium iodide at room temperature for 30 min. In order to assess the phosphorylation of p90RSK, cells were incubated overnight at 4°C in rabbit polyclonal phospho-p90RSK1 antibody (Cell Signaling Tech., Danvers, MA). After a brief wash in PBS containing 0.5% BSA, coverslips were incubated in Alexa Fluor 488 anti-rabbit secondary antibody (1 μg/ml) and 25 μg/ml propidium iodide at room temperature for 30 min. For both experiments, after a last wash, the slips were washed and mounted onto glass slides using gelvatol. After overnight drying, the slides were imaged for total fluorescence using a Zeiss Axioplan confocal laser-scanning microscope. Each image was scanned along the Z-axis in 7–10 sectional planes with 0.43 μm steps (512 × 512 pixels per sectional plane). Following imaging, representative images were quantified for green fluorescence (with at least 15 cells per field) using Adobe Photoshop ver. 6.0 and then graphed using Microsoft Excel.
In vivo MAP kinase luciferase assay
ERK/MAPK phosphorylation and activation of downstream substrate ELK1 in NR6WT murine fibroblasts was quantified using an MAPK in vivo kinase assay kit (Clontech, Mountain View, CA) according to the manufacturer’s protocol. Briefly, a pTet-ELK vector (50 ng/rxn) expressing a fusion protein with the functional domain of ELK and Tet repressor (TetR) domain and a luciferase reporter vector, pTRE-Luc (0.5 μg/rxn), containing a tet-responsive element (TRE) upstream of the luciferase gene, were transiently co-transfected into NR6WT cells seeded into 6-well plates using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as the transfecting agent. pTet-Neg (50 ng/rxn) and pTet-Off (50 ng/rxn) vectors, negative and positive control plasmids respectively co-transfected with pTRE-Luc, were also used in parallel. At 4 h post transfection, cells were washed in PBS and incubated with quiescent medium containing 0.25% dialyzed FBS for 3 h. Increasing concentrations of either EGF (10, 1, and 0.1 nM) or Ten14 (1, 0.1, and 0.01 μM) were incubated for 15 min, 60 min, and 4 h. Following incubation in serum free medium, cells were harvested on ice and assayed for luciferase expression using Luciferase™ Reporter Assay System (Promega, Madison, WI) according to the manufacturer’s instructions. Light emission was measured for 20 sec with LB Lumat 9505 luminometer (Berthold Tech., Oakridge, TN). The experiment was also performed either in the presence of 10 μM PD153035, a reversible inhibitor for EGFR, or in the presence of 2 μg/ml doxycycline which inhibits translation of the vectors, with both conditions serving as negative controls.
Phosphorylation assay
NR6WT cells, grown to 80% confluence, quiesced for 24 h, and then were stimulated with indicated levels of EGF or Ten14 alone for 5 min at 37°C or 4°C. Cells were then lysed with sample buffer and separated on 7.5–10% SDS–PAGE and immunoblotted for the indicated proteins. These were PLCγ1 tyrosyl-phosphorylated at either Y783 or Y1253 (Santa Cruz, CA), ERK/MAPK dually phosphorylated (Upstate Biotechnology, Boston, MA), or ∝-actin (Sigma, St Louis, MO).
Results
Ten14 exhibits differential activation of signaling cascades downstream of EGFR due to surface restriction of receptor
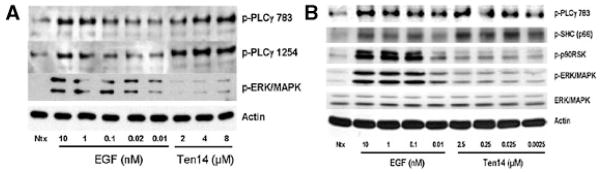
We have previously demonstrated that at least some of the EGFR-mediated signaling pathways stimulating cell migration and proliferation are separable (Chen et al., 1994a). These diverge at the immediate and intermediate post-receptor stage, with motility requiring direct phosphorylation and activation of PLCγ (Chen et al., 1994b) and indirect activation of m-calpain secondary to plasma membrane-associated ERK (Glading et al., 2000). As the low affinity and rapid off-rate of Ten14 detaches it prior to internalization of EGFR and restricts active EGFR to the cell surface (Iyer et al., 2007), we investigated whether such binding results in a biochemical activation profile different from that of classical soluble ligands such as EGF. Both PLCγ and ERK/MAPK were robustly activated and modulated in a dose-dependent manner in response to EGF, as expected (Fig. 1). For Ten 14, we observed strong activation of PLCγ as determined by phosphorylation at both the activation site, Y783, and the IP3 formation site, Y1254 (Nishibe et al., 1990; Kim et al., 1991). However, the phosphorylated, active ERK remained at near basal levels. Phosphorylation of the cytosolic target of ERK, p90RSK, was similarly absent. As the majority of active ERK derives from endosome-associated ERK (Haugh et al., 1999a), this dichotomy in signaling suggested that Ten14 binding leads to parsing of the signaling cascade downstream of EGFR.
Fig. 1.
Ten14 stimulates PLCγ1 preferentially over ERK/MAPK. Quiesced NR6WT cells were stimulated with indicated doses of EGF or Ten14 for 5 min at 37°C. Cells were then lysed, proteins separated through SDS–PAGE gels, and immunoblotted for the indicated proteins. Shown are representative blots of at least four for each analysis. A: Both Ten14 and EGF simulate PLCγ1 to equivalent levels at comparable concentrations of ligand. However, ERK/MAPK is weakly phosphorylated even at the high concentrations of Ten14. B: PLCγ1 and SHC are robustly activated even by nanomolar levels of Ten14. However, unlike EGF, the phosphorylation of ERK/MAPK and p90RSK remains weak even at micromolar concentrations of Ten14.
We had previously demonstrated that Ten14 fails to drive internalization of EGFR even at high ligand concentrations (Iyer et al., 2007). Therefore, we proposed that this differential activation of molecules downstream of EGFR was due to pools of active EGFR compartmentalized at the cell surface in response to Ten14. The biochemical profile observed in response to Ten14 models similar ERK signaling as seen after inhibition of EGFR internalization via mutation of the endocytic pathway or artificial obstruction (Vieira et al., 1996; Daaka et al., 1998; Pierce et al., 2000; Tong et al., 2000; Di Fiore and De Camilli, 2001). Therefore, we assessed the signaling activation profiles in response to EGF under physical conditions that limit EGFR internalization (Fig. 2A). At 4°C, the biochemical signaling profile of EGF was strikingly similar to Ten14 at 37°C, with weak activation of ERK even at saturating levels of EGF (10 nM). This difference was not a result of impaired signal transduction through EGFR as evinced by the phosphorylation of PLCγ and Src homologous and collagen-like (SHC) protein which is the initial, immediate post-receptor adaptor leading to ERK activation. Thus, the differential signaling profile seemed to be driven primarily by the restriction of active EGFR at the cell surface. In order to verify this effect of compartmentalized receptor activation and signaling, we assessed localization of total and activated EGFR for cells treated with increasing concentrations of EGF at 4°C. Not surprisingly, we found that active EGFR now compartmentalized primarily on the cell surface with EGF treatment (Fig. 2C,D), supporting our contention that the differential response at 4°C is driven due to the inability of EGFR to be internalized. It is important to note that the phosphorylated form of PLCγ has been shown to associate mainly with surface-associated EGFR (Haugh et al., 1999b; Matsuda et al., 2001). Also, we infer that the ERK being activated by Ten14, albeit at low levels, is part of pool of membrane-proximal ERK, and this could result in activation of signaling cascades qualitatively distinct from endosomal ERK/MAPK signaling (Glading et al., 2001).
Fig. 2.
Ten14 exerts its effects mainly by the compartmentalization of EGFR at the cell surface. NR6WT cells were quiesced, and then stimulated with indicated doses of EGF for the set time points or 5 min at 37°C and 4°C.Cells were then lysed, proteins separated through SDS–PAGE gels, and immunoblotted for the indicated proteins. Shown are representative blots of at least four for each analysis. For immunofluorescence studies, NR6WT cells were treated with EGF at 4°C and assayed for EGFR localization. A: Over a 5 min period, EGF leads to robust dose-dependent activation of ERK/MAPK at 37°C, accompanied by phosphorylation of PLCγ1 and SHC. However, at 4°C, the activation profile for EGF-treated cells changes, with increased phosphorylation of PLCγ1 and SHC and very low levels of ERK/MAPK activation. This profile is strikingly is similar to that observed with Ten14 at 37°C (see Fig. 1B). B: A differential activation profile is observed for EGF-treated cells over a period of 30 min, where the robust activation of ERK/MAPK seen at 37°C is lost at 4°C, but increased phosphorylation of PLCγ1 is observed. C,D: For cells treated with EGF at 4°C, active EGFR is localized predominantly at the cell surface, suggesting that the effects observed with Ten14 at 37°C and EGF at 4°C (right part) is driven mainly through compartmentalization of active EGFR at the plasma membrane.
Ten14 differentially activates divergent signaling from ERK downstream of EGFR
In order to delineate the effects of such divergent EGFR-mediated signaling at the cell surface in response to Ten14, we selected key downstream molecules that would transduce the differential signaling effects of ERK depending on signaling locale. Signaling downstream from ERK has been shown to bifurcate at the cell surface, with plasma membrane-associated ERK leading to activation of m-calpain (Glading et al., 2001) and cytosolic ERK predominantly activating p90RSK and ELK1 (Gille et al., 1995; Brunet et al., 1999), with the latter transiting to the nucleus (Brunet et al., 1999; Hochholdinger et al., 1999).
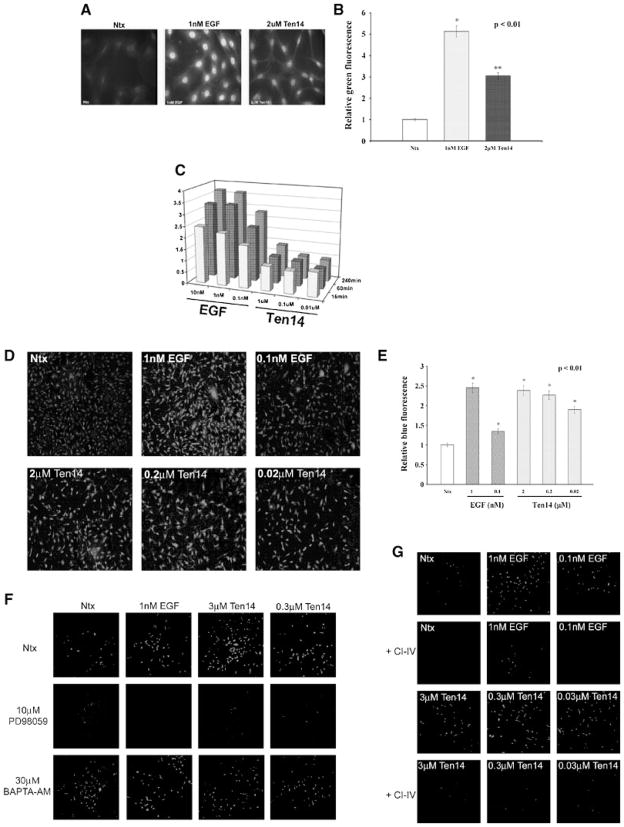
We assessed the activation of p90RSK (Fig. 3A,B) and ELK1 (Fig. 3C), both transcription factors that signal to promote cell proliferation in response to robust activation of ERK, particularly that of endosomal ERK (Wang et al., 2002). EGF resulted in strong activation of ELK1 as expected, as measured by the expression of the luciferase reporter that was placed under the action of ELK1. However, we observed only near basal levels of ELK1 activity for all concentrations of Ten14 over a 4 h time period. Using a reversible pharmacologic inhibitor of active EGFR (PD153035), we observed a downregulation of ELK1 activation for both EGF and Ten14, confirming that ELK1 activation occurred in an EGFR-dependent manner in our system (data not shown). Moreover, we observed weak activation of p90RSK for Ten14 as assessed by immunofluorescence and immunoblotting (see Fig. 1).
Fig. 3.
Differential activation of signaling effectors by Ten14 is preferential for motility-associated events. A,B: Quiesced NR6WT cells plated on cover-slips were assayed for phosphorylated p90RSK. (A) We observe much lower levels of active p90RSK with Ten14 as compared to EGF. Shown are representative immunofluorescent images (grayscale) of three separate experiments. (B) EGF increased phosphorylated p90RSK staining 5.14 ±0.25-fold versus 3.04 ±0.09-fold for Ten14 (P < 0.05) as determined by quantification of fluorescent intensity of 15 randomly selected cells in one randomly selected experiment. C: NR6WT cells transiently co-transfected with the pTet-ELK and pTRE-LUC reporter vectors were incubated with increasing concentrations of either EGF (10, 1, 0.1 nM) or Ten14 (1, 0.1, 0.01 μM) over increasing periods of time, with control plates receiving no ligand. Cells were harvested and assayed for luciferase expression and data plotted as a value relative to basal levels. A dose response was observed over time for EGF, with even the lowest levels activating ELK robustly. However, Ten14 did not stimulate activation of ELK over basal levels. Shown is mean, normalized to luciferase activity in serum-free media for each time point, of experiments at least four time points for each stimulation/time point (each in triplicate); s.e.m. is <10% of value and are not shown to limit visual clutter. At all time points Ten 14 is statistically different from EGF, and only at the highest concentration (1μM Ten14) is Ten14 statistically different from background (P < 0.05). D,E: Cells were incubated with 50 μM BOC-LM-CMAC for 15 min at 37°C. Various concentrations of EGF and Ten14 were added and cells were incubated at 37°C for 15 min. Calpain activity was assessed by fluorescence microscopy. (D) We observe robust activation of calpain even at 0.02 μM, whereas calpain activity for 0.1 nM is lower, suggesting sustained activation of calpain with Ten14. Shown are representative immunofluorescent images of five separate experiments. (E) EGF at its highest concentration (1 nM) increased BOC fluorescence 2.45 ±0.09-fold which is similar to the 2.38 ±0.08-fold for Ten14 (P is not significantly different from each other though P < 0.01 compared to control for both ligands) as determined by quantification of fluorescent intensity of 15 randomly selected cells in one randomly selected experiment. However, at lower concentrations of each ligand, Ten14 stimulation led to greater BOC fluorescence than EGF (P < 0.01 comparing the two ligands). F: Cells were preincubated with either PD98059 or BAPTA for 1 h prior to addition of BOC-LM-CMAC and ligand treatment, and then assayed for calpain activity. PD98059 decreased the overall calpain activity observed for both ligands, whereas BAPTA did not have any significant effects. This suggests that the calpain cleavage observed in Fig. 3D is predominantly through ERK-mediated activation of m-calpain and not mu-calpain (imaged at 10× magnification). G: In order to confirm the ligand-induced activation of m-calpain, NR6WT were pretreated with CI-IV prior to the addition of BOC-LM-CMAC. After treatment with different ligand concentration, calpain activity was assessed. We observed significantly lower levels of calpain activation for cells treated with CI-IV, confirming that m-calpain was involved.
Interestingly, an important molecule in the motility-associated pathway downstream from ERK, m-calpain, was robustly activated in response to Ten14 (Fig. 3D,E). Boc-LM-CMAC is cleaved by both the calcium-sensitive mu-calpain and the phosphorylation-activated m-calpain forms of intracellular calpain (Glading et al., 2000). Therefore, we delineated the contributions of each isoform to overall Boc-LM-CMAC cleavage using pharmacologic inhibitors. We saw a reduction in overall Boc-LM-CMAC cleavage when cells were pretreated with PD98059, a specific ERK inhibitor, but no reduction in the signal when cells were preincubated with BAPTA, an intracellular calcium chelator (Fig. 3F). This suggests that m-calpain plays a predominant role, with mu-calpain not being an important contributor to the overall Ten14-mediated calpain activity via ERK. CI-IV, a selective inhibitor of m-calpain had similar effects to PD98059, confirming our results (Fig. 3G). Also, increased m-calpain activation was observed for EGF-treated cells at 4°C (data not shown), resulting from an increased pool of active ERK at the cell surface due to restriction of EGFR (see Fig. 2D). These data support a model of surface-restricted signaling of EGFR in response to Ten14 leading to preferential activation of molecules along the motility cascade as opposed to the mitogenic pathways.
Ten14 preferentially activates cell migration over proliferation
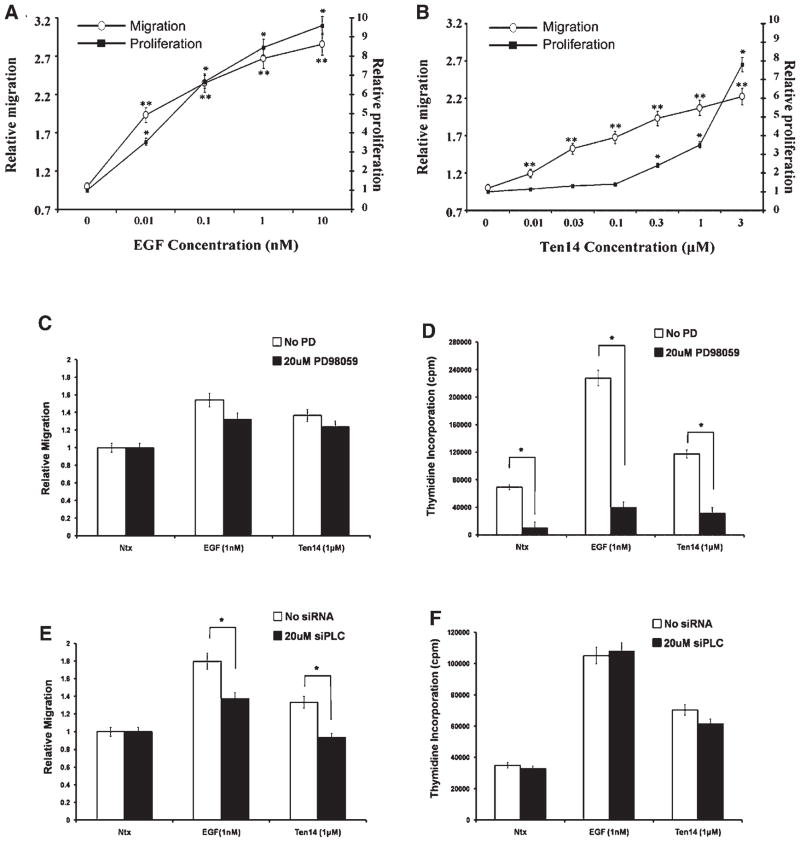
The foregoing data would suggest that cell responses would be different upon EGFR activation by EGF or Ten14 (Fig. 4). This should be most pronounced at limiting concentrations of ligand. As we titered out EGF, both motility and proliferation decreased in parallel. However, for Ten 14, motility was effected at concentrations that failed to stimulate proliferation over basal levels (Fig. 4B). Additionally we used both pharmacologic and biologic inhibitors against ERK and PLCγ1, respectively, to identify their contributions to the overall biophysical response. We observed that the inhibitor against ERK/MAPK were able to negate the proliferative effects of both Ten14 and EGF, but did not significantly affect migration (Fig. 4C,D). On the other hand, the inhibitor against PLCγ1 did not decrease proliferation, but significantly retarded Ten14-mediated migration (Fig. 4E,F). This suggested that Ten14 exerted its effects on the overall biophysical response by parsing the signaling of downstream molecules such as PLCγ1 and ERK, directed mainly by the compartmentalization of EGFR at the cell surface.
Fig. 4.
Ten14 activates migration at levels that fail to stimulate proliferation. Quiesced NR6WT cells were exposed to various concentrations of ligand. Proliferation (filled squares) and motility (open circles) were assessed as described and values plotted. Alternatively, cells were either incubated with PD 98059 or transected with siRNA against PLCγ1 before assessing migration and proliferation. A: For cells treated with EGF, we observed a linear dose response for both migration (open circles) and proliferation (filled squares) over different concentrations. Shown are mean ±s.e.m., normalized to treatment with diluent alone, of experiments performed at least 5 times (in triplicate). *for proliferation and **for migration denotes P < 0.05 compared to diluent alone. B: In the case of Ten14, we observed migration at levels of ligand that fail to promote proliferation, suggesting a separation in the migration/proliferation profile. However, at very high doses, the proliferative effects of Ten14 are enhanced. Shown are mean ±s.e.m., normalized to treatment with diluent alone, of experiments performed at least 5 times (in triplicate). *for proliferation and **for migration denotes P < 0.05 compared to diluent alone. C,D: Cells incubated with ERK/MAPK inhibitor PD98059 show a decrease in proliferation but no significant effects on proliferation for cells treated with EGF or Ten14 (P < 0.05). E,F: However, cells treated with siRNA against PLCγ1 demonstrated a significant decrease in migration without significant effects on proliferation, suggesting that the enhanced migration observed at subsaturating doses of ligand (see B) may be mediated mainly through activation of PLCγ1 (P < 0.05).
Though ERK-mediated cell proliferation can be driven by strong endosomal ERK/MAPK signaling, the signals required to generate mitogenesis need only be above a threshold and exigent over a long period of time (Reddy et al., 1998; Jones and Kazlauskas, 2001), and can be achieved by surface-restricted EGFR (Ebisuya et al., 2005). This would explain the increase in cell proliferation at higher concentrations of Ten14, where the threshold required for proliferation is being met more effectively than at lower levels of Ten14.
Discussion
EGFL repeats of tenascin-C have been shown to regulate a number of cellular responses including counteradhesion, cell rounding, growth suppression, and apoptosis in various cell types (Jones and Jones, 2000). Herein, we presented data demonstrating that activation of EGFR by an ultralow affinity matrikine signals to induce fibroblast motility preferentially over mitogenesis. This separation of cellular signaling is noted not only in the cell response but also in the biochemical switches that are key for these responses. What distinguishes the ultralow affinity matrikines, the EGFL repeats of tenascin-C and laminin (Swindle et al., 2001; Schenk et al., 2003), from the classic high affinity soluble peptide ligands, EGF, TGFα, and related ligands, is the mode of binding. These matrikines bind only transiently to the receptor, with the result that neither receptor nor ligand undergoes ligand-induced internalization (Iyer et al., 2007). Thus, essentially all of the EGFR signaling occurs from the plasma membrane locale. Based on the parsing of the signaling, we propose that plasma membrane-associated signaling of EGFR is preferential for motility. This finding provides a new mechanism by which a cell can select responses from otherwise pleiotropic signals.
The mode of binding of EGFL repeats to EGFR is akin to the interaction observed with other low affinity receptor classes such as the intergrin family of receptors binding to fibronectin. Although the biophysical response elicited in the latter case is adhesion (as opposed to migration with EGFL repeats), avidity of multiple ligand-receptor pairs rather than individual affinity is the critical property that drives the interaction between fibronectin and integrins (Carman and Springer, 2003). Interestingly, the interaction of EGFL repeats in ECM proteins, and certainly tenascin C, with EGFR could be potentiated by the fact that these matrix constituents have integrin-interacting domains. As such, by binding to two types of cell surface receptors simultaneously, the EGFL repeat–EGFR interaction may be prolonged. As studies have shown that cell responses depend not only on the identity of receptor activated, but also on the temporal nature of activation, this could have implications for the biological outcome of such receptor binding. While intriguing, these temporally integrated assessments lie beyond the scope of this article.
Herein, we have discussed the contribution of ERK/MAPK signaling to cell motility in terms of plasma membrane localized fraction that activates m-calpain. This does not reflect the total contribution that this ubiquitous intermediary signaling molecule(s) makes to cell motility. During haptokinetic motility, ERK/MAPK signaling drives MLC-actuated transcellular contractility required to bring the tail forward (Elicieri et al., 1998; Cheresh et al., 1999). Furthermore, the membrane-activated pool of ERK/MAPK does not signal exclusively to m-calpain activation and motility. Surface-activated EGFR can drive mitogenesis in a manner requiring ERK/MAPK, likely due to reaching a time-integrated threshold of signaling (Wells et al., 1990a,b; Vieira et al., 1996).
It goes without saying that many other molecules such as the GTPases Rho, Rac & Cdc42, and the protein focal adhesion kinase (FAK) may also be involved as part of the post receptor signal transduction pathways that contribute toward the overall response that we observe with EGFL repeats in our system. The fact that we are able to account for the observed responses (Fig. 4) using only PLCγ and ERK qualifies these molecules as critical rate-limiting factors in EGFL repeat signaling via EGFR, as expected from literature (Ridley et al., 2003; Hautaniemi et al., 2005; Kharait et al., 2006). In support of this, inhibitors against ERK and PLCγ were able to negate the effects on migration and proliferation observed in response to Ten14 (see Fig. 4). However, assaying for the other aforementioned molecules such as Cdc42 and its effect on cytoskeletal reorganization may further the understanding of the action of EGFL repeats, particularly in situations such as wound-healing and metastasis, where multiple factors influence the eventual outcome.
While we support the postulate that it is the plasma membrane locale that is preferential for motility, we must note that other differences may account for this outcome preference. Non-internalizing EGFR, due to elimination of AP-2-binding sites (Chen et al., 1989) or abrogation of dynamin-mediated internalization (Vieira et al., 1996) are fully capable of driving proliferation (Vieira et al., 1996; Wells et al., 1990a,b), though motility could not be determined in these cells due to lack of activation of key molecular switches such as PLCγ (Chen et al., 1994b). Further, not only does the transient ligandation result in predominantly plasma membrane-associated signaling, but matrikines also produce a highly staccato mode of signaling with the receptor avoiding activation-related downregulation (Iyer et al., 2007). These latter properties may also channel the signaling toward specific pathways. However, we cannot distinguish between these properties and the plasma membrane locales at present, as ligand or receptor engineering are not capable of separating these ligand-receptor binding properties. Physically tethering ligand may address this issue and is being actively pursued.
The mode of matrikine signaling is physiologically relevant, as the EGFL-containing tenascin-C displays a very discrete and transient pattern of expression during embryogenesis, wound healing, and tumor progression ( Jones and Jones, 2000). This pattern coincides with situations of increased motility of adherent cells. In such scenarios, EGFL repeats may also potentially bind multiple receptors simultaneously, thereby increasing the effective local concentration of these matrikines. This tonic increase of EGFL repeats may have a cumulative effect leading to an increase in the apparent affinity, thereby leading to enhanced physiological effects. In addition, EGFL-repeats of laminin are released as biologically relevant matrikines in vivo, where they stimulate the release of matrix metalloproteases during mammary gland involution, and potently trigger EGFR-mediated cell migration without significant activation of ERK/MAPK (Schenk et al., 2003). Thus, the appearance of tenascin-C would imply enhanced migration, whether physiological during (neo)organogenesis or pathological during tumor invasion and dissemination (Wells, 2000). Our finding not only sheds light on a basic biological question of how cells respond differently via the same receptor but also holds promise for tissue-engineering approaches to alter pathological wound repair and tumor invasion.
Acknowledgments
Contract grant sponsor: National Institute of General Medical Sciences (USA).
We thank Samantha L. Hess, Ali Jiwani, Christelle Akati, and Caitlin Q. Marlow for insightful discussions and protein purification assistance. We acknowledge Jean-Francois Hamel and BPEC for advice and biotechnology assistance. This work was supported by grants from the National Institute of General Medical Sciences (NIH).
Literature Cited
- Bhalla US, Iyengar R. Emergent properties of networks of biological signaling pathways. Science. 1999;283:381–387. doi: 10.1126/science.283.5400.381. [DOI] [PubMed] [Google Scholar]
- Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouyssegur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999;18:664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke P, Schooler K, Wiley HS. Regulation of epidermal growth factor receptor signaling by endocytosis and intracellular trafficking. Mol Biol Cell. 2001;12:1897–1910. doi: 10.1091/mbc.12.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman CV, Springer TA. Integrin avidity regulation: Are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15:547–556. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Chen WS, Lazar CS, Lund KA, Welsh JB, Chang CP, Walton GM, Der CJ, Wiley HS, Gill GN, Rosenfeld MG. Functional independence of the epidermal growth factor receptor from a domain required for ligand-induced internalization and calcium regulation. Cell. 1989;59:33–43. doi: 10.1016/0092-8674(89)90867-2. [DOI] [PubMed] [Google Scholar]
- Chen P, Gupta K, Wells A. Cell movement elicited by epidermal growth factor receptor requires kinase and autophosphorylation but is separable from mitogenesis. J Cell Biol. 1994a;124:547–555. doi: 10.1083/jcb.124.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Xie H, Sekar MC, Gupta K, Wells A. Epidermal growth factor receptor-mediated cell motility: Phospholipase C activity is required, but mitogen-activated protein kinase activity is not sufficient for induced cell movement. J Cell Biol. 1994b;127:847–857. doi: 10.1083/jcb.127.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Murphy-Ullrich JE, Wells A. A role for gelsolin in actuating epidermal growth factor receptor-mediated cell motility. J Cell Biol. 1996a;134:689–698. doi: 10.1083/jcb.134.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Xie H, Wells A. Mitogenic signaling from the egf receptor is attenuated by a phospholipase C-gamma/protein kinase C feedback mechanism. Mol Biol Cell. 1996b;7:871–881. doi: 10.1091/mbc.7.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh DA, Leng J, Klemke RL. Regulation of cell contraction and membrane ruffling by distinct signals in migrating cells. J Cell Biol. 1999;146:1107–1116. doi: 10.1083/jcb.146.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, Lefkowitz RJ. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- Di Fiore PP, De Camilli P. Endocytosis and signaling: An inseparable partnership. Cell. 2001;106:1–4. doi: 10.1016/s0092-8674(01)00428-7. [DOI] [PubMed] [Google Scholar]
- Ebisuya M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: Mechanisms for providing signaling specificity. J Cell Sci. 2005;118:2997–3002. doi: 10.1242/jcs.02505. [DOI] [PubMed] [Google Scholar]
- Elicieri BP, Klemke R, Stromblad S, Cheresh DA. Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J Cell Biol. 1998;140:1255–1263. doi: 10.1083/jcb.140.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb MH, Shaw PE. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 1995;14:951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glading A, Chang P, Lauffenburger DA, Wells A. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J Biol Chem. 2000;275:2390–2398. doi: 10.1074/jbc.275.4.2390. [DOI] [PubMed] [Google Scholar]
- Glading A, Uberall F, Keyse SM, Lauffenburger DA, Wells A. Membrane proximal ERK signaling is required for M-calpain activation downstream of epidermal growth factor receptor signaling. J Biol Chem. 2001;276:23341–23348. doi: 10.1074/jbc.M008847200. [DOI] [PubMed] [Google Scholar]
- Haugh JM, Huang AC, Wiley HS, Wells A, Lauffenburger DA. Internalized epidermal growth factor receptors participate in the activation of p21(ras) in fibroblasts. J Biol Chem. 1999a;274:34350–34360. doi: 10.1074/jbc.274.48.34350. [DOI] [PubMed] [Google Scholar]
- Haugh JM, Schooler K, Wells A, Wiley HS, Lauffenburger DA. Effect of epidermal growth factor receptor internalization on regulation of the phospholipase C-gamma1 signaling pathway. J Biol Chem. 1999b;274:8958–8965. doi: 10.1074/jbc.274.13.8958. [DOI] [PubMed] [Google Scholar]
- Hautaniemi S, Kharait S, Iwabu A, Wells A, Lauffenburger DA. Modeling of signal-response cascades using decision tree analysis. Bioinformatics. 2005;21:2027–2035. doi: 10.1093/bioinformatics/bti278. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Baier G, Nogalo A, Bauer B, Grunicke HH, Uberall F. Novel membrane-targeted ERK1 and ERK2 chimeras which act as dominant negative, isotype-specific mitogen-activated protein kinase inhibitors of Ras-Raf-mediated transcriptional activation of c-fos in NIH 3T3 cells. Mol Cell Biol. 1999;19:8052–8065. doi: 10.1128/mcb.19.12.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer AKV, Tran KT, Borysenko CW, Cascio M, Camacho CJ, Blair HC, Bahar I, Wells A. Tenascin cytotactin epidermal growth factor-like repeat binds epidermal growth factor receptor with low affinity. J Cell Physiol. 2007;211:748–758. doi: 10.1002/jcp.20986. [DOI] [PubMed] [Google Scholar]
- Jones FS, Jones PL. The tenascin family of ECM glycoproteins: Structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn. 2000;218:235–259. doi: 10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Jones SM, Kazlauskas A. Growth-factor-dependent mitogenesis requires two distinct phases of signalling. Nat Cell Biol. 2001;3:165–172. doi: 10.1038/35055073. [DOI] [PubMed] [Google Scholar]
- Jones PL, Crack J, Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the av b3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol. 1997;139:279–293. doi: 10.1083/jcb.139.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharait S, Dhir R, Lauffenburger D, Wells A. Protein kinase Cdelta signaling downstream of the EGF receptor mediates migration and invasiveness of prostate cancer cells. Biochem Biophys Res Commun. 2006;343:848–856. doi: 10.1016/j.bbrc.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Kim HK, Kim JW, Zilberstein A, Margolis B, Kim JG, Schlessinger J, Rhee SG. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-gamma 1 phosphorylation on tyrosine residues 783 and 1254. Cell. 1991;65:435–441. doi: 10.1016/0092-8674(91)90461-7. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Paterson HF, Rodriguez R, Fensome AC, Ellis MV, Swann K, Katan M. Real time fluorescence imaging of PLC gamma translocation and its interaction with the epidermal growth factor receptor. J Cell Biol. 2001;153:599–612. doi: 10.1083/jcb.153.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibe S, Wahl MI, Hernandez-Sotomayor SM, Tonks NK, Rhee SG, Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science. 1990;250:1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Maudsley S, Daaka Y, Luttrell LM, Lefkowitz RJ. Role of endocytosis in the activation of the extracellular signal-regulated kinase cascade by sequestering and nonsequestering G protein-coupled receptors. Proc Natl Acad Sci USA. 2000;97:1489–1494. doi: 10.1073/pnas.97.4.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk DB. Epidermal growth factor receptor-stimulated intestinal epithelial cell migration requires phospholipase C activity. Gastroenterology. 1998;114:493–502. doi: 10.1016/s0016-5085(98)70532-3. [DOI] [PubMed] [Google Scholar]
- Reddy CC, Wells A, Lauffenburger DA. Comparative mitogenic potencies of EGF and TGF alpha and their dependence on receptor-limitation versus ligand-limitation. Med Biol Eng Comput. 1998;36:499–507. doi: 10.1007/BF02523222. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Rocks O, Peyker A, Bastiaens PI. Spatio-temporal segregation of Ras signals: One ship, three anchors, many harbors. Curr Opin Cell Biol. 2006;18:351–357. doi: 10.1016/j.ceb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Schenk S, Quaranta V. Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol. 2003;13:366–375. doi: 10.1016/s0962-8924(03)00129-6. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hintermann E, Bilban M, Koshikawa N, Hojilla C, Khokha R, Quaranta V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindle CS, Tran KT, Johnson TD, Banerjee P, Mayes AM, Griffith L, Wells A. Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol. 2001;154:459–468. doi: 10.1083/jcb.200103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong XK, Hussain NK, Adams AG, O’Bryan JP, McPherson PS. Intersectin can regulate the Ras/MAP kinase pathway independent of its role in endocytosis. J Biol Chem. 2000;275:29894–29899. doi: 10.1074/jbc.M004096200. [DOI] [PubMed] [Google Scholar]
- Tran KT, Griffith L, Wells A. Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair Regen. 2004;12:262–268. doi: 10.1111/j.1067-1927.2004.012302.x. [DOI] [PubMed] [Google Scholar]
- Tran KT, Lamb P, Deng JS. Matrikines and matricryptins: Implications for cutaneous cancers and skin repair. J Dermatol Sci. 2005;40:11–20. doi: 10.1016/j.jdermsci.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- Wang Y, Pennock S, Chen X, Wang Z. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Mol Cell Biol. 2002;22:7279–7290. doi: 10.1128/MCB.22.20.7279-7290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A. EGF receptor. Int J Biochem Cell Biol. 1999;31:637–643. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- Wells A. Tumor invasion: Role of growth factor-induced cell motility. Adv Cancer Res. 2000;78:31–101. doi: 10.1016/s0065-230x(08)61023-4. [DOI] [PubMed] [Google Scholar]
- Wells A, Welsh JB, Lazar CS, Wiley HS, Gill GN, Rosenfeld MG. Ligand-induced transformation by a non-internalizing EGF receptor. Science. 1990;247:962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- Wells A, Welsh JB, Lazar CS, Wiley HS, Gill GN, Rosenfeld MG. Ligand-induced transformation by a noninternalizing epidermal growth factor receptor. Science. 1990;247:962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]