INTRODUCTION
Eosinophilic esophagitis (EE) is an increasingly recognized disorder characterized by an abnormal accumulation of eosinophils in the esophageal mucosa in patients with symptoms of refractory gastroesophageal reflux and dysphagia.1 While the pathogenesis remains unknown, initial studies in mice exposed to the aeroallergen Aspergillus fumigatus suggested a role for T cells and IL-5.2,3 Recent studies of RNA in human biopsy specimens have suggested a role for IL-13 and eotaxin-3 in recruitment of eosinophils.4 Once present, eosinophils may act in an autocrine fashion to perpetuate inflammation and decrease epithelial barrier function.5 An allergic trigger for this inflammation is suspected since a large proportion of patients have identifiable allergic sensitivities. 6,7 In spite of the high prevalence of specific allergic sensitivities and associated atopic diagnoses frequently found among EE patients, the relationship between EE and allergic sensitization is not straightforward.
The role of allergic sensitization in EE seems to mimic that which is seen in atopic dermatitis and asthma rather than traditional food allergy. To start with, the inciting food or inhaled allergen cannot be identified from the patient’s history. Individuals with EE, who have IgE antibody to food allergens, do not usually report immediate symptoms such as oral allergy syndrome, urticaria, or anaphylaxis. Though seasonal fluctuation in symptoms and eosinophil counts has been observed, the role of inhalant allergic triggers remains less clear.8,9 The mechanism of EE may not be a result of preformed mediator release from cross linking of mast cell bound IgE antibodies, and food (and inhalant) sensitivities have not been found in some of the patients. However, almost all patients have been shown to improve with complete dietary avoidance.10,11 This makes the proper identification of allergic triggers highly desirable.
Typically serum IgE antibody measurements have not been used in the measurement of food sensitivities in pediatric EE patients.1 The objective of the present study is to characterize the allergic sensitization found in pediatric EE patients by measuring specific IgE antibodies to common food and inhalant allergens (using CAP FEIA) and comparing the results with those of patch testing to foods and standard epicutaneous skin tests to foods and inhalants.
METHODS
Between January, 2007 and June 2009, patients referred to the Allergy Clinic at Nationwide Children’s Hospital (Columbus, Ohio) with biopsies positive for eosinophilic esophagitis (EE), as measured by ≥15 eosinophils/hpf, were randomly approached for participation in a cross sectional study. All parents (patients) (n=55) agreed to participate; however, blood could not be drawn on two patients, so they were not included in the analysis. This study was approved by the IRB, and all parents (and participants) provided written informed consent (and assent).
Questionnaires were administered to document EE symptoms and treatment as well as associated allergic diagnoses. Standard epicutaneous skin prick allergy tests were performed to a panel of 16 foods (egg, milk, wheat, soy, peanut, cashew, shrimp, beef, chicken, pork, rye, oat, corn, peas, tomato, and potato) and 38 common inhalant allergens using Sharp-Test Applicators (Panatrex, Placentia, CA).11 A wheal response ≥3 mm (in greatest diameter) larger than the negative control with surrounding erythema was considered positive. Total IgE and specific IgE antibodies to the eight most common foods identified by testing (egg, milk, wheat, soy, peanut, cashew, beef, and rye) and eight inhalant allergens (dust mite, cat, dog, mold mix, birch, timothy, weed mix, and ragweed) were measured using CAP FEIA (Phadia, Uppsala, Sweden). Milk proteins (α-lactalbumin, β-lactoglobulin, and casein), bromelain, Bet v 2 (profilin) and Candida albicans were also measured. Specific IgE to cross reactive carbohydrate determinants (CCD) and Helicobacter pylori were measured using Streptavidin CAP.12 Specific IgE measurements >0.35 IU/ml were classified as positive.
At a separate visit, patch testing to foods was performed regardless of the results of skin prick tests and serum IgE measurements (n=44). Patch testing materials were prepared using approximately 2 g dry food in 2 ml saline (milk, soy, egg, peanut, wheat, corn, oat, rice, rye, white potato, chicken, beef, pork, and lamb).13 Single ingredient baby food vegetables and fruits were used undiluted. The semisolid food preparations were placed in 12 mm Finn Chambers (Allerderm, Phoenix, AZ) secured on the back for 48 hours. Skin responses were read at 72 hours (the same investigator interpreted all of the tests). The results were graded incrementally based on the presence (or absence) of erythema and number of papules observed at each site. For this study, a response that included papules was considered positive.
The outcome of interest was evidence of sensitization based on the results of skin prick, CAP FEIA, and patch tests. Sensitization was classified as IgE mediated if either skin prick or CAP FEIA was positive. A positive patch test was considered a non-IgE mediated sensitization. Geometric mean titers of specific IgE to foods were measured and compared using ANOVA. The correlation between total IgE and titers of specific IgE was evaluated using Spearman rank. Kruskall Wallis X2 was used to evaluate the relationships between age, esophageal eosinophil counts, peripheral blood eosinophil counts, and total IgE in the subgroups of patients we identified (i.e. non-IgE sensitized, cow’s milk sensitized, and those with multiple food and pollen sensitivities). Statistical analyses were performed using SPSS 17 (Chicago, IL).
RESULTS
The demographic profile of the pediatric eosinophilic esophagitis (EE) patients included in this analysis is similar to that reported from other groups of patients that have been studied.14 Our patients ranged in age from 7 months to 18 years, with a median age at diagnosis of 9.0 years. A predilection for male gender was observed (75%), and a history of allergy medication use or diagnosis of asthma was reported by 70% of patients. The most frequently observed EE symptoms were dysphagia (51%), abdominal pain (45%), and vomiting (45%). Food impaction was reported by 36% of patients. Esophageal eosinophil counts ranged from 15 to 230/hpf, median 50/hpf. The median peripheral blood eosinophil count was 390/mm3 (Table 1).
Table 1.
Comparisons of atopic parameters† in three different phenotypic groups of pediatric EE patients.
| Patient group* |
|||||
|---|---|---|---|---|---|
| Parameter | Overall (n=53) |
Non-IgE sensitized (n=15) |
Cow’s milk sensitized§ (n=22) |
Multiple sensitivities§ (n=17) |
p value‡ |
| age (y) | 9 (2.9,13.6) | 9 (1.9,14) | 4.5 (2.2,11) | 13 (8.5,16) | 0.01 |
| esophageal eos/hpf | 50 (35,80) | 36 (30,89) | 50 (41,71) | 55 (41,71) | 0.6 |
| peripheral blood eos/mm3 | 390 (200,690) | 350 (130,530) | 460 (240,730) | 300 (200,540) | 0.3 |
| total IgE (IU/ml) | 64 (22,230) | 13 (6.8,28) | 120 (32,250) | 230 (150,480) | 0.001 |
| presented with obstruction (#) | 19 | 7 | 5 | 6 | ---- |
Results are presented as median (interquartile range).
Five patients did not fit any classification group. They had specific IgE to one or two foods or inhalants only. For this group median age= 4.6y, median esophageal eos=85/hpf, median peripheral blood eos=483mm3, and median total IgE=49 IU/ml, and one patient presented with obstructive symptoms.
Six patients had both specific IgE to cow’s milk and multiple sensitivities. They are included in the number of patients shown for each group but were not included in the statistical analysis for comparison among groups.
Statistical comparisons were made using Kruskall Wallis X2.
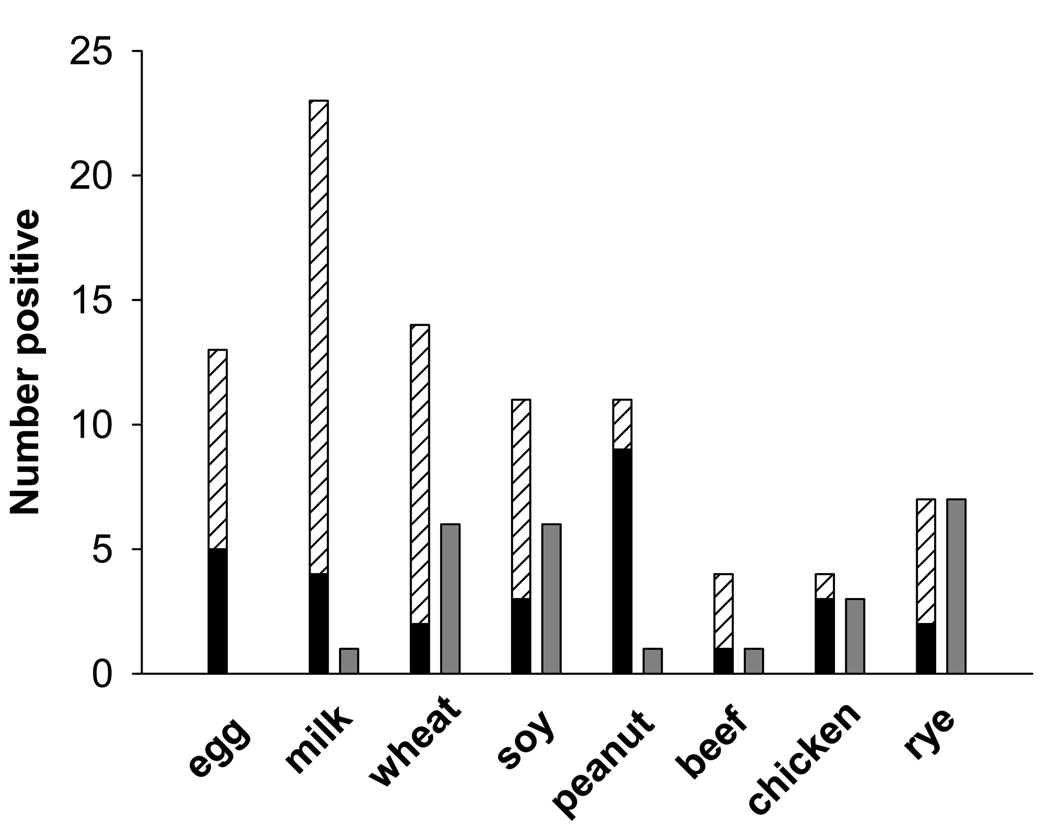
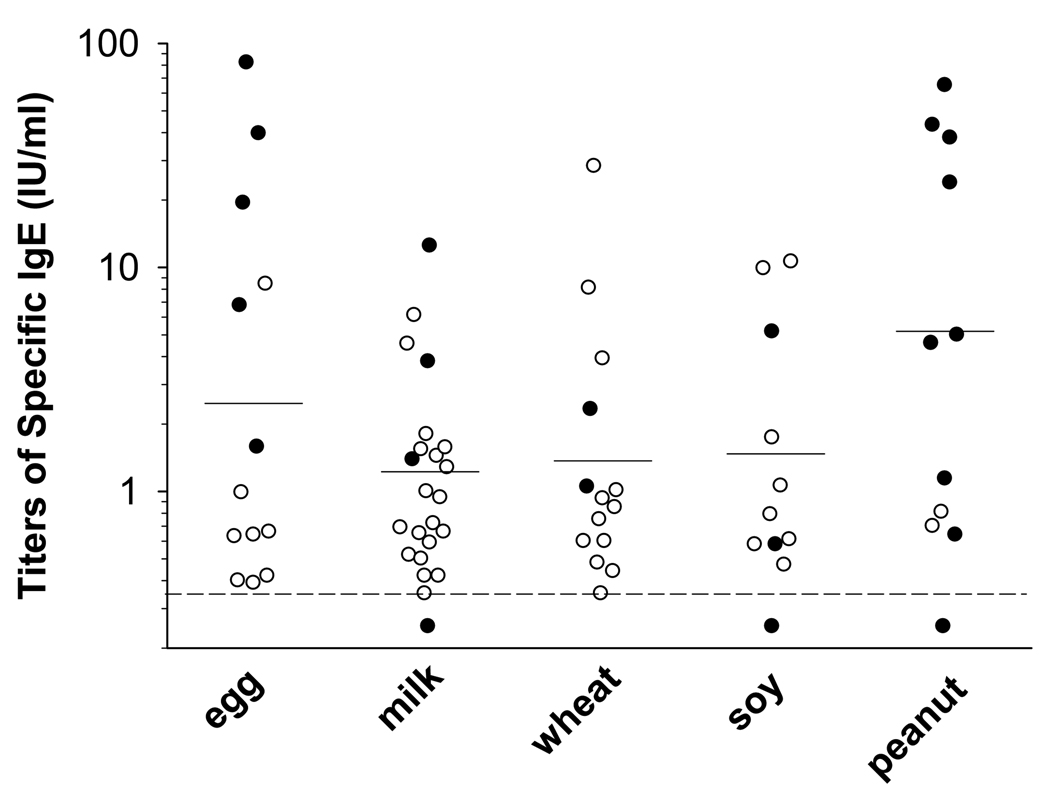
We evaluated IgE responses to food by both skin prick testing and serum specific IgE antibody measurement. Serum assays as compared with skin prick testing detected more positives, particularly with regard to milk, wheat, soy, and egg (Fig 1). Overall serum IgE measurements identified a previously undiagnosed food sensitivity in 42% of patients. The additional sensitivity most frequently recognized with serum assays was milk (36%). The prevalence of milk sensitivity identified by using both testing methods was 43%. The geometric mean titer of specific IgE to each food was low with egg at 2.4 IU/ml, milk at 1.2 IU/ml, wheat at 1.3 IU/ml, soy at 1.5 IU/ml, and peanut at 5.5 IU/ml. However in some cases, skin prick testing was negative and the titer of specific IgE measured was high (Fig 2). Furthermore, specific IgE titers were high relative to total IgE and for wheat, soy, and peanut, specific IgE was related to total IgE (Spearman r = 0.45, 0.57, and 0.61 respectively, p<0.01 for each).
FIG 1.
Food sensitivity using different testing methods. For milk, egg, wheat and soy, serum specific IgE measurement (hatched bars) detected more positives than skin prick testing (black bars) or patch testing (gray bars). Note that for each of the foods milk, soy, and peanut there was one patient who had a positive skin test but specific IgE <0.35 IU/ml.
FIG 2.
Titers of specific IgE to foods. In the cases of milk, wheat and soy, some patients with high titers of specific IgE had negative skin tests (open circles). Serum measurements in patients with positive skin tests are represented by filled circles.
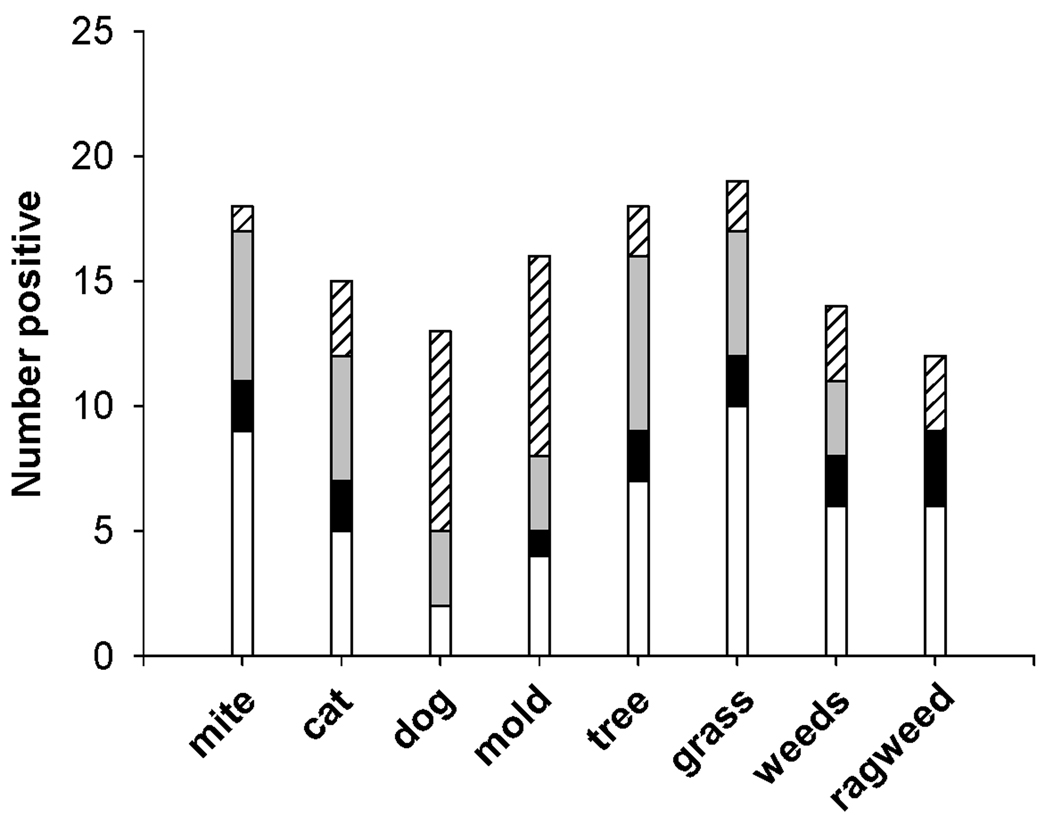
Inhalant sensitivities were measured using skin prick tests and a screening panel of specific IgE tests. Even in this pediatric population, inhalant sensitivity was found as frequently as foods (Fig 3). In keeping with that, multiple sensitization was common; the mean number of IgE mediated sensitivities per individual was 3.5. In spite of the finding of frequent multiple sensitivities, levels of total IgE for the whole group were not markedly elevated (median 64 IU/ml). Seventeen patients (32 %) had a cluster of multiple sensitivities (≥4) that included pollens, soy, grains, peanut, and/or tree nuts. When compared with the non-IgE sensitized and cow’s milk sensitized groups, the patients with multiple pollen and food sensitivities tended to be older and to have higher total IgE (Kruskall Wallis X2, p=0.01 and p<0.001 respectively) (Table 1).
FIG 3.
Inhalant sensitivities were measured by skin testing (all patients) and serum specific IgE measurement (in a subgroup). Patients with a positive skin test who did not have specific IgE testing are indicated by white bars. Patients in whom specific IgE was measured are represented by filled bars: black (skin test positive and serum testing negative), light gray (both tests positive), or hatched (skin test negative and serum testing positive). The number of positives detected by each testing technique varied by allergen.
Because of recent evidence that IgE antibody to both plant derived and mammalian derived cross reactive carbohydrate determinants (CCD) can give rise to positive serum assays with negative skin tests, we also tested the sera for specific IgE to CCD. The sera were tested for MUXF3 (using bromelain), galactose alpha-1,3-galactose (using cetuximab), and N-glycolylneuraminic acid. Two different patients had specific IgE to bromelain, and only one patient had specific IgE to galactose alpha-1,3-galactose (Table 2).
Table 2.
Prevalence of specific IgE to to cross reactive carbohydrate determinants (CCD) and infectious agents of the gastrointestinal tract.
| Non-IgE sensitized | Cow’s milk sensitized± | Multiple sensitivities¥ | |
|---|---|---|---|
| CCD≠ | |||
| MUXF3-bromelain | 0 | 1(0)‡ | 1(1) |
| Alpha-gal-cetuximab | 0 | 0(0) | 1(1) |
| H. pylori | 0 | 2(2) | 1(1) |
| C. albicans | 0 | 1(1) | 4(1) |
Seven serum samples with specific IgE to milk (>0.35 IU/ml) were tested for specific IgE to individual milk proteins (α-lactalbumin, β-lactoglobulin, and casein). All seven samples had measurable IgE to two or three proteins.
In seven subjects with multiple pollen sensitivities, specific IgE to profilin was measured and was negative.
IgE antibody to N-glycolylneuraminic acid was also measured. No positives were found.
Number in brackets indicates the number of the positive patients having a serum IgE measurement of Class 2 or above.
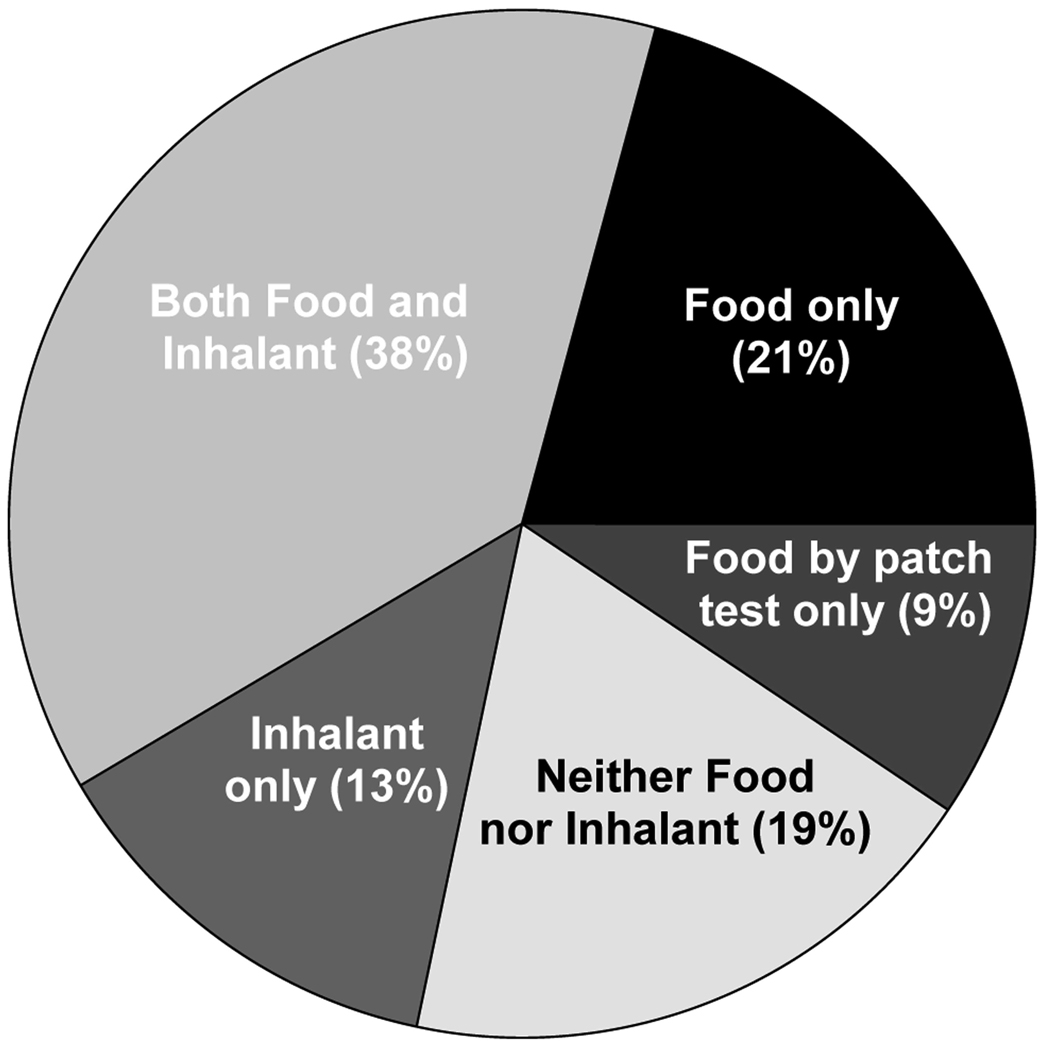
Patch testing was performed to identify non-IgE mediated food sensitivities. The results did not correlate with those found using skin or serum testing. Overall, patch tests were interpreted as positive in 17 patients (39%). The most common sensitivities noted by patch testing were rye (7/44), wheat (6/44), and soy (6/44). Other grains and meats were occasionally positive (Fig 1). Five patients who were otherwise non-sensitized had at least one positive patch test (Fig 4). The patients who were food sensitive by patch test(s) only (9%) were classified with the patients who had neither food nor inhalant sensitivities (19%) as non-IgE sensitized (Fig 4). Total IgE levels among the non-IgE sensitized patients were significantly lower (median 13 IU/ml) when compared with patients who had positive skin tests or serum IgE assays [median 150 IU/ml (Mann Whitney U 55, p<0.001)].
FIG 4.
Prevalence of allergic sensitivities among all EE patients as measured by using three available testing methods- serum specific IgE measurement, skin prick testing, and atopy patch testing.
Because of continuing confusion about the causes of EE and the presence of children without identifiable sensitization, we assayed sera for specific IgE to common commensal agents of the gastrointestinal tract. We selected C. albicans because it is a common organism in the esophagus and application of steroids to the esophagus is a known cause of increased Candidal growth. We also included H. pylori because of the high prevalence of H. pylori infection in patients with gastritis.15 Two sera were positive for specific IgE to H. pylori and five sera were positive for specific IgE to C. albicans (Table 2). None of the subjects classified as non-IgE sensitized had a positive test to either agent.
This was not designed as a treatment study; however, after finding a high prevalence of specific IgE to milk, clinical significance was examined retrospectively. For subjects with milk sensitivity, medical charts were reviewed for treatment recommendations as well as response to therapy (Table 3). Dietary avoidance of milk was recommended in 13 patients who had specific IgE to milk. Nine of these patients/parents reported clinical improvement and attributed it to the removal of milk from the diet. Repeat endoscopy and biopsy revealed <15 eos/hpf in 5/7 patients and showed improvement in 6/7 patients. Confounding factors included the common finding of multiple allergic sensitivities and concurrent use of medications.
Table 3.
Characteristics of patients with serum IgE antibody to milk in whom a milk elimination diet was attempted≠.
| Treatments |
|||||
|---|---|---|---|---|---|
| Patient | Specific IgE IU/ml(SPT*) |
Other allergic sensitivities‡ | Inhaled steroid§ | Clinical response to milk avoidance |
(Previous bx) Repeat bx |
| 1 | 12.5(4 mm) | >5 foods and inhalants | Y | no change | ND† |
| 2 | 6.12 | >5 foods and inhalants | Y | symptoms better | ND |
| 3 | 4.56 | beef, dust mite | N | no change | ND |
| 4 | 3.8(6 mm) | soy | N | no change | ND |
| 5 | 1.54 | egg | Y (stopped) | symptoms better | (60) 0 eos/hpf |
| 6 | 1.44 | none | Y | symptoms better | (26) 11 eos/hpf |
| 7 | 1.39(3 mm) | cat | N | symptoms better | ND |
| 8 | 1.28 | egg, wheat, rye | Y | symptoms better | (150) 1 eos |
| 9 | 1 | >5 inhalants | N | symptoms better | (160) 11 eos/hpf |
| 10 | 0.94 | cat | Y | symptoms better | (40) 25 eos/hpf |
| 11 | 0.59 | cat, grass | Y | no change | ND |
| 12 | 0.52 | >5 foods and inhalants | Y | symptoms better | (55) 50 eos/hpf |
| 13 | 0.42 | none | Y | symptoms better | (75) 0 eos/hpf |
Eight patients had serum IgE to milk >0.35; however, milk elimination was not attempted. These patients are not presented in this table.
Skin prick test response in patients with a positive result (mm of wheal in greatest diameter).
Other sensitivities identified by positive skin test or serum specific IgE.
Inhaled steroid was administered without a spacer and patients were instructed to swallow the medication.
ND indicates not done.
DISCUSSION
As a group, EE patients have an atopic phenotype with associated allergic rhinitis and/or asthma as well as frequent allergic sensitivities.1,6,7,16 Some investigators have suggested the importance of food triggers and others inhalants.9,2,16 The results of this study emphasize that a large proportion of pediatric EE patients have specific IgE to milk and that subgroups of EE patients can be identified and distinguished based on IgE responses. In this cohort of pediatric patients, three clinical groups were observed- non-IgE sensitized, milk sensitized, and those with multiple sensitivities (i.e. molds/ pollens/ grains/ legumes). Serum specific IgE measurements were helpful in detecting additional food sensitivities. However, even with extension of the battery of tests to foods, inhalants, CCD, and commensal organisms; there was a significant group of patients (20–30%) with no detectable sensitivity.
Serum specific IgE measurement as performed by CAP FEIA detected low levels of specific IgE to milk in 36% of patients studied. Furthermore in all serum samples with IgE to milk >0.35 IU/ml that were tested, specific IgE to two or more individual milk proteins (i.e. α-lactalbumin, β-lactoglobulin, and casein) was found. By contrast, only four patients had a positive standard skin prick test to this allergen. By using skin prick and patch testing, cow’s milk sensitivity, as a cause of symptoms, has been difficult to exclude in other groups of patients with EE. A negative predictive value of only 41% was reported when using the combination of skin prick and patch testing to diagnose milk sensitivity.17 Serum IgE measurement improves detection of milk sensitization. In our patients, specific IgE to milk ranged from 0.42 to 12.5 IU/ml. Clinically relevant titers in EE are not known; however, low levels of specific IgE to milk have been found to be relevant in patients with typical IgE mediated food hypersensitivity.18 Only for levels of specific IgE antibody to milk <0.8 IU/ml was the negative predictive value for having a clinical reaction >95%. Our study was not designed to evaluate response to treatment. However, the majority of patients who instituted a milk avoidance diet as a result of serum IgE measurement stated that this decreased their symptoms. Biopsy improvement was confirmed in most of the patients who had endoscopy. These data suggest that serum IgE antibody measurement may be helpful in the diagnosis of cow’s milk sensitivity in EE patients and that a subsequent trial of cow’s milk avoidance may provide clinical benefit.
As in studies of adults with EE, many of our patients with sensitivity to pollen by skin test also had low levels of specific IgE to wheat and soy.19 Though one small study in adults suggested that a six week elimination diet of grains (wheat and rye) did not result in decreased frequency of EE symptoms, the significance of these low levels remains unclear.20 It is possible that the low levels that we found using specific IgE measurement are consistent with existing data that suggest that most patients, even those without detected allergic sensitivities, respond to a strict elimination diet or a six food elimination diet that includes the foods most frequently associated with sensitization.8,21 It is also possible that low levels of serum specific IgE may be a reflection of local production of IgE in EE. There is now accumulating evidence for local production of IgE in tissues. Vicario et al detected mature IgE mRNA using RT-PCR on esophageal biopsies from EE patients.22 Recently it has been observed that B cell isotype switching can occur independent of interaction with CD40L.23 The molecule B cell activating factor (BAFF), which is of the TNF superfamily and produced by a variety of cells including epithelial cells and dendritic cells, can act as a second signal for class switching in the absence of CD40L interaction. Upregulation of BAFF has been measured in BAL fluid of asthmatic and allergic patients in response to allergen challenge.24 In the same study, eosinophils were the cells that accumulated most after challenge.24
One third of our patients had multiple pollen and mold sensitivities that were often associated with grain and legume sensitivity. This could not be explained by nonspecific binding as a result of elevated total IgE. In fact total IgE levels were only moderate as in earlier studies of allergic rhinitis.25 In spite of the relatively unimpressive total IgE measurements among this population with EE, total IgE was useful for distinguishing the subgroup of individuals without IgE mediated sensitization that had significantly lower levels. Furthermore, the predominance of inhalant sensitivities to molds and pollens in itself is striking. Studies of asthma have shown dust mite and cat to be the relevant associated allergic sensitivities.26,27 By contrast, pollen and mold sensitivities are more frequently associated with allergic rhinitis. Among this group of EE patients living in the Midwest with exposure to a variety of inhalant allergens including dust mite, pets, molds, and pollens, the prevalence of mold and pollen sensitivity is high compared with sensitivity to indoor allergens.
The consistent pattern of multiple associated sensitivities suggested that cross reactive carbohydrate IgE antibodies to foods and inhalants could be relevant among EE patients. Carbohydrate specific IgE has been reported for two basic types of glycans- N-linked and O-linked.28 N-glycans consist of a core that includes two N-acetyl-glucosamines and a mannose with additional possible linkages of α(1,3)-fucose and β(1,2)-xylose. These non-mammalian substitutions are highly immunogenic in humans and their presence in a wide variety of plants and invertebrates leads to a high degree of cross-reactivity. Clinically a subset of grass pollen allergic patients has been found to have IgE to peanut attributed to cross reactive carbohydrate determinants (CCD).29 Specific IgE to CCD was not a common feature in our patients (only three patients had detectable levels). We measured IgE to several plant N-linked glycans as well as the non-primate α-gal linkage.30 Alpha-gal has been linked to both anaphylaxis and delayed responses to mammalian derived food allergens.31 In the absence of specific IgE to CCD in this population, alternative explanations for the multiple associated sensitivities would be IgE antibody to cross reacting proteins or the independent development of IgE antibodies to many different epitopes. Interestingly, none of the seven serum samples taken from patients with multiple sensitization that we tested had specific IgE to profilin.
One of the limitations of this study, and most other studies of EE, is that food challenges were not performed to evaluate the eosinophilic response to food. Such an approach would be difficult for a variety of reasons. First, since EE patients often have a delayed response to foods, it would be hard to predict the timing of a response to food challenge. Another related issue is that a patient would have to be controlled (symptom free) to isolate a response to a single food. Furthermore, most patients had multiple food sensitivities and would require numerous procedures to assess individual food responses. Even the alternative of using skin biopsy after patch tests, commonly used with the atopy patch test in adults, has been considered too invasive in children.
EE is a mixed model of allergic disease which shares some features of atopic dermatitis and asthma as well as some features of allergic rhinitis. Similar to patients with allergic rhinitis, EE patients have total IgE usually within the normal range and frequent pollen and mold sensitivities. On the other hand, the delayed response to allergen exposure and suspected barrier defect are more consistent with atopic dermatitis and asthma. The results of this study suggest that a more directed approach to food avoidance may be beneficial. This could be accomplished by the addition of serum specific IgE testing to standard skin prick testing in order to obtain a full sensitivity profile. Once obtained, classification of an individual patient into the appropriate allergic grouping could be used to guide initial treatment recommendations.
Acknowledgments
Funding: National Institutes of Health grant K23AI059317and Davis Bremer Grant/The Ohio State University
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
| Study design | Data generation | Data analysis | Manuscript | |
|---|---|---|---|---|
| Erwin | X | X | X | X |
| James | X | X | X | |
| Gutekunst | X | X | X | |
| Russo | X | X | X | |
| Kelleher | X | X | X | |
| Platts-Mills | X | X | X | X |
References
- 1.Furuta GT, Liacouras CA, Collin MH, Gupta SK, Justinich C, Putnam PE, et al. Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–1363. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107:83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra A, Schlotman J, Wang M, Rothenberg ME. Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. J Leukocyte Biol. 2007;81:916–924. doi: 10.1189/jlb.1106653. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard C, Mingler MK, Vicario M, Abonia JP, Wu YY, Collins M, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292–1300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Fillon S, Robinson ZD, Colgan SP, Furuta GT. Epithelial function in eosinophilic gastrointestinal diseases. Immunol Allergy Clin North Am. 2009;29:171–178. doi: 10.1016/j.iac.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Rothenburg ME. Eosinophilic gastrointestinal disorders (EGID) J Allergy Clin Immunol. 2004;113:11–28. doi: 10.1016/j.jaci.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 7.Orenstein SR, Shalaby TM, Di Lorenzo C, Putnam PE, Sigurdsson L, Kocoshis SA. The Spectrum of pediatric eosinophilic esophagitis beyond infancy: A clinical series of 30 children. Am J Gastroenterol. 2000;95:1422–1430. doi: 10.1111/j.1572-0241.2000.02073.x. [DOI] [PubMed] [Google Scholar]
- 8.Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol. 2003;112:796–797. doi: 10.1016/s0091-6749(03)01715-9. [DOI] [PubMed] [Google Scholar]
- 9.Onbasi K, Sin AZ, Doganavsargil B, Onder GF, Bor S, Sebik F. Eosinophil infiltration of the oesophageal mucosa in patients with pollen allergy during the season. Clin Exp Allergy. 2005;35:1423–1431. doi: 10.1111/j.1365-2222.2005.02351.x. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol. 2003;98:777–782. doi: 10.1111/j.1572-0241.2003.07390.x. [DOI] [PubMed] [Google Scholar]
- 11.Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002;109:363–368. doi: 10.1067/mai.2002.121458. [DOI] [PubMed] [Google Scholar]
- 12.Erwin EA, Custis NJ, Satinover SM, Perzanowski MS, Woodfolk JA, Crane J, et al. Quantitative measurement of IgE antibodies to purified allergens using streptavidin linked to a high-capacity solid phase. J Allergy Clin Immunol. 2005;115:1029–1035. doi: 10.1016/j.jaci.2004.12.1131. [DOI] [PubMed] [Google Scholar]
- 13.Spergel JM, Andrews T, Brown-Whitehorn TF, Beausoleil JL, Liacouras CA. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol. 2005;95:336–343. doi: 10.1016/S1081-1206(10)61151-9. [DOI] [PubMed] [Google Scholar]
- 14.Assa’ad AH, Putnam PE, Collins MH, Akers RM, Jameson SC, Kirby CL, et al. Pediatric patients with eosinophilic esophagitis: An 8-year follow-up. J Allergy Clin Immunol. 2007;119:731–738. doi: 10.1016/j.jaci.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 15.Warren JR, Marshall BJ. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulcer ulceration. Lancet. 1983;i:1273–1275. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 16.Penfield JD, Lang DM, Goldblum JR, Lopez R, Falk GW. The role of allergy evaluation in adults with eosinophilic esophagitis. J Clin Gastroenterol. 2010;44:22–27. doi: 10.1097/MCG.0b013e3181a1bee5. [DOI] [PubMed] [Google Scholar]
- 17.Spergel JM, Brown-Whitehorn T, Beausoleil JL, Shuker M, Liacouras CA. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:509–511. doi: 10.1016/j.jaci.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997;100:444–451. doi: 10.1016/s0091-6749(97)70133-7. [DOI] [PubMed] [Google Scholar]
- 19.Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2008;6:531–535. doi: 10.1016/j.cgh.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 20.Simon D, Straumann A, Wenk A, Spichtin H, Simon HU, Braathen LR. Eosinophilic esophagitis in adults- no clinical relevance of wheat and rye sensitizations. Allergy. 2006;61:1480–1483. doi: 10.1111/j.1398-9995.2006.01224.x. [DOI] [PubMed] [Google Scholar]
- 21.Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, et al. Effect of six-food elimination diet of clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097–1102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Vicario M, Blanchard C, Stringer KF, Collins Mh, Mingler MK, Ahrens A, et al. Local B cells and IgE production in the esophageal mucosa in eosinophilic esophagitis patients. Gut. 2010;59:12–20. doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BlyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato A, Xiao H, Chustz RT, Liu MC, Schleimer RP. Local release of B cell-activating factor of the TNF family after segmental allergen challenge of allergic subjects. J Allergy Clin Immunol. 2009;123:369–375. doi: 10.1016/j.jaci.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 26.Erwin EA, Wickens K, Custis NJ, Woodfolk JA, Barry D, Crane J, et al. Cat and dust mite sensitivity and tolerance in relation to wheezing among children raised with high exposure to both allergens. J Allergy Clin Immunol. 2005;115:74–79. doi: 10.1016/j.jaci.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Sears MR, Burrow B, Flannery EM, Herbison GP, Holdaway MD. Atopy in childhood. I. Gender and allergen related risks for development of hay fever and asthma. Clin Exp Allergy. 1993;23:941–948. doi: 10.1111/j.1365-2222.1993.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 28.van Ree R. Carbohydrate epitopes and their relevance for the diagnosis and treatment of allergic diseases. Int Arch Allergy Immunol. 2002;129:189–197. doi: 10.1159/000066770. [DOI] [PubMed] [Google Scholar]
- 29.van der Veen MJ, van Ree R, Aalberse RC, Akkerdaas J, Koppelman SJ, Jansen HM, et al. Poor biologic activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. J Allergy Clin Immunol. 1997;100:327–334. doi: 10.1016/s0091-6749(97)70245-8. [DOI] [PubMed] [Google Scholar]
- 30.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 1008;358:1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Commins SP, Platts-Mills TAE. Anaphylaxis syndromes related to a new mammalian cross-reactive carbohydrate determinant. J Allergy Clin Immunol. 2009;124:652–657. doi: 10.1016/j.jaci.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]