FK 506, a powerful, new immunosuppressive agent, is currently undergoing clinical trials in transplant patients. Like cyclosporine (CyA), FK 506 suppresses interleukin-2 production and receptor expression on T cells.1,2 Additionally, both FK 506 and CyA are highly lipophilic.3 CyA is mainly transported by lipoproteins in plasma.4 The objective of the present study is to characterize the distribution of FK 506 in various plasma components.
MATERIALS AND METHODS
Materials
FK 506 powder drug, FK 506 peroxidase conjugate (FK-POD), and anti-FK 506 monoclonal antibody was a gift from Fujisawa Pharmaceutical Co., Ltd, Osaka, Japan. Goat anti-mouse polyclonal antibody was obtained from Atlantic Antibodies (Stillwater, MN). All necessary reagents were prepared with analytical grade chemicals. Sample extractions were performed using the Sep-Pak Cartridge Column (Waters, Milford, MA) method with an ALLTECH Manifold Assembly. Extracted samples were plated on a Linbro EIA-II Plus 96-well microtiter plate and absorbance readings were made using a Titertek Multiskan Plus microtiter plate reader (Flow Laboratories, McLean, VA) at 492 nm. Ultracentrifugations were performed in a Beckman L8-80 ultracentrifuge using a Ti80 rotor.
Methods
The distribution of FK 506 in plasma lipoproteins in vivo was studied in 13 transplant patients receiving an oral dose of FK 506 twice a day. Blood specimens, representing trough levels in these individuals, were warmed to 37°C for 1 hour, the erythrocytes were then separated from the plasma by centrifugation for 15 minutes at 1500 g and 37°C. The plasma samples from each patient were pooled and the lipoproteins were separated by ultracentrifugation using a sequential density gradient. Plasma very low-density lipoprotein ([VLDL] 1.006–1.019 g/mL), low-density lipoprotein ([LDL] 1.019–1.063 g/mL), and high-density lipoprotein ([HDL] 1.063–1.21 g/mL) were separated by standard differential ultracentrifugation techniques using appropriate density gradients.5 The remaining fraction was termed lipoprotein deficient serum (LPDS). The distribution of FK 506 among each fraction was determined using a two-step enzyme-linked immunoabsorption assay (ELISA) that utilizes a double antibody reaction.6 A standard curve was produced by the addition of FK 506, in methanol, to blood bank plasma to give final concentrations of 0, 0.1, 0.5, 1.0, 2.0, 5.0, and 10.0 ng/mL. The standards and patient samples were extracted using Sep-Pak columns. The bound FK 506 was eluted with methanol and then evaporated to dryness under nitrogen. The dried samples were then analyzed by the method described by Tamura et al.6 Plasma lipoprotein cholesterol in each of the patient lipoprotein fractions was measured by the Cobas Bio Chemical Analyzer (Roche, Nutley, NJ).
RESULTS
The distribution of FK 506 (Table 1) in plasma VLDL was 11 ± 3%; LDL, 3 ± 3%; HDL, 21 ± 8%; and the remaining in lipoprotein deficient (LPDS) fraction, 64 ± 8%. The plasma lipoprotein fractions were also analyzed for the relative percent distribution of cholesterol (Table 2). VLDL contained 12 ± 7%; LDL, 57 ± 8%; HDL, 27 ± 10%; and LPDS, 4 ± 2%. The FK 506 plasma concentration ranged from 0.25 to 12.8 ng/mL. However, there was no systemic change in the percent distribution of FK 506 into various plasma components indicating linear uptake over this concentration range.
Table 1.
Distribution of FK 506 in Plasma Lipoproteins in Transplant Patients
| Patient No. | FK 506 (ng/mL) |
Percentage of Distribution of Plasma Concentration |
|||
|---|---|---|---|---|---|
| Plasma | VLDL | LDL | HDL | LPDS | |
| 1 | 12.8 | 9 | 7 | 21 | 63 |
| 2 | 19.0 | 12 | 9 | 19 | 60 |
| 3 | 1.1 | 10 | 7 | 26 | 57 |
| 4 | 2.1 | 9 | 3 | 29 | 59 |
| 5 | 1.4 | 9 | 5 | 30 | 56 |
| 6 | 7.9 | 15 | 5 | 20 | 60 |
| 7 | 0.4 | 8 | ND | 26 | 66 |
| 8 | 1.8 | 9 | ND | 28 | 61 |
| 9 | 0.5 | 11 | ND | 15 | 72 |
| 10 | 0.3 | 8 | ND | 20 | 72 |
| 11 | 1.0 | 16 | ND | 21 | 62 |
| 12 | 0.7 | 11 | ND | NO | 84 |
| 13 | 1.9 | 11 | 5 | 19 | 65 |
| Mean = SD | 11 ± 3 | 3 ± 3 | 21 ± 8 | 64 ± 8 | |
Abbreviation: ND, nondetectable.
Table 2.
Distribution of Cholesterol in Plasma Lipoproteins in Transplant Patients
| Patient No. | Cholesterol (mg/dL) |
Percentage of Distribution of Plasma Concentration |
|||
|---|---|---|---|---|---|
| Plasma | VLDL | LDL | HDL | LPDS | |
| 1 | 103 | 13 | 62 | 22 | 3 |
| 2 | 127 | 18 | 67 | 13 | 2 |
| 3 | 117 | 11 | 51 | 34 | 4 |
| 4 | 128 | 7 | 58 | 32 | 3 |
| 5 | 99 | 11 | 62 | 24 | 3 |
| 6 | 107 | 10 | 73 | 14 | 3 |
| 7 | 122 | 8 | 44 | 45 | 3 |
| 8 | 150 | 14 | 55 | 29 | 2 |
| 9 | 112 | 10 | 50 | 35 | 5 |
| 10 | 133 | 5 | 51 | 41 | 3 |
| 11 | 162 | 28 | 48 | 22 | 2 |
| 12 | 169 | 22 | 52 | 18 | 8 |
| 13 | 76 | 2 | 63 | 30 | 5 |
| Mean ± SD | 12 ± 7 | 57 ± B | 27 ± 10 | 4 ± 2 | |
DISCUSSION
Using conventional differential ultracentrifugation methods with density gradients, we separated the plasma lipoproteins from the pooled serum of 13 transplant patients. The plasma lipoprotein fractions were then analyzed for the average percent distribution of both FK 506 and cholesterol. The distribution of FK 506 was highest in the LPDS fraction of the plasma (Fig 1). In contrast, the average percent distribution of cholesterol was found to be lowest in this fraction (Fig 1). This finding is particularly interesting in view of the belief that FK 506 is similar to CyA in its lipophilic properties as well as its mechanism of immunosuppressive activity. The majority of plasma CyA is associated with LDL and HDL where the average percent distribution of cholesterol is highest (Fig 1).4 These observations show that FK 506 and CyA have significantly different distributions in plasma; additionally, this suggests that the mechanism of transport in plasma may be different for the two compounds. We propose that plasma FK 506 is mainly associated with plasma proteins, such as albumin and or alpha-1 acid glycoprotein (AAG).
Fig 1.
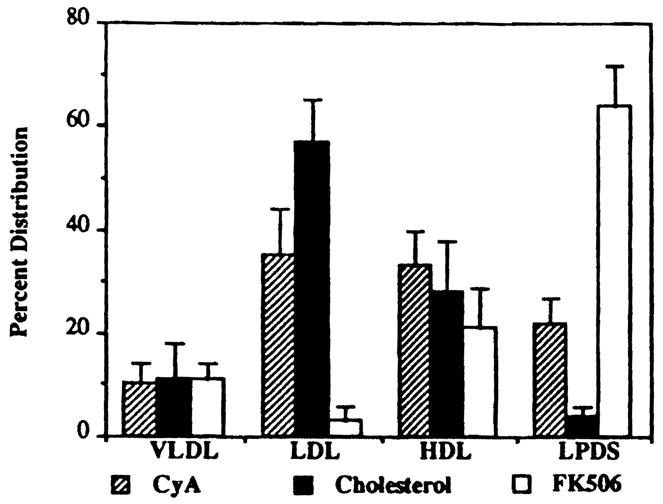
Average percentage of distribution of FK 506, CyA, and cholesterol.
These results indicate that changes in lipoprotein concentrations in transplant patients are not likely to alter FK 506 distribution in plasma, while those of albumin and AAG are more likely to change the extent of binding of FK 506 to plasma proteins.
Acknowledgments
This project was funded in part by the Pathology Education and Research Foundation.
References
- 1.Zeevi A, Eiras G, Burckart G, et al. Transplant Proc. 1990;22(suppl 1):60. [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett WM, Norman DJ. Ann Rev Med. 1986;37:215. doi: 10.1146/annurev.me.37.020186.001243. [DOI] [PubMed] [Google Scholar]
- 3.Venkataramanan R, Jain AB, Cadoff E, et al. Transplant Proc. 1990;22(Suppl 1):52. [PMC free article] [PubMed] [Google Scholar]
- 4.Gureki J, Warty V, Sanghvi A. Transplant Proc. 1985;17:1997. [PubMed] [Google Scholar]
- 5.Havel RJ, Eder HA, Bragdon JH. J Clin Invest. 1955;34:1345. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamura K, Kobayashi M, Hashimoto K, et al. Transplant Proc. 1987;19(suppl 6):23. [PubMed] [Google Scholar]


