Abstract
Purpose
The capacity of a preexisting coating of Escherichia coli 83972 to reduce catheter colonization by Enterococcus faecalis 210 was investigated. Enterococcus was chosen for these trials since it is a common urinary pathogen in patients with an indwelling urinary catheter.
Materials and Methods
Each experiment tested 3 growth conditions. Group 1 or E. coli plus Enterococcus catheters were exposed to E. coli 83972 for 24 hours and then to Enterococcus for 30 minutes. Group 2 or E. coli alone catheters were incubated in E. coli for 24 hours and then in sterile broth for 30 minutes. Group 3 or Enterococcus alone catheters did not undergo the initial incubation with E. coli before the 30-minute incubation with Enterococcus: All catheters were then incubated in sterile human urine for 24 hours. Catheters were washed with saline and cut into 5, 1 cm. segments. Each segment was sonicated and the sonication fluid was diluted and plated. The results of each of the 5 segments were averaged and the set of experiments was repeated 7 times.
Results
A preexisting coating of E. coli 83972 reduced catheter colonization by E. faecalis 210 more than 10-fold. Enterococcus alone catheters had a median of 9.7 × 105 enterococci per cm., whereas E. coli plus Enterococcus catheters had a median of 0.38 × 105 enterococci per cm. (p = 0.016).
Conclusions
Pre-inoculating urinary catheters with E. coli 83972 significantly impedes catheter colonization by Enterococcus: These promising in vitro results prompt the clinical investigation of this particular application of bacterial interference.
Keywords: urinary tract, urinary tract infections, Escherichia coli, catheters, indwelling, antibiosis
Urinary tract infection is the most common infection in patients with a chronic indwelling bladder catheter.1, 2 Although the closed catheter system delays bacterial entry into the urinary tract, bacteriuria still develops in the majority of patients within 2 weeks of continuous catheter use.3, 4 Essentially all patients with an indwelling urinary catheter for 30 days or less have bacteriuria, commonly with more than 1 organism.1, 5, 6 In many cases this bacteriuria is asymptomatic but when catheter associated urinary tract infection develops, the infection is often difficult to treat.7 In the absence of symptoms bacteria in the bladder are sometimes considered benign colonizers and often remain untreated.
The concept of using nonpathogenic bacteria for preventing symptomatic infection is known as bacterial interference.8, 9 The finding of Lindberg et al that asymptomatic bacteriuria in girls did not cause renal damage10 was the first step toward using bacterial interference for preventing symptomatic urinary tract infection. Subsequently studies revealed that treating asymptomatic bacteriuria in girls led to a change in urinary flora and would increase the risk of acute pyelonephritis.11 Studies in elderly subjects, patients with spinal cord injury and women indicate that treating asymptomatic bacteriuria in these populations is neither beneficial nor effective.12–15 Allowing asymptomatic bladder colonization to persist to prevent colonization by a pathogenic strain may be a passive approach to bacterial interference.14
A more active approach to bacterial interference involves intentionally colonizing the bladder with a nonpathogenic organism to prevent infection by more virulent bacteria. In a prospective nonrandomized trial inducing deliberate bladder colonization by the nonpathogenic Escherichia coli 83972 strain in patients with spinal cord injury and neurogenic bladder significantly reduced the rate of symptomatic urinary tract infection.16 Of 44 spinal cord injured patients who achieved bladder colonization longer than 1 month in duration after deliberate bladder inoculation with E. coli 83972, 30 experienced a 50-fold reduction in the rate of symptomatic urinary tract infection compared with the baseline rate of urinary tract infection before the study period.16 However, the major limitation of this bladder inoculation study was the difficulty of establishing long-term colonization of this organism. Of 93 inoculation attempts only 47 (51%) were successful. Also, E. coli 83972 often cleared spontaneously from the bladder. Pre-inoculating urinary catheters with E. coli 83972 before placing them may enhance the protective effect of bacterial interference by 2 means. The persistence of E. coli 83972 in the bladder may be improved and the established presence of this nonpathogenic strain of E. coli may reduce the likelihood of pathogenic organisms establishing catheter colonization, bladder colonization and perhaps symptomatic urinary tract infection. With this goal in mind we investigated whether previous inoculation of urinary catheters with E. coli 83972 would reduce catheter colonization by Enterococcus faecalis in vitro. Enterococcus was chosen because enterococci are a common cause of urinary tract infection in patients with a chronic indwelling catheter.4 In addition, the increasing prevalence of antibiotic resistant enterococci suggests that a nonpharmaceutical approach to the control of this pathogen would be useful.17
MATERIALS AND METHODS
Bacteria
E. coli 83972, serotype ON:KN (N = nontypable) was initially isolated from the urine of a girl with a 3-year history of asymptomatic bacteriuria with stable renal function.18 This strain was selected because of its documented ability to persist in the urinary tract without causing disease. E. faecalis 210 was initially isolated from the urine of a patient with catheter related urinary tract infection. Each strain was stored at −80C in 10% glycerol culture and then passaged on trypticase soy agar II with 5% sheep blood (BBL, Cockeysville, Maryland). Just before performing the catheter studies bacteria were retrieved from the trypticase soy agar plates and cultured overnight in trypticase soy broth (Difco, Sparks, Maryland). Bacteria were harvested by centrifugation and re-suspended in sterile saline to a concentration of 108 colony-forming units per ml.
Experimental variables and controls
Each experiment tested 3 growth conditions. Group 1 or E. coli plus Enterococcus catheters were exposed to E. coli 83972 for 24 hours and then to Enterococcus for 30 minutes. Group 2 or E. coli alone catheters were incubated in E. coli for 24 hours and then in sterile broth for 30 minutes. Group 3 or Enterococcus alone catheters did not undergo the initial incubation with E. coli before the 30-minute incubation with Enterococcus (fig. 1).
Fig. 1.
Experimental protocol. TSB, trypticase soy broth
Catheter preparation
Foley catheters used in this experiment were composed of latex with a hydrophilic coating (Bardex, Covington, Georgia). Pilot trials had demonstrated optimal adherence of E. coli 83972 to this catheter material compared with silicone (data not shown). All catheters were 12Fr with a 5 cc ribbed balloon. Under sterile conditions each end of each catheter was removed and discarded. The remaining catheter shaft was cut into 8 cm. pieces. The balloon lumen of each piece was plugged at the 2 ends with sterile pipette tips and sealed with flame, leaving only the main catheter lumen open. The catheter pieces were placed into sterile 50 cc centrifuge tubes containing trypticase soy broth.
Bacterial interference trial
For growth condition 1 catheter pieces were placed into sterile 50 ml. centrifuge tubes containing trypticase soy broth. E. coli 83972 was added to each tube at a starting concentration of 105 colony-forming units per ml. The catheter pieces were incubated for 24 hours at 37C in the E. coli suspension. Catheter pieces previously exposed to E. coli 83972 were next placed in a suspension of 104 colony-forming units per ml. E. faecalis in trypticase soy broth at 37C without washing. After 30 minutes of incubation in the Enterococcus suspension the catheter pieces were removed and placed immediately in filter sterilized human urine at 37C. The catheters were incubated in urine at 37C for 24 hours (fig. 1).
Under growth condition 2 the initial 24-hour incubation in E. coli 83972 was done according to the same procedure but the broth in the 30-minute incubation step was sterile. In contrast, group 3 catheters remained untouched during the initial 24-hour incubation of groups 2 and 3 catheters, and during the 30-minute incubation step they were placed in trypticase soy broth containing 104 per ml. E. faecalis. All groups 1 to 3 catheters were transferred to sterile urine after the 30 minutes incubation step and incubated for 24 hours at 37C. Urine was obtained from a single male donor and filter sterilized by passage through a 0.22 μm. filter.
Catheter harvest
Under sterile conditions the 8 cm. catheter pieces were removed from the tubes and flushed with 60 cc sterile saline using a needle and syringe. The 2 ends of each piece were cut off and the balloon lumen was flushed with 30 cc saline. The catheter was then gently agitated in 3 separate saline baths. Each lumen was rapidly flushed with air. Five 1-cm. segments were cut from each catheter. Each 1 cm. segment was placed in 1 cc saline and sonicated at 55,000 Hz. for 10 minutes in a water bath sonicator (Buehler Scientific, Evanston, Illinois).19 Sonication fluid was diluted to 1:102, and 100 μl. aliquots of original and diluted sonication fluid were inoculated onto blood and colistin-nalidixic acid agar plates to determine plate counts. Gram staining was done to verify the types of organisms recovered. A sample of the urine in each centrifuge tube was also diluted and plated to assess final bacterial concentration and purity.
Statistical analysis
Data on the 5, 1 cm. segments were averaged to determine the mean colony-forming units per cm. per catheter. Each set of experiments was repeated 7 times. The Wilcoxon 2-tailed signed rank test was done for all comparisons using commercially available software.
RESULTS
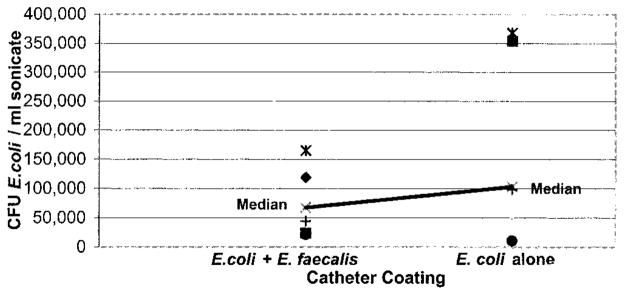
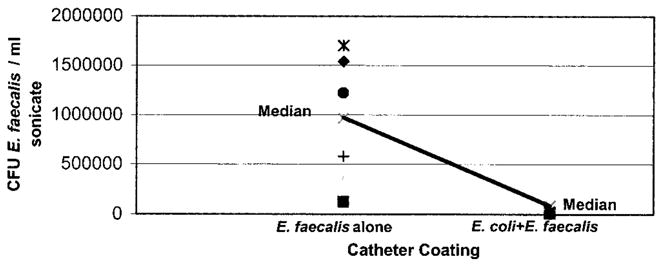
The preexisting coating of E. coli 83972 reduced catheter colonization by E. faecalis 210 by a more than 10-fold difference (figs. 2 and 3). Enterococcus alone catheters had a median of 9.7 × 105 enterococci per cm., whereas E. coli plus Enterococcus catheters had a median of 0.38 × 105 enterococci per cm. (p = 0.016). The number of E. coli found on the catheters after incubation for 24 hours in urine was similar regardless of the presence or absence of Enterococcus (median 6.7 × 104 versus 9.9 × 104 colony-forming units E. coli per cm., p = 0.47, fig. 4).
Fig. 2.
Catheters pre-inoculated with E. coli 83972 had significantly fewer enterococci on surfaces than those without previous exposure to E. coli (p = 0.016). Each point represents data from 1 of 7 trials, indicating average enterococci per ml. sonicate fluid recovered from 5, 1 cm. segments cut from single catheter. CFU, colony-forming units.
Fig. 3.

Slightly overgrown E. faecalis 210 showing dramatic reduction in number of enterococcal colonies retrieved from catheters pre-inoculated with E. coli 83972. A, colistin-naladixic acid plate with fewer colonies received 100 μl. sonicate fluid from 1 cm. segment of E. coli plus Enterococcus catheter. Colistin-naladixic acid was chosen to eliminate E. coli. B, blood agar plate with more colonies received 100 μl. sonicate fluid from 1 cm. segment of Enterococcus alone catheter. Enterococcal colony counts did not differ in blood agar and colistin-naladixic acid plates.
Fig. 4.
Exposure of E. coli coated catheters to Enterococcus did not significantly affect number of E. coli recovered from catheters (p = 0.47). Each point represents data from 1 of 7 trials, indicating average E. coli per ml. sonicate fluid recovered from 5, 1 cm. segments cut from single catheter. CFU, colony-forming units.
We also compared the final concentrations of E. coli 83972 and E. faecalis 210 in the urine. In tubes containing E. coli plus Enterococcus catheters the median concentration of E. coli 83972 was 1.2 × 108 colony-forming units per ml. urine, while the median concentration of E. coli 83972 in tubes containing E. coli alone catheters was 1.1 × 108 colony-forming units per ml. (p = 1.0, fig. 5). These results indicate that E. faecalis did not inhibit the growth of E. coli 83972 in urine.
Fig. 5.
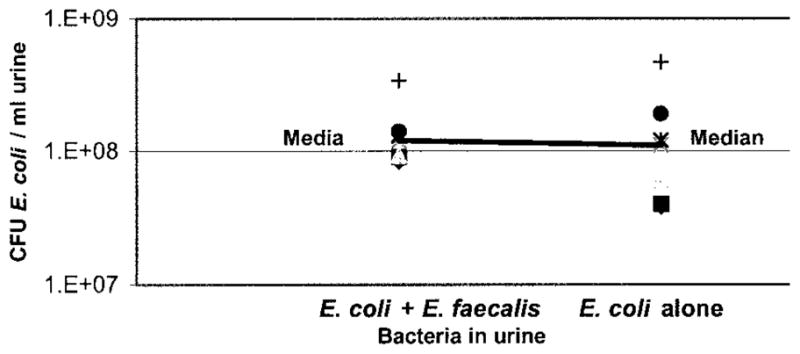
Whether catheter was exposed to Enterococcus before placement in urine did not affect final concentration of E. coli in urine (p = 1). Each point represents data from 1 of 7 trials, indicating colony-forming units (CFU) of E. coli per ml. urine after catheter incubation in urine for 24 hours.
However, the presence of E. coli in the urine appeared to inhibit enterococcal growth in urine, although this trend did not reach statistical significance. Tubes containing Enterococcus alone catheters had a median concentration of 5.3 × 107 enterococci per ml. urine, while tubes with E. coli plus Enterococcus catheters had a median concentration of 0.86 × 107 enterococci per ml. urine (p = 0.063, fig. 6).
Fig. 6.
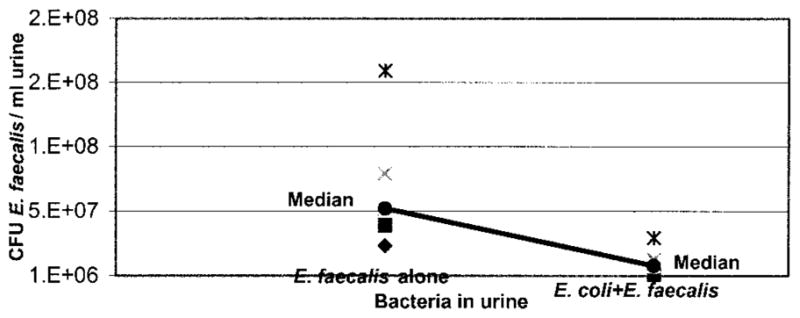
When E. coli 83972 was present on catheter surface when catheter was placed in urine, fewer enterococci were recovered from urine 24 hours later. This trend did not reach statistical significance because only 5 of 7 trials provided reliable results of final urinary Enterococcus concentration (p = 0.063). Each point represents data from 1 of 5 trials, indicating colony-forming units (CFU) of E. faecalis per ml. urine after catheter incubation in urine for 24 hours.
DISCUSSION
The greater than 10-fold reduction in catheter colonization by E. faecalis 210 when the catheter had a pre-existing coating of E. coli 83972 indicates that E. coli 83972 can effectively interfere with enterococcal growth on a urinary catheter. Although in these experiments we exposed E. coli coated catheters to a relatively high concentration of enterococci (1 × 104 colony-forming units per ml.), E. coli still significantly impeded colonization by enterococci. In addition, the presence of Enterococcus did not affect the concentration of E. coli on the catheter surface. Thus, Enterococcus did not appear to replace E. coli on the catheter surface, at least during the 24-hour incubation period. Catheter related urinary tract infection may arise when a small number of organisms gain entry and then multiply to high concentrations in the bladder.2, 20 Our results suggest that a urinary catheter with a preexisting coating of E. coli is likely to be effective for preventing a small number of pathogenic organisms from colonizing the catheter and the bladder, eventually causing urinary tract infection.
Our preliminary results also indicate that E. coli 83972 inhibits growth of E. faecalis 210 in urine. However, the presence of E. faecalis did not affect the growth rate of E. coli 83972 in urine. These findings prompted us to study further the ability of E. coli 83972 to inhibit enterococcal growth. E. coli 83972 was inoculated with a stab into solid medium, grown overnight, killed with chloroform and then overlaid with a soft agar containing E. faecalis 210.21 Using this stab overlay method we found that killed E. coli 83972 inhibited the growth of E. faecalis 210 in a zone around the stab. These observations indicate that E. coli 83972 synthesizes a factor or factors that inhibit the growth of E. faecalis. Elucidating the molecular mechanism of the growth inhibition of Enterococcus by E. coli 83972 is planned to be a subject of our future experiments.
Although to our knowledge the mechanism of the inhibition of Enterococcus by E. coli is not currently known, the inhibitory effect of Lactobacillus on Enterococcus has been well studied. In a parallel plate flow chamber Lactobacillus was able to displace adherent E. faecalis from hydrophobic and hydrophilic substrata.22 Many strains of Lactobacillus produce biosurfactants or microbial products that affect the surface tension at liquid-air interfaces.23 Several isolated biosurfactants were able to slow the initial deposition rates and decrease the number of adherent cells of uropathogenic E. faecalis to hydrophilic glass and to hydrophobic silicone rubber in a parallel plate flow chamber.23–25 Molecular analysis of the biosurfactant produced by lactobacilli revealed that most of the anti-enterococcal activity was attributable to a 29 kDa. protein that is thought to act as a adhesin.26 Therefore, the same proteins that help lactobacilli bind to host components, such as the extracellular matrix, may also help to exclude binding by pathogenic organisms.
While these in vitro studies with Lactobacillus suggest that the scientific mechanisms underlying bacterial interference can be elucidated, Lactobacillus has several limitations as an agent of bacterial interference for preventing urinary tract infection in vivo. Lactobacillus does not colonize the bladder and is found exclusively on the female urogenital epithelium.27, 28 Thus, Lactobacillus may not be an effective agent of bacterial interference in men or in patients with an indwelling catheter.
E. coli 83927 may have broader applicability as an agent of bacterial interference for preventing urinary tract infection. In prospective nonrandomized human studies bladder inoculation with E. coli 83972 has proved to be safe and effective for protecting against symptomatic urinary tract infection.16, 29 We anticipate that inserting bladder catheters with pre-formed coating of E. coli 83972 may be more successful than direct inoculation for establishing and maintaining bladder colonization with E. coli 83972.
This particular approach to bacterial interference using urinary catheters pre-inoculated with E. coli 83972 is designed to avoid the use of antibiotics and their attendant complications. Clinical trials of antimicrobial agents for irrigating the catheter or bladder,30, 31 disinfecting the collection bag32, 33 or cleansing the urethral meatus34, 35 have yielded disappointing results. Antibiotics can suppress the development of bacteriuria in patients with short-term indwelling catheters but in those who are chronically catheterized antibiotics favor the development of highly resistant urinary flora.1, 4 Furthermore, using antibiotics in all patients with a urinary catheter would increase the incidence of adverse drug reactions and supra-infections, such as Clostridium difficile colitis.1 Since catheter associated urinary tract infection is a major cause of morbidity and mortality in patients with an indwelling catheter,1, 2 a new approach for preventing urinary tract infection in this population is urgently needed.
The encouraging results of this initial in vitro study invite further investigations in vitro and in vivo. In vitro the ability of a pre-formed biofilm of E. coli 83972 to prevent catheter colonization by bacterial genera other than Enterococcus merits investigation. In vivo a pilot clinical trial is planned to examine the safety and efficacy of placing urinary catheters with a preexisting coating of E. coli 83972 in patients with neurogenic bladder and recurrent urinary tract infection. If pilot trials of this approach to bacterial interference are successful in the spinal cord injured population, the trials can be extended to other catheter dependent populations, including geriatric patients and nursing home residents.
CONCLUSIONS
This in vitro study demonstrated that a pre-existing coating of E. coli 83972 on the surface of urinary catheters significantly impeded colonization by Enterococcus faecalis 210. This promising approach to bacterial interference invites further clinical investigation.
Acknowledgments
Supported by the Veterans Administration, Paralyzed Veterans of America and Spinal Cord Society.
References
- 1.Warren J. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11:609. doi: 10.1016/s0891-5520(05)70376-7. [DOI] [PubMed] [Google Scholar]
- 2.Cardenas D, Hooten T. Urinary tract infection in persons with spinal cord injury. Arch Phys Med Rehabil. 1995;76:272. doi: 10.1016/s0003-9993(95)80615-6. [DOI] [PubMed] [Google Scholar]
- 3.Warren J. Nosocomial urinary tract infections. In: Mandell G, Bennett J, Dolin R, editors. Principles and Practice of Infectious Diseases. 5. Philadelphia: Churchill Livingstone; 2000. p. 3028. [Google Scholar]
- 4.Stover S, Lloyd K, Waites K, et al. Urinary tract infection in spinal cord injury. Arch Phys Med Rehabil. 1989;70:47. [PubMed] [Google Scholar]
- 5.Stamm W. Catheter-associated urinary tract infections: epidemiology, pathogenesis, and prevention. Am J Med. 1991;91:3B. doi: 10.1016/0002-9343(91)90345-x. [DOI] [PubMed] [Google Scholar]
- 6.Warren J, Tenney J, Hoopes J, et al. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982;146:719. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]
- 7.Darouiche R. Infections in patients with spinal cord injury. In: Mandell G, Bennett J, Dolin R, editors. Principles and Practice of Infectious Disease. 5. Philadelphia: Churchill Livingstone; 2000. p. 3159. [Google Scholar]
- 8.Sprunt K, Leidy G. The use of bacterial interference to prevent infection. Can J Microbiol. 1988;34:332. doi: 10.1139/m88-061. [DOI] [PubMed] [Google Scholar]
- 9.Bibel D. Bacterial interference, bacteriotherapy, and bacterio-prophylaxis. In: Aly R, Shinefield H, editors. Bacterial Interference. Boca Raton: CRC Press; 1982. p. 1. [Google Scholar]
- 10.Lindberg U, Claesson I, Hanson L, et al. Asymptomatic bacteriuria in schoolgirls: VIII. Clinical course during a 3-year follow-up. J Pediatr. 1978;92:194. doi: 10.1016/s0022-3476(78)80003-1. [DOI] [PubMed] [Google Scholar]
- 11.Hansson S, Jodal U, Lincoln K, et al. Untreated asymptomatic bacteriuria in girls: II-effect of phenoxymethylpenicillin and erythromycin given for intercurrent infections. Br Med J. 1989;298:856. doi: 10.1136/bmj.298.6677.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolle L, Bjornson J, Harding G, et al. Bacteriuria in elderly institutionalized men. N Engl J Med. 1983;309:1420. doi: 10.1056/NEJM198312083092304. [DOI] [PubMed] [Google Scholar]
- 13.Nicolle L. Asymptomatic bacteriuria in the elderly. Infect Dis Clin N Am. 1997;11:647. doi: 10.1016/s0891-5520(05)70378-0. [DOI] [PubMed] [Google Scholar]
- 14.Darouiche R, Hull R. Bacterial interference for prevention of urinary tract infection: an overview. J Spinal Cord Med. 2000;23:136. doi: 10.1080/10790268.2000.11753521. [DOI] [PubMed] [Google Scholar]
- 15.Tencer J. Asymptomatic bacteriuria-a long-term study. Scand J Urol Nephrol. 1988;22:31. doi: 10.1080/00365599.1988.11690380. [DOI] [PubMed] [Google Scholar]
- 16.Darouiche RO, Donovan WH, Del Terzo M, et al. Pilot trial of bacterial interference for preventing urinary tract infection. Urology. 2001;58:339. doi: 10.1016/s0090-4295(01)01271-7. [DOI] [PubMed] [Google Scholar]
- 17.Norris A, Reilly J, Edelstein P, et al. Chloramphenicol for the treatment of vancomycin-resistant enterococcal infections. Clin Infect Dis. 1995;20:1137. doi: 10.1093/clinids/20.5.1137. [DOI] [PubMed] [Google Scholar]
- 18.Andersson P, Engberg I, Lidin-Janson G, et al. Persistence of Escherichia coli bacteriuria is not determined by bacterial adherence. Infect Immunol. 1991;59:2915. doi: 10.1128/iai.59.9.2915-2921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherertz R, Raad I, Belani A, et al. Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J Clin Microbiol. 1990;28:76. doi: 10.1128/jcm.28.1.76-82.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunin C, Steele C. Culture of the surfaces of urinary catheters to sample urethral flora and study the effect of antimicrobial therapy. J Clin Microbiol. 1985;21:902. doi: 10.1128/jcm.21.6.902-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monk M, Clowes R. Transfer of the colicin I factor in Escherichia coli K12 and its interaction with the F fertility factor. J Gen Microbiol. 1964;36:365. doi: 10.1099/00221287-36-3-365. [DOI] [PubMed] [Google Scholar]
- 22.Millsap K, Reid G, Van der Mei H, et al. Displacement of Enterococcus faecalis from hydrophobic and hydrophilic substrata by Lactobacillus and Streptococcus spp. as studied in a parallel plate flow chamber. App Environ Microbiol. 1994;60:1867. doi: 10.1128/aem.60.6.1867-1874.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velraeds M, Van der Mei H, Reid G, et al. Inhibition of initial adhesion of uropathogenic Enterococcus faecalis by biosurfactants from Lactobacillus isolates. App Environ Microbiol. 1996;62:1958. doi: 10.1128/aem.62.6.1958-1963.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velraeds M, Van der Mei H, Reid G, et al. Inhibition of initial adhesion of uropathogenic Enterococcus faecalis to solid substrata by an adsorbed biosurfactant layer from Lactobacillus acidophilus. Urology. 1997;49:790. doi: 10.1016/S0090-4295(97)00065-4. [DOI] [PubMed] [Google Scholar]
- 25.Velraeds M, Van de Belt-Gritter B, Van der Mei H, et al. Interference in initial adhesion of uropathogenic bacteria and yeasts to silicone rubber by a Lactobacillus acidophilus biosurfactant. J Med Microbiol. 1998;47:1081. doi: 10.1099/00222615-47-12-1081. [DOI] [PubMed] [Google Scholar]
- 26.Heinemann C, Van Hylckama Vleigh J, Janssen D, et al. Purification and characterization of a surface-binding protein from Lactobacillus fermentum RC-14 that inhibits adhesion of Enterococcus faecalis 1131. FEMS Microbiol Lett. 2000;190:177. doi: 10.1111/j.1574-6968.2000.tb09282.x. [DOI] [PubMed] [Google Scholar]
- 27.Reid G, Bruce A, McGroaty J, et al. Is there a role for lacto-bacilli in prevention of urogenital and intestinal infections? Clin Microbiol Rev. 1990;3:335. doi: 10.1128/cmr.3.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagberg L, Bruce A, Reid G, et al. Colonization of the urinary tract with bacteria from the normal fecal and urethral flora in patients with recurrent urinary tract infections. In: Kass E, Svanborg Eden C, editors. Host Parasite Interactions in the Urinary Tract. Chicago: University of Chicago Press; 1989. p. 289. [Google Scholar]
- 29.Hull R, Rudy D, Donovan W, et al. Urinary tract infection prophylaxis using Escherichia coli 83972 in spinal cord injured patients. J Urol. 2000;163:1. [PubMed] [Google Scholar]
- 30.Bastable J, Peel R, Birch D, et al. Continuous irrigation of the bladder after prostatectomy: its effect on post-prostatectomy infection. Br J Urol. 1977;49:689. doi: 10.1111/j.1464-410x.1977.tb04554.x. [DOI] [PubMed] [Google Scholar]
- 31.Warren J, Platt R, Thomas R, et al. Antibiotic irrigation and catheter-associated urinary-tract infection. N Engl J Med. 1978;299:570. doi: 10.1056/NEJM197809142991103. [DOI] [PubMed] [Google Scholar]
- 32.Thompson R, Haley C, Searchy M, et al. Catheter-associated bacteriuria: failure to reduce attack rates using periodic instillations of a disinfectant into urinary drainage systems. JAMA. 1984;251:747. doi: 10.1001/jama.251.6.747. [DOI] [PubMed] [Google Scholar]
- 33.Gillespie W, Jones J, Teasdale C, et al. Does the addition of disinfectant to urine drainage bags prevent infection in catheterized patients? Lancet. 1983;1:1037. doi: 10.1016/s0140-6736(83)92657-0. [DOI] [PubMed] [Google Scholar]
- 34.Huth T, Burke J, Larsen R, et al. Randomized trial of meatal care with silver sulfadiazine cream for the prevention of catheter-associated bacteriuria. J Infect Dis. 1992;165:14. doi: 10.1093/infdis/165.1.14. [DOI] [PubMed] [Google Scholar]
- 35.Classen D, Larsen R, Burke J, et al. Daily meatal care for prevention of catheter-associated bacteriuria: results using polyantibiotic cream. Infect Control Hosp Epidemiol. 1991;12:157. doi: 10.1086/646309. [DOI] [PubMed] [Google Scholar]