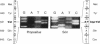Abstract
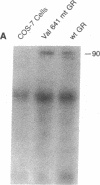
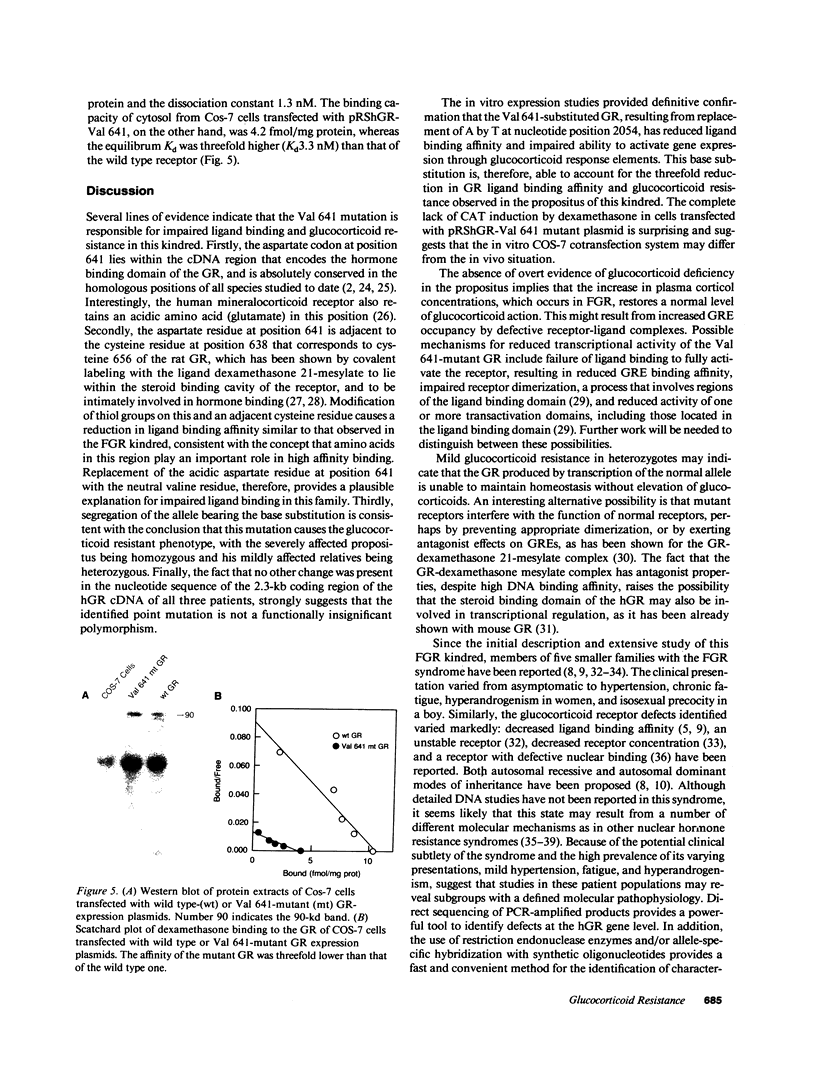
Familial glucocorticoid resistance is a hypertensive, hyperandrogenic disorder characterized by increased serum cortisol concentrations in the absence of stigmata of Cushing's syndrome. Our previous studies of the first reported kindred showed a two- to threefold reduction in glucocorticoid receptor-ligand binding affinity in the propositus, and a lesser reduction in affinity in his mildly affected son and nephew. Glucocorticoid receptor cDNA from these three patients was amplified by polymerase chain reaction and sequenced. The cDNA nucleotide sequence was normal, except for nucleotide 2054, which substituted valine for aspartic acid at amino acid residue 641. The propositus was homozygous while the other relatives were heterozygous for the mutation. COS-7 monkey kidney cells were cotransfected with expression vectors for either wild type or Val 641-mutant receptors, together with the reporter plasmid pMMTV-CAT. Dexamethasone increased chloramphenicol acetyltransferase activity in cells expressing wild type receptor, but had no effect in cells expressing Val 641-mutant receptors, despite similar receptor concentrations, as indicated by Western blotting. The binding affinity for dexamethasone of the Val 641-mutant receptor was threefold lower than that of the wild type receptor. These results suggest that glucocorticoid resistance in this family is due to a point mutation in the steroid-binding domain of the glucocorticoid receptor.
Full text
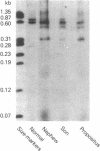
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accili D., Frapier C., Mosthaf L., McKeon C., Elbein S. C., Permutt M. A., Ramos E., Lander E., Ullrich A., Taylor S. I. A mutation in the insulin receptor gene that impairs transport of the receptor to the plasma membrane and causes insulin-resistant diabetes. EMBO J. 1989 Sep;8(9):2509–2517. doi: 10.1002/j.1460-2075.1989.tb08388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza J. L., Weinberger C., Cerelli G., Glaser T. M., Handelin B. L., Housman D. E., Evans R. M. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987 Jul 17;237(4812):268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Brönnegård M., Werner S., Gustafsson J. A. Primary cortisol resistance associated with a thermolabile glucocorticoid receptor in a patient with fatigue as the only symptom. J Clin Invest. 1986 Nov;78(5):1270–1278. doi: 10.1172/JCI112711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chrousos G. P., Vingerhoeds A. C., Loriaux D. L., Lipsett M. B. Primary cortisol resistance: a family study. J Clin Endocrinol Metab. 1983 Jun;56(6):1243–1245. doi: 10.1210/jcem-56-6-1243. [DOI] [PubMed] [Google Scholar]
- Chrousos G. P., Vingerhoeds A., Brandon D., Eil C., Pugeat M., DeVroede M., Loriaux D. L., Lipsett M. B. Primary cortisol resistance in man. A glucocorticoid receptor-mediated disease. J Clin Invest. 1982 Jun;69(6):1261–1269. doi: 10.1172/JCI110565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen M., Northrop J. P., Ringold G. M. The mouse glucocorticoid receptor: mapping of functional domains by cloning, sequencing and expression of wild-type and mutant receptor proteins. EMBO J. 1986 Oct;5(10):2513–2522. doi: 10.1002/j.1460-2075.1986.tb04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke U., Foellmer B. E. The glucocorticoid receptor gene is in 5q31-q32 [corrected]. Genomics. 1989 May;4(4):610–612. doi: 10.1016/0888-7543(89)90287-5. [DOI] [PubMed] [Google Scholar]
- Giguère V., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Functional domains of the human glucocorticoid receptor. Cell. 1986 Aug 29;46(5):645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. E., Wilson J. D. The syndromes of androgen resistance. N Engl J Med. 1980 Jan 24;302(4):198–209. doi: 10.1056/NEJM198001243020404. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Weinberger C., Ong E. S., Cerelli G., Oro A., Lebo R., Thompson E. B., Rosenfeld M. G., Evans R. M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985 Dec 19;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M. R., Malloy P. J., Kieback D. G., Kesterson R. A., Pike J. W., Feldman D., O'Malley B. W. Point mutations in the human vitamin D receptor gene associated with hypocalcemic rickets. Science. 1988 Dec 23;242(4886):1702–1705. doi: 10.1126/science.2849209. [DOI] [PubMed] [Google Scholar]
- Iida S., Gomi M., Moriwaki K., Itoh Y., Hirobe K., Matsuzawa Y., Katagiri S., Yonezawa T., Tarui S. Primary cortisol resistance accompanied by a reduction in glucocorticoid receptors in two members of the same family. J Clin Endocrinol Metab. 1985 May;60(5):967–971. doi: 10.1210/jcem-60-5-967. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Kadowaki H., Taylor S. I. A nonsense mutation causing decreased levels of insulin receptor mRNA: detection by a simplified technique for direct sequencing of genomic DNA amplified by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Jan;87(2):658–662. doi: 10.1073/pnas.87.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts S. W., Poldermans D., Zweens M., de Jong F. H. Familial cortisol resistance: differential diagnostic and therapeutic aspects. J Clin Endocrinol Metab. 1986 Dec;63(6):1328–1333. doi: 10.1210/jcem-63-6-1328. [DOI] [PubMed] [Google Scholar]
- Linder M. J., Thompson E. B. Abnormal glucocorticoid receptor gene and mRNA in primary cortisol resistance. J Steroid Biochem. 1989 Feb;32(2):243–249. doi: 10.1016/0022-4731(89)90259-8. [DOI] [PubMed] [Google Scholar]
- Lipsett M. B., Chrousos G. P., Tomita M., Brandon D. D., Loriaux D. L. The defective glucocorticoid receptor in man and nonhuman primates. Recent Prog Horm Res. 1985;41:199–247. doi: 10.1016/b978-0-12-571141-8.50009-3. [DOI] [PubMed] [Google Scholar]
- Malchoff C. D., Javier E. C., Malchoff D. M., Martin T., Rogol A., Brandon D., Loriaux D. L., Reardon G. E. Primary cortisol resistance presenting as isosexual precocity. J Clin Endocrinol Metab. 1990 Feb;70(2):503–507. doi: 10.1210/jcem-70-2-503. [DOI] [PubMed] [Google Scholar]
- Marcelli M., Tilley W. D., Wilson C. M., Wilson J. D., Griffin J. E., McPhaul M. J. A single nucleotide substitution introduces a premature termination codon into the androgen receptor gene of a patient with receptor-negative androgen resistance. J Clin Invest. 1990 May;85(5):1522–1528. doi: 10.1172/JCI114599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld R., Rusconi S., Godowski P. J., Maler B. A., Okret S., Wikström A. C., Gustafsson J. A., Yamamoto K. R. Genetic complementation of a glucocorticoid receptor deficiency by expression of cloned receptor cDNA. Cell. 1986 Aug 1;46(3):389–399. doi: 10.1016/0092-8674(86)90659-8. [DOI] [PubMed] [Google Scholar]
- Miller N. R., Simons S. S., Jr Steroid binding to hepatoma tissue culture cell glucocorticoid receptors involves at least two sulfhydryl groups. J Biol Chem. 1988 Oct 15;263(29):15217–15225. [PubMed] [Google Scholar]
- Nawata H., Sekiya K., Higuchi K., Kato K., Ibayashi H. Decreased deoxyribonucleic acid binding of glucocorticoid-receptor complex in cultured skin fibroblasts from a patient with the glucocorticoid resistance syndrome. J Clin Endocrinol Metab. 1987 Aug;65(2):219–226. doi: 10.1210/jcem-65-2-219. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Takeda K., Ain K., Ceccarelli P., Nakai A., Seino S., Bell G. I., Refetoff S., DeGroot L. J. Generalized resistance to thyroid hormone associated with a mutation in the ligand-binding domain of the human thyroid hormone receptor beta. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8977–8981. doi: 10.1073/pnas.86.22.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons S. S., Jr, Miller P. A., Wasner G., Miller N. R., Mercier L. Inverse correlation between dexamethasone 21-mesylate agonist activity and sensitivity to dexamethasone for induction of tyrosine aminotransferase in rat hepatoma cells. J Steroid Biochem. 1988 Jul;31(1):1–7. doi: 10.1016/0022-4731(88)90198-7. [DOI] [PubMed] [Google Scholar]
- Simons S. S., Jr, Pumphrey J. G., Rudikoff S., Eisen H. J. Identification of cysteine 656 as the amino acid of hepatoma tissue culture cell glucocorticoid receptors that is covalently labeled by dexamethasone 21-mesylate. J Biol Chem. 1987 Jul 15;262(20):9676–9680. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tomita M., Brandon D. D., Chrousos G. P., Vingerhoeds A. C., Foster C. M., Fowler D., Loriaux D. L., Lipsett M. B. Glucocorticoid receptors in Epstein-Barr virus-transformed lymphocytes from patients with glucocorticoid resistance and a glucocorticoid-resistant New World primate species. J Clin Endocrinol Metab. 1986 Jun;62(6):1145–1154. doi: 10.1210/jcem-62-6-1145. [DOI] [PubMed] [Google Scholar]
- Usala S. J., Tennyson G. E., Bale A. E., Lash R. W., Gesundheit N., Wondisford F. E., Accili D., Hauser P., Weintraub B. D. A base mutation of the C-erbA beta thyroid hormone receptor in a kindred with generalized thyroid hormone resistance. Molecular heterogeneity in two other kindreds. J Clin Invest. 1990 Jan;85(1):93–100. doi: 10.1172/JCI114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoeds A. C., Thijssen J. H., Schwarz F. Spontaneous hypercortisolism without Cushing's syndrome. J Clin Endocrinol Metab. 1976 Nov;43(5):1128–1133. doi: 10.1210/jcem-43-5-1128. [DOI] [PubMed] [Google Scholar]