Abstract
Rationale
There is an extensive literature showing that the CB1 cannabinoid receptor antagonist rimonabant (SR141716) decreases alcohol consumption in animals, but little is known about its effects in human alcohol drinkers.
Methods
In this study, 49 nontreatment-seeking heavy alcohol drinkers participated in a 3-week study. After a 1-week baseline, participants received either 20 mg/day of rimonabant or placebo for 2 weeks under double-blind conditions. During these 3 weeks, participants reported their daily alcohol consumption by telephone. Subsequently, they participated in an alcohol self-administration paradigm in which they received a priming dose of alcohol followed by the option of consuming either eight alcohol drinks or receiving $3.00 for each nonconsumed drink. Endocrine measures and self-rating scales were also obtained.
Results
Rimonabant did not change alcohol consumption during the 2 weeks of daily call-ins. Similarly, the drug did not change either alcohol self-administration or endocrine measures during the laboratory session.
Conclusion
We conclude that the daily administration of 20 mg of rimonabant for 2 weeks has no effect on alcohol consumption in nontreatment-seeking heavy alcohol drinkers.
Keywords: Rimonabant (SR141716), Alcohol, Heavy drinkers, CB1 receptor antagonist, Alcohol self-administration, Endocannabinoids
Introduction
Endocannabinoids act at CB1 receptors which are expressed in both the brain (i.e., hypothalamus, cingulate, amygdala nuclei, nucleus accumbens, septum, and cerebellum), as well as in the periphery (i.e., adipose tissue, liver, and gastrointestinal tract; Pacher et al. 2006). Given the association of endocannabinoids with brain-reward circuits (DiMarzo and Matias 2005), there is a great deal of interest in examining how endocannabinoids might influence alcohol consumption.
Animal studies show that voluntary alcohol drinking in rats is increased following the acute (Colombo et al. 2002; Gallate et al. 1999) as well as chronic administration of CB1 receptor agonists (Lopez-Moreno et al. 2004), whereas treatment with rimonabant (SR141716) decreases voluntary alcohol intake (Arnone et al. 1997; Colombo et al. 1998). Animal models designed to approximate human alcohol consumption show that rimonabant administration is effective in decreasing the operant self-administration of alcohol by rodents, where the animals’ motivation to drink is assessed by the amount of work they are willing to perform to receive alcohol (Economidou et al. 2006; Freedland et al. 2001; Hansson et al. 2007). The rebound increase in alcohol consumption typically seen in animals following alcohol deprivation is also attenuated by rimonabant treatment, and the administration of rimonabant to Wistar rats, prior to being exposed to alcohol cues that had been previously associated with alcohol, reduces alcohol-seeking behavior (Cippitelli et al. 2005). Conversely, potentiation of endocannabinoid transmission within the prefrontal cortex increases alcohol self-administration and preference (Hansson et al. 2007).
Similar to the effects of pharmacological blockade of CB1 receptors, the genetic deletion of the CB1 receptor in mice also results in reduced preference for and intake of alcohol (Hungund et al. 2003; Naassila et al. 2004; Poncelet et al. 2003; Thanos et al. 2005; Wang et al. 2003). In the study by Wang et al. (2003), the high ethanol preference of the wild-type mice declined with age such that the older mice drank no more than their CB1 receptor deficient littermates. In the old wild-type mice, rimonabant no longer affected ethanol intake. CB1 receptor densities and endocannabinoid levels in different brain regions were similar in young and old wild-type mice. However, there was a selective reduction in CB1 receptor coupling in the limbic forebrain of old versus young mice (Wang et al. 2003). Since the limbic forebrain contains reward centers, such as the nucleus accumbens and the anterior cingulate cortex, these findings suggest that endocannabinoids could be involved in mediating the rewarding effects of ethanol. These findings are in agreement with an independent study in wild-type C57B1/6J mice showing that the ethanol-induced increase in dopamine release in the nucleus accumbens can be blocked by rimonabant and is absent in the CB1 knockout mice (Hungund et al. 2003). Rimonabant also inhibited transient dopamine surges in the nucleus accumbens of freely moving rats, elicited not only by ethanol but also by nicotine and cocaine (Cheer et al. 2007) suggesting an obligatory role for endocannabinoids in the activation of the dopaminergic reward pathway by addictive substances.
Although there is an extensive literature showing that the CB1 cannabinoid receptor antagonist rimonabant decreases alcohol consumption in animals, there is only one recently published study (Soyka et al. 2008) examining the effect of rimonabant (20 mg/day for 12 weeks) on the rate of relapse among recently detoxified alcohol-dependent individuals. Of interest, this study failed to find a significant difference between rimonabant and placebo for the time to first drink and relapse to heavy drinking. Potential confounds of this study included the fact that the study had a high placebo response rate and was conducted at multinational sites. In order to expand upon the effect of rimonabant in humans, we conducted a phase I/II clinical trial to test the hypothesis that nontreatment-seeking heavy alcohol drinkers taking rimonabant, compared with those taking placebo, would consume fewer drinks in a naturalistic outpatient setting as well as during an alcohol self-administration paradigm (ASA).
A secondary objective of the study was to examine the effect of chronic CB1 antagonism on plasma endocrine measures prior to and during the ASA. A previous study (O’Malley et al. 2002) employed a similar alcohol self-administration paradigm as used here to study the effects of naltrexone on alcohol craving and on neuroendocrine measures following alcohol self-administration. That study found that naltrexone increased plasma adrenal corticotropic hormone (ACTH)/cortisol levels relative to placebo both under baseline conditions and following alcohol. Cortisol levels were inversely related to urge to drink, leading the authors to conclude that a naltrexone-induced increase in hypothalamic–pituitary–adrenal (HPA) activity may mediate, or be a marker for, its ability to reduce alcohol craving (O’Malley et al. 2002). Pharmacological blockade or genetic ablation of CB1 receptors also leads to increased HPA activity in rodents (Steiner and Wotjak 2008). This led us to hypothesize that participants taking rimonabant would have elevated plasma ACTH/cortisol levels compared to placebo.
Materials and methods
Participants
Men and women, between 21 and 45 years of age who consumed 20–50 drinks per week, were recruited from the greater Washington, D.C. area to participate in a pharmacological study examining the efficacy of rimonabant to decrease alcohol consumption. Because the effect of rimonabant on alcohol consumption in mice is related to age (Wang et al. 2003), we excluded older participants. The study protocol was approved by the National Institute of Mental Health Institutional Review Board. Initial assessment included a comprehensive medical and psychiatric examination. For ethical reasons, all participants were not seeking treatment for their alcohol consumption. Participants were required to have a body mass index (BMI) of 18–30, not be taking any medications, and have a negative urine drug screen for illicit drugs. All participants tested negative for hepatitis and HIV; minor elevations in liver functions (i.e., twice normal) were permitted. Participants agreed to abstain from the use of illicit drugs for the duration of the study. Participants were required to have a normal 12-lead electrocardiogram (EKG) with the following parameters (PR <210 ms, QRS <120 ms, QTcB males <430 ms, QTcB females <450 ms). Participants with major psychiatric illnesses such as bipolar, schizophrenia, or severe depression associated with suicide attempts were excluded. Participants who had a history of withdrawal symptoms which resulted in a score of 8 or above on the Clinical Institute Withdrawal Assessment (Sullivan et al. 1989) instrument were also excluded.
Study design
Prior to entering the study, each participant was extensively queried about their alcohol consumption to ascertain eligibility for the study. Their alcohol consumption over the past 3 months was assessed using timeline follow-back (TLFB; Sobell and Sobell 1992). Diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV) diagnoses (American Psychiatric Association 1994) were also determined. Participants received no feedback about their alcohol consumption, except to inform them that they fulfilled the requirements for the study. Participants were asked to call the clinic every day for 21 days prior to the ASA and report their alcohol consumption for the previous day on an answering machine. The daily call-ins were intended to capture the participants’ alcohol consumption in a naturalistic setting.
Following a 1-week baseline evaluation, participants were randomized according to a double-blind design to receive either 20 mg/day of rimonabant or placebo for 2 weeks. Participants were evaluated regularly for medication side effects, changes in laboratory values and EKG, and illicit drugs. After being on rimonabant or placebo for 14 days, the ASA was performed. On the day of the ASA, participants were asked to take their medication at 8:00 a.m. in the morning before coming to the National Institute on Alcohol Abuse and Alcoholism (NIAAA) outpatient clinic. Patients were required to return their study medication bottle for a pill count. At 12:00 noon, blood for rimonabant levels and routine laboratory measures including urine drug screen were obtained. Participants then had a light lunch.
Alcohol self-administration paradigm
The ASA session was initiated at 3:45 p.m. Using the self-administration paradigm previously used to demonstrate effects of naltrexone on alcohol drinking (O’Malley et al. 2002), participants received an oral priming dose of ethanol designed to raise the blood alcohol level (BAL) to an average of 0.03 g/dl and to elicit a desire to consume alcohol. This priming dose was given 50 min before the participants entered into the self-administration period of the paradigm. Participants were seated in a comfortable lounge chair located in a private patient care room devoid of a bed. Participants had access to selected magazines and music options. An intravenous catheter was inserted to provide venous access for blood sampling with minimal disturbance to the participant. Researchers only interacted with the participant to obtain rating scales, blood samples, and to administer the trays of alcoholic beverages.
During the self-administration period, participants were presented with a tray containing four drinks (each drink was designed to increase the BAL by 0.015 g/dl). The participant was given the opportunity of consuming each drink or receiving $3 for each drink not consumed. At the end of 1 h, the tray was removed. Following a 10-min break, a second tray identical to the first tray was given to the participant. By giving two separate trays, we prevented the rapid consumption of eight drinks in a short period of time. The Biphasic Alcohol Effects Scale (BAES) and Alcohol Urge Questionnaire (AUQ) rating scales as well as blood for alcohol, ACTH, and cortisol plasma concentrations were obtained prior to the priming drink and at 10, 20, 30, 40, 50, 80, 110, 120, 150, and 180 min after the priming drink. Following the ASA, the participants spent the night on the NIAAA inpatient unit and were discharged the following morning.
Each participant returned to the NIAAA outpatient clinic for two follow-up visits (7 days post-ASA and 29 days post-ASA) to assess any negative sequelae from their participation in the study. If indicated, participants were counseled about their alcohol consumption and offered referrals for alcohol treatment. Routine laboratory measures including urine drug screens were also obtained.
Study medication, rimonabant (SR141716)
Participants received 20 mg of rimonabant or placebo once a day for 14 days. This dose was safe and effective in previous studies in obese patients (see Investigator brochure). The plasma terminal half-life is between 6 to 9 days in young normal weight adult subjects; maximum plasma concentrations of the drug are reached 1 to 3 h after drug administration. Once a day, dosing was instituted because of the prolonged plasma half-life and animal studies showing that food intake is altered by rimonabant for up to 15 h (McLaughlin et al. 2003). Rimonabant was administered for 14 days to obtain meaningful data on outpatient alcohol consumption. In a study of rimonabant in obesity (PDY3255), the drug had a significant effect on body weight after just 7 days.
Results and statistical analysis
Forty-four participants were randomized (35 males, nine females). Five participants were excluded from analysis due to abnormal electrocardiogram (i.e., prolonged QTC), noncompliance, positive urinalysis for illicit drugs, and fainting during the alcohol self-administration study. Participant characteristics are described in Tables 1 and 2. Statistical analyses were performed using Statistica (Statsoft I 2005). The level of significance was set at 0.05. There were no significant differences between treatment groups for age (F(1, 37)=0.00, p=0.96), BMI (F(1, 37)=0.48, p=0.49), baseline weight (F(1, 37)=1.6, p=0.21), and TLFB monthly drinks (F(1, 37)=3.22, p=0.08). There was also no difference between the groups for weight loss from baseline to ASA (F(1, 36)=0.05, p=0.82).
Table 1.
Participant characteristics
| Placebo (n=21) | Rimonabant(n=18) | |
|---|---|---|
| Age (years±SE) | 31.5±1.75 | 31.6±1.92 |
| Males | 16 | 15 |
| Females | 5 | 3 |
| Participants meeting (DSM-IV) criteria for | ||
| Alcohol dependence | 15 | 15 |
| Alcohol abuse | 3 | 1 |
| Number of smokers | 11 | 6 |
| Weight at baseline (kg±SE) | 75.4±2.5 | 80.4±3.1 |
| Weight loss (baseline to ASA; kg±SE) | 0.59±0.4 | 0.47±0.4 |
| 90 days timeline follow-back | ||
| Number of drinking days prior to enrollment | 64.5±4.4 | 69.8±3.8 |
| Average number of drinks per drinking day | 6.5±0.4 | 7.3±0.7 |
Table 2.
Participant characteristics (called-in daily number of drinks (average±SE))
| Mean±SE | Minimum | Maximum | Mean±SE | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| Days1–8 | 5.5±0.5 | 2.1 | 11.0 | 5.7±0.8 | 2.3 | 15.5 |
| Days 9–15 | 4.9±0.4 | 2.4 | 9.0 | 4.9±0.5 | 1.5 | 10.4 |
| Days 16–21 | 4.6±0.5 | 1.8 | 12.3 | 5.0±0.5 | 1.3 | 9.7 |
Daily call-ins
The self-reported daily number of drinks were averaged for the baseline period (days 1–8), for the first week on rimonabant or placebo (days 9–15), and for the second week on rimonabant or placebo (days 16–21; see Tables 1 and 2). A repeated-measures analysis with an adjustment for the average number of the drinks per day for the baseline period (prior to randomization) was performed with the dependent variables being the weekly average number of drinks (days 9–15, and 16–21) and treatment group (rimonabant or placebo) as the grouping variable. There was no significance treatment effect (F(1, 36)=0.05, p=0.83), time effect (F(1, 36)=0.36, p=0.55), or interaction (F(1, 36)=0.46, p=0.50).
Rimonabant drug levels
All of the participants assigned to receive rimonabant had a positive drug level ranging from 138 to 350 ng/ml measured on the day of the ASA session. Returned medication bottles indicated that all of the patients had taken their study medication.
Side effects
The Visual Analog Scale (VAS) was utilized to monitor drug side effects each time the patient came to the outpatient clinic. T tests performed on the VAS scores obtained on the day of the ASA session showed no significant differences for drug side effects. Throughout the study, none of the participants reported feelings of depression or suicidal thoughts. One of the participants taking rimonabant had an angry outburst, and one of the participants taking placebo had a panic attack several days after the medication phase was completed.
Alcohol self-administration paradigm
Priming dose period
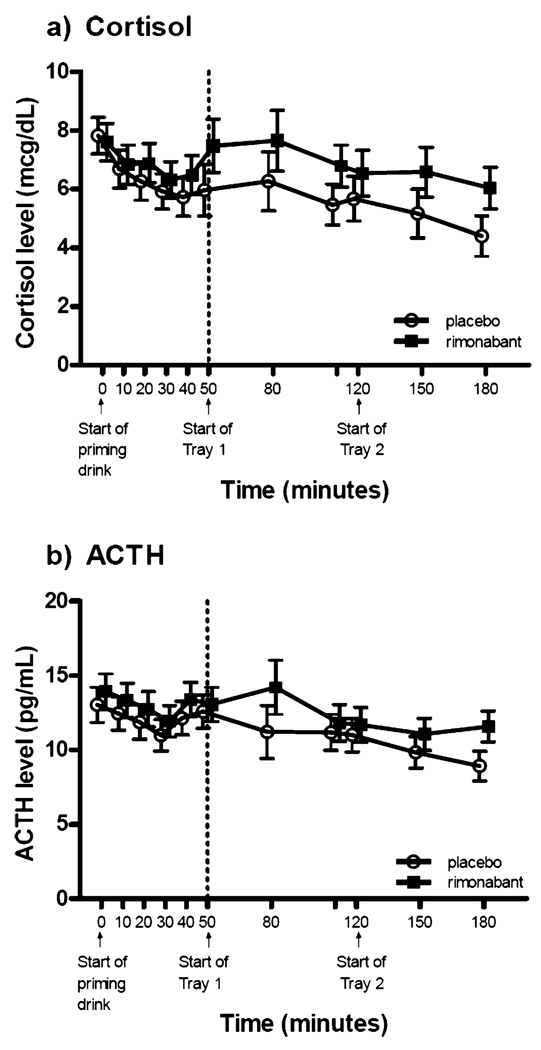
Repeated-measures analysis of variance was performed using times (in minutes) 10 through 50 as the repeated-measures and baseline values (prior to the priming drink) as the covariates. The BALs showed a significant time effect (F(4, 140)=16.21, p<0.0001) and no treatment or interaction effect (F(1, 35)=0.02, p=0.90; F(4, 140)=0.83, p=0.51, respectively). There was no significant treatment, time, or interaction effect for ACTH or cortisol (see Fig. 1).
Fig. 1.
Hormone levels obtained during the alcohol self-administration paradigm. a Plasma cortisol levels for both the priming dose period and the alcohol self-administration period showed no significant treatment, time, or interaction effect. b Plasma ACTH levels showed no treatment, time, or interaction effect for the priming dose period; the alcohol self-administration period showed a significant time effect (F(4, 136)=5.18, p<0.001) but no treatment or interaction effect (vertical bars denote ±standard errors)
The Alcohol Urge Questionnaire and Biphasic Alcohol Effects Scale stimulant and sedative subscales showed no significant treatment or interaction effect. A significant time effect was found for the BAES stimulant subscale (F(4, 128)=2.49, p=0.046). The time effect was not found for the BAES sedative subscale or the AUQ.
Alcohol self-administration period
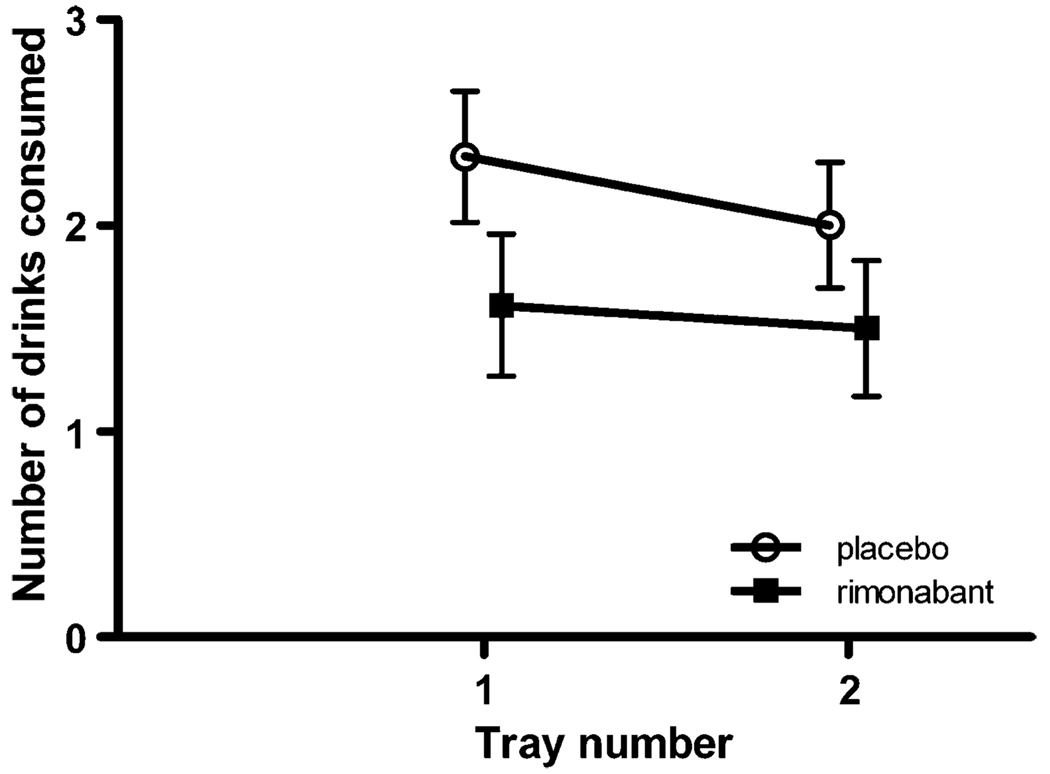
We performed a repeated-measures analysis of variance with the number of drinks consumed on tray 1 and tray 2 as the repeated measure and treatment as the between-groups measure. There was no difference in the number of drinks consumed between those taking rimonabant or placebo (F(1, 37)=1.98, p=0.17). There was also no time or interaction effect (F(1, 37)=2.18, p=0.15; F(1, 37)=0.55, p=0.46, respectively; see Fig. 2).
Fig. 2.
Number of drinks consumed during alcohol self-administration period: number of drinks consumed from tray 1 and tray 2 show no significant treatment, time, or interaction effect
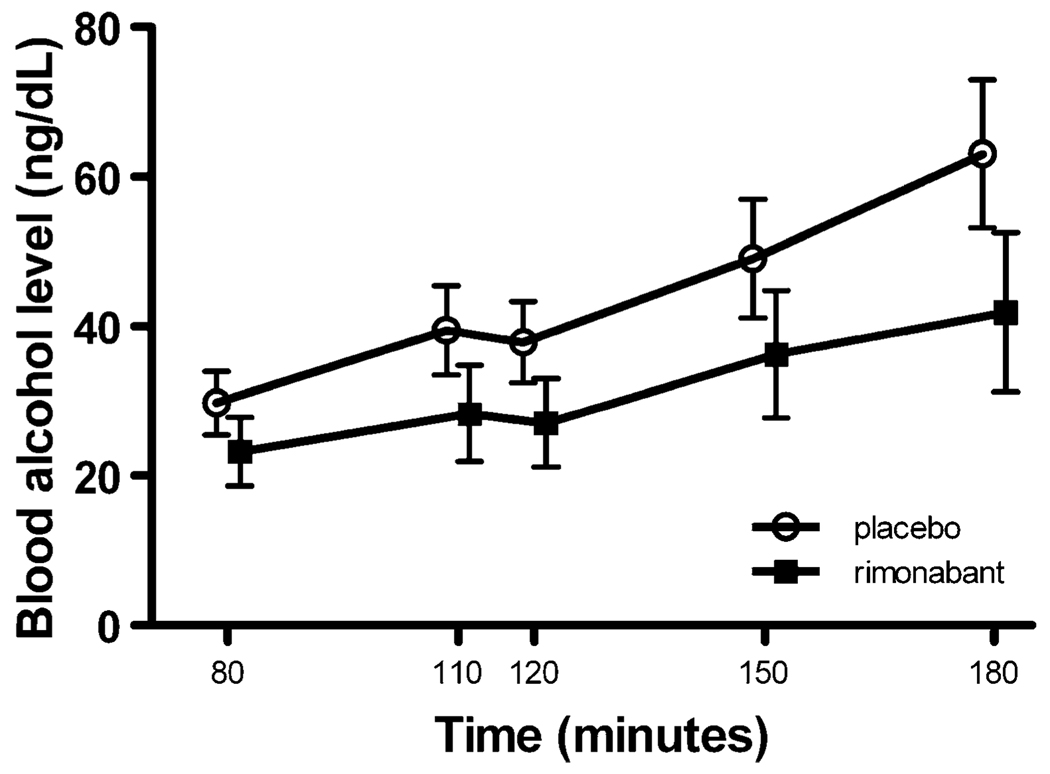
A repeated-measures analysis of variance was performed with the repeated measure factor being time points 80 through 180 min. To reduce the error of variance in our analysis, the 50-min time point (the start of tray 1) was used as the covariate for all other measures obtained during the alcohol self-administration period. There were no significant treatment effects or interactions for the measures BAL, ACTH, cortisol, AUQ, BAES stimulant subscale, or BAES sedative subscale. BAL (F(4, 144)=3.87, p=0.005; see Fig. 3) and ACTH (F(4, 136)=5.18, p<0.001) showed a significant time effect (see Fig. 1).
Fig. 3.
BALs obtained during the alcohol self-administration period: BALs showed a significant time effect (F(4, 144)=3.87, p=0.005) but no treatment or interaction effect. The BAL at time 50 min (start of tray 1) was used as the covariate for the time points 80 through 180 min (covariate mean BAL was 17.7 ng/dl)
Additional analyses were performed to examine correlations (using Spearman’s rank correlation coefficients) within our data using the maximum value determined for each of the repeated measure variables utilized in this study. There was no significant correlation between age or any of the endocrine measures and number of drinks consumed during the ASA. Total number of drinks during the ASA was not correlated (r=−0.01) with the blood level of rimonabant of participants in the drug treatment group. The total number of drinks during the ASA was not significantly correlated (r=0.13) with the TLFB monthly drinks. The total number of drinks consumed during the ASA was not significantly correlated with blood endocrine measures during the ASA.
The average AUQ score during priming was significantly correlated (Spearman’s rank correlation, r=0.65 in rimonabant group and r=0.57 in the placebo group, p<0.01) with the total number of drinks during the ASA. The average BAES stimulant and sedation subscale scores during priming were not significantly correlated with the total number of drinks during the ASA.
Discussion
The goal of this study was to test, in a controlled hospital environment as well as in an outpatient setting, the ability of a novel compound to reduce alcohol consumption. Since numerous animal studies have shown that antagonism at the CB1 receptor site reduces voluntary alcohol consumption (Colombo et al. 1998; Wang et al. 2003), motivation to consume alcohol (Economidou et al. 2006), and the amount of alcohol consumed following periods of abstinence (Cippitelli et al. 2005), rimonabant was judged to be an excellent candidate compound. The primary measure to assess drug efficacy was the total number of drinks consumed during the ASA. This paradigm was previously proven successful in detecting a difference in alcohol consumption between naltrexone and placebo (O’Malley et al. 2002).
The ASA failed to detect a significant difference between rimonabant and placebo for any of the outcome measures. These measures included the number of drinks consumed following the priming drink, alcohol-induced changes in ACTH, cortisol, and peak alcohol concentrations, as well as self-reported ratings on the BAES and AUQ. The positive correlation between the average AUQ scores following the priming drink and the total number of drinks consumed during the ASA substantiates the validity of the drinking paradigm. However, it is possible that the priming drink obscured our ability to determine if rimonabant influenced the likelihood of the participant taking the first drink.
The study also included an outpatient component which would approximate drug efficacy in the participant’s natural setting, i.e., number of drinks consumed during the 2 weeks on study medication. Results showed that there was no significant difference in the number of drinks consumed between treatment groups. After their participation in the daily call-ins and the ASA, some participants reported that performing the TLFB and calling into the clinic every day affected their alcohol consumption. Of interest, approximately 50% of the participants reported that at the end of the study, they realized they had an alcohol problem and desired to decrease their consumption. None of the measures obtained in the protocol predicted which of the participants would be motivated to change their drinking behaviors.
It is unlikely that the lack of drug effect is due to inadequate compliance. The drug levels measured in the rimonabant treatment group are consistent with levels found in normal volunteers and obese patients following 20 mg/day of rimonabant in other studies (Turpault et al. 2006). Given that the effect size (D) was only 0.28, it is possible that a larger number of participants could result in sufficient power to yield a significant drug effect. Given the small sample size, it is not possible to determine whether sex had any effect on the treatment outcome. The fact that a number of animal studies showed a significant effect of rimonabant on alcohol consumption is consistent with the fact that laboratory studies tend to yield larger effect sizes than clinical treatment trials. For example, a number of animal studies showed efficacy of naltrexone and acamprosate, yet not all human studies showed clinical efficacy (Rosner et al. 2008). Also, the dose of rimonabant used in this study (20 mg) causes incomplete blockade of CB1 receptors (Huestis et al. 2001). In contrast, the higher doses used in animal studies (3–10 mg/kg), which resulted in reduced alcohol intake, caused near-complete receptor occupancy. Therefore, it is possible that at a higher dose, rimonabant may significantly reduce the desire to drink. Unfortunately, the psychiatric profile of rimonabant makes the use of higher doses problematic in humans.
This limitation may also be the main reason of the differential effectiveness of naltrexone and rimonabant on reducing alcohol intake, even when tested in the same laboratory drinking paradigm (O’Malley et al. 2002). Both compounds are believed to inhibit reward-related signaling in the mesolimbic reward pathway, and in animal models, both were found to reduce drinking in the same dose range (1–10 mg/kg). In human studies, naltrexone has been found effective in reducing drinking at doses of 50 to 150 mg, and there is evidence that μ opioid receptor occupancy is near maximal even at the 50 mg dose (Weerts et al. 2008), which is different from the partial CB1 receptor blockade achieved by 20-mg rimonabant. This could suggest that for reducing the motivation to drink, one may need to maximally inhibit reward-related signaling by either opioid peptides or endocannabinoids.
It should be noted that the results of this study were obtained in heavy alcohol drinkers who had no desire to decrease their alcohol consumption. It is possible that rimonabant may have had a significant effect in treatment-seeking alcoholics. However, this seems unlikely given the results of a recent study by Soyka et al. (2008), showing that rimonabant did not have a significant effect on relapse rate in recently detoxified alcoholics.
In summary, we found no effect on alcohol consumption in nontreatment-seeking heavy alcohol drinkers when rimonabant was administered at a dosage of 20 mg/day for 14 days. This result is particularly important since the endocannabinoid system has been viewed as a potential target for the pharmacological treatment of patients with alcohol dependence (Heilig and Egli 2006).
Acknowledgment
Special thanks to Niloofar Ghassemzedeh, B.S. and Kathryn Rice, B.A. for many hours of collecting data for this manuscript and thanks to Shellie-Anne Levy, B.A. for assisting with data presentation.
Footnotes
Ethical standards Approval for the study was obtained from the National Institute of Mental Health Institutional Review Board and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Signed informed consent was obtained by the Principal Investigator (D.T. George) or his designee from all participants prior to study involvement.
Disclosure/conflict of interest D.T. George, M.D. has full control of all primary data and agrees to allow the journal to review the data if requested. No author has any commercial or financial involvement that would represent a conflict of interest. Stephanie S. O’Malley, PhD, is a member of the American College of Neuropsychopharmacology task force, Alcohol Clinical Trials Initiative, which is sponsored by Eli Lilly, Janssen, Schering Plough, Lundbeck, and Alkermes. She is also on the Scientific Review panel for the Hazelden Foundation. Raafat Bishai, M.D., is employed by Sanofi-Aventis, and Sanofi-Aventis provided technical support and rimonabant. All work performed by government employees was done in accord with their official duties; therefore, the contents of this manuscript are considered to be within public domain.
Contributor Information
David Ted George, Email: tedg@mail.nih.gov, Laboratory of Clinical and Translational Studies, National Institute on Alcohol Abuse and Alcoholism, 10 Center Drive, 10 CRC-Hatfield Center, Rm. 1-5330, MSC 1108, Bethesda, MD 20892-1108, USA.
David W. Herion, Laboratory of Clinical and Translational Studies, National Institute on Alcohol Abuse and Alcoholism, 10 Center Drive, 10 CRC-Hatfield Center, Rm. 1-5330, MSC 1108, Bethesda, MD 20892-1108, USA
Cheryl L. Jones, Laboratory of Clinical and Translational Studies, National Institute on Alcohol Abuse and Alcoholism, 10 Center Drive, 10 CRC-Hatfield Center, Rm. 1-5330, MSC 1108, Bethesda, MD 20892-1108, USA
Monte J. Phillips, Laboratory of Clinical and Translational Studies, National Institute on Alcohol Abuse and Alcoholism, 10 Center Drive, 10 CRC-Hatfield Center, Rm. 1-5330, MSC 1108, Bethesda, MD 20892-1108, USA
Jacqueline Hersh, Laboratory of Clinical and Translational Studies, National Institute on Alcohol Abuse and Alcoholism, 10 Center Drive, 10 CRC-Hatfield Center, Rm. 1-5330, MSC 1108, Bethesda, MD 20892-1108, USA.
Debra Hill, Laboratory of Clinical and Translational Studies, National Institute on Alcohol Abuse and Alcoholism, 10 Center Drive, 10 CRC-Hatfield Center, Rm. 1-5330, MSC 1108, Bethesda, MD 20892-1108, USA.
Markus Heilig, Laboratory of Clinical and Translational Studies, National Institute on Alcohol Abuse and Alcoholism, 10 Center Drive, 10 CRC-Hatfield Center, Rm. 1-5330, MSC 1108, Bethesda, MD 20892-1108, USA.
Vijay A. Ramchandani, Laboratory of Clinical and Translational Studies, National Institute on Alcohol Abuse and Alcoholism, 10 Center Drive, 10 CRC-Hatfield Center, Rm. 1-5330, MSC 1108, Bethesda, MD 20892-1108, USA
Christopher Geyer, Clinical Center Nursing, NIH, Bethesda, MD, USA.
David E. Spero, Clinical Center Nursing, NIH, Bethesda, MD, USA
Erick D. Singley, Laboratory of Clinical and Translational Studies, National Institute on Alcohol Abuse and Alcoholism, 10 Center Drive, 10 CRC-Hatfield Center, Rm. 1-5330, MSC 1108, Bethesda, MD 20892-1108, USA
Stephanie S. O’Malley, School of Medicine, Yale University, New Haven, CT, USA
Raafat Bishai, International Clinical Development, Sanofi-Aventis, Malvern, PA, USA.
Robert R. Rawlings, Laboratory of Clinical and Translational Studies, National Institute on Alcohol Abuse and Alcoholism, 10 Center Drive, 10 CRC-Hatfield Center, Rm. 1-5330, MSC 1108, Bethesda, MD 20892-1108, USA
George Kunos, Email: gkunos@mail.nih.gov, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD, USA; NIAAA, 5625 Fishers Ln, MSC 9413, Rm. 2S-24, Rockville, MD 20892-9413, USA.
References
- American Psychiatric Association. Washington, DC: American Psychiatric Association; Diagnostic and statistical manual of mental disorders. (4th edn.) 1994
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le FG. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Hansson AC, Del AI, Sommer W, Heilig M, Massi M, Bermudez-Silva FJ, Navarro M, Ciccocioppo R, de Fonseca FR. Cannabinoid CB1 receptor antagonism reduces conditioned reinstatement of ethanol-seeking behavior in rats. Eur J NeuroSci. 2005;21:2243–2251. doi: 10.1111/j.1460-9568.2005.04056.x. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Fa M, Guano L, Lobina C, Loche A, Reali R, Gessa GL. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol. 1998;33:126–130. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, Carai MM, Gessa L. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacology. 2002;159:181–187. doi: 10.1007/s002130100887. [DOI] [PubMed] [Google Scholar]
- DiMarzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, Trabace L, Ciccocioppo R. Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology (Berl) 2006;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Sharpe AL, Samson HH, Porrino LJ. Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 2001;25:277–282. [PubMed] [Google Scholar]
- Gallate JE, Saharov T, Mallet PE, McGregor IS. Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur J Pharmacol. 1999;370:233–240. doi: 10.1016/s0014-2999(99)00170-3. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Bermudez-Silva FJ, Malinen H, Hyytia P, Sanchez-Vera I, Rimondini R, de Rodriguez FF, Kunos G, Sommer WH, Heilig M. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology. 2007;32:117–126. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [see comment] [DOI] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Gonzalez-Cuevas G, de Rodriguez FF, Navarro M. Long-lasting increase of alcohol relapse by the cannabinoid receptor agonist WIN 55, 212-2 during alcohol deprivation. J Neurosci. 2004;24:8245–8252. doi: 10.1523/JNEUROSCI.2179-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, Betz AJ, Ishiwari K, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology. 2004;46:243–253. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary–adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [Review] [1332 refs] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncelet M, Maruani J, Calassi R, Soubrie P. Overeating, alcohol and sucrose consumption decrease in CB1 receptor deleted mice. Neurosci Lett. 2003;343:216–218. doi: 10.1016/s0304-3940(03)00397-5. [DOI] [PubMed] [Google Scholar]
- Rosner S, Leucht S, Lehert P, Soyka M. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol (Oxf) 2008;22:11–23. doi: 10.1177/0269881107078308. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Soyka M, Koller G, Schmidt P, Lesch OM, Leweke M, Fehr C, Gann H, Mann KF. Cannabinoid receptor 1 blocker rimonabant (SR 141716) for treatment of alcohol dependence: results from a placebo-controlled, double-blind trial. J Clin Psychopharmacol. 2008;28:317–324. doi: 10.1097/JCP.0b013e318172b8bc. [DOI] [PubMed] [Google Scholar]
- Statsoft I. STATISTICA (data analysis software system) Tulsa: Statsoft I; 2005. [Google Scholar]
- Steiner MA, Wotjak CT. Role of the endocannabinoid system in regulation of the hypothalamic–pituitary–adrenocortical axis. Prog Brain Res. 2008;170:397–432. doi: 10.1016/S0079-6123(08)00433-0. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-AR) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND. Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res. 2005;164:206–213. doi: 10.1016/j.bbr.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Turpault S, Kanamaluru V, Lockwood GF, Bonnet D, Newton J. Rimonabant pharmacokinetics in healthy and obese subjects. Clin Pharmacol Ther. 2006;79:50. [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci U S A. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Kim YK, Wand GS, Dannals RF, Lee JS, Frost JJ, McCaul ME. Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects. Neuropsychopharmacology. 2008;33:653–665. doi: 10.1038/sj.npp.1301440. [DOI] [PubMed] [Google Scholar]