Abstract
Objectives
The presence of a nonpathogenic organism on the surface of a urinary catheter might impede catheter colonization by pathogens and thus prevent urinary tract infection. Previously, we reported that preinoculating urinary catheters with a nonpathogenic strain of Escherichia coli (83972) significantly impeded catheter colonization in vitro by gram-positive bacteria (Enterococcus faecalis). To explore this phenomenon further, we investigated in vitro whether E. coli 83972 could likewise inhibit catheter colonization by gram-negative and fungal uropathogens (Providencia stuartii, uropathogenic E. coli, and Candida albicans).
Methods
For each of the three uropathogens tested, we examined three different incubation conditions: (a) E. coli plus uropathogen catheters were exposed to E. coli 83972 for 24 hours and then to a uropathogen for 30 minutes; (b) E. coli-alone catheters were incubated with E. coli for 24 hours and then in sterile broth for 30 minutes; and (c) uropathogen-alone catheters were incubated in sterile broth for 24 hours before the 30-minute incubation with the uropathogen. All catheters were subsequently incubated in sterile human urine for 24 hours. Catheters were then rinsed and sonicated to determine the numbers of adherent organisms per centimeter.
Results
Pre-exposure of the catheter to E. coli 83972 in all cases significantly reduced the number of uropathogens colonizing the catheter surfaces.
Conclusions
E. coli 83972 significantly impedes catheter colonization by all tested bacterial and fungal pathogens. The broad applicability of this particular approach to bacterial interference in vitro invites further exploration in vivo.
Most individuals with spinal cord injury (SCI) have a neurogenic bladder, and the resulting urinary stasis and bladder catheterization predispose them to recurrent urinary tract infection (UTI). Now that individuals with SCI have normal or near-normal life expectancies, secondary infectious complications, such as UTI, have become major causes of morbidity and mortality.1 Currently, few, if any, measures are effective at preventing UTI in persons with a neurogenic bladder and an indwelling urinary catheter.
Bacterial interference, or using nonpathogenic bacteria to prevent symptomatic infection,2 may offer a solution to the problem of recurrent UTI in chronically catheterized individuals. Essentially, all patients with an indwelling urinary catheter for 30 days or longer have bacteriuria, frequently with more than one organism.3–5 Studies in elderly subjects, patients with SCI, and adult women indicate that treatment of asymptomatic bacteriuria in these populations is neither beneficial nor effective.2,6–8 Allowing asymptomatic bladder colonization to persist in order to prevent colonization by a pathogenic strain may constitute a passive approach to bacterial interference.2
A more active approach to bacterial interference involves intentionally colonizing the bladder with a nonpathogenic organism to prevent infection with more virulent bacteria. Prospective clinical trials have demonstrated that direct bladder inoculation with a nonpathogenic strain of E. coli (83972) is safe and protective in persons with SCI.9,10 However, the direct bladder inoculation process was cumbersome and frequently required multiple attempts to induce colonization. Furthermore, we wished to address the problem of recurrent UTI at the level at which catheter-associated UTI begins—the formation of biofilm. Implanted urinary catheters rapidly acquire a complex, three-dimensional biofilm composed of bacteria, their extracellular products, and components deposited from bodily fluids. The pathogenic organisms in a biofilm continually seed the bladder, leading to bacteriuria and/or UTI.3 Because biofilm formation on a wet-implanted device such as a urinary catheter is nearly impossible to prevent,11,12 we propose instead to manipulate the adherent microbial flora. We ultimately plan to use preinoculated Foley catheters as a means to colonize the human bladder with E. coli 83972 and thus prevent UTI.
With this goal in mind, we investigated the interference capacities of E. coli 83972 in vitro. As we have previously reported, preinoculating urinary catheters with E. coli 83972 significantly impeded catheter colonization by the gram-positive organism Enterococcus faecalis.13 To explore this interference phenomenon further, we investigated in vitro whether E. coli 83972 could likewise inhibit catheter colonization by gram-negative and fungal uropathogens, including Providencia stuartii, uropathogenic lactose-negative E. coli, and Candida albicans. These three organisms were specifically chosen because they are major causes of bacteriuria and/or UTI in chronically catheterized persons. 14,15
MATERIAL AND METHODS
Bacteria
E. coli 83972, serotype ON:KN (N = nontypeable), lactose positive, was initially isolated from the urine of a young girl who had had asymptomatic bacteriuria for 3 years with stable renal function.16 This bacterial strain was selected because of its documented ability to persist in the urinary tract without causing disease. The P. stuartii, uropathogenic lactose-negative E. coli, and C. albicans strains were isolated from the urine of patients with symptomatic UTI. All strains were stored at −80°C in a 10% glycerol culture. Bacterial strains were then passaged on either trypticase soy agar with 5% sheep blood (BBL, Cockeysville, Md) or MacConkey agar (BBL). The C. albicans strain was passaged on Sabouraud agar (Becton Dickinson, Sparks, Md). Bacteria were retrieved from the agar plates and cultured overnight in trypticase soy broth (TSB; Becton Dickinson). A sample of the overnight culture was adjusted to ~108 colony-forming unit (cfu)/mL.
Catheter Preparation
Commercially available, 12F Foley catheters composed of latex with a hydrophilic coating were used in this study (Bardex, Covington, Ga). Under sterile conditions, the catheter shaft was cut into 7-cm pieces. The balloon lumen of each piece was plugged on both ends with sterile pipette tips and sealed with flame. The purpose of sealing the balloon channel was to leave only the main catheter lumen accessible to organisms, such as is the case when a catheter is placed in the human bladder. The catheter pieces were placed in sterile 50-mL centrifuge tubes containing TSB.
Experimental Variables and Controls
Each set of experiments included one experimental catheter (incubated first with E. coli 83972 and subsequently with a uropathogen), and two control catheters (one incubated only with E. coli 83972 and one incubated only with the uropathogen). Six to nine sets of experiments were performed with each of the three uropathogens tested. The three catheters in each set were incubated at 37°C as follows: (a) E. coli plus uropathogen catheters were exposed to E. coli 83972 in TSB for 24 hours at a starting concentration of 105 cfu/mL and then were transferred without washing to a tube containing a uropathogen in TSB at a concentration of 105 cfu/mL for 30 minutes; (b) E. coli-alone catheters were incubated for 24 hours with E. coli 83972 at a starting concentration of 105 cfu/mL and then transferred to sterile broth for 30 minutes; and (c) uropathogen-alone catheters were incubated in sterile broth for 24 hours before the 30-minute incubation with a uropathogen. After the 30-minute incubation step, all catheter pieces were removed, placed immediately in filter-sterilized human urine, and incubated in urine at 37°C for 24 hours. Thus, biofilm formation by the challenge uropathogens occurred in urine, as would occur in vivo. The urine was obtained from a single male donor and was filter sterilized by passage through a 0.22-μm filter.
The purpose of incubating the E. coli 83972-coated catheters for 30 minutes with uropathogens in broth was to standardize the catheter exposure to the uropathogens. Because the organisms used in these trials grow better in broth than in urine, catheter seeding by both E. coli 83972 and the challenge uropathogens was performed in broth (TSB).
Catheter Harvest
Under sterile conditions, the 7-cm catheter pieces were removed from the tubes and flushed with saline. Each catheter piece was then gently agitated in three consecutive saline baths. The purpose of the rinsing procedure was to remove residual urine and loosely attached organisms from the surface of the catheters. Three 1-cm segments were cut from each catheter. Each 1-cm segment was placed in 1 mL of saline and sonicated at 55,000 Hz for 10 minutes in a water bath sonicator (Buehler Scientific, Evanston, Ill) at room temperature.17 The sonication fluid was diluted to 1:103, and 100 μL aliquots of the original and diluted sonication fluid were inoculated onto agar plates to determine the plate counts. Gram staining of selected colonies was done to verify the types of organisms. Samples of the urine in each centrifuge tube were also diluted and spread on agar plates to assess the final bacterial concentrations and purity. For each tested uropathogen, this set of experiments described above was repeated up to nine times.
Statistical Analysis
For the purposes of statistical comparison, the median of the colony counts from the 1-cm catheter segments was used as the value for each catheter. The Wilcoxon two-tailed signed rank test was used for all comparisons using Statistical Analysis System software.
RESULTS
The pre-existing coating of E. coli 83972 significantly reduced catheter colonization by all tested uropathogens (Fig. 1). P. stuartii-alone catheters had a median of 3.6×103 cfu/cm of P. stuartii, and the E. coli plus P. stuartii catheters had a smaller median of 1.9×102 cfu/cm of P. stuartii (P=0.03, Wilcoxon signed rank test). For the uropathogenic lactose-negative E. coli catheters, 1.11 × 104 cfu/cm were found on the catheter surface when the catheter had not been previously exposed to E. coli 83972 versus only 8.5 × 101 cfu/cm when the catheter had been pre-exposed to E. coli 83972 (P = 0.03). For C. albicans catheters, 2.8 × 103 cfu/cm and 10 cfu/cm were found in the absence and presence of E. coli 83972, respectively (P = 0.02). In the cases of P. stuartii and lactose-negative E. coli, preinoculation of the catheters with E. coli 83972 reduced the concentration of uropathogens on the catheter surface in all six sets of experiments. A similar observation was noted in eight of nine sets of experiments with C. albicans. In no case did incubation with uropathogens significantly reduce the number of E. coli 83972 recovered from the catheter surface.
FIGURE 1.
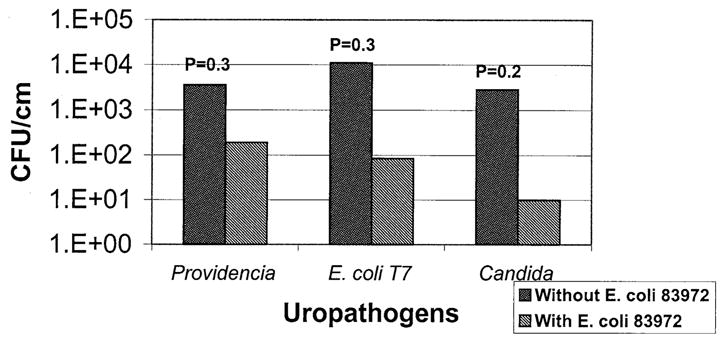
E. coli 83972 inhibits catheter colonization by uropathogens. Data from six to nine sets of experiments for each tested uropathogen are represented. Gram-negative and fungal uropathogens were significantly less likely to colonize urinary catheters with a pre-existing coating of E. coli 83972 than control catheters without E. coli 83972.
COMMENT
Catheter-related UTI can arise when a few organisms gain entry to the urinary tract and then multiply to high concentrations in the bladder.18,19 The results of this in vitro study suggest that a urinary catheter with a pre-existing coating of E. coli 83972 is likely to be effective at inhibiting even a relatively large number of pathogenic organisms belonging to multiple different genera from colonizing the catheter and thus gaining access to the bladder. Two aspects of these results are particularly encouraging. First, E. coli 83972 protected against catheter colonization by a variety of uropathogens, including gram-positive,13 gram-negative, and fungal uropathogens, and second, exposure of the E. coli 83972-coated catheters to uropathogens did not diminish the numbers of E. coli 83972 recovered from the catheter surface.
The potentially broad-spectrum protection afforded by E. coli 83972 is particularly important, because it may help avoid the common clinical scenario in which suppression of one species of uropathogen allows other strains to flourish. Indeed, the failure to suppress all strains of uropathogens is the major pitfall to the long-term use of antimicrobial agents for UTI prevention in chronically catheterized patients. When prophylactic antibiotics are given to patients with a neurogenic bladder and chronic bacteriuria, the colonizing flora rapidly shift from antibiotic-susceptible organisms to antibiotic-resistant organisms.20,21 Using a broadly interfering agent such as E. coli 83972 to prevent UTI may avoid this problem of supercolonization with resistant flora. Another potential benefit of using bacterial interference rather than antimicrobial agents for UTI prevention may be that patients take fewer courses of antibiotics and thus experience fewer adverse drug effects. Our bacterial interference approach to UTI prevention is designed to decrease the use of antimicrobial agents and their attendant complications.
We found that recovery of E. coli 83972 from the catheters was not significantly affected by the presence or absence of uropathogens in the urine. The implication of this finding is that E. coli 83972 may persist on the surface of urinary catheters when inserted into patients, whose bladders may contain multiple species of uropathogens. If catheter colonization with E. coli 83972 proves to be durable in vivo, the E. coli 83972 on the catheter surface may serve as a nidus to continually seed the bladder with this protective strain. Currently, a pilot clinical trial is underway to examine the safety and efficacy of placing urinary catheters with a pre-existing coating of E. coli 83972 in SCI patients with a neurogenic bladder and recurrent UTI.
E. coli 83972 may have several advantages as an agent of bacterial interference for prevention of UTI compared with Lactobacillus, an organism that has been extensively studied. In vitro, various species of Lactobacillus can block adherence of uropathogens to vaginal epithelial cells, secrete antibacterial agents, and even produce an antifungal compound.22–24 In vivo, deliberate intravaginal instillation of Lactobacillus casei GR-1 into 5 women with recurrent UTI reduced colonization of the urogenital epithelium by coliforms but not by enterococci. 25 A subsequent study in 41 adult women with recurrent UTI suggested that giving intravaginal Lactobacillus suppositories after completion of antibiotic therapy helped restore the indigenous vaginal flora and reduced the recurrence rate of UTI.26 Although the work with Lactobacillus is promising, Lactobacillus does not colonize the bladder and is found exclusively on the female urogenital epithelium.26,27 Therefore, this approach using Lactobacillus may not be applicable to men or to patients with indwelling catheters. Because our target population is individuals with a neurogenic bladder secondary to SCI, many of whom are male and require indwelling catheters, E. coli 83972 is probably a better agent of bacterial interference for our purposes.
The mechanisms underlying the interference effects of E. coli 83972 are not known. Possible mechanisms include spatial constraints, competition for locally available nutrients, and secretion of inhibitory agents by E. coli 83972.28 Because the effect of bacterial interference manifests on the catheter surface, the characteristics of the biofilm formed by E. coli 83972 likely play a central role in its ability to act as an agent of bacterial interference. Elucidating the molecular mechanism of growth inhibition of uropathogens by E. coli 83972 will be a subject of future experiments.
We also do not know whether the observed decrease in the numbers of uropathogens attached to catheter surfaces will translate in vivo to decreased numbers of free-floating uropathogens in patients’ urine. One of the aims of the planned in vivo trial of E. coli 83972-coated catheters will be to compare the numbers and types of organisms recovered from catheter surfaces to the numbers and types of organisms present in the urine.
CONCLUSIONS
The results of this in vitro study demonstrated that a pre-existing coating of E. coli 83972 on the surface of urinary catheters significantly impedes colonization by a variety of uropathogens. This promising approach to bacterial interference invites additional clinical investigation.
Acknowledgments
This study was supported by the Department of Veterans Affairs Rehabilitation Research and Development Service Merit Review B2125-RA, Paralyzed Veterans of America grant 302, and USPHS grant HD42014.
References
- 1.Darouiche R. Infections in patients with spinal cord injury. In: Mandell G, Bennett J, Dolin R, editors. Principles and Practice of Infectious Disease. Philadelphia: Churchill Livingstone; 2000. pp. 3159–3163. [Google Scholar]
- 2.Darouiche R, Hull R. Bacterial interference for prevention of urinary tract infection: an overview. J Spinal Cord Med. 2000;23:136–141. doi: 10.1080/10790268.2000.11753521. [DOI] [PubMed] [Google Scholar]
- 3.Stamm W. Catheter-associated urinary tract infections: epidemiology, pathogenesis, and prevention. Am J Med. 1991;91:65S–71S. doi: 10.1016/0002-9343(91)90345-x. [DOI] [PubMed] [Google Scholar]
- 4.Warren J. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11:609–622. doi: 10.1016/s0891-5520(05)70376-7. [DOI] [PubMed] [Google Scholar]
- 5.Warren J, Tenney J, Hoopes J, et al. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982;146:719–723. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]
- 6.Nicolle L, Bjornson J, Harding G, et al. Bacteriuria in elderly institutionalized men. N Engl J Med. 1983;309:1420–1425. doi: 10.1056/NEJM198312083092304. [DOI] [PubMed] [Google Scholar]
- 7.Nicolle L. Asymptomatic bacteriuria in the elderly. Infect Dis Clin North Am. 1997;11:647–662. doi: 10.1016/s0891-5520(05)70378-0. [DOI] [PubMed] [Google Scholar]
- 8.Tencer J. Asymptomatic bacteriuria—a long-term study. Scand J Urol Nephrol. 1988;22:31–34. doi: 10.1080/00365599.1988.11690380. [DOI] [PubMed] [Google Scholar]
- 9.Hull R, Rudy D, Donovan W, et al. Urinary tract infection prophylaxis using Escherichia coli 83972 in spinal cord injured patients. J Urol. 2000;163:872–877. [PubMed] [Google Scholar]
- 10.Darouiche R, Donovan W, Del Terzo M, et al. Pilot trial of bacterial interference for preventing urinary tract infection. Urology. 2001;58:339–344. doi: 10.1016/s0090-4295(01)01271-7. [DOI] [PubMed] [Google Scholar]
- 11.Destedt J, Wollin T, Reid G. Biomaterials used in urology: current issues of biocompatibility, infection, and encrustation. J Endourol. 1998;12:493–500. doi: 10.1089/end.1998.12.493. [DOI] [PubMed] [Google Scholar]
- 12.Costerton J, Stewart P, Greenberg E. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 13.Trautner B, Darouiche R, Hull R, et al. Pre-inoculation of urinary catheters with Escherichia coli 83972 inhibits catheter colonization by Enterococcus faecalis. J Urol. 2002;167:375–379. [PMC free article] [PubMed] [Google Scholar]
- 14.Warren JW, Tenney JH, Hoopes JM, et al. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982;146:719–723. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]
- 15.Kauffman C, Vazquez J, Sobel J, et al. Prospective multicenter surveillance study of funguria in hospitalized patients. Clin Infect Dis. 2000;30:14–18. doi: 10.1086/313583. [DOI] [PubMed] [Google Scholar]
- 16.Andersson P, Engberg I, Lidin-Janson G, et al. Persistence of Escherichia coli bacteriuria is not determined by bacterial adherence. Infect Immun. 1991;59:2915–2921. doi: 10.1128/iai.59.9.2915-2921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherertz R, Raad I, Belani A, et al. Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J Clin Microbiol. 1990;28:76–82. doi: 10.1128/jcm.28.1.76-82.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardenas D, Hooten T. Urinary tract infection in persons with spinal cord injury. Arch Phys Med Rehabil. 1995;76:272–280. doi: 10.1016/s0003-9993(95)80615-6. [DOI] [PubMed] [Google Scholar]
- 19.Kunin C, Steele C. Culture of the surfaces of urinary catheters to sample urethral flora and study the effect of antimicrobial therapy. J Clin Microbiol. 1985;21:902–908. doi: 10.1128/jcm.21.6.902-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waites K, Canupp K, Brookings E, et al. Effect of oral ciprofloxacin on bacterial flora of perineum, urethra, and lower urinary tract in men with spinal cord injury. J Spinal Cord Med. 1999;22:192–198. doi: 10.1080/10790268.1999.11719569. [DOI] [PubMed] [Google Scholar]
- 21.Reid G, Howard L. Effect of uropathogens of prophylaxis for urinary tract infection in spinal cord injured patients: preliminary study. Spinal Cord. 1997;35:605–607. doi: 10.1038/sj.sc.3100456. [DOI] [PubMed] [Google Scholar]
- 22.Magnusson J, Schnurer J. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl Environ Microbiol. 2001;67:1–5. doi: 10.1128/AEM.67.1.1-5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernet-Camard M, Lievin V, Brassart D, et al. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl Environ Microbiol. 1997;63:2747–2753. doi: 10.1128/aem.63.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osset J, Bartolome R, Garcia E, et al. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J Infect Dis. 2001;183:485–491. doi: 10.1086/318070. [DOI] [PubMed] [Google Scholar]
- 25.Bruce A, Reid G. Intravaginal instillation of lactobacilli for prevention of recurrent urinary tract infections. Can J Microbiol. 1988;34:339–343. doi: 10.1139/m88-062. [DOI] [PubMed] [Google Scholar]
- 26.Reid G, Bruce A, Taylor M. Influence of three-day antimicrobial therapy and lactobacillus vaginal suppositories on recurrence of urinary tract infections. Clin Ther. 1992;14:11–16. [PubMed] [Google Scholar]
- 27.Hagberg L, Bruce A, Reid G, et al. Colonization of the urinary tract with bacteria from the normal fecal and urethral flora in patients with recurrent urinary tract infection. In: Kass E, Svanborg-Eden C, editors. Host Parasite Interactions in the Urinary Tract. Chicago: University of Chicago Press; 1989. pp. 194–197. [Google Scholar]
- 28.Watnick P, Kolter R. Biofilm, city of microbes. J Bacteriol. 2000;182:2675–2679. doi: 10.1128/jb.182.10.2675-2679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


