Abstract
During the 2001 anthrax attacks, public health agencies faced operational and communication decisions about the use of antibiotic prophylaxis and the anthrax vaccine with affected groups, including postal workers. This communication occurred within an evolving situation with incomplete and uncertain data. Guidelines for prophylactic antibiotics changed several times, contributing to confusion and mistrust. At the end of 60 days of taking antibiotics, people were offered an additional 40 days' supply of antibiotics, with or without the anthrax vaccine, the former constituting an investigational new drug protocol. Using data from interviews and focus groups with 65 postal workers in 3 sites and structured interviews with 16 public health professionals, this article examines the challenges for public health professionals who were responsible for communication with postal workers about the vaccine. Multiple factors affected the response, including a lack of trust, risk perception, disagreement about the recommendation, and the controversy over the military's use of the vaccine. Some postal workers reacted with suspicion to the vaccine offer, believing that they were the subjects of research, and some African American workers specifically drew an analogy to the Tuskegee syphilis study. The consent forms required for the protocol heightened mistrust. Postal workers also had complex and ambivalent responses to additional research on their health. The anthrax attacks present us with an opportunity to understand the challenges of communication in the context of uncertain science and suggest key strategies that may improve communications about vaccines and other drugs authorized for experimental use in future public health emergencies.
In 2001, Bacillus anthracis sent through the United States Postal Service (USPS) caused 22 cases of anthrax, including 5 deaths.1 The attack led to the closing of the Trenton Postal Processing and Distribution Center (PDC) in New Jersey and the Brentwood PDC in Washington, DC;2–4 anthrax contamination was found in 21 postal facilities, including the Morgan Central Postal Facility in New York City.5
Initially, 10,000 people with either a suspected or confirmed exposure to anthrax were recommended to receive the antibiotic ciprofloxacin for 60 days; later it was recommended that they receive doxycycline6 (see Figure 1). Individuals reported adverse effects from both drugs, which became the major self-reported reason for discontinuing treatment.12 In December 2001, the Centers for Disease Control and Prevention (CDC) offered several at-risk groups—individuals in the Brentwood and Hamilton PDCs, American Media, Inc., and the Senate office building, where exposure to high doses was possible, and those who did not complete 60 days of antibiotics9—the option of 40 additional days of antibiotics, or an anthrax vaccine with 40 days of antibiotics, the latter constituting an investigational new drug protocol (IND).6,10 Offering the vaccine as an IND required informed consent. Additionally, CDC required that those taking the vaccine participate in a follow-up study.13 The CDC asked people to consult with their physicians about their decision.10 Only 199 of the 1,727 people who received the 40 days of antibiotics took the vaccine, which was a 3-dose course over 4 weeks10—different from the 6-dose pre-exposure course offered to military personnel.11
FIG. 1.
Background on Antibiotic Prophylaxis and Anthrax Vaccine
In this article, we focus on the reactions of postal workers and public health professionals to the anthrax vaccine following the 2001 letter attacks. This case study provides some critical insights that are relevant to today's discussion of novel medical countermeasures, particularly in the context of the Project BioShield Act of 2004, which allows the FDA to approve the emergency use of such products to enable rapid distribution.14,15 It is likely that the FDA will use its Emergency Use Authorization (EUA) in a future emergency.15 This article expands on the existing literature by examining the challenges of communicating about an experimental vaccine in the midst of a real bioterrorist attack, characterized by uncertainty and distrust, and in a significantly complex communication environment with competing messages.
Factors Affecting Vaccine Uptake
The offer of an anthrax vaccine during a bioterrorist attack represented a new challenge for public health professionals. Most existing literature focuses on typical vaccine-preventable diseases, finding that key factors that affect vaccine uptake include clear recommendations from physicians, the public's subjective risk (including hazard and outrage factors), social networks, and belief in the vaccine's safety.16–21 The literature also documents factors associated with reluctance to accept a vaccine, including failure to attend to outrage factors, concerns about vaccine safety, mistrust and fears about motivations for vaccination, and perceived lack of a clear recommendation.17 Few studies speak to the unusual circumstances posed by the anthrax vaccine, such as the issues surrounding its IND status, postexposure prophylaxis, and the patient's subjective risk perception 60 days after the attack.
The literature on willingness to accept the smallpox vaccination documents the most relevant factors, including: incomplete knowledge of the risk of an attack; limited knowledge about adverse effects; perceived risk of adverse effects and desire to observe what happened to early vaccinees; belief that benefits did not outweigh risks and that vaccinations were not necessary; complex weighing of perceived risk of smallpox versus the risk from the vaccine; potential decisional conflict; and concerns about compensation.22–24 In contrast, studies have found that vaccine acceptance is positively affected by perceived benefit of vaccination, perceived greater risk and worry about bioterrorism, positive beliefs about vaccines in general, and female gender.23,24 In an anthrax vaccine study, laboratory workers who declined the vaccine were more likely to perceive a low risk of contracting anthrax, to be very concerned about the vaccine's safety, and to be less likely to trust the information in the Vaccine Information Statement.25
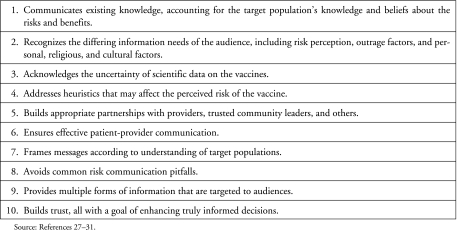
Faced with the 2001 bioterrorist attack, public health agencies implemented crisis and emergency risk communication (CERC) strategies with specific audiences, including postal workers. CERC requires that (1) decisions must occur in a compressed timeframe; (2) the decision may be irreversible; (3) the outcome may be uncertain; and (4) the information necessary for that decision may be incomplete or uncertain.26(p6) In the case of the anthrax vaccine, all of these conditions were present. Although literature exists on effective risk communication for vaccines in general (see Figure 2), there are few studies specifically about the anthrax vaccine. In one U.S. Army study, inadequate risk communication and education about the anthrax vaccine resulted in a reduced ability to address controversy and problems with trust and credibility.32
FIG. 2.
Components of Effective Risk Communication on Vaccines
In the anthrax attacks, studies have found that individuals who adhered to their 60-day antibiotic protocol were more likely to enroll in the IND, suggesting that adherence may be a surrogate for risk perception12 and that risk perception and adherence may ultimately depend on patient-physician contact, with patients being more likely to adhere to prescriptions when advised by their doctors to do so.33 Some evidence exists that postal workers perceived the IND as experimentation33,34 and that those who chose to be vaccinated had a higher level of trust in public health professionals than did those who refused.34
In such circumstances, trust—comprised of caring and empathy, competence and expertise, dedication and commitment, and honesty and openness—is vital to the success of crisis and emergency risk communication.35–39 Other researchers also consider fiduciary responsibility, absence of bias, predictability, and fairness as critical to trust.35,40,41 Equally important are the factors that diminish trust, including “disagreement among experts, lack of coordination among organizations, an unwillingness to acknowledge risks, unwillingness to disclose information, perceived irresponsibility in managing risk and insensitivity of authorities to the public's need for dialogue.”42(p208)
In the anthrax attacks, trust in and credibility for public health agencies were reduced by uncertainty, concerns about equity between postal workers and Senate staff, agency disagreements, and mixed and changing messages.33,34,42–47 In an earlier study, we also found that the preexisting contentious workplace relationship between postal labor and management contributed to the communication difficulties.42
The complex challenges of the anthrax attacks offer insights into the policy, operational, and communication decisions that would be necessary in a future emergency and raise particular issues to be considered in the context of an Emergency Use Authorization.
Methods
This research used a qualitative case study design to explore the impact of the anthrax attacks on postal workers at the Brentwood PDC, the Trenton PDC, and the Morgan Facility. We analyzed data from key informant interviews, individual interviews, and focus groups with postal workers (Table 1)and 16 interviews with public health professionals (8 from CDC and 8 from state or local health departments). The specific aim was to understand the communication regarding the vaccine. Given this methodology, this article reports on the experiences of those interviewed and does not attempt to generalize in any statistical sense to the whole population of postal workers or public health professionals.
Table 1.
Postal Worker Key Informant, Individual Interview, and Focus Group Participants
| Participants | Key Informants | Individual Interviews | Focus Groups |
|---|---|---|---|
| Craft | |||
| Clerk | 3 | 7 | 26 |
| Maintenance | 2 | 3 | 0 |
| Mail handler | 2 | 10 | 9 |
| Technician | 1 | 0 | 0 |
| Motor vehicles | 1 | 0 | 1 |
| Tenure with USPS | |||
| Range | 15-35 years | 6-38 years | 6-38 years |
| Mean | 23 years | 15 years | 25 years |
| Age | |||
| Range | 40-63 years | 32-62 years | 38-69 years |
| Mean | 53 years | 45 years | 51 years |
| Race | |||
| African American | 7 | 12 | 26 |
| White | 2 | 4 | 9 |
| Hispanic | 0 | 1 | 1 |
| African American- | 0 | 3 | 0 |
| Hispanic | |||
| Gender | |||
| Female | 0 | 12 | 16 |
| Male | 9 | 8 | 20 |
| Number of interviews or focus groups per site | |||
| Washington, DC | 3 | 4 | 1 |
| New Jersey | 2 | 0 | 3 |
| New York | 4 | 16 | 2 |
Source: Reference 42.
We used an intentional sampling method to reach both postal workers and public health professionals in all sites. Key informant interviews, which were generally with leaders in the workplace or unions, were conducted first, followed by individual interviews and focus groups. Key informants did not participate in focus groups; their interviews served to provide critical contextual issues and contributed to the evolution of final interview and focus group guides. Similar research questions were asked of postal workers in all interviews and focus groups, including an open-ended question about reactions to the vaccine, which was followed by probes to learn more. Public health professionals were asked about communication and reactions to the vaccine. In most sessions, the topic of the vaccine also surfaced in response to other questions. Because the study did not focus on vaccine uptake, we did not include a specific question about vaccine use. However, 3 participants volunteered that they had taken either a full or partial course of vaccine.
Two researchers reviewed the transcripts of the interviews and focus groups separately, developing basic categories or codes that described major topics of discussion. All coded categories were further examined in order to identify core themes. A third researcher, not involved in data collection or early analysis, reviewed all transcripts, using a key word search, to confirm previous analyses. Additionally, we reviewed the coded categories to determine the extent that themes converged or differed across the samples (key informants, individual worker, focus group, and public health professionals). We found that most themes spanned across the types of interviews and were echoed in the focus groups. Additionally, there were similarities between postal workers and public health professionals. Some discussions by public health professionals were related to their specific role as a professional and their observations of the postal workers; these are presented in a separate section. (For a detailed description of the methods, see Quinn and colleagues.42)
Results
Across all sites, themes emerged that can inform future crisis and emergency risk communication. In some cases, public health professionals offered confirmation of the perspectives of postal workers. Confusion and frustration with the changing treatment recommendations created a context in which many postal workers were distrustful before the recommendation for the vaccine was issued. Agency disagreements about the vaccine, lack of perceived risk, physician recommendations, and media coverage of the military's controversy about the anthrax vaccine also affected the response. Additionally, fears about experimentation, confusion about the implications of the required informed consent, and postal workers' resentment of perceived different treatment of Senate staff shaped reactions to the vaccine.
Trust and Credibility
Previous research has documented factors that affected trust during the anthrax attacks.33,34,42,46 Postal workers talked at length about trust and how it affected their decisions regarding treatment and acceptance of the vaccine. In the aftermath of the attacks, postal workers' trust had diminished over time. Thus, when the vaccine was offered, public health officials had already lost trust and credibility. One postal worker voiced this common theme: “They lied to us before …. I would never take the vaccine because of the mistrust that they [public health] already put down ….” The preexisting tense workplace, with postal workers' distrust of USPS management, influenced their view of public health agencies and affected their willingness to consider the vaccine:
Certain people said, “No, I am not going to take it.” Some were very afraid … but the vast majority refused to do it because they didn't trust it. They didn't trust because the environment they were in. They knew management would do anything to get this mail out
There was also a sense that many questions about the vaccine had remained unanswered:
When the CDC came back and said “either take more antibiotics or take this vaccine,” it was a whole different story. My sense is that there were unanswered questions, there were concerns about the dosages and the long-term effects of the antibiotics, and some people were very sick.
…not enough facts, not enough testing of the vaccine on people. It has only been used on a few military personnel. I didn't trust them. I could not trust them to put that disease in my body purposely even though it's a very small dose and it was supposed to build up my immunity
Information-Seeking Behavior
The events leading up to the vaccine offer and the existing distrust created a situation where postal workers were skeptical of information provided by public health professionals, especially about the vaccine. Taking the vaccine was viewed as a critical health decision; therefore, postal workers sought information from multiple outside sources. They were sophisticated in information seeking, not only comparing information from different sources, but some going so far as to contact expert scientists for second opinions. Additionally, in one site, union officials reported that they were contacted by several individuals, ranging from family members of the military who had taken the vaccine to elected officials, all of whom recommended against the vaccine.
Consequently, individual decision making became more complex as postal workers found contradictory opinions about the vaccine, which added to the confusion and fear.
You were getting information that was contradictory to what they already said and then information from the outside, of other friends, of people that are hooked up with some of the universities around here start talking and you want to go whoa, nothing agreed with what they said. Then it got a little scary.
Uncertainty and conflicting opinions created confusion for postal workers that was further aggravated when public health agencies disagreed on recommendations about the vaccine. The New Jersey and Washington, DC, health departments openly opposed the use of the vaccine in the media. Postal workers were unsure of whose advice to follow:
Here are two people that know what they are talking about, and they are disagreeing about whether people should get shots or not. That kind of scared me right there because these are people that should be on the same page … they took it to the media and once you take it to the media and the people can see and hear what is going on, people are going to be upset.
Consequently, postal workers reacted with skepticism, as evidenced in this comment:
The New Jersey Health Department was against it; CDC was for it; they are telling me that it's based on some study with monkeys. What are you comparing me with some monkeys for?
Several postal workers asked the public health professionals for their personal opinions on the vaccine and were appreciative when they received a straightforward answer. One postal worker said,
I really appreciate the fact when the question was asked to the doctor, would she take the vaccine, and her answer was definitely no. I would rather hear that than to hear her say yes. At least she was honest enough to say no. Because of that, I can now draw a conclusion.
The Military's Use of the Anthrax Vaccine
The controversy over the military's use of the anthrax vaccine influenced perceptions of the vaccine and decision making. These comments illustrate the influence:
I found an article in Time magazine where military people that had taken it [the vaccine] were having all kinds of health problems. Some of the men could no longer produce babies, women were having certain deformities. I was reading all these negative things … and all I kept thinking was this medication [antibiotics] has already caused me to have bronchitis and an enlarged heart
60 Minutes had done a thing a couple of months before on TV about career Navy pilots who gave up their commission. They waited their whole lives to become a career Navy pilot. Now what is this telling you? Other people besides my doctor and me and 1,500 postal employees are worried about this vaccination. There are guys that gave up their career and disobeyed a direct order from their military superior because they said, “Forget it.”
While many postal workers shared that this information, along with their distrust, was enough for them to refuse the vaccine, others reported that the difference in the vaccine protocol caused alarm. Postal workers were aware that the military offered a 6-dose pre-exposure vaccine protocol, but they were being offered a postexposure, 3-dose IND protocol. They were concerned about the divergence and the lack of communication about the different protocols.
I did my own research. There was discrepancy in terms of how many shots you would get versus what was recommended to the military. There were 6 shots in the military. It just seemed like the program wasn't in sync with what the military got as opposed to what we would get. Is this something new and why did you change something or is this better or worse?
Perceptions of the Informed Consent Form
The use of a consent form, which is required for an investigational protocol, also eroded confidence in accepting the vaccine. Postal workers believed that they would be taking personal risks in using the vaccine without clear answers about the benefits to them:
The form said that if anything happened, I got to take it at my own risk. So now, why are you giving me stuff that can possibly injure me? Why am I risking something when I thought the purpose of this was to prevent risk? This type of thing lowers your hope of receiving protection from the people who should provide that for you.
This is an IND, investigational new drug, because FDA says it has to be given that way because that is the nature of doing it. So you are using me as an experimental animal. What do you mean I signed away my rights? If I have a problem, there is no financial help. If I have a problem, you're telling me I can sue the government, big deal.
These reactions contributed to postal workers' fears about being the subjects of experimentation.
Fears about Experimentation
The perceived lack of communication about the vaccine protocol further substantiated postal workers' beliefs that they were the subjects of an experiment. One New Jersey postal worker remarked: “Yeah, the Trenton lab rats. They made up t-shirts and a lot of people were wearing them. We felt like we were lab rats. We're like an experiment, some kind of government experiment.”48 Across all locations, postal workers used terms such as “lab monkey” and “guinea pigs”:
Basically, the bottom line is that we people felt like we were guinea pigs, like we were experimented on or used as test subjects.
First, you are telling us that cipro could kill it. Now you are telling us that this other medication is going to kill it. You are using us for guinea pigs.
… [T]his is CDC. I'm sure they looked at us this way. This is a great group here. We got old, young, physically very fit, physically not very fit, people with diabetes, people with heart conditions, and we're getting a chance to observe them all and see what happens when they take doxycycline.
Postal workers gave historical examples of experimentation as they discussed their own concerns about being test subjects, including other experiments by the military, other vaccines previously used on military personnel, and their suspicions about vaccines as the source of HIV transmission. While the theme of experimentation cut across racial lines, African American postal workers in 4 interviews and 1 focus group made the analogy to the Tuskegee syphilis study:
Same thing they did in the syphilis experiment, they want to use a large section of people to do their experiment on.
… giving a little more information whereas with the Tuskegee study, no information was given
In one site, where the analogy to the Tuskegee study arose more frequently, one postal worker's distrust extended to the present study for which he was being interviewed: “The only reason that I can believe that CDC decided to fund this study is very simple. We stopped them from using postal workers as guinea pigs for their vaccine.”
Although concerns about experimentation were prevalent and affected decision making about the vaccine, it is also noteworthy that many postal workers did not perceive themselves to be at risk by late December 2001. A common response was, “They did offer it [vaccine] to me, but I didn't feel like I needed it.” Postal workers also felt that the antibiotics gave them enough protection: “I thought the antibiotics were enough on my body and I figured I didn't need any more medicine after 60 days of antibiotics. I figured the vaccine—I didn't need it.” Others reported that their physicians recommended against the vaccine.
Vaccine Uptake
Few of the participants who were interviewed reported taking the vaccine, although some reported that they knew postal workers who had taken it. One postal worker who did take the vaccine reported that, because of workplace practices in his facility, he believed he might still be at risk. Another was a military veteran who reported that he had been accustomed to vaccination in his military experience. However, he also reported that, once he had begun the vaccine protocol, he began to hear other information that made him less confident in his decision.
Reactions to Other Research After the Anthrax Attacks
Postal workers talked about the monitoring activities and scientific research conducted by CDC after the attack.12,49 They were ambivalent about these activities. Some postal workers expressed a desire for research on long-term effects of the antibiotic use or the anthrax exposure.
I believe there should be some surveillance on our health, genuine surveillance.
You are the Centers for Disease Control. You need to find out why all these people are having all these problems all of the sudden.
Others expressed hostility toward these research activities:
Even today, we still get calls from CDC, and they are not asking us our problems and what to do about these problems. They are compiling their information so we feel like lab monkeys. We feel like parts of an experiment, and this has got most of us very pissed off.
They [CDC] are calling your house. They want to talk to you about how you're doing. You never examined me. You didn't take any blood test. You gave me some pills and now you are going to call me every year.
This concept of follow-up studies was foreign to many postal workers, and their lack of understanding was compounded by distrust and confusion. This is the context in which the effort to conduct follow-up studies may have contributed to concerns about the Tuskegee study.
A repeated theme was the need for scientific agencies, including the CDC, to disseminate results to those people enrolled in their studies. Some believed that the CDC only appeared when it needed subjects for its studies and that results were never disseminated, leading to a knowledge gap.
I am sure they probably spend millions of dollars doing research, probably have a congressional panel on anthrax right now, but the information will be five years away.
Get the information there. Get the information now. Let us know if you made changes. I understand maybe for security reasons you don't want to let everybody know, but at least let us know that you are still alive. Where is CDC after all of this anthrax? We don't know if they are still there. We don't know if they are doing tests; we don't even know what kind of safeguard they put in after this. They said they got some new machine that eradicates all the mail. How is that affecting us? We don't know if that stuff is hurting us or just killing the anthrax.
The considerable ambivalence about research voiced by postal workers left public health agencies with a difficult dilemma.
Public Health Professionals' Reactions and Observations
Public health professionals confirmed much of what we learned from postal workers about the vaccine. Some echoed that postal workers did not see themselves as at risk by late December 2001. A few noted the impact of the lingering perception about differential treatment between Senate staff and postal workers; a public health professional observed its impact:
Everyone was trying to figure out whether they should take it or shouldn't take it. Then, to add insult to injury, the folks on Capitol Hill were told if they took the vaccine, they would have follow-up care and the folks who were in Brentwood were told “you've got to sign a big 9-page release form and if you have any problems, typically you are on your own.” That was the single biggest debacle. That was much worse than either the nasal swabs or the medication problems, because that one we could have avoided.
Two public health professionals spoke specifically about the Tuskegee syphilis study. In the fall of 2001, the comparison to the Tuskegee study had also been reported in the media.50–53 One public health professional was troubled by the mention of the study in the vaccine discussion:
Somebody came in there [Brentwood] and told these people, “Just like Tuskegee, they are experimenting on you guys.” What a harmful thing that is to do. What it does is create a huge barrier…. You have to debunk something they've accepted. It's not an easy task.
However, another public health professional understood how this contributed to distrust of follow-up studies:
People did not understand why the CDC was following up on them, and so the link between that and Tuskegee, that's not hard to make…. Tuskegee is not a remote concept to African Americans
Public health professionals shared the unique perspective of being involved in a system that was charged with offering treatment options. Several questioned whether the vaccine was offered for political reasons rather than from scientific necessity:
That was a public health message: it was unnecessary. The doxy or cipro were sufficient. Then HHS made a political decision to give everybody [vaccine] injections, which most of us recognize as being a response to the congressional staffers who wanted more guarantee than antibiotics. We [had] told people, swore on their mother's grave, that it wasn't necessary to take injections. All you needed to do was take your antibiotic, because that's what the scientists told us all the way through and that's what evidence proved. Then, all of a sudden, you got this policy that said if you would like to take a vaccine injection, we have them available to you.
I don't think there is a scientific basis for doing this. If you look carefully, even though the CDC didn't come out and say Ivan [Walks] is right, nobody came out and supported the vaccine. There were no doctors who came out and said this vaccine is a good idea, people ought to take it. It didn't happen because the decision to release the vaccine was not a decision that, in my opinion, was supported by the scientific evidence.
The uncertain scientific evidence, changing recommendations, and the concern over high levels of exposure contributed significantly to the difficulty in communication, and placed public health professionals in a difficult position:42,54
Everyone at CDC felt it should be taken by people who had really high risk exposures, but they made the decision to offer it to anyone who wanted it. That was kind of a weird thing to be going to a facility where no one had contracted anthrax and, according to what we were originally thinking, nobody even really needed antibiotics and now we're offering them the vaccine. It was a series of very confusing messages, and if you are a postal worker and you doubt everything that is being told to you, you are trying to confidently read between the lines. As transparent as we were trying to be … with whatever the state of affairs was and whatever our knowledge was, we knew that we didn't know everything and I think that was very dissatisfying.
Despite the difficulties, some public health professionals viewed the offer of continued antibiotics and the vaccine as another opportunity to engage postal workers. In some sites, these December clinics provided more attention to postal workers' information needs and more individualized care. One public health professional reported, “On the later shifts, there were more pharmacists so people actually were getting counseled by a pharmacist.” Several public health professionals acknowledged that providing opportunities for postal workers to make decisions about their own health bolstered their sense of control. One public health professional reflected:
In the face of bioterrorism or any terrorism, people are absolutely terrified. Giving them any sense of control over anything, even if it's a choice of whether or not to take antibiotics, ends up doing a tremendous amount of mental health good. I think that ultimately giving people the choices with the vaccine was a very good idea. Although people didn't take the antibiotics, my sense was the fact that they got it and they had it in their hand and they felt like they could take it if they wanted. That made a huge difference. That's traditionally not good public health practice, but in the setting of terrorism, that worked well.
Discussion and Implications
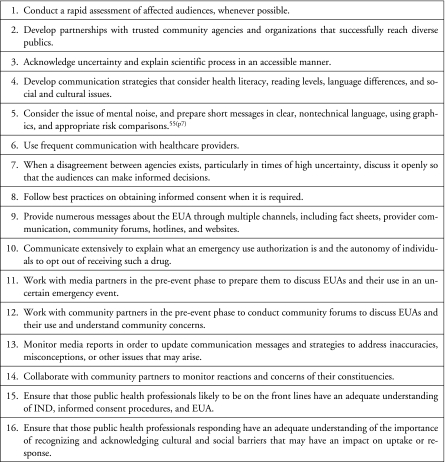
There are important lessons from the response to the anthrax vaccine, which may inform communication in the case of a pandemic or bioterrorist event. We recommend several strategies to improve communication in such situations (Figure 3).
FIG. 3.
Figure 3. Recommendations for Communication about INDs and EUA Products during a Pandemic or Bioterrorist Event
The media context at the time of the vaccine offer may have exacerbated the communication challenges. There was significant negative coverage of the lack of a definitive recommendation by CDC and scientific disagreements with the vaccine offer expressed by other health departments, other health professionals, and labor unions.52,56–66 Newspaper coverage referred to the vaccine offer as “experimental” and reported the workers' fear of being “guinea pigs,” as well as pointing to the analogy to the Tuskegee syphilis study.51,57,58,62,63,66–72 Less frequently was there a discussion of who was at risk and proximity to areas of high risk.51,73–75 This certainly suggests the need to work intensively with media to help ensure that public health messages are communicated accurately, as well as monitoring media reports to improve messages.
At the time, the IND was the only way to get an unapproved product, or an approved product for an unapproved use, to people in an emergency.15 For all INDs, the FDA provides general requirements for informed consent stating clearly that the consent form would not release the investigator or sponsor of liability for negligence. However, postal workers' comments suggest that they believed they were releasing the CDC from liability. This was reinforced by the media when on December 20 CNN stated, “Individuals who opt to receive the vaccine would have to sign an informed-consent form, a move that would essentially relieve the manufacture [sic] of any liability.”26 This echoes findings from an earlier study of African Americans, where participants understood the purpose of an informed consent procedure but many believed it to be tantamount to signing away their rights.53
In a future public health emergency, it may be necessary to invoke the EUA, which does not require informed consent. This would likely present challenges similar to the anthrax or smallpox vaccine. The FDA recommends that a fact sheet with information on the EUA product be provided to the public.14 The individual recipient can refuse an unapproved treatment protocol, but there is no written consent. This makes the government's response to an emergency potentially more efficient and swift, but it raises the question of how informed the public will be when receiving the EUA product. Public health professionals will need to be highly vigilant to ensure that the fact sheet provides information in an accessible manner and highlights the potential risks of the EUA product. Additionally, information about the EUA will need to be accessible through multiple channels, in different languages, and at different literacy levels. It will also be critical that CDC communicates with healthcare providers on the rationale and use of the EUA. Moreover, it will be essential to work with the media to make coverage of the EUA, its risks and benefits, and its rationale as clear as possible.
Since we know that difficulties in ensuring adequate informed consent during research are already well documented,53,76 we expect that in the mental noise of an emergency, there will be problems in communicating about an EUA. To some extent, Flory and Emanuel offer guidance from their review of interventions to improve informed consent.57 They found that using standard consent forms in conjunction with 2 meetings with a health professional to discuss consent is the most promising method for increasing understanding. Although this would be difficult in the context of mass vaccination or EUA, public health professionals could use multiple channels, such as written materials appropriate for the populations affected, involving community partners as educators, and holding community forums, to increase understanding of the EUA product. Quinn elsewhere recommends a set of relevant strategies for working specifically with minority communities to prepare for emergencies.77 Additionally, it is critical that the public fully understands their option for refusal of an EUA.
Fears of experimentation are rooted in the legacy of the Tuskegee syphilis study, which remains a cultural symbol in the African American community.53 It is possible that if an IND or EUA is necessary, Tuskegee will be raised as a “red flag” by some. In 2001, public health professionals were uncomfortable talking about the Tuskegee study, as one participant indicated, and unable to effectively address the nuanced concerns underlying its being raised. Clearly, the study is not the only obstacle, yet it cannot be dismissed. Therefore, it is essential that public health professionals know about the Tuskegee study, understand its significance, and demonstrate the skills to address the concerns about experimentation and trust that are at the root of the legacy. We would even assert that public health professionals working with INDs or EUA products in minority communities proactively raise the Tuskegee study themselves and make the distinctions between that study and the IND/EUA product. Although many would see this as risky, we would argue that, in fact, opening that dialogue fosters trust, enables the public to ask questions, and demonstrates cultural competence. It is helpful if a rapid assessment can uncover what Covello refers to as “hidden symbolism” and broader cultural considerations.55 In addition, we must be cognizant that fear of experimentation cuts across racial lines and is likely to be a factor with an EUA.
In crisis situations, it is likely that uncertainty will contribute to different opinions about the use of a vaccine or an IND/EUA product. In 2001, public health professionals were unprepared for the vaccine recommendation, and agencies did not have consensus about its use, which contributed to further distrust and suspicion among postal workers. It is highly likely that, in a future event, uncertainty will create a similar situation in which professionals disagree, potentially leading to an erosion of trust. In a discussion of the question of “speaking with one voice,” Clarke and colleagues offer some useful guidance.78 They argue that, in times of great uncertainty and with highly diverse audiences, having multiple voices may actually be useful. We concur, with the provision that the professionals or agencies in disagreement join together to discuss in public the rationale and processes by which they come to their conclusions. Creating an open forum in which audiences can understand more about the science and decision-making processes can foster trust and enhance the public's ability to make informed decisions about an IND/EUA product.
The rate of anthrax vaccine uptake was very low.79 The percentage of people at risk who receive any vaccine is determined in large part by their perceived risk of getting the disease, as well as their perception regarding the vaccine's safety.16,20,25 Other predictors of vaccine uptake were physicians' recommendations and having an acquaintance who had been vaccinated.20 A physician's advice and support from friends and family influenced adherence among those exposed to anthrax.33 These data reinforce the critical importance of targeted communication with clinical providers in order to strengthen their recommendations for people at risk. Additionally, at the beginning of the anthrax attacks, the “outrage” experienced by people at risk was significant, leading to heightened perceived risk.80 After 5 deaths, there were no further illnesses or fatalities. Over time, postal workers were given more information on the side effects from the antibiotics than to possible risks from exposure to the anthrax spores. At this point, the “hazard” and “outrage” components of risk were low, leading to a very low perception of risk from anthrax among most people. Effective crisis and emergency risk communication at this juncture could have increased the hazard component of perceived risk and contributed to higher rates of adherence to antibiotics and vaccine uptake.
Tensions resulting from perceived unfairness and inequity between the postal workers and Senate staff continued during the vaccine period.52,60,68,75,81 This likely fueled the ongoing distrust and reduced uptake of the vaccine. Addressing issues of equity and fairness is essential to building trust in preparation for future events, particularly in light of existing literature on perceived discrimination in bioterrorist or pandemic events.
It is fundamentally important that CDC and other public health agencies continue efforts to repair the breach in trust created during the anthrax attacks. One potential avenue for repairing trust is to disseminate results from related research through the channels of postal unions and the USPS management. This approach recognizes the importance of responding to audience needs and using appropriate messengers.
There are several important limitations to this study. Although we recruited aggressively for the study, there could be inherent bias in those who chose to participate. While some participants indicated whether they had taken or not taken the vaccine, not all provided that information. Generalizability cannot be understood in a statistical sense, but the themes presented in this article were heard across the 3 sites and different data collection methods.
We can be certain that in the future we will grapple with communicating about the use of novel countermeasures, such as an experimental or off-label vaccine or drug, in the midst of a rapidly evolving emergency. Public health agencies must begin now to build trust and educate diverse publics, before the uncertainty and time pressures of that emergency create major obstacles for communication. Failure to start now to engage and educate can lead to unnecessary risk, disease, and deaths, whether in a pandemic or a bioterrorist attack.
Acknowledgments
The authors wish to thank the postal workers and public health professionals who kindly shared their time and insights with us. We also wish to acknowledge our colleague, Carol McAllister, who died during the preparation of this article. Dr. McAllister offered valuable insights into qualitative research, for which we are grateful. This study was supported by grant #S2136-21/21 from the CDC/ASPH Cooperative Agreement. Drs. Quinn and Kumar are currently supported by the Research Center of Excellence in Minority Health Disparities at the Center for Minority Health, University of Pittsburgh, funded by the National Center on Minority Health and Health Disparities, NIH (2P60MD000207-07, S. Thomas, PI).
References
- 1.Jernigan DB. Raghunathan PL. Bell BP, et al. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis. 2002 Oct;8(10):1019–1028. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S.Centers for Disease Control and Prevention. Evaluation of Bacillus anthracis contamination inside the Brentwood Mail Processing and Distribution Center—District of Columbia, October 2001. MMWR Morb Mortal Wkly Rep. 2001;50(50):1129–1133. [Google Scholar]
- 3.Greene CM. Reefhuis J. Tan C, et al. Epidemiologic investigations of bioterrorism-related anthrax, New Jersey, 2001. Emerg Infect Dis. 2002 Oct;8(10):1048–1055. doi: 10.3201/eid0810.020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan CG. Sandhu HS. Crawford DC, et al. Surveillance for anthrax cases associated with contaminated letters, New Jersey, Delaware, and Pennsylvania, 2001. Emerg Infect Dis. 2002 Oct;8(10):1073–1077. doi: 10.3201/eid0810.020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Government Accountability Office. U.S. Postal Service: Better Guidance Is Needed to Ensure an Appropriate Response to Anthrax Contamination. Washington, DC: GAO; 2004. [Google Scholar]
- 6.U.S. Centers for Disease Control and Prevention. Use of anthrax vaccine in response to terrorism: supplemental recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2002;51(45):1024–1026. [PubMed] [Google Scholar]
- 7.Inglesby TV. Henderson DA. Bartlett JG, et al. Anthrax as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 1999 May 12;281(18):1735–1745. doi: 10.1001/jama.281.18.1735. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Centers for Disease Control and Prevention. Interim guidelines for investigation of and response to Bacillus anthracis exposures. MMWR Morb Mortal Wkly Rep. 2001;50(44):987–990. [PubMed] [Google Scholar]
- 9.U.S. Centers for Disease Control and Prevention. CDC responds: an update on treatment options for postal and other workers exposed to anthrax [transcript] http://emergency.cdc.gov/DocumentsApp/Anthrax/12212001/postalworkers.pdf. Dec 27, 2001. [Nov 2;2008 ]. http://emergency.cdc.gov/DocumentsApp/Anthrax/12212001/postalworkers.pdf
- 10.U.S. Centers for Disease Control and Prevention. Notice to readers: additional options for preventive treatment of persons exposed to inhalational anthrax. MMWR Morb Mortal Wkly Rep. 2001;50(50):1142–1151. [Google Scholar]
- 11.U.S. Department of Defense. Anthrax vaccine immunization program. http://www.anthrax.osd.mil/vaccine/schedule.asp. [Nov 2;2008 ]. http://www.anthrax.osd.mil/vaccine/schedule.asp
- 12.Shepard CW. Soriano-Gabarro M. Zell ER, et al. Antimicrobial postexposure prophylaxis for anthrax: adverse events and adherence. Emerg Infect Dis. 2002 Oct;8(10):1124–1132. doi: 10.3201/eid0810.020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Centers for Disease Control and Prevention. Backgrounder and advisory. http://www.cdc.gov/od/oc/media/pressrel/b011221.htm. Dec 21, 2001. [Mar 28;2008 ]. http://www.cdc.gov/od/oc/media/pressrel/b011221.htm
- 14.U.S. Food and Drug Administration. Guidance Emergency Use Authorization of Medical Products. Rockville, MD: Office of Counterterrorism Policy and Planning; 2007. http://www.fda.gov/oc/guidance/emergencyuse.html. [Nov 2;2008 ]. http://www.fda.gov/oc/guidance/emergencyuse.html
- 15.Nightingale SL. Prasher JM. Simonson S. Emergency Use Authorization (EUA) to enable use of needed products in civilian and military emergencies, United States. Emerg Infect Dis. 2007;13(7):1046–1051. doi: 10.3201/eid1307.061188. [DOI] [PubMed] [Google Scholar]
- 16.Brewer NT. Hallman WK. Subjective and objective risk as predictors of influenza vaccination during the vaccine shortage of 2004-2005. Clin Infect Dis. 2006 Dec 1;43(11):1379–1386. doi: 10.1086/508466. [DOI] [PubMed] [Google Scholar]
- 17.Burgess DC. Burgess MA. Leask J. The MMR vaccination and autism controversy in United Kingdom 1998-2005: inevitable community outrage or a failure of risk communication? Vaccine. 2006 May 1;24(18):3921–3928. doi: 10.1016/j.vaccine.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 18.Goins WP. Schaffner W. Edwards KM. Talbot TR. Healthcare workers' knowledge and attitudes about pertussis and pertussis vaccination. Infect Control Hosp Epidemiol. 2007 Nov;28(11):1284–1289. doi: 10.1086/521654. [DOI] [PubMed] [Google Scholar]
- 19.Petts J. Niemeyer S. Health risk communication and amplification: learning from the MMR vaccination controversy. Health Risk Soc. 2004;6(1):7–23. [Google Scholar]
- 20.Samoff E. Dunn A. VanDevanter N. Blank S. Weisfuse IB. Predictors of acceptance of hepatitis B vaccination in an urban sexually transmitted diseases clinic. Sex Transm Dis. 2004 Jul;31(7):415–420. doi: 10.1097/01.olq.0000130533.53987.78. [DOI] [PubMed] [Google Scholar]
- 21.Skowronski DM. Pielak K. Remple VP, et al. Adult tetanus, diphtheria and pertussis immunization: knowledge, beliefs, behavior and anticipated uptake. Vaccine. 2004 Dec 2;23(3):353–361. doi: 10.1016/j.vaccine.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 22.Benin AL. Dembry L. Shapiro ED. Holmboe ES. Reasons physicians accepted or declined smallpox vaccine, February through April, 2003. J Gen Intern Med. 2004 Jan;19(1):85–89. doi: 10.1111/j.1525-1497.2004.36005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everett WW. Coffin SE. Zaoutis T. Halpern SD. Strom BL. Smallpox vaccination: a national survey of emergency health care providers. Acad Emerg Med. 2003 Jun;10(6):606–611. doi: 10.1111/j.1553-2712.2003.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 24.Kwon N. Raven MC. Chiang WK, et al. Emergency physicians' perspectives on smallpox vaccination. Acad Emerg Med. 2003 Jun;10(6):599–605. doi: 10.1111/j.1553-2712.2003.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 25.Fowler GL. Baggs JM. Weintraub ES. Martin SW. McNeil MM. Gust DA. Factors influencing laboratory workers' decisions to accept or decline anthrax vaccine adsorbed (AVA): results of a decision-making study in CDC's anthrax vaccination program. Pharmacoepidemiol Drug Saf. 2006 Dec;15(12):880–888. doi: 10.1002/pds.1302. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds B. Atlanta, GA: Centers for Disease Control and Prevention; 2002. Introduction to Crisis and Emergency Risk Communication. Crisis and Emergency Risk Communication; pp. 1–12. [Google Scholar]
- 27.Calman KC. Communication of risk: choice, consent, and trust. Lancet. 2002 Jul 13;360(9327):166–168. doi: 10.1016/S0140-6736(02)09421-7. [DOI] [PubMed] [Google Scholar]
- 28.Leask J. Vaccination and risk communication: summary of a workshop, Arlington Virginia, USA, 5-6 October 2000. J Paediatr Child Health. 2002 Apr;38(2):124–128. doi: 10.1046/j.1440-1754.2002.00791.x. [DOI] [PubMed] [Google Scholar]
- 29.MacIntyre CR. Leask J. Immunization myths and realities: responding to arguments against immunization. J Paediatr Child Health. 2003 Sep–Oct;39(7):487–491. doi: 10.1046/j.1440-1754.2003.t01-1-00200.x. [DOI] [PubMed] [Google Scholar]
- 30.Stoto MA. Evans G. Bostrom A. Vaccine risk communication. Am J Prev Med. 1998 Apr;14(3):237–239. doi: 10.1016/s0749-3797(97)00059-7. [DOI] [PubMed] [Google Scholar]
- 31.Waisbord S. Larson H. Why Invest in Communication for Immunization: Evidence and Lessons Learned. A joint publication of the Health Communication Partnership based at Johns Hopkins Bloomberg School of Public Health/Center for Communication Programs (Baltimore) and the United Nations Children's Fund. New York: 2005. [Google Scholar]
- 32.Freeman BD. Carlisle, PA: US Army War College; 2001. The Importance of Health Risk Communication in the Creation of the Anthrax Vaccine Immunization Program. [Google Scholar]
- 33.Stein BD. Tanielian TL. Ryan GW. Rhodes HJ. Young SD. Blanchard JC. A bitter pill to swallow: nonadherence with prophylactic antibiotics during the anthrax attacks and the role of private physicians. Biosecur Bioterror. 2004;2(3):175–185. doi: 10.1089/bsp.2004.2.175. [DOI] [PubMed] [Google Scholar]
- 34.Blanchard JC. Haywood Y. Stein BD. Tanielian TL. Stoto M. Lurie N. In their own words: lessons learned from those exposed to anthrax. Am J Public Health. 2005 Mar;95(3):489–495. doi: 10.2105/AJPH.2004.038349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Covello VT. Peters RG. Wojtecki JG. Hyde RC. Risk communication, the West Nile virus epidemic, and bioterrorism: responding to the communication challenges posed by the intentional or unintentional release of a pathogen in an urban setting. J Urban Health. 2001 Jun;78(2):382–391. doi: 10.1093/jurban/78.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratzan SC. Communicating risk: from crisis to calm. J Health Commun. 2005 Mar;10(2):103–104. doi: 10.1080/10810730590915083. [DOI] [PubMed] [Google Scholar]
- 37.Shore DA. Communicating in times of uncertainty: the need for trust. J Health Commun. 2003;8(Suppl 1):13–14. doi: 10.1080/713851977. [DOI] [PubMed] [Google Scholar]
- 38.Sorensen J. Risk communication and terrorism. Biosecur Bioterror. 2004;2(3):229–231. doi: 10.1089/bsp.2004.2.229. [DOI] [PubMed] [Google Scholar]
- 39.The Working Group on “Governance Dilemmas” in Bioterrorism Response. Leading during bioattacks and epidemics with the public's trust and help. Biosecur Bioterror. 2004;2(1):25–40. doi: 10.1089/153871304322964318. [DOI] [PubMed] [Google Scholar]
- 40.Peters RG. Covello VT. McCallum DB. The determinants of trust and credibility in environmental risk communication: an empirical study. Risk Anal. 1997 Feb;17(1):43–54. doi: 10.1111/j.1539-6924.1997.tb00842.x. [DOI] [PubMed] [Google Scholar]
- 41.Trettin L. Musham C. Is trust a realistic goal of environmental risk communication? Environ Behav 2000. 2000 May 1;32(3):410–426. [Google Scholar]
- 42.Quinn SC. Thomas T. McAllister C. Postal workers' perspectives on communication during the anthrax attack. Biosecur Bioterror. 2005;3(3):207–215. doi: 10.1089/bsp.2005.3.207. [DOI] [PubMed] [Google Scholar]
- 43.Gursky E. Inglesby TV. O'Toole T. Anthrax 2001: observations on the medical and public health response. Biosecur Bioterror. 2003;1(2):97–110. doi: 10.1089/153871303766275763. [DOI] [PubMed] [Google Scholar]
- 44.Joellenbeck LM. Hernandez LM. The Institute of Medicine's independent scientific assessment of Gulf War health issues. Mil Med. 2002 Mar;167(3):186–190. [PubMed] [Google Scholar]
- 45.North CS. Pollio DE. Pfefferbaum B, et al. Concerns of Capitol Hill staff workers after bioterrorism: focus group discussions of authorities' response. J Nerv Ment Dis. 2005 Aug;193(8):523–527. doi: 10.1097/01.nmd.0000172598.82779.12. [DOI] [PubMed] [Google Scholar]
- 46.Quinn S. Thomas T. McAllister C. Lessons from the 2001 anthrax attack: a conceptual model for crisis and emergency risk communication. In: Seeger M, editor; Sellnow T, editor; Ulmer R, editor. Crisis Communication and the Public Health. Cresskill, NJ: Hampton Press; 2008. pp. 23–42. [Google Scholar]
- 47.Sandman PM. Anthrax, bioterrorism, and risk communication: guidelines for action. 2001. http://www.psandman.com/col/part1.htm. [Nov 2;2008 ]. http://www.psandman.com/col/part1.htm
- 48.Trenton Metro Area Local American Postal Workers Union. AFL-CIO. Lab rat corner: anthrax. http://trentonmetroarealocal.com/lab_rat_corner.html. [Nov 2;2008 ]. http://trentonmetroarealocal.com/lab_rat_corner.html
- 49.Jefferds MD. Laserson K. Fry AM, et al. Adherence to antimicrobial inhalational anthrax prophylaxis among postal workers, Washington, D.C., 2001. Emerg Infect Dis. 2002 Oct;8(10):1138–1144. doi: 10.3201/eid0810.020331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altman L. The doctor's world: in offering anthrax vaccine, officials admit to unknowns. New York Times. Dec 25, 2001.
- 51.Connolly C. Goldstein A. Anthrax vaccine plan sows confusion; D.C. advises workers against treatment. Washington Post. 2001 Dec 20;:A01. [Google Scholar]
- 52.Connolly C. Vaccine plan revives doubts on anthrax policy. Washington Post. Dec 24, 2001. p. A01.
- 53.Freimuth VS. Quinn SC. Thomas SB. Cole G. Zook E. Duncan T. African Americans' views on research and the Tuskegee syphilis study. Soc Sci Med. 2001 Mar;52(5):797–808. doi: 10.1016/s0277-9536(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 54.U.S. Centers for Disease Control and Prevention. Telebriefing transcript MMWR and anthrax update with Drs. Koplan, Sokas and Sanderson. Dec 20, 2001. www.cdc.gov/od/oc/media/transcripts/t011220.htm. [Nov 2;2008 ]. www.cdc.gov/od/oc/media/transcripts/t011220.htm
- 55.Covello VT. Best practices in public health risk and crisis communication. J Health Commun. 2003;8(Suppl 1):5–8. doi: 10.1080/713851971. discussion 148–151. [DOI] [PubMed] [Google Scholar]
- 56.Blum J. Anthrax widespread at Brentwood; spores scattered farther through postal center than thought. Washington Post. Dec 21, 2001. p. A20.
- 57.Blum J. Postal worker vaccinations to begin today; some Brentwood employees wary of experimental precaution against anthrax. Washington Post. Dec 27, 2001. p. B2.
- 58.Connolly C. Goldstein A. Anthrax exposure estimates increased; first Capitol Hill aides receive vaccine shots. Washington Post. Dec 21, 2001. p. A1.
- 59.Connolly C. Workers exposed to anthrax shun vaccine; low participation is blamed on confusing signals from U.S. health authorities. Washington Post. 2002 Jan 8;:A6. [Google Scholar]
- 60.Goldstein A. Postal workers to receive vaccine advice; health officials use video, doctor visits to sort out confusion over vaccine. Washington Post. Dec 22, 2001. p. A11.
- 61.Goldstein A. Dewar H. Anthrax vaccine proves hard sell; postal employees opt for more pills. Washington Post. Dec 23, 2001. p. A18.
- 62.Hanley R. A nation challenged: New Jersey; postal workers hesitant on anthrax vaccination. New York Times. Dec 22, 2001.
- 63.Haughney C. Anthrax vaccine rejected. Washington Post. Jan 4, 2002. p. A13.
- 64.A muddled message on anthrax vaccine [editorial] New York Times. Dec 20, 2001.
- 65.Rosenbaum D. A nation challenged: the disease: frustration at health agency over critics of anthrax policy. New York Times. Dec 21, 2001.
- 66.Twomey S. Vaccine offer draws few postal workers; shots available as supplement to drugs in effort to prevent anthrax outbreaks. Washington Post. Dec 28, 2001. p. A6.
- 67.Connolly C. CDC gets Pentagon's anthrax vaccine; officials to use medicine to treat those at risk for infection. Washington Post. Dec 13, 2001. p. A10.
- 68.Stolberg S. Rosenbaum D. A nation challenged; U.S. will offer anthrax shots for thousands. New York Times. Dec 19, 2001.
- 69.Connolly C. Vedantam S. U.S. offers anthrax vaccine to thousands; preparation used by military is unlicensed, controversial. Washington Post. Dec 19, 2001. p. A3.
- 70.Stewart B. Union head objects to anthrax vaccine program. New York Times. Dec 25, 2001.
- 71.Stolberg S. A nation challenged: steps against anthrax; civilians are reluctant to join US test of anthrax vaccine. New York Times. Jan 8, 2002.
- 72.Double exposure [editorial] Washington Post. Dec 19, 2001. p. A38.
- 73.Vaccine offer raises new questions [editorial] Washington Post. Dec 23, 2001. p. A20.
- 74.Vedantam S. Anthrax vaccine mulled for up to 3,000 exposed; ‘experimental’ use likely to be controversial. Washington Post. Dec 16, 2001. p. A33.
- 75.Vedantam S. Connolly C. Anthrax vaccine urged for Hill staff; health officials want inoculations to start this week. Washington Post. 2001:A1. [Google Scholar]
- 76.Flory J. Emanuel E. Interventions to improve research participants' understanding in informed consent for research: a systematic review. JAMA. 2004 Oct 6;292(13):1593–1601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 77.Quinn S. Crisis and emergency risk communication in a pandemic: a model for building capacity and resilience of minority communities. Health Promotion Practice. 2008;9(4Suppl):18S–25S. doi: 10.1177/1524839908324022. [DOI] [PubMed] [Google Scholar]
- 78.Clarke L. Chess C. Holmes R. O'Neill KM. Speaking with one voice: risk communication lessons from the US anthrax attacks. Journal of Contingencies and Crisis Management. 2006;14:160–169. [Google Scholar]
- 79.Martin SW. Tierney BC. Aranas A, et al. An overview of adverse events reported by participants in CDC's anthrax vaccine and antimicrobial availability program. Pharmacoepidemiol Drug Saf. 2005 Jun;14(6):393–401. doi: 10.1002/pds.1085. [DOI] [PubMed] [Google Scholar]
- 80.Sandman PM. Hazard versus outrage in the public perception of risk. In: Covello V, editor. Effective Risk Communication: The Role and Responsibility of Government and Nongovernmental Organizations. New York: Plenum Press; 1989. pp. 45–49. [Google Scholar]
- 81.Rosenbaum D. Stolberg S. A nation challenged: the vaccine; as U.S. offers anthrax shots, safety debate begins again. New York Times. Dec 20, 2001.