Abstract
Congenital defects are a major complication of diabetic pregnancy, and the leading cause of infant death in the first year of life. Caudal dysgenesis, occurring up to 200-fold more frequently in children born to diabetic mothers, is a hallmark of diabetic pregnancy. Given that there is also an at least 3-fold higher risk for heart defects and neural tube defects, it is important to identify the underlying molecular mechanisms for aberrant embryonic development.
We have investigated gene expression in a transgenic mouse model of caudal dysgenesis, and in a pharmacological model using situ hybridization and quantitative real-time PCR. We identify altered expression of several molecules that control developmental processes and embryonic growth. The results from our models point towards major implication of altered Wnt signaling in the pathogenesis of developmental anomalies associated with embryonic exposure to maternal diabetes.
1. Introduction
Maternal diabetes is associated with increased risk for congenital abnormalities, and congenital malformations are the leading cause of infant death in the first year (Martin et al., 2008). The most prominent congenital malformations associated with diabetic pregnancies are cardiovascular defects, neural tube defects and caudal dysgenesis. The malformations in the caudal region involve lumbar and more distal vertebrae and are associated with neurulation defects, including neural tube defects; in more severe cases, legs and anal-rectal structures are also malformed. Caudal dysgenesis has the strongest association with diabetes, occurring up to 200 times more frequently in infants of diabetic mothers than in other infants (Mills, 1982). The molecular mechanisms affected by maternal diabetes in the developing embryo are currently unknown, and thus the pathogenic processes causing the developmental abnormalities of diabetic embryopathy are not well understood.
Recent studies in experimental animals have shown that in embryos exposed to conditions of maternal diabetes, several developmental control genes are abnormally expressed. For example, decreased expression of Pax3 in embryos from diabetic dams has been linked to neural tube defects (Phelan et al., 1997). Maternal diabetes also increased the expression of Hoxb5 in developing lungs of rats (Jacobs et al., 1998). Embryos exposed to the combination of maternal diabetic milieu and retinoic acid exhibit decreased expression of Wnt3a associated with an increased incidence of caudal dysgenesis (Chan et al., 2002; Leung et al., 2004). These results suggest a mechanism where the tissue-specific defects in diabetic embryopathy result from altered expression of molecules that play a role in patterning and development of those embryonic tissues.
We have recently compared gene expression profiles of diabetes-exposed and control embryos at embryonic day 10.5, by using whole-genome Affymetrix microarrays (Pavlinkova et al., submitted). Over 30% of genes we identified encode transcription factors, chromatin modifying proteins and components of signaling pathways that impinge on transcription, supporting the idea that maternal diabetes de-regulates tissue specific gene expression programs in the developing embryo. Yet, because we did these studies on whole embryos, they provide only limited information on the involvement of any of the identified genes in pathogenesis of specific developmental defects in diabetic embryopathy, such as caudal dysgenesis. We therefore used a caudal dysgenesis model, the Isl1-transgenic mouse (Muller et al., 2003), to investigate the expression of developmentally relevant genes. Because the initial experiments established a possible role for Wnt3a in the caudal dysgenesis model, we also investigated the expression of Wnt signaling pathway molecules in mouse embryos exposed to maternal diabetes during pregnancy. We included in our analyses several genes that were not represented on the original microarrays but were implicated in developmental defects from studies on mutant mice. Supplementing and extending our microarray approach, the present study corroborates the involvement of the Wnt signaling pathway in the elevated risk for the birth defects of diabetic embryopathy.
2. Methods
2.1. Experimental animals
Isl1 transgenic embryos were generated using the VP-16-based binary transgenic system (Muller et al., 2003) (see Figure 1, Panel G). Briefly, the transactivator (TA) transgenic lines express the viral transactivator VP16 under control of the Hoxc8 promoter, and transresponder (TR) transgenic lines carry the cDNA from the rat Isl1 gene linked to the 360 bp fragment from the immediate early gene promoter of the ICP4 gene of herpes simplex virus. The TR transgene is activated only when combined in the same animal with a TA transgene. TA only animals were used as controls, and genotyping was performed as described previously (Muller et al., 2003).
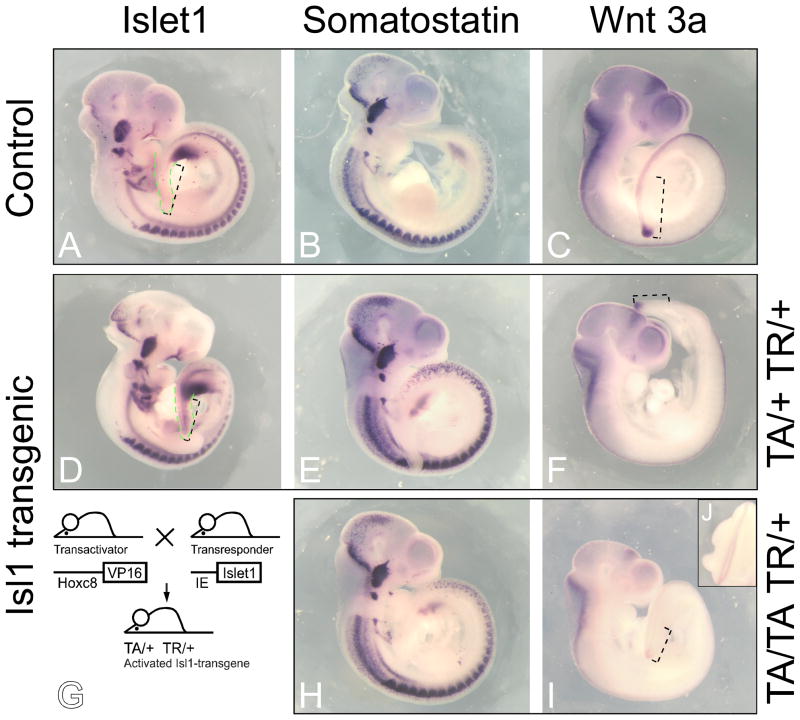
Figure 1. Expression of Sst and Wnt3a in Hoxc8-Isl1 transgenic embryos.
The expression patterns of Isl1, Sst and Wnt3a in the E10.5 transgenic embryos were analyzed using whole mount in situ hybridization. Panels A–C: control embryos transgenic for the TA transgene. Panels D–F and H–J: Isl1-transgenics generated according to the breeding scheme in Panel G. Signals for Sst expression were more intense in the transgenic embryos (Panels E, H, compared to Panel B), and this appeared throughout the entire Sst expression domain. Wnt3a expression was decreased in the tail bud and the neural tube in transgenic embryos hemizygous for both TA and TR (TA/+ TR/+, Panel F) compared to controls transgenic for transactivator only (TA/+ +/+, Panel D). Compound transgenic embryos with transgenic loci in excess of hemizygosity (TA/TA TR/+, Panel I) exhibited greater reduction of Wnt3a expression compared to TA/+TR/+ embryos (Panel F). Green stippled lines mark the posterior region in Isl1 hybridized embryos (Panels A and D); increased signal intensity in the posterior region of the Isl1-transgenic embryo indicates overexpression of the Isl1-transgene, as expected. Black stippled lines mark length of distance between hindlimb bud and tip of tail; in both Isl1-transgenic embryos, the tail region is shorter (as previously published in Muller et al., 2003). Panel J depicts defective neural tube closure in the lumbar region of the embryo shown in Panel I.
Diabetes was induced in female FVB mice (aged 7–9 weeks) before pregnancy by two intraperitoneal injections of 100 mg/kg body weight Streptozotocin in 50 mM sodium citrate buffer at pH 4.5 (STZ; Sigma, St. Louis, MO) within a one-week interval. Glucose levels in whole blood were measured using a Glucometer (Bayer, Tarrytown, NJ); the animals had ad libitum access to a standard diet (Harlan-Teklad LM-485). The dams were set up for mating no earlier than 7 days after the last injection, and the day of detection of a vaginal plug was counted as day 0.5 of gestation. All embryos used for the experiments were isolated from dams that were classified as diabetic when the blood glucose levels exceeded 250 mg/dl from the day of mating through the day of embryo harvest. The average blood glucose levels were 148 mg/dl (±18) before STZ treatment, 337 mg/dl (±79) on the day of mating, and 528 mg/dl (±70) on the day of embryo harvest (n=11). The sizes of litters at E10.5 from diabetic dams were not significantly different from controls (10.06 ± 1.103 n=17 vs. 9.636 ± 0.8557 n=11; ns).
2.2. Quantitative Real-time PCR
Quantitative Real-Time PCR (qRT-PCR) was performed using an ABI Prism 7000 instrument (Applied Biosystems, Foster City, CA) on cDNA samples from individual whole diabetes-exposed embryos (5 litters) and controls (4 litters) or pools of 4–5 control embryos (each pool consisted of individuals from only one litter) isolated at E10.5. Total RNA was isolated using TrizolR (Invitrogen, Carlsbad, CA). RNA samples (5 μg) were subjected to reverse transcription using Superscript II (Invitrogen, Carlsbad, CA). The PCR reactions and quantifications were described previously (Kruger et al., 2006) with the following modifications: the initial AmpliTaq activation at 95°C for 10 minutes was followed by 40 cycles at 95°C for 15 seconds and 1 minute at 60°C. The values for detection above threshold level (Ct) for each gene were determined relative to measurements of Polymerase Σ 4 (PolΣ4) cDNA in independent reactions with aliquots of the same sample. Normalized Ct values (CtGENE − CtPolΣ4) were compared between groups of diabetes-exposed and control embryos using an unpaired two-tailed t-test with assumption of unequal variance (Microsoft Excel Analysis Pack). Primer sets were designed to exclude amplification of potentially contaminating genomic DNA by positioning of the amplicons across exon-exon junctions. Primer sequence positions are listed in Table 1.
Table 1. Primers used for quantitative RT-PCR assays.
| Gene symbol | RefSeq ID | Forward Primer starting position | sequence | Reverse primer starting position | sequence | Amplification rate |
|---|---|---|---|---|---|---|
| Apc | NM_007462 | 1175 | TTCTAGCGGCACGCACTCT | 1292 | TCGTGACATATCGTCCTTATCATGA | 1.9 |
| Ctnnb1 | NM_007614 | 1736 | ACCCAACGGCGCACCT | 1843 | CCGAGCAAGGATGTGGAGA | 2.0 |
| Dkk1 | NM_010051 | 511 | CCCGGGAACTACTGCAAAAA | 597 | TTCAATGATGCTTTCCTCAATTTC | 2.0 |
| Hoxc8 | NM_010466 | 595 | CAACACTAACAGTAGCGAAGGACAAG | 727 | CAAGGTCTGATACCGGCTGTAAGT | 1.8 |
| Isl1 | NM_021459 | 826 | AAGCGGTGCAAGGACAAGA | 972 | GTTAGCCTGTAAACCACCATCATGT | 2.0 |
| Lrp5 | NM_008513 | 713 | CTGCGACGGTGAGGCC | 839 | GAAGGAGTCACACTGTTGCTTGA | 2.0 |
| Pax3 | NM_008781 | 241 | AAAAAGGCTAAACACAGCATCGA | 320 | TCAATATCGGAGCCTTCATCTGA | 1.8 |
| Pax4 | NM_011038 | 1156 | CAGGCAGATGTTCCAGTGACA | 1223 | GAGGGATTGGCAGTCCCAGTA | 1.9 |
| Pax6 | NM_013627 | 1293 | AGTGAATGGGCGGAGTTATGAT | 1427 | GGAACTTGGACGGGAACTGA | 2.0 |
| Sst | NM_009215 | 185 | CCCAGACTCCGTCAGTTTCTG | 302 | GGGCATCATTCTCTGTCTGGTT | 2.0 |
| Wnt3a | NM_009522 | 422 | TGGCCCTGTTCTGGACAAAG | 493 | CTGCACAGGAGCGTGTCACT | 1.8 |
| Wnt5a | NM_009524 | 916 | TTCTGTCTTTGGCAGGGTGAT | 990 | ACCCCAGCTGCGCTCA | 1.8 |
2.3. In situ hybridization
In situ hybridizations were performed using whole embryos at E10.5 and E9.5 (free of any extraembryonic membranes) as described (Hogan et al., 1994). Briefly, embryos were fixed in 4% Paraformaldehyde (PFA) in phosphate-buffered saline (PBS) at 4°C for 24 hours. Embryos were incubated with riboprobes at 63°C for 24 hours. After washing, the bound digoxigenin-labeled riboprobe was detected by antidigoxigenin-antibody coupled to alkaline phosphatase (AP; Roche Applied Science, Indianapolis, IN) and BM Purple as the substrate for AP (Roche Applied Science, Indianapolis, IN). Tissue staining in embryos was photographed using a Leica MZ9.5 stereomicroscope and Kodak M290 camera.
3. Results and Discussion
Although the teratogenic effects of maternal diabetes are well documented, the causes of specific developmental anomalies in diabetic embryopathy remain elusive. Diabetes-induced morphogenetic defects have been associated with diverse processes, including increased apoptosis (Gareskog et al., 2007; Reece et al., 2005), altered lipid metabolism (Wentzel et al., 1999), oxidative stress (Loeken, 2004), and changes in gene expression (Phelan et al., 1997; Reece et al., 2006) in the embryo and yolk sac (Reece et al., 1994). Experimental animal models to date have focused on type I diabetes, but it is possible that some mechanisms may be shared in type II diabetes, since, at least in human type II diabetic pregnancies, similar birth defects have been reported. The current study provides a detailed examination of the expression of developmentally important molecules, which play a role in the patterning and development of specific embryonic tissues.
3.1 Wnt gene expression in the Isl1 transgenic model of caudal dysgenesis
Our group has previously generated the Isl1 transgenic mice that exhibit profound caudal growth defects, resembling human caudal dysgenesis syndrome (Muller et al., 2003). To analyze the relationship between the caudal dysgenesis phenotype in our transgenic mouse model and caudal dysgenesis in diabetic embryopathy, we investigated the expression of two molecules for which preliminary measurements by quantitative RT-PCR had indicated altered expression: Somatostatin mRNA appeared increased, and Wnt3a mRNA decreased in caudal regions dissected from Isl1-transgenic embryos at 10.5 days of development (Treece, Kruger and Kappen, unpublished observations).
Isl1, a LIM homeodomain transcription factor, is required for normal embryonic development, since embryos lacking Isl1 die by embryonic day 10.5 (Pfaff et al., 1996). Loss-of-function studies in the mouse revealed crucial roles for Isl1 in motor neuron, cardiac and limb development (Cai et al., 2003; Pfaff et al., 1996).
Furthermore, Isl1 is required for the development of the mesenchyme of the dorsal pancreatic bud and for endocrine cell differentiation in the pancreas (Ahlgren et al., 1997). In somatostatin producing rat islet cell lines, Isl1 binds to the somatostatin gene enhancer, and together with CREB, regulates somatostatin gene transcription (Leonard et al., 1992; Vallejo et al., 1992). Thus, in Isl1-transgenic embryos, increased Somatostatin (Sst) expression is consistent with the Sst locus being a target for the Isl1 transcription factor. Wnt3a has previously been demonstrated in mouse mutants to have a role in caudal development, and it was reported as downregulated in embryos exposed to maternal diabetes and retinoic acid, and may be responsible for the increased rate of tail defects under these conditions (Chan et al., 2002; Shum et al., 1999).
To determine whether the misregulation of Sst and/or Wnt3a could be responsible for the caudal growth defects in Isl1-transgenics, we performed in situ hybridizations. Figure 1 shows that Isl1, as expected when driven off Hoxc8 regulatory sequences (Muller et al., 2003), is expressed in the posterior region, in particular caudal to the hindlimb bud, at higher levels in the transgenic compared to the control embryo (Panels A, D). Yet, there did not appear to be a region-specific increase in Sst expression in the posterior; instead, the in situ hybridization signals for Sst appear to be more intense throughout the Sst expression domain in the transgenics (Panels E, H) compared to control (Panel B). While this makes a primary involvement of Sst in the caudal growth defects in Isl1-transgenics unlikely, the overall increased Sst levels may explain the reduced size of Isl1-transgenic embryos, as Sst plays a role in developmental and cellular growth regulation (reviewed in (Anderson et al., 2004)).
Wnt3a expression was clearly reduced in Isl1-transgenic embryos (Panels F, J), preferentially in the region posterior to the hindlimb. Although not completely repressed, the reduced Wnt3a expression coincided with reduced tail length (compare transgenics in Panels F and I to controls in Panels A and C), and, in the embryo with the highest transgene expression, was associated with defective neural tube closure in the posterior region (see inset J). This is consistent with the finding that in the skeleton, severity of the Isl1-induced phenotype was also dependent on transgene dosage (Muller et al., 2003). Thus, reduced Wnt3a expression in the Isl1-transgenic embryos could be responsible for the developmental defects seen in these mice (Muller et al., 2003), namely shortened tails, absent tails, and spina bifida, which occurs with a frequency of 9/97 (3.3%) at E11.5. Taken together our results suggest that Isl1-induced reduction of Wnt3a expression, and hence decreased activity of the Wnt signaling pathway, may play a major role in Isl1-induced developmental defects that resemble caudal dysgenesis syndrome. These results prompted us to further investigate the expression of genes involved in posterior development and/or Wnt signaling in embryos exposed to the conditions of maternal diabetes during pregnancy.
3.2. Expression of developmental regulatory genes in embryos from diabetic pregnancies
The occurrence of phenotypes that resemble diabetic embryopathy, namely neural tube defects and caudal dysgenesis, in Isl1-overexpressing mice suggested to us that one potential mechanism by which maternal diabetes might be causing developmental defects could be through the upregulation of pancreatic transcription factors, of which Isl1 is one example. We therefore assayed, in embryos exposed to conditions of maternal diabetes during pregnancy, the expression of Isl1 and two other prominent pancreatic transcription factors, Pax4 and Pax6. Pax4 and Pax6 play important roles in pancreas organogenesis (Sosa-Pineda et al., 1997; St-Onge et al., 1997), and dysregulation of Pax4 and Pax6 are correlated with diabetes and developmental defects. In humans, mutations in the Pax4 gene are associated with type 2 diabetes (Mauvais-Jarvis et al., 2004; Shimajiri et al., 2001; Shimajiri et al., 2003), and mutations in Pax6 are linked to glucose intolerance (Yasuda et al., 2002), and dysregulation of Pax6 leads to a reduction of islet cells in the pancreas and diabetes (Ashery-Padan et al., 2004; Yamaoka et al., 2000). Using qRT-PCR, we measured the expression of these Pax genes and Isl1 in whole diabetes-exposed embryos at E10.5. The expression of Pax4 and Pax6 was not significantly different in diabetes-exposed embryos compared to controls (Figure 2). For Isl1, the comparison did not reach statistical significance; however, significantly increased (P=0.009) expression (by 1.7-fold) was observed in subsequent microarray assays (Pavlinkova et al., submitted). Thus, with the exception of potentially upregulated Isl1 expression, maternal diabetes does not appear to lead to generalized activation of pancreatic transcription factors at the E10.5 timepoint assayed here. However, it is still possible that maternal diabetes could affect pancreatic transcription factors at earlier stages of development, or that interference with development of the pancreas as a metabolic organ in the embryo at later stages (Jensen, 2004) may contribute to “fetal origins of adult disease” (Barker et al., 1993; Eriksson et al., 2006; Fall et al., 1998), in particular diabetes.
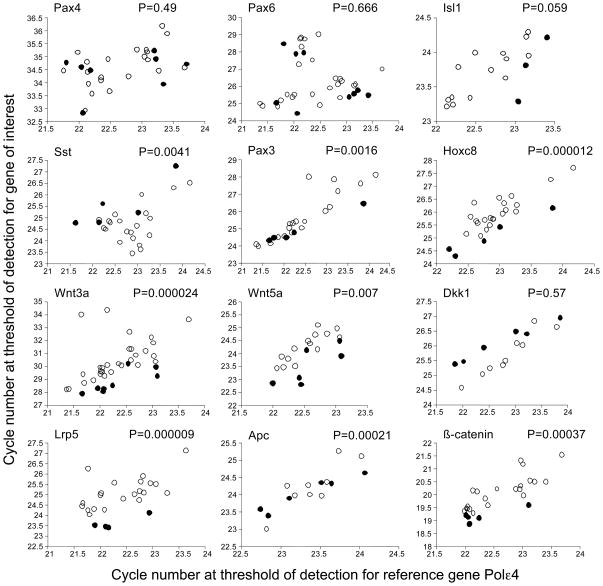
Figure 2. Expression of selected developmental control and Wnt pathway genes in diabetes-exposed embryos.
Expression levels were measured by quantitative Real-Time PCR in relation to the expression levels of the reference gene Polymerase Σ 4 in the same samples. Samples were from independent diabetes-exposed embryos without overt developmental defects, and control samples were pools of 4–5 embryos from normal pregnancies. Each open circle represents an individual diabetes-exposed embryo; each closed circle represents a measurements for a control sample. Greater values on the axes reflect lower levels of expression; hence, data points towards the top of each graph represent lower expression of the gene of interest, data points towards the bottom of each graph represent higher expression levels. For the majority of genes where a statistically significantly different distribution of data points was observed (P-values from two-tailed t-tests are noted) between diabetes-exposed and control embryos, expression levels are reduced under conditions of maternal diabetes; the exception is Sst, whose expression levels are increased in diabetes-exposed embryos. Expression levels were normalized to expression levels of PolΣ4 for calculation of the fold magnitude of change (see text).
Interestingly, our assays demonstrate increased expression levels for Somatostatin mRNA in diabetes-exposed embryos by 2.2-fold, and recent microarray data (2.1-fold increase, P=0.008) agree with this finding. This would be consistent with increased Isl1 expression, although due to the variation in measurements within the diabetes-exposed group, direct correlations at the level of individual embryos could not be made (data not shown). Nevertheless, the finding of increased Sst expression is intriguing, since progeny that were exposed to maternal diabetes during pregnancy are also smaller at birth, although they eventually grow to the same size as unexposed age matched controls (data not shown). Thus, the overall decreased growth, without overt developmental delay, could be mediated by elevated levels of somatostatin. Although there were no developmental defects reported for somatostatin-deficient mice (Low et al., 2001; Zeyda et al., 2001), somatostatin is a well-known inhibitor of cellular growth in normal and tumor cells (Pyronnet et al., 2008). Because changes in Sst levels are detected downstream of maternal diabetes and downstream of Isl1 in the transgenic model, our results implicate Somatostatin either as a causal effector or predisposing factor in the pathogenesis of phenotypes associated with diabetic embryopathy. Predisposition could involve gene-gene or gene-environment interactions, such as aberrant expression of other genes, e.g. Wnt3a, prolonged metabolic imbalance, or altered placental function in diabetic pregnancy (Salbaum and Kappen, unpublished observations).
However, we found significant effects of diabetes-exposure on gene expression levels for several other genes: the expression of Pax3 was significantly decreased in exposed embryos by an average 1.5-fold, consistent with the reduction of Pax3 at E8.5 reported by others (Phelan et al., 1997), and supporting our hypothesis that expression of developmental control genes is altered by maternal diabetes. Consistent with this idea, we found that exposure decreased by 1.6-fold on average the expression levels for Hoxc8, a patterning gene involved in development of ectodermal and mesodermal derivatives in the posterior region (LeMouellic et al., 1992). The decreased levels of Hoxc8 may be causally involved in or predispose to posterior defects, such as caudal dysgenesis. In this regard, it is of significance that we consistently detect decreased levels (by 3.1-fold) of Wnt3a, a gene well known to be required for proper caudal development, defects of which are highly characteristic for diabetic embryopathy. Mice with null mutation of Wnt3a (Takada et al., 1994), or with a hypomorphic allele of Wnt3a (in the vestigial tail mutant, vt (Greco et al., 1996)), exhibit axial truncations associated with extensive caudal cell death. Thus, we have identified two known developmental regulatory molecules for posterior development as altered in their expression in the embryo by maternal diabetes. These results, for the first time, provide evidence for potential molecular mechanisms involved in caudal growth defects in diabetic embryopathy.
3.3. Expression of Wnt pathway genes in embryos exposed to maternal diabetes
The implication from our results of Wnt3a signaling in both the caudal dysgenesis and the diabetic embryopathy models led us to investigate other components of the Wnt signaling pathway: Wnt5a was selected because its deficiency is associated with caudal dysgenesis (Yamaguchi et al., 1999), and Lrp5 is a Wnt co-receptor with Lrp6 (Kelly et al., 2004), whose targeted mutation phenotype also includes axis truncation (Pinson et al., 2000). Dickkopf 1, an inhibitory ligand for Lrp6 (Niehrs, 2006), was chosen as it can act as an inhibitor of Lrp5-mediated Wnt signaling during gastrulation (MacDonald et al., 2004), and a hypomorphic allele of Dkk1 ameliorates the axis truncations with loss of Lrp6 (MacDonald et al., 2004). Apc is a known signal transducer in the canonical Wnt pathway (Huelsken and Birchmeier, 2001; Logan and Nusse, 2004), which leads to stabilization of β-catenin and its translocation into the nucleus where it, together with proteins of the TCF family (Brantjes et al., 2002), acts as a transcription factor and regulates the read-out at the transcriptional level of Wnt signaling (Willert and Jones, 2006). Our initial microarray analyses indicated that β-catenin, Frzb, and Apc transcripts were decreased in embryos exposed to maternal diabetes (Table 2), while probes for Wnt3a, Wnt5a, Lrp5, and Dkk1 were either not present on the microarray chip, or the signal was below detection in diabetes-exposed embryos.
Table 2. Initial microarray results (Affymetrix 430A 2.0 chips).
Wnt pathway gene expression in a comparison between diabetes-exposed and control embryos as detected by microarray hybridization. Experimental details are published elsewhere (Pavlinkova et al., submitted); statistical analysis was done in GeneSpring and CyberT (P-values given here). Several of the Wnt pathway genes under study here were not represented on the 430A array.
| Gene symbol | Affymetrix probe ID | RefSeq Transcript ID | P value (CyberT) | Fold change | Gene name |
|---|---|---|---|---|---|
| Ctnnb1 | 1430533_a_at | NM_007614 | 0.0003 | −1.52 | catenin (cadherin associated protein), beta 1 |
| Apc | 1450056_at | NM_007462 | 0.0096 | −1.79 | adenomatosis polyposis coli |
| Frzb | 1448424_at | NM_011356 | 0.0274 | −1.53 | frizzled-related protein |
Interestingly, with the exception of Dkk1, all components of the Wnt signalling pathway exhibit lower expression levels in diabetes-exposed embryos. This suggests that the signal transduction cascade is de-regulated at many steps in the pathway and implicates multiple genes in the Wnt signaling pathway in predisposition or effector function in birth defects in diabetic embryopathy. The levels of expression were reduced on average by 3.1-fold for Wnt3a, 1.6-fold for Wnt5a, 2.8-fold for Lrp5, and 1.6-fold for Apc. Transcription of beta-catenin was reduced by 1.44 fold on average, and this would be expected to aggravate low β-catenin levels due to protein instability. Thus, our results show that extra- as well as intra-cellular components at all levels of the Wnt signal transduction cascade are transcriptionally de-regulated in embryos affected by maternal diabetes, and thus implicate Wnt signaling as a major mechanism in the pathogenesis of diabetic embryopathy.
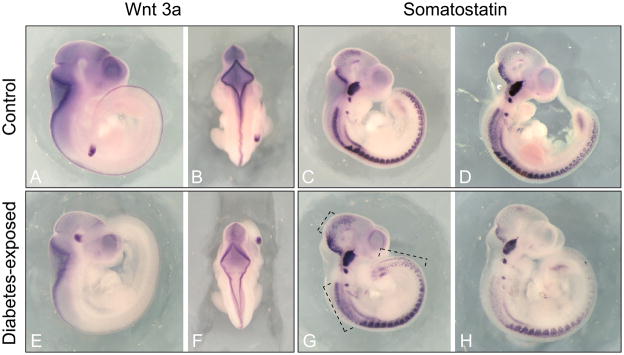
In order to investigate whether the alterations in expression can be assigned to specific tissues in the developing embryo, we performed in situ hybridization analysis in embryos from diabetic and normal pregnancies. For Wnt3a, we detected decreased expression along its expression domain in the dorsal nervous system of exposed embryos, and, in one specimen, we observed delayed closure of the hindbrain (Figure 3 Panel F). The extent of the expression domain of Wnt3a in the tail bud of exposed embryos appeared to be slightly reduced (compare Panels A and E). The development of these tissues is frequently affected in diabetic embryopathy, and decreased expression of Wnt3a in these tissues might increase the predisposition to neural tube or caudal defects associated with diabetic embryopathy. For Somatostatin, the hybridization patterns in normal embryos were very comparable (see Panels C and D) while two different patterns of hybridization signals were found in diabetes-exposed embryos: there was either a broadening of the expression domain within the nervous system in the midbrain and cervical regions of the nervous system and an extension of expression towards the posterior in the caudal region (see Panel G), or a relative reduction of expression in these same regions (see Panel H). This appeared to be unrelated to developmental progression of the embryos (note comparable cranial ganglia and brain vesicle development in all 4 Sst-hybridized specimen), but likely reflects the variation between individuals in quantitative levels of gene expression as measured in the quantitative RT-PCR assays (see Figure 2). A functional role for somatostatin in development of the nervous system at midgestation has not been investigated, but our results suggest that cells of the developing nervous system are the likely source of altered somatostatin production in the embryo under conditions of exposure to maternal diabetes.
Figure 3. Decreased expression of Wnt3a in diabetes-exposed embryos.
Expresssion patterns of Wnt3a (Panels A, B, E, F) and Sst (Panels C, D, G, H) were visualized by in situ hybridization on whole mounts specimen of diabetes-exposed and control embryos at E10.5. Wnt3a expression was reduced in both the tail bud and the neural tube of embryos from diabetic pregnancies compared to control embryos. Two patterns were observed for Sst expression in diabetes-exposed embryos: Panel G shows increased expression in midbrain, cervical and caudal areas (marked by black stippled line) relative to other regions in the embryo; Panel H shows relative decrease of Sst expression in those areas. Closure of the hindbrain appears delayed in Panel F.
4. Summary and Future Directions
Using the Isl1-transgenic model of caudal dysgenesis, we identify the Wnt signaling pathway as a potential pathogenic mechanism in the caudal growth defects, and altered Somatostatin expression as a possible contribution to the risk for diabetic embryopathy. Based upon this animal model, we had originally hypothesized that hyperglycemia in the mother during diabetic pregnancy might activate Isl1 and other pancreatic transcription factors, and that such deregulation might play a role in developmental abnormalities. Our quantitative measurements of gene expression levels for the pancreatic transcription factors Pax4 and Pax6, however, indicate that their expression in the embryo is unaffected by maternal diabetes, and therefore, do not support this proposition. Instead, we identify the developmental control genes Pax3 and Hoxc8 as altered in the diabetes-exposure paradigm. Homozygous mutations in Pax3 result in severe neural crest and neural tube closure defects in the Splotch mouse (Epstein et al., 1991), and decreased expression would likely predispose embryos to a higher risk for birth defects. Decreased expression of Pax3 at E8.5 in embryos exposed to maternal diabetes has been reported previously (Phelan et al., 1997), and our findings at E10.5 are consistent. A direct test for the hypothesis that Pax3 reduction elevates the risk for neural tube defects was performed by exposing Pax3 mutant heterozygotes, whose expression level is genetically reduced, to maternal diabetes, and indeed, elevated incidence of neural tube closure defects was demonstrated (Machado et al., 2001). Mechanistic studies on the role of Hoxc8 in diabetic embryopathy are lacking to date, and the functional role of altered Somatostatin expression during embryogenesis in the diabetic pregnancy paradigm also remains to be investigated.
The major outcome of this study is the implication of impaired Wnt signalling downstream of Isl1 and in conditions of maternal diabetes. Wnt-signaling is known to be critical for caudal development (Catala, 2002; Takada et al., 1994), and a reduction of Wnt3a expression was previously reported to sensitize mice for caudal defects when retinoic acid is administered (Chan et al., 2002; Shum et al., 1999). Our results show for the first time that Isl1 overexpression in the caudal region critically affects this pathway. It is currently unknown whether Wnt3a expression can be directly regulated by binding of Isl1 to regulatory regions in the Wnt3a locus and repression of transcription. Alternatively, reduced Wnt3a expression in our transgenic model may reflect the increased cell death in the posterior region of these transgenic mice (Muller et al., 2003). Interestingly, Wnt3a has recently been shown to exert a stimulatory effect for self-renewal and inhibitory effect on differentiation of Isl1-positive progenitor cells in the cardiovascular system (Qyang et al., 2007). Thus, it is also possible that reduced Wnt3a levels reduce proliferation of the Isl1-expressing cells in the posterior region.
Hoxc8 has also been shown to be involved in regulation of cell proliferation (Lei et al., 2006; Maulbecker and Gruss, 1993), and its downregulation in embryos exposed to maternal diabetes points to the intriguing possibility that a feedback regulatory loop may exist between the regulation of cell proliferation and Wnt3a expression in caudal growth of the embryo. Hyperglycemia during pregnancy can then be hypothesized to disrupt the balance of signals in this feedback loop, and this hypothesis will be experimentally tested in future experiments.
Our results implicate reduced expression of various components of the Wnt signaling pathway at extra- and intra-cellular levels and in regulation of β-catenin dependent transcription. In this regard, it is intriguing that in a second independent microarray experiment that included a much larger number of genes on the chip (Pavlinkova et al., submitted), we have identified additional components of Wnt signaling as altered in diabetes-exposed embryos (Table 3). While not yet validated by independent expression assays, all results implicate reduced gene expression, consistent with our RT-PCR data. These findings, combined with our results reported here, position Wnt signaling as a major intersection of abnormally regulated pathways in diabetic embryopathy. In summary, our results suggest several possible molecular pathways that may be involved in mediating the increased susceptibility to congenital malformations associated with diabetic pregnancies through altered expression of developmental control genes.
Table 3. Wnt pathway gene expression in diabetes-exposed embryos assayed by Affymetrix microarray (430 2.0).
Gene expression levels for selected known Wnt pathway genes in a comparison between diabetes-exposed and control embryos at day 10.5 of gestation. Details are published elsewhere (Pavlinkova et al., submitted). Signals for expression of Wnt3a, probes for which are represented on the 430 assay) were below the limit of detection in all samples. It is noteworthy that all components of the Wnt signaling pathway exhibit decreased expression in diabetes-exposed embryos (D) compared to controls (N), as reflected in the negative fold-change values.
| Gene symbol | Affymetrix probe ID | P value (CyberT) | Fold change D vs. N | Gene name |
|---|---|---|---|---|
| Apc | 1450056_at | 0.022 | −3.6 | adenomatosis polyposis coli |
| Ctnnb1 | 1430533_a_at | 0.039 | −12.9 | catenin (cadherin associated protein), beta 1 |
| Dixdc1 | 1444395_at | 0.009 | −7.3 | DIX domain containing 1 |
| Dvl2 | 1417207_at | 0.002 | −2.5 | dishevelled 2, dsh homolog (Drosophila) |
| Frzb | 1448424_at | 0.013 | −3.0 | frizzled-related protein |
| Fzd1 | 1422985_at | 0.039 | −7.2 | frizzled homolog 1 (Drosophila) |
| Fzd2 | 1418532_at | 0.012 | −2.7 | frizzled homolog 2 (Drosophila) |
| Fzd4 | 1449416_at | 0.045 | −11.3 | frizzled homolog 4 (Drosophila) |
| Fzd7 | 1450044_at | 0.015 | −2.2 | frizzled homolog 7 (Drosophila) |
| Gsk3b | 1451020_at | 0.039 | −2.3 | glycogen synthase kinase 3 beta |
| Sfrp1 | 1416594_at | 0.002 | −5.1 | secreted frizzled-related protein 1 |
| Wnt5a | 1448818_at | 0.042 | −2.1 | wingless-related MMTV integration site 5A |
| Wnt7b | 1420892_at | 0.0066 | −2.5 | wingless-related MMTV integration site 7B |
Acknowledgments
We thank Dana S’aulis for technical assistance with animal husbandry and in situ hybridization, and Dr. Claudia Kruger for consultation on determination of primer amplification rates. This work was supported by NIH grant RO1-HD37804 to C.K. and supplement RO1-HD37804-S1 to G.P.
References
- Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- Anderson LL, Jeftinija S, Scanes CG. Growth hormone secretion: molecular and cellular mechanisms and in vivo approaches. Exp Biol Med. 2004;229:291–302. doi: 10.1177/153537020422900403. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Zhou X, Marquardt T, Herrera P, Toube L, Berry A, Gruss P. Conditional inactivation of Pax6 in the pancreas causes early onset of diabetes. Dev Biol. 2004;269:479–488. doi: 10.1016/j.ydbio.2004.01.040. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (noninsulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- Brantjes H, Barker N, van Es J, Clevers H. TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling. Biol Chem. 2002;383:255–261. doi: 10.1515/BC.2002.027. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catala M. Genetic control of caudal development. Clin Genet. 2002;61:89–96. doi: 10.1034/j.1399-0004.2002.610202.x. [DOI] [PubMed] [Google Scholar]
- Chan BW, Chan KS, Koide T, Yeung SM, Leung MB, Copp AJ, Loeken MR, Shiroishi T, Shum AS. Maternal diabetes increases the risk of caudal regression caused by retinoic acid. Diabetes. 2002;51:2811–2816. doi: 10.2337/diabetes.51.9.2811. [DOI] [PubMed] [Google Scholar]
- Epstein DJ, Vekemans M, Gros P. Splotch (Sp2H), a mutation affecting development of the mouse neural tube, shows a deletion within the paired homeodomain of Pax-3. Cell. 1991;67:767–774. doi: 10.1016/0092-8674(91)90071-6. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Osmond C, Kajantie E, Forsen TJ, Barker DJ. Patterns of growth among children who later develop type 2 diabetes or its risk factors. Diabetologia. 2006;49:2853–2858. doi: 10.1007/s00125-006-0459-1. [DOI] [PubMed] [Google Scholar]
- Fall CH, Stein CE, Kumaran K, Cox V, Osmond C, Barker DJ, Hales CN. Size at birth, maternal weight, and type 2 diabetes in South India. Diabet Med. 1998;15:220–227. doi: 10.1002/(SICI)1096-9136(199803)15:3<220::AID-DIA544>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Gareskog M, Cederberg J, Eriksson UJ, Wentzel P. Maternal diabetes in vivo and high glucose concentration in vitro increases apoptosis in rat embryos. Reprod Toxicol. 2007;23:63–74. doi: 10.1016/j.reprotox.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Greco TL, Takada S, Newhouse MM, McMahon JA, McMahon AP, Camper SA. Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev. 1996;10:313–324. doi: 10.1101/gad.10.3.313. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Costantini F, Lacy E. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Huelsken J, Birchmeier W. New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev. 2001;11:547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Jacobs HC, Bogue CW, Pinter E, Wilson CM, Warshaw JB, Gross I. Fetal lung mRNA levels of Hox genes are differentially altered by maternal diabetes and butyrate in rats. Pediatr Res. 1998;44:99–104. doi: 10.1203/00006450-199807000-00016. [DOI] [PubMed] [Google Scholar]
- Jensen J. Gene regulatory factors in pancreatic development. Dev Dyn. 2004;229:176–200. doi: 10.1002/dvdy.10460. [DOI] [PubMed] [Google Scholar]
- Kelly OG, Pinson KI, Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- Kruger C, Talmadge C, Kappen C. Expression of folate pathway genes in the cartilage of Hoxd4 and Hoxc8 transgenic mice. Birth Defects Res A Clin and Mol Teratol. 2006;76:216–229. doi: 10.1002/bdra.20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Juan AH, Kim MS, Ruddle FH. Identification of a Hoxc8-regulated transcriptional network in mouse embryo fibroblast cells. Proc Natl Acad Sci USA. 2006;103:10305–10309. doi: 10.1073/pnas.0603552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMouellic H, Lallemand Y, Brulet P. Homeosis in the mouse induced by a null mutation in the Hox-3.1 gene. Cell. 1992;69:251–264. doi: 10.1016/0092-8674(92)90406-3. [DOI] [PubMed] [Google Scholar]
- Leonard J, Serup P, Gonzalez G, Edlund T, Montminy M. The LIM family transcription factor Isl-1 requires cAMP response element binding protein to promote somatostatin expression in pancreatic islet cells. Proc Natl Acad Sci US A. 1992;89:6247–6251. doi: 10.1073/pnas.89.14.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung MB, Choy KW, Copp AJ, Pang CP, Shum AS. Hyperglycaemia potentiates the teratogenicity of retinoic acid in diabetic pregnancy in mice. Diabetologia. 2004;47:515–522. doi: 10.1007/s00125-004-1350-6. [DOI] [PubMed] [Google Scholar]
- Loeken MR. Free radicals and birth defects. J Matern Fetal Neonatal Med. 2004;15:6–14. doi: 10.1080/14767050310001650662. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Low MJ, Otero-Corchon V, Parlow AF, Ramirez JL, Kumar U, Patel YC, Rubinstein M. Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest. 2001;107:1571–1580. doi: 10.1172/JCI11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Adamska M, Meisler MH. Hypomorphic expression of Dkk1 in the doubleridge mouse: dose dependence and compensatory interactions with Lrp6. Development. 2004;131:2543–2552. doi: 10.1242/dev.01126. [DOI] [PubMed] [Google Scholar]
- Machado AF, Zimmerman EF, Hovland DN, Jr, Weiss R, Collins MD. Diabetic embryopathy in C57BL/6J mice. Altered fetal sex ratio and impact of the splotch allele. Diabetes. 2001;50:1193–1199. doi: 10.2337/diabetes.50.5.1193. [DOI] [PubMed] [Google Scholar]
- Martin JA, Kung HC, Mathews TJ, Hoyert DL, Strobino DM, Guyer B, Sutton SR. Annual summary of vital statistics: 2006. Pediatrics. 2008;121:788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- Maulbecker CC, Gruss P. The oncogenic potential of deregulated homeobox genes. Cell Growth & Differentiation. 1993;4:431–441. [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Smith SB, Le May C, Leal SM, Gautier JF, Molokhia M, Riveline JP, Rajan AS, Kevorkian JP, Zhang S, Vexiau P, German MS, Vaisse C. PAX4 gene variations predispose to ketosis-prone diabetes. Hum Mol Genet. 2004;13:3151–3159. doi: 10.1093/hmg/ddh341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JL. Malformations in infants of diabetic mothers. Teratology. 1982;25:385–394. doi: 10.1002/tera.1420250316. [DOI] [PubMed] [Google Scholar]
- Muller YL, Yueh YG, Yaworsky PJ, Salbaum JM, Kappen C. Caudal dysgenesis in Isl-1 in transgenic mice. FASEB J. 2003;17:1349–1351. doi: 10.1096/fj.02-0856fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron dependent step in interneuron differentiation. Cell. 1996;84:309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- Phelan SA, Ito M, Loeken MR. Neural tube defects in embryos of diabetic mice: role of the Pax-3 gene and apoptosis. Diabetes. 1997;46:1189–1197. doi: 10.2337/diab.46.7.1189. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Pyronnet S, Bousquet C, Najib S, Azar R, Laklai H, Susini C. Antitumor effects of somatostatin. Mol Cell Endocrinol. 2008 Feb 13; doi: 10.1016/j.mce.2008.02.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, Caron L, Wu X, Clarke J, Taketo MM, Laugwitz KL, Moon RT, Gruber P, Evans SM, Ding S, Chien KR. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Reece EA, EP, CH, Wu Y-K, Naftolin F. The Yolk Sac Theory: Closing the Circle on Why Diabetes-Associated Malformations Occur. J Soc Gynecol Investig. 1994;1:3–13. [PubMed] [Google Scholar]
- Reece EA, Ji I, Wu YK, Zhao Z. Characterization of differential gene expression profiles in diabetic embryopathy using DNA microarray analysis. Am J Obstet Gynecol. 2006;195:1075–1080. doi: 10.1016/j.ajog.2006.05.054. [DOI] [PubMed] [Google Scholar]
- Reece EA, Ma XD, Zhao Z, Wu YK, Dhanasekaran D. Aberrant patterns of cellular communication in diabetes-induced embryopathy in rats: II, apoptotic pathways. Am J Obstet Gynecol. 2005;192:967–972. doi: 10.1016/j.ajog.2004.10.592. [DOI] [PubMed] [Google Scholar]
- Shimajiri Y, Sanke T, Furuta H, Hanabusa T, Nakagawa T, Fujitani Y, Kajimoto Y, Takasu N, Nanjo K. A missense mutation of Pax4 gene (R121W) is associated with type 2 diabetes in Japanese. Diabetes. 2001;50:2864–2869. doi: 10.2337/diabetes.50.12.2864. [DOI] [PubMed] [Google Scholar]
- Shimajiri Y, Shimabukuro M, Tomoyose T, Yogi H, Komiya I, Takasu N. PAX4 mutation (R121W) as a prodiabetic variant in Okinawans. Biochem Biophys Res Commun. 2003;302:342–344. doi: 10.1016/s0006-291x(03)00176-1. [DOI] [PubMed] [Google Scholar]
- Shum AS, Poon LL, Tang WW, Koide T, Chan BW, Leung YC, Shiroishi T, Copp AJ. Retinoic acid induces down-regulation of Wnt-3a, apoptosis and diversion of tail bud cells to a neural fate in the mouse embryo. Mech Dev. 1999;84:17–30. doi: 10.1016/s0925-4773(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature. 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Vallejo M, Penchuk L, Habener JF. Somatostatin gene upstream enhancer element activated by a protein complex consisting of CREB, Isl-1-like, and alpha-CBF-like transcription factors. Journal of Biological Chemistry. 1992;267:12876–12884. [PubMed] [Google Scholar]
- Wentzel P, Welsh N, Eriksson UJ. Developmental damage, increased lipid peroxidation, diminished cyclooxygenase-2 gene expression, and lowered prostaglandin E2 levels in rat embryos exposed to a diabetic environment. Diabetes. 1999;48:813–820. doi: 10.2337/diabetes.48.4.813. [DOI] [PubMed] [Google Scholar]
- Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20:1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yamaoka T, Yano M, Yamada T, Matsushita T, Moritani M, Ii S, Yoshimoto K, Hata J, Itakura M. Diabetes and pancreatic tumours in transgenic mice expressing Pax 6. Diabetologia. 2000;43:332–339. doi: 10.1007/s001250050051. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Kajimoto Y, Fujitani Y, Watada H, Yamamoto S, Watarai T, Umayahara Y, Matsuhisa M, Gorogawa S, Kuwayama Y, Tano Y, Yamasaki Y, Hori M. PAX6 mutation as a genetic factor common to aniridia and glucose intolerance. Diabetes. 2002;51:224–230. doi: 10.2337/diabetes.51.1.224. [DOI] [PubMed] [Google Scholar]
- Zeyda T, Diehl N, Paylor R, Brennan MB, Hochgeschwender U. Impairment in motor learning of somatostatin null mutant mice. Brain Res. 2001;906:107–114. doi: 10.1016/s0006-8993(01)02563-x. [DOI] [PubMed] [Google Scholar]