Abstract
Ca2+ triggers many forms of exocytosis in different types of eukaryotic cells, for example synaptic vesicle exocytosis in neurons, granule exocytosis in mast cells, and hormone exocytosis in endocrine cells. Work over the last two decades has shown that synaptotagmins function as the primary Ca2+-sensors for most of these forms of exocytosis, and that synaptotagmins act via Ca2+-dependent interactions with both the fusing phospholipid membranes and the membrane fusion machinery. However, some forms of Ca2+-induced exocytosis may utilize other, as yet unidentified Ca2+-sensors, for example, slow synaptic exocytosis mediating asynchronous neurotransmitter release. In the following overview, we will discuss the synaptotagmin-based mechanism of Ca2+-triggered exocytosis in neurons and neuroendocrine cells, and its potential extension to other types of Ca2+-stimulated exocytosis for which no synaptotagmin Ca2+-sensor has been identified.
Introduction
Ca2+-induced exocytosis initiates many forms of intercellular communication, as exemplified by synaptic transmission, which begins with Ca2+-triggered synaptic vesicle exocytosis that mediates neurotransmitter release (Fig. 1) [1]. Similarly, neuroendocrine cells secrete hormones by Ca2+-induced exocytosis [2], mast cells release their granule contents upon stimulation by Ca2+-controlled exocytosis [3], and even in T-lymphocytes, Ca2+-triggered exocytosis is functionally essential [4]. The question of how Ca2+ triggers exocytosis was first raised by Bernhard Katz’s seminal discovery that Ca2+ induces synaptic vesicle exocytosis, and thereby initiates synaptic transmission [5]. However, not much progress was made in this question until the discovery of synaptotagmin-1 (Syt1) as a candidate Ca2+-sensor for synaptic exocytosis [6]. Work from many laboratories has provided overwhelming evidence that Syt1 and its homologs function as the primary Ca2+-sensors in most forms of exocytosis, and has elucidated the principal mechanism by which synaptotagmin operates [1]. However, as described below, this work has also raised important new questions about the role of Ca2+ in regulating membrane traffic. The present review focuses on the cell biology of Ca2+-triggered exocytosis in neurons and endocrine cells, and tries to relate the emerging synaptotagmin Ca2+-sensor paradigm to these new unanswered questions.
Figure 1. Synaptic and endocrine Ca2+-triggered exocytosis.
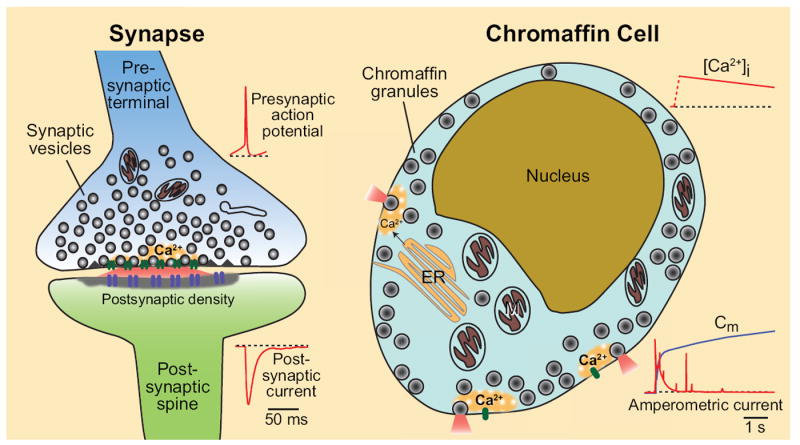
At a synapse (left), neurotransmitters are packaged into small synaptic vesicles, which are docked at the active zone adjacent to voltage-dependent Ca2+-channels. A presynatpic action potential (insert) gates Ca2+-influx into the terminal, thereby triggering vesicle exocytosis. The released transmitters produce a postsynaptic current (insert) which can be recorded by whole-cell patch clamping. In endocrine cells (right), hormones are packaged into LDCVs, which are generally not docked. Upon sustained increases in cytosolic Ca2+, as obtained during stimulation or Ca2+-uncaging (insert), exocytosis is triggered with a significantly slower time course than at a synapse, as measured by amperometry or capacintance (Cm; insert). Note that Ca2+-channel and release sites are not tightly coupled in endocrine cells. ER, endoplasmic reticulum; M, mitochondrion. Traces are shown purely for demonstration purposes.
Synaptic exocytosis
In presynaptic nerve terminals, neurotransmitters are packaged into small synaptic vesicles, and released by Ca2+-triggered exocytosis of synaptic vesicles at the presynaptic active zone (Fig. 1). Three different modes of neurotransmitter release exist (Fig. 2 A):
Figure 2. Synaptic vesicle exocytosis detected by whole-cell patch clamp recordings.
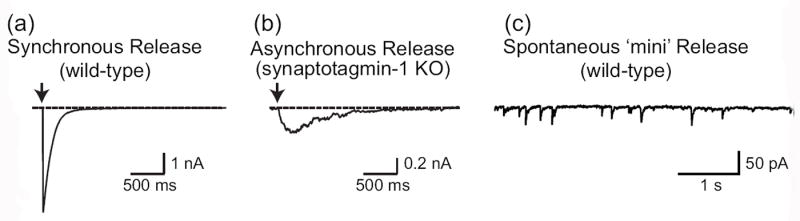
Images depict representative traces of postsynaptic currents illustrating the three different forms of synaptic exocytosis: evoked synchronous release from wild-type synapses (a), evoked asynchronous release from Syt1-deficient synapses (b) and spontaneous mini release (c). Note that asynchronous release also can be recorded in some wild-type neurons upon high-frequency stimulation.
Evoked synchronous release initiates within a millisecond after an action potential induces Ca2+-influx into a presynaptic terminal [8]. Fast synchronous release measured as the postsynaptic response can be fitted by a double exponential function, and thus can be arbitrarily subdivided into a fast and a slow phase [9].
Evoked asynchronous release sets in with a delay after an action potential, and is normally negligible [10], either because it is outcompeted by the synchronous release mechanism [11], or because the synchronous release mechanism (i.e., synaptotagmin and complexin, see below) suppresses asynchronous release [12]. However, asynchronous release becomes a dominant form of release in some synapses during high-frequency trains of action potentials, particularly in inhibitory synapses [13-15].
Spontaneous ‘mini’ release, finally, represents the exocytosis of single vesicles [16] that is independent of action potentials, but nevertheless largely Ca2+-dependent [17]. Spontaneous release is induced by resting Ca2+-concentrations, or may be stimulated by stochastic Ca2+-channel openings and/or Ca2+-sparks via Ca2+-influx from internal Ca2+-stores [18].
All three forms of synaptic exocytosis likely perform important physiological functions. Evoked synchronous release represents the primary mode of intercellular communication between neurons, rendering its importance obvious. Bursts of activity are often seen in neurons, suggesting that these trigger asynchronous release which may shape neural function at the circuit level [19]. Spontaneous release may improve the signal to noise ratio at the systems level [20], and/or to contribute to the development and maintenance synapses [21].
In central synapses, synaptic vesicles exist in functionally different pools [22], although this may not be true for neuromuscular synapses [23]. All types of synaptic exocytosis probably utilize the same release-ready pool of vesicles (the so-called readily-releasable pool [RRP]), which accounts only for a small percentage of the total vesicle pool [22]. An operational definition of the RRP is the amount of release triggered by application of hypertonic sucrose, which induces Ca2+-independent exocytosis of all synaptic vesicles in the RRP [24, 25]. The morphological correlate of the RRP is unclear; RRP vesicles are distinct from the synaptic vesicles that are morphologically docked at the active zone because the number of docked vesicles does not correlate with the RRP size [26], and a point mutation that activates the SNARE-protein syntaxin-1B simultaneously increases the number of docked vesicles but decreases the RRP size [27].
Endocrine exocytosis
Hormonal exocytosis of endocrine cells operates on large dense-core vesicles (LDCVs) that are probably similar to neuropeptide LDCVs in neurons. LDCV exocytosis has been studied mostly in adrenal chromaffin cells and in pancreatic β-cells, where Ca2+-triggered exocytosis operates in three phases, referred to as the fast, slow and sustained phase [28,29]. The three phases of LDCV exocytosis have been attributed to different vesicle pools, but it is uncertain whether these pools represent physically distinct types of vesicles, or simply different functional states. In addition, spontaneous exocytosis of LDCVs has also been described in chromaffin cells, and also appears to be regulated by Ca2+ [30].
Even the fast phase of LDCV exocytosis is much slower than both synchronous and asynchronous synaptic exocytosis (Fig. 1). Nevertheless, LDCV and synaptic exocytosis are very similar, as they appear to use the same Ca2+-triggering mechanisms (see discussion below), and differ primarily in how synaptic vesicles and LDCVs are docked and prepared for fusion (i.e., primed).
Synaptotagmins as Ca2+-sensors for exocytosis
Synaptotagmins are synaptic and secretory vesicle proteins (although some isoforms may be on the plasma membrane) that contain a single N-terminal transmembrane region, and two C-terminal Ca2+-binding C2-domains [6]. 16 mammalian synaptotagmin isoforms were identified, 8 of which bind Ca2+ with distinct apparent Ca2+-affinities (Syt1-3, Syt5-7, Syt9, and Syt10 [31-33], Table 1). Synaptotagmins are highly conserved evolutionarily; even all invertebrates express multiple isoforms. However, synaptotagmins are absent from plants and unicellular eukaryotes, suggesting that they emerged coincidentally with animals during evolution.
Table 1.
Properties of synaptotagmins
| Protein | Class | Localization | Ca2+-binding | Special properties | Function | References |
|---|---|---|---|---|---|---|
| Syt 1 | A | Synaptic vesicles LDCVs | Yes | N-terminal N-glycosylation Phospholipids and SNARE binding | Ca2+-sensor for fast exocytosis | 6,10,12,31-33, 39-43,74 |
| Syt2 | A | Synaptic vesicles LDCVs? | Yes | N-terminal N-glycosylation Phospholipid and SNARE binding | Ca2+-sensor for fast exocytosis | 8,9,31-33,43, 44,54,74 |
| Syt3 | C | Plasma membrane | Yes | Disulfide bonds at N-terminus Phospholipid binding | Ca2+-sensor for exocytosis? | 31-33,74 |
| Syt4 | D | Postsynaptic? | No* | Aspartate to Serine substitution in C2A domain loops | unknown*; not essential for survival | 73,74 |
| Syt5 | C | Plasma membrane | Yes | Disulfide bonds at N-terminus Phospholipid bindings | Ca2+-sensor for exocytosis? | 31-33,74 |
| Syt6 | C | Plasma membrane | Yes | Disulfide bonds at N-terminus Phospholipid binding | Ca2+-sensor for exocytosis? | 31,74 |
| Syt7 | B | LDCVs Synapses? | Yes | Multiple splicing isoforms Phospholipid and SNARE binding | Ca2+-sensor for LDCV exocytosis | 47-50,74 |
| Syt8 | F | primarily GIT tract | No | Only Syt isoform that is expressed at highest levels outside of brain | Not known | 31,74 |
| Syt9 | A | Synaptic vesicles | Yes | Phospholipid and SNARE binding | Ca2+-sensor for fast exocytosis | 43,74 |
| Syt10 | C | Plasma membrane | Yes | Disulfide bonds at N-terminus Phospholipid binding | Ca2+-sensor for exocytosis? | 31,74 |
| Syt11 | D | Unknown | No | Similar to Syt4, but more abundant | unknown | 74 |
| Syt12 | F | Synaptic vesicles | No | Phosphorelated by PKA | May regulate miniature release | 31,74,75 |
| Syt13 | F | Not known | No | Not known | 31,74 | |
| Syt14 | E | Not known | No | Syt14-16 form a group of related and evolutionarily conserved isoforms | Not known | 76 |
| Syt15 | E | Not known | No | Syt14-16 form a group of related and evolutionarily conserved isoforms | Not known | 76 |
| Syt16 | E | Not known | No | Syt14-16 form a group of related and evolutionarily conserved isoforms | Not known | 76 |
Synaptotagmins are defined by the presence of an N-terminal transmembrane region, and two C-terminal C2-domains. Each synaptotagmin protein is encoded by a different gene; some are differentially spliced (e.g., Syt7). Classes of synaptotagmins are proposed defined by sequence homologies, conservation, and functions
Syt4 binds to Ca2+ in Drosophila and has been implicated in postsynaptic exocytosis.
Synaptic and endocrine exocytosis are mediated by the same fusion machinery composed of SNARE- and SM-proteins as other membrane fusion reactions – in fact, this fusion machinery was discovered at the synapse [34]. SNARE-proteins catalyze fusion by forming a complex that bridges the two fusing membranes, forcing these membranes together, whereas SM-proteins promote fusion by an unknown but essential mechanism. Ca2+-binding to synaptotagmin triggers exocytosis by operating on this fusion machinery with the help of an ancillary protein called complexin. Thus, only six proteins – three SNARE-proteins, one SM-protein (Munc18-1), one synaptotagmin, and complexin – form the core of the Ca2+-triggered exocytosis machinery (Fig. 2B), constituting a molecular clockwork that exhibits an amazing simplicity which we refer to as the synaptotagmin paradigm [34].
Both C2-domains of synaptotagmin bind Ca2+; C2-domains were first shown to represent Ca2+-binding domains in Syt1 [35]. C2-domains are janus-faced domains with 2-3 Ca2+-binding sites on top, and a Ca2+-independent surface on the bottom. Ca2+-binding induces simultaneous synaptotagmin-binding to both the fusing phospholipid membranes, and the assembling SNARE complex (Fig. 3). Complexin activates SNARE-complexes prior to synaptotagmin action, and clamps fusion by preventing complete SNARE-complex assembly until Ca2+ binds to synaptotagmin [36,37]. Complexin performs these actions by binding to SNARE complexes, and synaptotagmin dislodges the complexin clamp (Fig. 3 step 5, [38]). Blocking Ca2+-binding to the C2B-domain blocks synchronous exocytosis [39], whereas blocking Ca2+-binding to the C2A-domain decreases exocytosis ~40%, and additionally decreases the apparent Ca2+-cooperativity of exocytosis ~40% [40]. Thus, the two C2-domains of synaptotagmins thus are not equivalent, but they cooperate with each other, with the C2B-domain playing the leading part. Nevertheless, mutations in the C2A-domain alter the overall apparent Ca2+-affinity of synaptotagmin and change the Ca2+-affinity of synaptic exocytosis correspondingly, an observation that provided the formal proof for the Ca2+-sensor of synaptotagmin in exocytosis [41,42]. Biochemically, Ca2+-binding to the C2B-domain is essential both for effective Ca2+-dependent phospholipid binding of synaptotagmin and for displacing the complexin clamp from SNARE complexes. Note that in the latter process, complexin may still remain associated with the SNARE complex, since it likely interacts with the complex via multiple mechanisms.
Figure 3. Model of the molecular steps mediated synaptic vesicle exocytosis (modified from [38]).
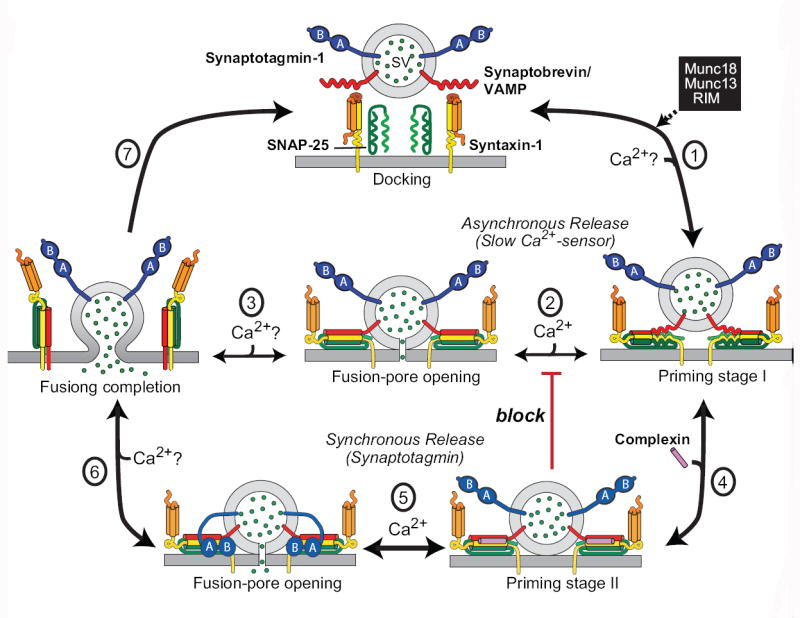
Synaptic vesicles are docked at the active zone of a presynaptic terminal with unassembled SNARE complexes (top)m and are then primed for release by partial SNARE-complex assembly that is catalyzed by Munc18, Munc13, and RIM (step 1). At least in inhibitory synapses, this priming process might be further modulated by ELKS2. The primed vesicles form the substrate for two main pathways of Ca2+-triggered neurotransmitter release: asynchronous release (steps 2 and 3), in which full assembly of SNARE complexes leads to fusion-pore opening followed by complete fusion (step 3); and synchronous release (steps 4,5 and 6), in which “superpriming” by binding of complexins to assembled SNARE complexes (step 4) activates and freezes SNARE complexes in a metastable state (referred to as priming stage II). This stage is then substrate for fast Ca2+-triggering of release when Ca2+-binding to Syt1 induces its binding to phospholipids and to SNARE complexes, with the latter reaction displacing complexin and resulting in fusion-pore opening (step 5) and full fusion (step 6). Both the synchronous and the asynchronous release pathway can mediate spontaneous ‘mini’ release, depending on the local Ca2+-microdomain. Synaptotagmin and complexin clamp (block, in red) the unidentified slow Ca2+-sensor which mediates the asynchronous release; this clamping is relieved when Ca2+ binds to Syt1, allowing competition between Syt1 and the asynchronous Ca2+-sensor during high-frequency stimulation [12,44].
Three synaptotagmins (Syt1, Syt2, and Syt9) function as Ca2+-sensors for synaptic exocytosis, but exhibit distinct expression patterns and properties [43]. Syt2 triggers release much faster than Syt1 and operates in synapses relying on fast signaling, such as the calyx of Held synapse or the neuromuscular junction [8-10,44]. Syt9 triggers release with a significantly slower timecourse than either Syt1 or Syt2, and is primarily expressed in the reward pathway [43].
In endocrine LDCV exocytosis, the major Ca2+-sensors are Syt1 and Syt7, another Ca2+-binding synaptotagmin [45-50]. Single deletions of Syt1 or Syt7 in chromaffin cells impair preferentially the fast or slow phase of LDCV exocytosis, respectively, suggesting that the two synaptotagmins mediate different phases, whereas double deletions of Syt1 and Syt7 block both phases [48]. Point mutations in Syt1 that change its Ca2+-affinity change the apparent Ca2+-affinity of exocytosis in chromaffin cells correspondingly, similar to the synapse [46]. Whereas at least Syt2 can substitute for Syt1 in chromaffin exocytosis [51], Syt7 cannot substitute for Syt1 in synaptic exocytosis [43], and Syt7 deletions have no significant effect on synaptic exocytosis [52]. The reason for this selectivity remains unclear, as Syt7 is very similar to Syt1, and only differs from Syt1 in lacking an N-terminal intravesicular N-glycosylation site and in exhibiting a higher apparent Ca2+-affinity [32]. In chromaffin cells, Syt1 may also be involved in docking of LDCVs since LDCV docking was impaired in cells from Syt1 KO mice [53]. However, the mechanism of this change remains unclear because synapses lacking Syt1 exhibit no major docking phenotype, and a similar docking phenotype was observed in chromaffin cells but not synapses in other mutant mice [27].
Synaptotagmins have also been implicated in non-neuronal and non-endocrine forms of Ca2+-induced exocytosis. In mast cells, Syt2 functions as the major Ca2+-sensor for exocytosis [54]. In fibroblasts, Syt7 may mediate Ca2+-triggered lysosome exocytosis [55], although this has been disputed [56]. It is notable that no definitive functions were described for 4 of the 8 Ca2+-binding synaptotagmin isoforms (i.e., Syt3, Syt5, Syt6, and Syt10). These synaptotagmins form a highly homologous subgroup that exhibit Ca2+-dependent phospholipid- and SNARE-binding similar to Syt1, Syt2, Syt7, and Syt9, and likely also function in exocytosis.
Dual-Ca2+-sensor model for synaptic exocytosis
The most precise definition of synaptic transmission was achieved at the giant calyx of Held synapse in the brainstem that allows simultaneous patching of pre- and postsynaptic cells [57,58]. Measurements in the calyx synapse provided estimates of the Ca2+-affinity (10-100 μM) and Ca2+-cooperativity (~5 Ca2+-ions) of neurotransmitter release [44,57,58]. As shown in mutant mice, this release is mediated by Syt2 as Ca2+-sensor [9,44].
Calyx synapses normally exhibit little asynchronous release, and produce almost only synchronous release even at stimulation frequencies of 100 Hz, making it impossible to analyze asynchronous release biophysically. In Syt2-deficient synapses, however, asynchronous release could be analyzed in isolation, uncontaminated by a more dominant synchronous release component [44]. Such analyses uncovered an asynchronous component of exocytosis in calyx synapses that displayed an apparent Ca2+-cooperativity of exocytosis of only ~2 Ca2+-ions, whereas synchronous release operated with an apparent Ca2+-cooperativity of ~5 Ca2+-ions, although both exhibited similar Ca2+-affinities (~40 μM). Because of the different Ca2+-cooperativities but similar Ca2+-affinities of synchronous and asynchronous exocytosis, low Ca2+-concentrations preferentially but incompletely activate asynchronous release in wild-type synapses, whereas higher Ca2+-concentrations, such as those achieved following an action potential, preferentially and completely activate synchronous release [44].
Based on the biophysical definition of asynchronous release, a dual-Ca2+-sensor model was proposed which at present provides the most precise description of evoked synaptic vesicle exocytosis for synapses [44]. The model assumes that the synchronous Ca2+-sensor synaptotagmin competes with an unknown asynchronous Ca2+-sensor, with the asynchronous Ca2+-sensor binding Ca2+ more slowly but at lower concentrations than the synchronous Ca2+-sensor. It should be noted that although the dual-Ca2+-sensor model is the best available, it does not take into account the intrinsic heterogeneity of synapse vesicles. Vesicles likely differ in their proximity to Ca2+-channels, which is a major determinant for the probability and speed of exocytosis [59]. Moreover, the number of SNARE complexes on a primed vesicle probably contributes to the Ca2+-sensitivity of this vesicle, but differs between vesicles [27]. Finally, synaptic vesicles exhibit size heterogeneity [60], resulting in variations in the postsynaptic signal. In addition, the dual-Ca2+-sensor model also does not include the possibility that at least at some synapses, synaptotagmin may inhibit the asynchronous Ca2+-sensor [12,17]. Thus, despite the fact that the dual-Ca2+-sensor model is currently the best available, it is far from perfect.
Synaptotagmin as a Ca2+-sensor for spontaneous release
At a synapse, lowering the extracellular Ca2+-concentration partially blocks spontaneous mini release; incubating synapses with membrane-permeable Ca2+-buffers, however, or infusing Ca2+-buffers into the calyx presynaptic terminal, blocks almost all spontaneous release [9,17]. These results suggested that the majority of spontaneous release is Ca2+-dependent, but raised the question what Ca2+-sensor mediates this effect. Interestingly, knockin mutations in Syt1 that change its apparent Ca2+-affinity caused corresponding effects on the frequency of spontaneous release. Specifically, when the apparent Ca2+-affinity of Syt1 is decreased ~2-fold by the R233Q mutation, the frequency of spontaneous release is decreased ~2-fold, whereas an increase in Ca2+-dependent SNARE-complex binding by the D232N mutation of Syt1 caused a correspondingly large increase in spontaneous release frequency [17]. These effects suggested that most spontaneous release is induced by Ca2+-binding to Syt1, with the Ca2+ derived from resting Ca2+-levels, Ca2+-influx via stochastically opening Ca2+-channels, or Ca2+-sparks. However, a recent study showed that deletion of another Ca2+-binding protein, Doc2, causes a partial decrease in mini frequency, indicating that this protein may contribute to the Ca2+-regulation of spontaneous release [61].
Strikingly, although point mutations in Syt1 modulate spontaneous release, deletion of Syt1 or Syt2 increase spontaneous release dramatically, despite blocking evoked synchronous release [9,12,44]. The ‘new’ spontaneous release in Syt1-deficient synapses is still Ca2+-dependent, but activated at lower extracellular Ca2+-concentrations with a lower apparent Ca2+-cooperativity than wild-type spontaneous release [17]. Thus, the increased spontaneous release in Syt1 KO synapses (or, for that matter, also in Syt2 KO synapses) exhibits the properties of asynchronous release as determined in the calyx synapse [44]. These results indicate Syt1 and Syt2 might generally inhibit asynchronous release. An alternative explanation is that spontaneous release represents a completely separate cell-biological pathway, as suggested by reports that evoked and spontaneous synaptic release are using different synaptic vesicle pool [62,63]. If so, the Syt1 and Syt2 deletions may not actually disinhibit the asynchronous Ca2+-sensor, but instead activate this separate pathway.
Other potential Ca2+-sensors for exocytosis
Which Ca2+-sensor mediates asynchronous release and other Ca2+-dependent types of exocytosis in which Syt1, Syt2, Syt7 and Syt9 don’t act as Ca2+-sensors? Naturally, prime candidates are the other four Ca2+-binding synaptotagmins that have no known function (Syt3, Syt5, Syt6, and Syt10). However, at least for asynchronous release, this candidacy is doubtful since these synaptotagmins bind Ca2+ via a mechanism akin to that of Syt1, with a likely Ca2+-binding stoichiometry of ~5, whereas asynchronous release exhibits a much lower apparent Ca2+-stoichiometry.
Two major classes of cytosolic Ca2+-binding proteins are known, EF-hand proteins and C2-domain containing proteins. Calmodulin, the most important EF-hand Ca2+-binding protein, appears to enhance neurotransmitter release in both excitatory and inhibitory synapses without directly participating in asynchronous exocytosis [64]. Numerous other EF-hand Ca2+-binding proteins are also expressed in brain, but most function as Ca2+-buffers or as Ca2+-regulated enzymes. Many C2-domain proteins are expressed in brain, most of which contain a single C2-domain and are involved in signal transduction. A smaller subset of C2-domain proteins contains multiple C2-domains, such as synaptotagmins (Fig. 4). Although the function of most of these multiple C2-domain proteins is unknown, the synaptotagmin paradigm suggests that at least some of them are involved in membrane traffic, rendering them candidates for exocytotic Ca2+-sensors.
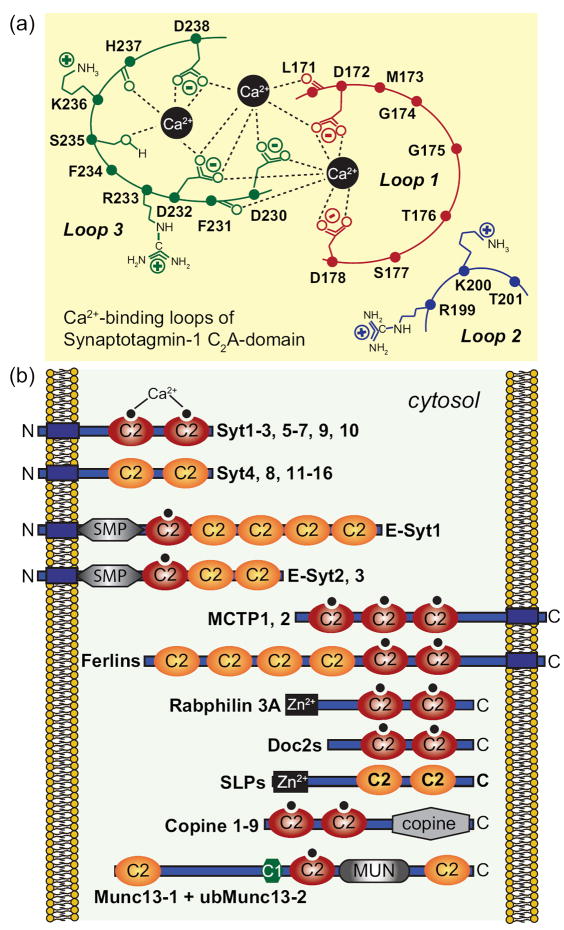
Figure 4. Structures of multiple C2-domain proteins.
A. Schematic diagram of the top Ca2+-binding loops of the Syt1 C2A-domain (modified from [41]). The C2A-domain has three top loops, of which loops 1 and 3 form three Ca2+-binding sites. Five aspartate residues, one serine residue and two backbone carbonyl groups coordinate the three bound Ca2+ ions. Residues are shown in single-letter aminio acid code and are identified by residue numbers corresponding to mouse Syt1.
B. Domain structures of proteins with multiple C2-domains that contain or lack a transmembrane domain. The mammalian genome encodes four classes of multiple C2-domain proteins containing transmembrane regions: synaptotagmins (Syt1-16; note that ‘Syt17’ does not contain a transmembrane region, but is membrane-anchored via palmitoylated cysteine residues), extended synaptotagmins (E-Syt1-3), multiple C2-domain and transmembrane proteins (MCTP1-2) and ferlins (including otoferlin, dysferlin, myoferlin and 2 other uncharacterized ferlin like proteins). Examples of soluble C2-domain containing proteins are also included. C2-domains with canonical Ca2+-binding consensus sequences are labeled as Ca2+-binding C2-domains, although Ca2+-binding has not yet been tested to all of these domains. C2-domains were assigned based on the conserved domain database of the NCBI; some proteins, especially ferlins, may have additional unpredicted C2-domains that do not precisely fit the consensus sequence, as well as alternative transcripts with fewer C2-domains.
Ferlins contain at least six C2-domains and a C-terminal transmembrane region; the two C-terminal C2-domains include canonical Ca2+-binding sequences. Ferlins are required for sperm exocytosis in C. elegans [65]. Of the 5 mammalian ferlins, dysferlin is involved in Ca2+-dependent exocytosis of repair vesicles in muscle [66], and otoferlin is required for Ca2+-dependent exocytosis in hair cells [67], although the mechanisms involved have not been defined.
MCTPs (multiple C2-domain transmembrane proteins) are evolutionarily conserved proteins with 3 C2-domains containing canonical Ca2+-binding sequences, and a C-terminal transmembrane region. The functions of MCTPs are unknown [68].
E-Syts (extended synaptotagmins) are also evolutionarily conserved transmembrane proteins. E-Syts contain an N-terminal transmembrane region like synaptotagmins, followed by a single SMP-domain and either five C2-domains (E-Syt1) or three C2-domains (E-Syt2 and E-Syt3). Only the N-terminal C2-domain contains the requisite Ca2+-binding sequence, and exhibits Ca2+-dependent phospholipid binding [69]. No functional data on E-Syts are available.
Synaptotagmin-like proteins (SLPs) represent a large and heterogeneous class of proteins with two C-terminal C2-domains but without transmembrane regions. An evolutionarily conserved subset of SLPs contains N-terminal Rab-binding sequences and zinc-finger domains (including rabphilin); another subset of SLPs that is not evolutionarily conserved lacks the N-terminal Zinc-finger domain (Doc2s, slp2c, slp3b). In vertebrate synapses, rabphilin is important for re-priming of synaptic vesicles [70]. Doc2 proteins appear to bind SNARE proteins and phospholipids tighter than Syt1, and deletion of Doc2α and Doc2β partly reduces spontaneous release [61]. In chromaffin cells, Doc2β plays a role in Ca2+-dependent priming and exocytosis [71], but the overall function of Doc2 proteins and other SLPs remains unclear.
Copines are soluble double C2-domain proteins of unknown function that bind to phospholipids [72]. Copines are evolutionarily conserved, and contain two N-terminal C2-domains with canonical Ca2+-binding sequences and a unique C-terminal domain.
Conclusions
A universal mechanism by which Ca2+-binding to synaptotagmins triggers exocytosis has emerged over the last decade. This mechanism mediates most Ca2+-triggered exocytosis using a pas-de-deux of synaptotagmins and complexin acting on SNARE complexes and phospholipid membranes. However, new intriguing questions have emerged. Are forms of exocytosis for which no synaptotagmin Ca2+-sensor has been identified, such as asynchronous release, mediated by an atypical synaptotagmin, or by novel Ca2+-sensor, for example one of the other multiple C2-domain proteins (Fig. 4)? How do the Ca2+-sensors for synchronous and asynchronous release intersect in synaptic exocytosis – do they compete, or do synaptotagmins and complexin inhibit the alternative pathway? What is the role of ferlins in exocytosis in Ca2+-triggered exocytosis? Answering these questions will significantly advance the field beyond the synaptotagmin paradigm.
Acknowledgments
We wish to thank Drs. Weiping Han and Jianyuan Sun for valuable discussions. Z.P.P. was supported by NARSAD Young Investigator Award and NIH/NINDS Epilepsy Training Grant 5T32NS007280.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 2.Bean AJ, Zhang X, Hokfelt T. Peptide secretion: what do we know? FASEB J. 1994;8:630–638. doi: 10.1096/fasebj.8.9.8005390. [DOI] [PubMed] [Google Scholar]
- 3.Lindau M, Gomperts BD. Techniques and concepts in exocytosis: focus on mast cells. Biochim Biophys Acta. 1991;1071:429–471. doi: 10.1016/0304-4157(91)90006-i. [DOI] [PubMed] [Google Scholar]
- 4.Randriamampita C, Trautmann A. Ca2+ signals and T lymphocytes; “New mechanisms and functions in Ca2+ signalling”. Biol Cell. 2004;96:69–78. doi: 10.1016/j.biolcel.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Katz B, Miledi R. Ionic requirements of synaptic transmitter release. Nature. 1967;215:651. doi: 10.1038/215651a0. [DOI] [PubMed] [Google Scholar]
- 6.Perin MS, Fried VA, Mignery GA, Jahn R, Südhof TC. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- 7.Borst JG, Helmchen F, Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J Physiol. 1995;489:825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pang ZP, Melicoff E, Padgett D, Liu Y, Teich AF, Dickey BF, Lin W, Adachi R, Südhof TC. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J Neurosci. 2006;26:13493–13504. doi: 10.1523/JNEUROSCI.3519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pang ZP, Sun J, Rizo J, Maximov A, Südhof TC. Genetic analysis of synaptotagmin 2 in spontaneous and Ca2+-triggered neurotransmitter release. EMBO J. 2006;25:2039–2050. doi: 10.1038/sj.emboj.7601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 11.Otsu Y, Shahrezaei V, Li B, Raymond LA, Delaney KR, Murphy TH. Competition between phasic and asynchronous release for recovered synaptic vesicles at developing hippocampal autaptic synapses. J Neurosci. 2004;24:420–433. doi: 10.1523/JNEUROSCI.4452-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maximov A, Südhof TC. Autonomous Function of Synaptotagmin 1 in Triggering Synchronous Release Independent of Asynchronous Release. Neuron. 2005;48:547–554. doi: 10.1016/j.neuron.2005.09.006. [DOI] [PubMed] [Google Scholar]
- ••13.Best AR, Regehr WG. Inhibitory regulation of electrically coupled neurons in the inferior olive is mediated by asynchronous release of GABA. Neuron. 2009;62:555–565. doi: 10.1016/j.neuron.2009.04.018.. This paper shows that the GABAergic input into inferior olive neurons from deep cerebellar nuclei was almost exclusively asynchronous, with little conventional synchronous release. Stimulation of above 10 Hz evoked asynchronous steady-state inhibitory current that depends on residual calcium signaling.
- ••14.Daw MI, Tricoire L, Erdelyi F, Szabo G, McBain CJ. Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread and target-cell independent. J Neurosci. 2009;29:11112–11122. doi: 10.1523/JNEUROSCI.5760-08.2009.. This study examined asynchronous release from cholecystokinin-containing hippocampal interneurons during trains of action potentials. The authors also studied the Ca2+-sensitivity of synchronous and asynchronous release from this synapse, and suggested that these two types of vesicle exocytosis are likely operated by different Ca2+-sensors.
- 15.Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- 16.Katz B, Miledi R. Tetrodotoxin and neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1967;167:8–22. doi: 10.1098/rspb.1967.0010. [DOI] [PubMed] [Google Scholar]
- ••17.Xu J, Pang ZP, Shin OH, Südhof TC. Synaptotagmin-1 functions as a Ca2+ sensor for spontaneous release. Nat Neurosci. 2009;12:759–766. doi: 10.1038/nn.2320.. This paper shows that spontaneous release is almost completely Ca2+-dependent, and is largely mediated by Ca2+-binding to Syt1 Moreover, the paper demonstrates that the increased frequency of spontaneous release in Syt1 knockout mice is due to a disinhibition of a secondary Ca2+-sensor that exhibits the properties of the Ca2+-sensor for asynchronous release. Thus, Syt1 performs dual functions in spontaneous release: it acts as a major Ca2+-sensor for mini release and also clamps a second sensor operates with a lower Ca2+-cooperativity.
- ••18.Llano I, González J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2009;3:1256–1265. doi: 10.1038/81781.. This paper reveals that intracellular presynaptic Ca2+-stores can drive spontaneous neurotransmitter release by generating spontaneous Ca2+-transients.
- •19.Harvey CD, Collman F, Dombeck DA, Tank DW. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature. 2009;461:941–946. doi: 10.1038/nature08499.. This study uses an interesting experimental paradigm in mice, namely virtual navigation, in conjunction with electrophysiology to describe how burst activity in hippocampal place cells during navigation encodes spatial information. The burst frequency of place cells when animal is in field is around 8 Hz, suggesting that asynchronous release probably is important at neural circuit level.
- 20.Stacey WC, Durand DM. Stochastic resonance improves signal detection in hippocampal CA1 neurons. J Neurophysiol. 2000;83:1394–1402. doi: 10.1152/jn.2000.83.3.1394. [DOI] [PubMed] [Google Scholar]
- 21.Yu CR, Power J, Barnea G, O’Donnell S, Brown HE, Osborne J, Axel R, Gogos JA. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–566. doi: 10.1016/s0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
- 22.Harata N, Pyle JL, Aravanis AM, Mozhayeva M, Kavalali ET, Tsien RW. Limited numbers of recycling vesicles in small CNS nerve terminals: implications for neural signaling and vesicular cycling. Trends Neurosci. 2001;24:637–643. doi: 10.1016/s0166-2236(00)02030-0. [DOI] [PubMed] [Google Scholar]
- 23.Rizzoli SO, Betz WJ. The structural organization of the readily releasable pool of synaptic vesicles. Science. 2004;303:2037–2039. doi: 10.1126/science.1094682. [DOI] [PubMed] [Google Scholar]
- 24.Hubbard JI, Jones SF, Landau EM. An examination of the effects of osmotic pressure changes upon transmitter release from mammalian motor nerve terminals. J Physiol. 1968;197:639–657. doi: 10.1113/jphysiol.1968.sp008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 26.Xu-Friedman MA, Harris KM, Regehr WG. Three-dimensional comparison of ultrastructural characteristics at depressing and facilitating synapses onto cerebellar Purkinje cells. J Neurosci. 2001;21:6666–6672. doi: 10.1523/JNEUROSCI.21-17-06666.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••27.Gerber SH, Rah JC, Min SW, Liu X, de Wit H, Dulubova I, Meyer AC, Rizo J, Arancillo M, Hammer RE, et al. Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science. 2008;321:1507–1510. doi: 10.1126/science.1163174.. In this paper, mice in which syntaxin-1 is constitutively ‘open’ and thus activated are used to examine the relationship of SNARE-complex assembly to Ca2+-triggered vesicle exocytosis. The results indentifies a function for the closed confirmation of syntaxin-1 in controlling fusion of vesicles in the RRP via interaction with SM proteins, and demonstrates that vesicle docking is not equivalent to formation of the RRP.
- 28.Voets T, Neher E, Moser T. Mechanisms underlying phasic and sustained secretion in chromaffin cells from mouse adrenal slices. Neuron. 1999;23:607–615. doi: 10.1016/s0896-6273(00)80812-0. [DOI] [PubMed] [Google Scholar]
- 29.Braun M, Ramracheya R, Johnson PR, Rorsman P. Exocytotic properties of human pancreatic beta-cells. Ann N Y Acad Sci. 2009;1152:187–193. doi: 10.1111/j.1749-6632.2008.03992.x. [DOI] [PubMed] [Google Scholar]
- 30.Lefkowitz JJ, Fogarty KE, Lifshitz LM, Bellve KD, Tuft RA, ZhuGe R, Walsh JV, Jr, De Crescenzo V. Suppression of Ca2+ syntillas increases spontaneous exocytosis in mouse adrenal chromaffin cells. J Gen Physiol. 2009;134:267–280. doi: 10.1085/jgp.200910285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, Ullrich B, Zhang ZZ, Anderson RGW, Brose N, Südhof TC. Ca2+-dependent and Ca2+-independent activities of neural and nonneural synaptotagmins. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 32.Sugita S, Shin O-H, Han W, Lao Y, Südhof TC. Synaptotagmins form hierarchy of exocytotic Ca2+-sensors with distinct Ca2+-affinities. EMBO J. 2002;21:270–280. doi: 10.1093/emboj/21.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhalla A, Tucker WC, Chapman ER. Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentration to SNARE-mediated membrane fusion. Mol Biol Cell. 2005;16:4755–4764. doi: 10.1091/mbc.E05-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davletov BA, Südhof TC. A single C2-domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid-binding. J Biol Chem. 1993;268:26386–26390. [PubMed] [Google Scholar]
- ••36.Giraudo CG, Garcia-Diaz A, Eng WS, Chen Y, Hendrickson WA, Melia TJ, Rothman JE. Alternative zippering as an on-off switch for SNARE-mediated fusion. Science. 2009;323:512–516. doi: 10.1126/science.1166500.. This article reports a simple mechanistic model that accounts for the clamping function of complexin, a model whereby the accessory α-helix of complexin inserts into the assembling, partially zippered SNARE complex to prevent its full assembly, and is displaced from this position by Syt1.
- ••37.Maximov A, Tang J, Yang X, Pang ZP, Südhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505.. Use genetics, RNAi knockdowns and electrophysiology, this study demonstrates that complexin functions in synaptic vesicle exocytosis by binding to assembling SNARE complexes and not individual SNARE proteins, and that this binding mediates both the clamping and activation functions of complex via distinct domains.
- 38.Tang J, Maximov A, Shin O-H, Dai H, Rizo J, Südhof TC. A Complexin/Synaptotagmin-1 Switch Controls Fast Synaptic Vesicle Exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 39.Mackler JM, Drummond JA, Loewen CA, Robinson IM, Reist NE. The C(2)B Ca(2+)-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature. 2002;418:340–344. doi: 10.1038/nature00846. [DOI] [PubMed] [Google Scholar]
- •40.Shin OH, Xu J, Rizo J, Südhof TC. Differential but convergent functions of Ca2+ binding to synaptotagmin-1 C2 domains mediate neurotransmitter release. Proc Natl Acad Sci U S A. 2009;106:16469–16474. doi: 10.1073/pnas.0908798106.. This study demonstrates that blocking Ca2+-binding to the C2A-domain of Syt1 changes the apparent Ca2+-cooperativity of exocytosis, thereby providing formal proof that Ca2+-binding to both C2-domains of Syt1 contributes to triggering exocytosis.
- 41.Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Südhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 42.Pang ZP, Shin O-H, Meyer AC, Rosenmund C, Südhof TC. A gain-of-function mutation in synaptotagmin-1 reveals a critical role of Ca2+-dependent SNARE-complex binding in synaptic exocytosis. J Neurosci. 2006;26:12556–12565. doi: 10.1523/JNEUROSCI.3804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •43.Xu J, Mashimo T, Südhof TC. Synaptotagmin-1, -2, and -9: Ca(2+) sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–581. doi: 10.1016/j.neuron.2007.05.004.. This paper demonstrated that among all synaptotagmins, only Syt1, Syt2, and Syt9 can functions as Ca2+-sensor for synaptic exocytosis in Syt1 knockout neurons, and additionally demosntrates that the three different synaptotagmin isoforms confer distinct properties onto Ca2+-triggered exocytosis.
- ••44.Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Südhof TC. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308.. This paper describes the biophysical properties of asynchronous exocytosis using photolysis of caged Ca2+ and Ca2+-imaging in the calyx of Held synapse, and establishes the most precise model of Ca2+-triggered exocytosis up-to-date.
- 45.Voets T, Moser T, Lund PE, Chow RH, Geppert M, Südhof TC, Neher E. Intracellular calcium dependence of large dense-core vesicle exocytosis in the absence of synaptotagmin I. Proc Natl Acad Sci U S A. 2001;98:11680–11685. doi: 10.1073/pnas.201398798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorensen JB, Fernandez-Chacon R, Südhof TC, Neher E. Examining synaptotagmin 1 function in dense core vesicle exocytosis under direct control of Ca2+ J Gen Physiol. 2003;122:265–276. doi: 10.1085/jgp.200308855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Wang P, Xu J, Gorelick F, Yamazaki H, Andrews N, Desir GV. Regulation of insulin secretion and GLUT4 trafficking by the calcium sensor synaptotagmin VII. Biochem Biophys Res Commun. 2007;362:658–664. doi: 10.1016/j.bbrc.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •48.Schonn JS, Maximov A, Lao Y, Südhof TC, Sorensen JB. Synaptotagmin-1 and -7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells. Proc Natl Acad Sci U S A. 2008;105:3998–4003. doi: 10.1073/pnas.0712373105.. The data presented in this paper indicate that Syt1 and Syt7 together mediate nearly all Ca2+-triggered exocytosis in chromaffin cells, but execute different phases of vesicle exocytosis.
- •49.Gustavsson N, Lao Y, Maximov A, Chuang JC, Kostromina E, Repa JJ, Li C, Radda GK, Südhof TC, Han W. Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc Natl Acad Sci U S A. 2008;105:3992–3997. doi: 10.1073/pnas.0711700105.. Using mouse genetics, the authors show that Syt7 functions as a Ca2+-sensor in insulin secreting β-cells, but also demonstrate that another Ca2+-sensor must exist to account for all of the Ca2+-triggered exocytosis observed.
- •50.Gustavsson N, Wei SH, Hoang DN, Lao Y, Zhang Q, Radda GK, Rorsman P, Südhof TC, Han W. Synaptotagmin-7 is a principal Ca2+ sensor for Ca2+ -induced glucagon exocytosis in pancreas. J Physiol. 2009;587:1169–1178. doi: 10.1113/jphysiol.2008.168005.. This study demonstrates that Syt7 functions as the major Ca2+-sensor for glucagon secretion in pancreatic α-cells.
- 51.Nagy G, Kim JH, Pang ZP, Matti U, Rettig J, Südhof TC, Sorensen JB. Different effects on fast exocytosis induced by synaptotagmin 1 and 2 isoforms and abundance but not by phosphorylation. J Neurosci. 2006;26:632–643. doi: 10.1523/JNEUROSCI.2589-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maximov A, Lao Y, Li H, Chen X, Rizo J, Sorensen JB, Südhof TC. Genetic analysis of synaptotagmin-7 function in synaptic vesicle exocytosis. Proc Natl Acad Sci U S A. 2008;105:3986–3991. doi: 10.1073/pnas.0712372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •53.de Wit H, Walter AM, Milosevic I, Gulyas-Kovacs A, Riedel D, Sorensen JB, Verhage M. Synaptotagmin-1 docks secretory vesicles to syntaxin-1/SNAP-25 acceptor complexes. Cell. 2009;138:935–946. doi: 10.1016/j.cell.2009.07.027.. The authors suggest that different from synaspes, adrenal chromaffin cells use a complex of Syt1 with SNARE proteins and Munc18-1 for vesicle docking.
- •54.Melicoff E, Sansores-Garcia L, Gomez A, Moreira DC, Datta P, Thakur P, Petrova Y, Siddiqi T, Murthy JN, Dickey BF, et al. Synaptotagmin-2 controls regulated exocytosis but not other secretory responses of mast cells. J Biol Chem. 2009;284:19445–19451. doi: 10.1074/jbc.M109.002550.. This paper provides the best evidence available for the function of synaptotagmins as Ca2+-sensors for exocytosis in non-neuronal and non-endocrine cells, by demonstrating that Syt2 mediates Ca2+-induced mast cell exocytosis.
- 55.Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW. Synaptotagmin VII regulates Ca(2+)-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol. 2000;148:1141–1149. doi: 10.1083/jcb.148.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jaiswal JK, Chakrabarti S, Andrews NW, Simon SM. Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol. 2004;2:E233. doi: 10.1371/journal.pbio.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bollmann JH, Sakmann B, Borst JG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- 58.Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- 59.Meinrenken CJ, Borst JG, Sakmann B. Local routes revisited: the space and time dependence of the Ca2+ signal for phasic transmitter release at the rat calyx of Held. J Physiol. 2003;547:665–689. doi: 10.1113/jphysiol.2002.032714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franks KM, Stevens CF, Sejnowski TJ. Independent sources of quantal variability at single glutamatergic synapses. J Neurosci. 2003;23:3186–3195. doi: 10.1523/JNEUROSCI.23-08-03186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •61.Groffen AJ, Martens S, Arazola RD, Cornelisse LN, Lozovaya N, de Jong AP, Goriounova NA, Habets RL, Takai Y, Borst JG, et al. Doc2b Is a High-Affinity Ca2+ Sensor for Spontaneous Neurotransmitter Release. Science. 2010;327:1614–1618. doi: 10.1126/science.1183765.. This paper reports that Doc2B, a cytosolic double C2 domain containing protein with higher Ca2+-affinity than Syt1, associates with phospholipids in the presence of Ca2+, binds to SNARE complexes, and promotes membrane fusion. In vivo data suggest that Doc2B partly contributes to spontaneous synaptic vesicle exocytosis.
- 62.Sara Y, Virmani T, Deak F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45:563–573. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- ••63.Fredj NB, Burrone J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci. 2009;12:751–758. doi: 10.1038/nn.2317.. This study used a novel technique to demonstrate that synaptic vesicles responsible for spontaneous release are distinct from synaptic vesicles that are released in response to action potentials.
- 64.Pang ZP, Cao P, Xu W, Südhof TC. Calmodulin Controls Synaptic Strength via Presynaptic Activation of CaM Kinase II. J Neurosci. 2010;30:4132–442. doi: 10.1523/JNEUROSCI.3129-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Washington NL, Ward S. FER-1 regulates Ca2+ -mediated membrane fusion during C. elegans spermatogenesis. J Cell Sci. 2006;119:2552–2562. doi: 10.1242/jcs.02980. [DOI] [PubMed] [Google Scholar]
- 66.Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 67.Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, et al. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 68.Shin OH, Han W, Wang Y, Südhof TC. Evolutionarily conserved multiple C2 domain proteins with two transmembrane regions (MCTPs) and unusual Ca2+ binding properties. J Biol Chem. 2005;280:1641–1651. doi: 10.1074/jbc.M407305200. [DOI] [PubMed] [Google Scholar]
- 69.Min SW, Chang WP, Südhof TC. E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proc Natl Acad Sci U S A. 2007;104:3823–3828. doi: 10.1073/pnas.0611725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deak F, Shin OH, Tang J, Hanson P, Ubach J, Jahn R, Rizo J, Kavalali ET, Südhof TC. Rabphilin regulates SNARE-dependent re-priming of synaptic vesicles for fusion. EMBO J. 2006;25:2856–2866. doi: 10.1038/sj.emboj.7601165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••71.Friedrich R, Groffen AJ, Connell E, van Weering JR, Gutman O, Henis YI, Davletov B, Ashery U. DOC2B acts as a calcium switch and enhances vesicle fusion. J Neurosci. 2008;28:6794–6806. doi: 10.1523/JNEUROSCI.0538-08.2008.. This paper utilizes chromaffin cells as a model system to study the function of Doc2B, showing that Doc2B functions as a priming factor and controls the fusogenicity of LDCVs.
- 72.Tomsig JL, Creutz CE. Copines: a ubiquitous family of Ca(2+)-dependent phospholipid-binding proteins. Cell Mol Life Sci. 2002;59:1467–1477. doi: 10.1007/s00018-002-8522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoshihara M, Adolfsen B, Galle KT, Littleton JT. Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science. 2005;310:858–863. doi: 10.1126/science.1117541. [DOI] [PubMed] [Google Scholar]
- 74.Südhof TC. Synaptotagmins: why so many? J Biol Chem. 2002;277:7629–7632. doi: 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- 75.Maximov A, Shin OH, Liu X, Sudhof TC. Synaptotagmin-12, a synaptic vesicle phosphoprotein that modulates spontaneous neurotransmitter release. J Cell Biol. 2007;176:113–124. doi: 10.1083/jcb.200607021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gustavsson N, Han W. Calcium-sensing beyond neurotransmitters: functions of synaptotagmins in neuroendocrine and endocrine secretion. Biosci Rep. 2009;29:245–259. doi: 10.1042/BSR20090031. [DOI] [PubMed] [Google Scholar]