Abstract
Approximately 500 million people worldwide are chronically infected with the hepatitis B virus (HBV) or hepatitis C virus (HCV), and are therefore at an increased risk for developing fatal liver diseases such as cirrhosis and hepatocellular carcinoma. The intracellular antiviral responses induced by interferon (IFN)-α/-β and/or IFN-γ play critical roles in the pathogenesis of HBV and HCV infection, and the function of IFN-λ in the host immune response to these viruses is beginning to be revealed. A better understanding of how IFN-λ influences HBV or HCV persistence is not only important for understanding the mechanisms of chronic virus infection, but also may lead to new approaches for improved antiviral therapies.
Introduction
Hepatitis B virus (HBV) and hepatitis C virus (HCV) remain important global public health problems, as the current therapies for these infections are limited by high cost, ineffectiveness in some patients, significant side effects, and viral resistance. Both HBV and HCV are sensitive to the antiviral activity of interferon (IFN)-λ in cell culture models of virus replication (Robek and others 2005; Zhu and others 2005; Doyle and others 2006; Marcello and others 2006; Hong and others 2007), but the interaction of these viruses with the IFN-λ response in a natural infection or therapeutic setting is less well understood. The IFN-λ response in vivo is likely complex as it may be influenced by factors not reflected in cell culture, such as host genetic variation, tissue-specific receptor expression, and expression of other pro- or anti-inflammatory cytokines in the liver. This review summarizes our current knowledge regarding the role of IFN-λ in the immune response to HBV and HCV. As a separate review in this issue is focused on the therapeutic use of pegylated (PEG)-IFN-λ for chronic HCV infection, this aspect is only addressed briefly here.
Interaction of HBV and HCV with the IFN-λ Response
Induction of IFN-λ by HBV and HCV
Despite the fact that HBV and HCV are both hepatotropic viruses that can establish chronic infections that persist for the lifetime of the host, there are substantial differences in the way in which these 2 viruses replicate their genomes and interact with the innate immune response (Wieland and Chisari 2005). HCV is a negative-strand RNA virus that induces expression of IFN-α/-β-stimulated genes in the liver after infection (Su and others 2002). However, activation of the IFN-α/-β response by HCV appears to be attenuated by the fact that the virus has evolved multiple mechanisms to block the induction of this pathway. The HCV NS3/4A protease inhibits IFN-β expression by blocking IRF-3 activation and cleaving the RIG-I and toll-like receptor signaling adapters IPS-1 and TRIF (Foy and others 2003, 2005; Li and others 2005). Another HCV nonstructural protein, NS2, inhibits activation of the IFN-β promoter through a different mechanism than that of NS3/4A (Kaukinen and others 2006). A third viral protein, NS5A, has also been implicated as an additional inhibitor of IFN-α/-β expression (Zhang and others 2005). Because IFN-α/-β and IFN-λ are both activated by similar stimuli (Coccia and others 2004) through a common molecular mechanism (Onoguchi and others 2007), it is likely that the viral immunomodulatory mechanisms that inhibit IFN-α/-β expression also block IFN-λ production. In fact, NS3/4A was shown to prevent the induction of both IFN-α/-β and IFN-λ when overexpressed in cell culture (Kaukinen and others 2006), and like IFN-α/-β, IFN-λ is expressed in peripheral blood mononuclear cells (PBMC), but not in the liver, of patients chronically infected with HCV (Mihm and others 2004).
In contrast to HCV, HBV does not induce a substantial IFN-α/-β response in the liver (Wieland and others 2004). However, HBV replication is sensitive to IFN-α/-β, as well as to the antiviral activity of IFN-γ produced by activated NK, NKT, and T cells in response to infection (Guidotti and others 1999). Like HCV, HBV may also employ methods of actively inhibiting the IFN-α/-β response, such as blocking STAT activation or interfering with MxA function (Foster and others 1991; Rosmorduc and others 1999). However, for HBV, other indirect mechanisms are also important for evasion of the IFN-α/-β response (Wieland and Chisari 2005). Unlike HCV, HBV genome synthesis occurs subsequent to viral capsid formation in the cytoplasm, thus shielding potential pathogen-associated molecular patterns in the viral DNA replication intermediates from recognition by cellular receptors. As with HCV, it is likely that the mechanisms employed by HBV to inhibit the IFN-α/-β response also allow the virus to evade the antiviral activity of IFN-λ, although this has not been examined in infected humans.
Mechanisms of antiviral activity
Although the inhibitory effects of IFN-λ have been observed against a wide variety of viruses, the molecular mechanisms behind these activities have not been fully described. Since IFN-α and IFN-λ induce nearly identical patterns of gene expression, the mechanism of viral inhibition is likely the same between the type I and III IFNs for most, if not all, viruses. We recently elucidated the molecular mechanism of IFN-λ-induced HBV inhibition, which we found to be common between IFN-λ, IFN-α/-β, and IFN-γ (Pagliaccetti and others 2010). All 3 IFN types inhibit HBV replication by preventing the assembly of viral RNA-containing capsids in the cytoplasm (Wieland and others 2005). Thus, although the exact IFN-stimulated gene (ISG) responsible for the inhibitory effect has yet to be identified, it is a factor subject to induction by all IFN types, including IFN-λ.
Recent studies have identified subtle differences in the kinetics of Jak-STAT activation and ISG expression between IFN-λ and IFN-α. Marcello and others (2006) found differences in STAT phosphorylation and in the gene expression profiles between IFN-α and IFN-λ over the course of 24 h in Huh-7 cells, and Maher and others (2008) found a more prolonged response when HaCaT cells were treated with IFN-λ compared with IFN-α/-β. Although the antiviral responses induced by IFN-λ are generally weaker than those activated by IFN-α, the evidence for a prolonged response may have implications for the therapeutic use of IFN-λ. A lower but sustained antiviral activity in hepatocytes could be preferable, considering that IFN-based therapies are administered over the course of many months.
Cooperative effects of cytokine combinations
Although each virus has evolved different strategies to avoid activating the IFN-α/-β response, both HCV and HBV are sensitive to the antiviral activity of IFN-α/-β, IFN-γ, and IFN-λ. This fact has prompted the investigation of the effect that combinations of these cytokines have on virus replication. These studies are important because multiple cytokines may be simultaneously expressed in the host response to the virus, and because of the potential therapeutic use of cytokine combinations. In the case of HCV, combinations of type I IFNs generally induce additive increases in virus inhibition compared with the individual cytokines alone, whereas combinations of IFN-α/-β and IFN-γ tend to have synergistic effects on HCV (Larkin and others 2003; Okuse and others 2005). These patterns of cooperative activity are not entirely unexpected given that all type I IFNs bind to the same receptor and, therefore, would not be expected to elicit greater than a dose-dependent additive effect in combination. In contrast, IFN-γ binds a separate receptor that activates a distinct signal transduction pathway and different pattern of gene expression that can synergize with the IFN-α/-β response.
However, the effect of combining IFN-α/-β with IFN-λ is harder to predict, because while the cytokines use different receptors, they activate nearly identical signal transduction cascades. Our group and others have shown that combinations of IFN-α/-β and IFN-λ increase the antiviral response to HCV in a manner that does not appear to be greater than an additive effect (Marcello and others 2006; Pagliaccetti and others 2008). We have also observed a synergistic increase in HCV inhibition when IFN-λ is combined with IFN-γ that correlated with enhanced IFN-stimulated gene expression (Pagliaccetti and others 2008), similar to the synergistic activities previously demonstrated for IFN-α and IFN-γ. However, these cooperative effects are dependent on the virus in question and therefore the specific IFN-induced proteins necessary to inhibit replication, as these general patterns also extend to vesicular stomatitis virus (VSV), yet no greater than additive effects on HBV replication were found when cells were treated with IFN-λ in combination with either IFN-α or IFN-γ (Pagliaccetti and others 2010).
IFN-λ Receptor Expression and In Vivo Activity
Compared with the IFN-α/-β receptor, which is ubiquitously expressed, the IFN-λ receptor displays a more restricted distribution. In cell culture, relative sensitivity to IFN-λ and IFN-α/-β correlates strongly with receptor expression (Meager and others 2005). With respect to hepatocytes, the human liver cancer cell lines HepG2 and Huh-7 both respond to IFN-λ and induce an antiviral response to viruses such as encephalomyocarditis virus (EMCV) and HCV (Sheppard and others 2003; Marcello and others 2006). In addition, HBV replication in c-met immortalized murine hepatocytes is highly sensitive to the antiviral activity of IFN-λ (Robek and others 2005).
Compared with studies on the antiviral activity of IFN-λ in cell culture, the number of reports on IFN-λ activity in vivo is relatively limited. IFN-λ antiviral activity in mice appears to be highly dependent on the tropism of the virus and the administration route of IFN-λ. Ank and others (2006) reported that administration of IFN-λ2 via intraperitoneal injection reduced HSV-2 infection in the liver, whereas intravenous injection of IFN-λ2 did not provide protection from infection by EMCV or lymphocytic choriomeningitis virus in the heart or spleen, respectively. In contrast, Mordstein and others (2008) observed that intraperitoneal injection of IFN-λ3 did not protect mice from infection by the hepatotropic virus Thogotovirus. Despite the lack of activity in the liver, protection from influenza A virus infection in the lungs was conferred by intranasal administration of IFN-λ3 (Mordstein and others 2008). Similarly, Sommereyns and others (2008) found low responses to IFN-λ3 in the mouse liver that correlated with low intrahepatic expression of the IFN-λ receptor. Consistent with a relatively low activity of IFN-λ in the mouse liver, in contrast to immortalized mouse hepatocytes (in which HBV replication is highly sensitive to IFN-λ), we have only found modest antiviral activity of IFN-λ when injected intravenously into HBV transgenic mice (Pagliaccetti and others 2010). These results highlight potential differences in IFN-λ activity in specific physiological contexts.
IFN-λ sensitivity and receptor expression in primary human brain and liver cells
A more complete characterization of the tissues in which the IFN-λ response is active is important for understanding the activity of this cytokine family in natural infections and for its therapeutic potential. We therefore compared the activity of IFN-λ in primary human liver and brain cells. Previous studies have shown that when the IFN-λ response is not robustly activated in the mouse brain (Sommereyns and others 2008), human neurons respond to the cytokine (Zhou and others 2009). A better understanding of the IFN-λ response in the brain and liver is important for 2 reasons. First, IFN-mediated noncytopathic mechanisms of controlling virus infection in the brain are critical for preventing the destruction of nondividing neurons (Griffin 2003), and the contribution of IFN-λ to this response is not characterized. Second, PEG-IFN-α therapy for chronic HCV infection can cause neuropsychiatric disorders, including depression and suicidal behavior (Raison and others 2005; Asnis and De La Garza 2006), and direct action of the cytokine on cells of the central nervous system may play a role in these effects, which may also limit the therapeutic use of PEG-IFN-λ.
We first examined the sensitivity of multiple brain cell types to IFN-λ, including primary neurons, astrocytes, choroid plexus (CP) epithelial and endothelial cells (ScienCell, Carlsbad, CA), and human brain cell cultures derived from epilepsy surgery (provided by A. van den Pol, Yale University). We found that as measured by activation of the ISGs MxA and IFI27, all brain cell types responded to IFN-λ1 (Fig. 1A). However, when compared with IFN-α, the level of ISG expression was much lower (typically 10- to 100-fold), especially in light of the fact that a higher concentration of IFN-λ was used to stimulate the cells (250 ng/mL versus 2 ng/mL of IFN-α). Further, while the antiviral response generated by 2 ng/mL of IFN-α completely protected astrocytes, neurons, and CP epithelial and endothelial cells from VSV infection, treatment with up to 250 ng/mL of IFN-λ1 did not block VSV-induced cell death (data not shown). In contrast to the brain cell types, similar levels of ISG expression were induced when primary hepatocytes were stimulated with either IFN-α or IFN-λ (Fig. 1A). Therefore, compared with IFN-α, IFN-λ may only play a minor role in protecting the brain from virus infection.
FIG. 1.
Interferon (IFN)-λ sensitivity and production in primary brain cells. (A) Induction of the representative IFN-stimulated genes MxA and IFI27 24 h after stimulation of primary astrocytes (Astro), choroids plexus (CP) endothelial (Endo) or epithelial (Epi) cells, neurons, or mixed brain cells with 2 ng (500 U)/mL IFN-α2a or 250 ng/mL IFN-λ1. Also shown is expression of MxA and IFI27 in primary hepatocytes (Hepat) under identical stimulation conditions. Gene expression is displayed as fold induction relative to untreated cells and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) measured by quantitative reverse-transcription PCR (RT-qPCR), and error bars indicate relative quantification maximum and minimum values. (B) Expression of IFN-λ1 or IFN-λ2/3 mRNA 6 h after infection with vesicular stomatitis virus at the indicated MOI. Gene expression is displayed as fold induction relative to uninfected cells normalized to GAPDH, as measured by RT-qPCR.
In addition to quantifying IFN-λ-mediated ISG expression, we also measured the ability of brain cells to produce IFN-λ after virus infection. Primary astrocytes, neurons, CP endothelial or epithelial cells, and brain cultures were infected with VSV, and expression of IFN-λ1 and IFN-λ2/3 was measured by quantitative reverse-transcription PCR (RT-qPCR) 6 h postinfection. Both IFN-λ1 and IFN-λ2/3 mRNA expression was highly induced in a multiplicity of infection (MOI)-dependent manner in both endothelial and epithelial cells from the CP, but not in astrocytes or neurons (Fig. 1B). A dose-dependent increase in IFN-λ expression was also found in the brain cell cultures (Fig. 1B), possibly due to the presence of microglia, a brain-resident antigen-presenting cell. Therefore, in contrast to the similar low-level response to IFN-λ found in all tested cell types, we found a marked difference in the ability of the cells to produce IFN-λ after VSV infection.
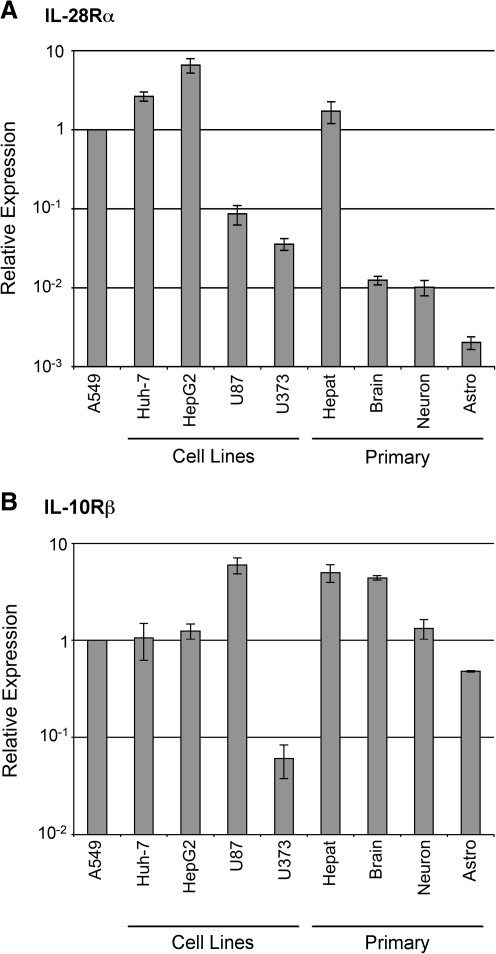
Like with the primary human brain cell types, we also found that the human glioblastoma cell lines U87 and U373 do not express high levels of ISGs after IFN-λ stimulation, in contrast to the hepatocellular carcinoma cell lines Huh-7 and HepG2, which, like primary human hepatocytes, respond relatively well to the cytokine (data not shown). We therefore compared the expression level of the IFN-λ receptor subunits IL-28Rα and IL-10Rβ in primary human cells and in hepatocyte and glia-derived cancer cell lines by RT-qPCR. Compared with the lung epithelial cell line A549, which responds well to IFN-λ (Kotenko and others 2003), the primary hepatocytes and hepatocellular carcinoma-derived cell lines expressed similar levels of IL-28Rα mRNA (Fig. 2A). In contrast, the glioblastoma cell lines and primary brain cells expressed levels of IL-28Rα mRNA that were between 20- and 1,000-fold less than the levels found in hepatocytes. Unlike IL-28Rα, IL-10Rβ expression was similar in all cell types tested with the exception of U373 cells, which expressed lower levels of this subunit (Fig. 2B). Therefore, the relatively low sensitivity of primary brain cell types to IFN-λ compared with hepatocytes correlates with expression of the IL-28Rα receptor subunit. These results may indicate that PEG-IFN-λ may elicit fewer of the neurological side effects that are associated with PEG-IFN-α therapy while still blocking virus replication in the liver.
FIG. 2.
IL-28Rα and IL-10Rβ expression in liver and brain cells. Expression of (A) IL-28Rα and (B) IL-10Rβ mRNA in the hepatocellular carcinoma cell lines Huh-7 and HepG2, the glioblastoma cell lines U87 and U373, and primary hepatocytes (Hepat), mixed brain cells, neurons, and astrocytes (Astro). Expression levels were measured by RT-qPCR, and are displayed as relative to the lung epithelial cell line A549. Data are displayed as the mean of 3 replicate measurements, and error bars indicate standard error of the mean.
IFN-λ Genetic Variation and HCV Pathogenesis
A number of recent genome-wide association studies have identified a genetic link between IFN-λ and multiple aspects of HCV infection and treatment. Single-nucleotide polymorphisms (SNPs) in the IL28B gene locus, which encodes IFN-λ3, are associated with both the spontaneous clearance of HCV and a sustained viral response after therapy (Ge and others 2009; Rauch and others 2010; Suppiah and others 2009; Tanaka and others 2009; Thomas and others 2009). Further, these polymorphisms correlate with differences in response to treatment among racial groups. The polymorphisms associated with poorer responses were found to be higher in African populations than in European populations, which correlate with the lower response rates of African Americans to PEG-IFN-α plus ribavirin treatment (Conjeevaram and others 2006).
Although the connection between IL28B variation and HCV infection outcome/therapy response is clear, the molecular mechanisms underlying this association are not. As postulated by the authors of these studies, there are a number of possibilities. First, 1 SNP located 3 kb upstream of the IL-28B gene is in linkage disequilibrium with a second variation within the IFN-λ3 coding region that may alter the activity of the protein (Ge and others 2009; Thomas and others 2009). Second, IFN-λ3 expression levels were found to be altered in patients harboring a second IL28B SNP, indicating the possibility that this variant occurs within a transcriptional regulatory region (Suppiah and others 2009; Tanaka and others 2009). Finally, because IFN-λ expression is regulated by IFN-α (Siren and others 2005; Ank and others 2006), it may play a role in amplifying the antiviral response activated in PEG-IFN-α therapy. Elucidating these mechanisms will be critical for understanding the role of IFN-λ in chronic HCV infection and therapy.
Perspectives for Future Directions
Despite the fact that much has been learned about the biological activity of IFN-λ, there is much that we still do not understand. Although tissue-specific expression of the receptor is increasingly becoming clear, the basis for this differential expression is mostly unknown and a number of questions remain. It will be important to understand the molecular basis for the transcriptional regulation of the IL-28Rα promoter, and to determine if receptor expression is influenced by tissue-specific transcription factors, the differentiation state of the cell, or by other inflammatory cytokines.
It will also be important to understand the basis for IL28B variation as a factor in therapy outcome for chronic HCV infection. IL28B variation is not the only host factor that has been associated with therapy outcome, and the interplay between these various factors needs to be defined. For example, it is well established that patients who fail to achieve a sustained viral response after PEG-IFN-α therapy tend to have a high baseline level of ISG expression in the liver before initiation of therapy (He and others 2006; Sarasin-Filipowicz and others 2008), and it is currently unclear how this observation is related to IL28B variation, or whether therapy with PEG-IFN-λ will be clinically beneficial in cases where PEG-IFN-α therapy has failed.
Finally, the clinical use of IFN-λ for other viral infections should continue to be explored. We and others have shown that IFN-λ is resistant to the activity of the secreted poxvirus immunomodulatory proteins that neutralize IFN-α/-β, and might therefore limit virus spread in vivo (Huang and others 2007; Bandi and others 2010; Del Mar Fernandez de Marco and others 2010). IFN-α is also an effective therapy for chronic HBV infection, but has the same disadvantages as its use for chronic HCV, namely, significant side effects. Antiviral drugs that are more effective and better tolerated have therefore mostly supplanted IFN-α for HBV therapy. However, viral resistance can be a problem with these drugs, so additional therapies are needed for HBV, and PEG-IFN-λ may be one such option.
Acknowledgments
Support for this work was provided by a grant from the Dana Foundation Neuroimmunology Program. N.E.P. was supported by PHS training grants T32 AI055403 and T32 AI007640, and a fellowship from the Anna Fuller Fund. The authors thank Nyree Maes and Minjung Han for technical assistance, Anthony van den Pol (Dept of Neurosurgery, Yale University) for providing brain cells, and the Yale Liver Center for providing primary hepatocytes.
Author Disclosure Statement
No competing financial interests exist.
References
- Ank N. West H. Bartholdy C. Eriksson K. Thomsen AR. Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80(9):4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnis GM. De La Garza R., 2nd Interferon-induced depression in chronic hepatitis C: a review of its prevalence, risk factors, biology, and treatment approaches. J Clin Gastroenterol. 2006;40(4):322–335. doi: 10.1097/01.mcg.0000210099.36500.fe. [DOI] [PubMed] [Google Scholar]
- Bandi P. Pagliaccetti NE. Robek MD. Inhibition of type III interferon activity by orthopoxvirus immunomodulatory proteins. J Interferon Cytokine Res. 2010;30(3):123–134. doi: 10.1089/jir.2009.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia EM. Severa M. Giacomini E. Monneron D. Remoli ME. Julkunen I. Cella M. Lande R. Uze G. Viral infection and toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol. 2004;34(3):796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- Conjeevaram HS. Fried MW. Jeffers LJ. Terrault NA. Wiley-Lucas TE. Afdhal N. Brown RS. Belle SH. Hoofnagle JH. Kleiner DE. Howell CD. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131(2):470–477. doi: 10.1053/j.gastro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Del Mar Fernandez de Marco M. Alejo A. Hudson P. Damon IK. Alcami A. The highly virulent variola and monkeypox viruses express secreted inhibitors of type I interferon. FASEB J. 2010;24(5):1479–1488. doi: 10.1096/fj.09-144733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SE. Schreckhise H. Khuu-Duong K. Henderson K. Rosler R. Storey H. Yao L. Liu H. Barahmand-pour F. Sivakumar P. Chan C. Birks C. Foster D. Clegg CH. Wietzke-Braun P. Mihm S. Klucher KM. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology. 2006;44(4):896–906. doi: 10.1002/hep.21312. [DOI] [PubMed] [Google Scholar]
- Foster GR. Ackrill AM. Goldin RD. Kerr IM. Thomas HC. Stark GR. Expression of the terminal protein region of hepatitis B virus inhibits cellular responses to interferons alpha and gamma and double-stranded RNA. Proc Natl Acad Sci U S A. 1991;88(7):2888–2892. doi: 10.1073/pnas.88.7.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy E. Li K. Sumpter R., Jr. Loo YM. Johnson CL. Wang C. Fish PM. Yoneyama M. Fujita T. Lemon SM. Gale M., Jr. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc Natl Acad Sci U S A. 2005;102(8):2986–2991. doi: 10.1073/pnas.0408707102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy E. Li K. Wang C. Sumpter R., Jr. Ikeda M. Lemon SM. Gale M., Jr. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science. 2003;300(5622):1145–1148. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- Ge D. Fellay J. Thompson AJ. Simon JS. Shianna KV. Urban TJ. Heinzen EL. Qiu P. Bertelsen AH. Muir AJ. Sulkowski M. McHutchison JG. Goldstein DB. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- Griffin DE. Immune responses to RNA-virus infections of the CNS. Nat Rev Immunol. 2003;3(6):493–502. doi: 10.1038/nri1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti LG. Rochford R. Chung J. Shapiro M. Purcell R. Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284(5415):825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- He XS. Ji X. Hale MB. Cheung R. Ahmed A. Guo Y. Nolan GP. Pfeffer LM. Wright TL. Risch N. Tibshirani R. Greenberg HB. Global transcriptional response to interferon is a determinant of HCV treatment outcome and is modified by race. Hepatology. 2006;44(2):352–359. doi: 10.1002/hep.21267. [DOI] [PubMed] [Google Scholar]
- Hong SH. Cho O. Kim K. Shin HJ. Kotenko SV. Park S. Effect of interferon-lambda on replication of hepatitis B virus in human hepatoma cells. Virus Res. 2007;126(1–2):245–249. doi: 10.1016/j.virusres.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Huang J. Smirnov SV. Lewis-Antes A. Balan M. Li W. Tang S. Silke GV. Putz MM. Smith GL. Kotenko SV. Inhibition of type I and type III interferons by a secreted glycoprotein from Yaba-like disease virus. Proc Natl Acad Sci U S A. 2007;104(23):9822–9827. doi: 10.1073/pnas.0610352104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukinen P. Sillanpaa M. Kotenko S. Lin R. Hiscott J. Melen K. Julkunen I. Hepatitis C virus NS2 and NS3/4A proteins are potent inhibitors of host cell cytokine/chemokine gene expression. Virol J. 2006;3:66. doi: 10.1186/1743-422X-3-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotenko SV. Gallagher G. Baurin VV. Lewis-Antes A. Shen M. Shah NK. Langer JA. Sheikh F. Dickensheets H. Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4(1):69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- Larkin J. Jin L. Farmen M. Venable D. Huang Y. Tan SL. Glass JI. Synergistic antiviral activity of human interferon combinations in the hepatitis C virus replicon system. J Interferon Cytokine Res. 2003;23(5):247–257. doi: 10.1089/107999003321829962. [DOI] [PubMed] [Google Scholar]
- Li K. Foy E. Ferreon JC. Nakamura M. Ferreon AC. Ikeda M. Ray SC. Gale M., Jr. Lemon SM. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci U S A. 2005;102(8):2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher SG. Sheikh F. Scarzello AJ. Romero-Weaver AL. Baker DP. Donnelly RP. Gamero AM. IFNalpha and IFNlambda differ in their antiproliferative effects and duration of JAK/STAT signaling activity. Cancer Biol Ther. 2008;7(7):1109–1115. doi: 10.4161/cbt.7.7.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcello T. Grakoui A. Barba-Spaeth G. Machlin ES. Kotenko SV. MacDonald MR. Rice CM. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131(6):1887–1898. doi: 10.1053/j.gastro.2006.09.052. [DOI] [PubMed] [Google Scholar]
- Meager A. Visvalingam K. Dilger P. Bryan D. Wadhwa M. Biological activity of interleukins-28 and −29: comparison with type I interferons. Cytokine. 2005;31(2):109–118. doi: 10.1016/j.cyto.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Mihm S. Frese M. Meier V. Wietzke-Braun P. Scharf JG. Bartenschlager R. Ramadori G. Interferon type I gene expression in chronic hepatitis C. Lab Invest. 2004;84(9):1148–1159. doi: 10.1038/labinvest.3700135. [DOI] [PubMed] [Google Scholar]
- Mordstein M. Kochs G. Dumoutier L. Renauld JC. Paludan SR. Klucher K. Staeheli P. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 2008;4(9):e1000151. doi: 10.1371/journal.ppat.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuse C. Rinaudo JA. Farrar K. Wells F. Korba BE. Enhancement of antiviral activity against hepatitis C virus in vitro by interferon combination therapy. Antiviral Res. 2005;65(1):23–34. doi: 10.1016/j.antiviral.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Onoguchi K. Yoneyama M. Takemura A. Akira S. Taniguchi T. Namiki H. Fujita T. Viral infections activate types I and III interferon genes through a common mechanism. J Biol Chem. 2007;282(10):7576–7581. doi: 10.1074/jbc.M608618200. [DOI] [PubMed] [Google Scholar]
- Pagliaccetti NE. Chu EN. Bolen CR. Kleinstein SF. Robek MD. Lambda and alpha interferon inhibit hepatitis B virus replication through a common molecular mechanism but with different in vivo activities. Virology. 2010;401(2):197–206. doi: 10.1016/j.virol.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccetti NE. Eduardo R. Kleinstein SH. Mu XJ. Bandi P. Robek MD. Interleukin-29 functions cooperatively with interferon to induce antiviral gene expression and inhibit hepatitis C virus replication. J Biol Chem. 2008;283(44):30079–30089. doi: 10.1074/jbc.M804296200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL. Demetrashvili M. Capuron L. Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19(2):105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A. Kutalik Z. Descombes P. Cai T. di Iulio J. Mueller T. Bochud M. Battegay M. Bernasconi E. Borovicka J. Colombo S. Cerny A. Dufour JF. Furrer H. Gunthard HF. Heim M. Hirschel B. Malinverni R. Moradpour D. Mullhaupt B. Witteck A. Beckmann JS. Berg T. Bergmann S. Negro F. Telenti A. Bochud PY. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure—a genome-wide association study. Gastroenterology. 2010;138(4):1338–1345. doi: 10.1053/j.gastro.2009.12.056. 1345.e1–7. [DOI] [PubMed] [Google Scholar]
- Robek MD. Boyd BS. Chisari FV. Lambda interferon inhibits hepatitis B and C virus replication. J Virol. 2005;79(6):3851–3854. doi: 10.1128/JVI.79.6.3851-3854.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmorduc O. Sirma H. Soussan P. Gordien E. Lebon P. Horisberger M. Brechot C. Kremsdorf D. Inhibition of interferon-inducible MxA protein expression by hepatitis B virus capsid protein. J Gen Virol. 1999;80(Pt 5):1253–1262. doi: 10.1099/0022-1317-80-5-1253. [DOI] [PubMed] [Google Scholar]
- Sarasin-Filipowicz M. Oakeley EJ. Duong FH. Christen V. Terracciano L. Filipowicz W. Heim MH. Interferon signaling and treatment outcome in chronic hepatitis C. Proc Natl Acad Sci U S A. 2008;105(19):7034–7039. doi: 10.1073/pnas.0707882105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard P. Kindsvogel W. Xu W. Henderson K. Schlutsmeyer S. Whitmore TE. Kuestner R. Garrigues U. Birks C. Roraback J. Ostrander C. Dong D. Shin J. Presnell S. Fox B. Haldeman B. Cooper E. Taft D. Gilbert T. Grant FJ. Tackett M. Krivan W. McKnight G. Clegg C. Foster D. Klucher KM. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4(1):63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- Siren J. Pirhonen J. Julkunen I. Matikainen S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J Immunol. 2005;174(4):1932–1937. doi: 10.4049/jimmunol.174.4.1932. [DOI] [PubMed] [Google Scholar]
- Sommereyns C. Paul S. Staeheli P. Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4(3):e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su AI. Pezacki JP. Wodicka L. Brideau AD. Supekova L. Thimme R. Wieland S. Bukh J. Purcell RH. Schultz PG. Chisari FV. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci U S A. 2002;99(24):15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suppiah V. Moldovan M. Ahlenstiel G. Berg T. Weltman M. Abate ML. Bassendine M. Spengler U. Dore GJ. Powell E. Riordan S. Sheridan D. Smedile A. Fragomeli V. Muller T. Bahlo M. Stewart GJ. Booth DR. George J. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41(10):1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- Tanaka Y. Nishida N. Sugiyama M. Kurosaki M. Matsuura K. Sakamoto N. Nakagawa M. Korenaga M. Hino K. Hige S. Ito Y. Mita E. Tanaka E. Mochida S. Murawaki Y. Honda M. Sakai A. Hiasa Y. Nishiguchi S. Koike A. Sakaida I. Imamura M. Ito K. Yano K. Masaki N. Sugauchi F. Izumi N. Tokunaga K. Mizokami M. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41(10):1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- Thomas DL. Thio CL. Martin MP. Qi Y. Ge D. O'Huigin C. Kidd J. Kidd K. Khakoo SI. Alexander G. Goedert JJ. Kirk GD. Donfield SM. Rosen HR. Tobler LH. Busch MP. McHutchison JG. Goldstein DB. Carrington M. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461(7265):798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S. Thimme R. Purcell RH. Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004;101(17):6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland SF. Chisari FV. Stealth and cunning: hepatitis B and hepatitis C viruses. J Virol. 2005;79(15):9369–9380. doi: 10.1128/JVI.79.15.9369-9380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland SF. Eustaquio A. Whitten-Bauer C. Boyd B. Chisari FV. Interferon prevents formation of replication-competent hepatitis B virus RNA-containing nucleocapsids. Proc Natl Acad Sci U S A. 2005;102(28):9913–9917. doi: 10.1073/pnas.0504273102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T. Lin RT. Li Y. Douglas SD. Maxcey C. Ho C. Lai JP. Wang YJ. Wan Q. Ho WZ. Hepatitis C virus inhibits intracellular interferon alpha expression in human hepatic cell lines. Hepatology. 2005;42(4):819–827. doi: 10.1002/hep.20854. [DOI] [PubMed] [Google Scholar]
- Zhou L. Wang X. Wang YJ. Zhou Y. Hu S. Ye L. Hou W. Li H. Ho WZ. Activation of toll-like receptor-3 induces interferon-lambda expression in human neuronal cells. Neuroscience. 2009;159(2):629–637. doi: 10.1016/j.neuroscience.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H. Butera M. Nelson DR. Liu C. Novel type I interferon IL-28A suppresses hepatitis C viral RNA replication. Virol J. 2005;2:80. doi: 10.1186/1743-422X-2-80. [DOI] [PMC free article] [PubMed] [Google Scholar]