Abstract
The glycosaminoglycan (GAG) content of engineered cartilage is a determinant of biochemical and mechanical quality. The ability to measure the degree to which GAG content is maintained or increases in an implant is therefore of importance in cartilage repair procedures. The gadolinium exclusion magnetic resonance imaging (MRI) method for estimating matrix fixed charge density (FCD) is ideally suited to this. One promising approach to cartilage repair is use of seeded injectable hydrogels. Accordingly, we assess the reliability of measuring GAG content in such a system ex vivo using MRI. Samples of the photo-polymerizable hydrogel, poly(ethylene oxide) diacrylate, were seeded with bovine chondrocytes (∼2.4 million cells/sample). The FCD of the constructs was determined using MRI after 9, 16, 29, 36, 43, and 50 days of incubation. Values were correlated with the results of biochemical determination of GAG from the same samples. FCD and GAG were found to be statistically significantly correlated (R2 = 0.91, p <0.01). We conclude that MRI-derived FCD measurements of FCD in injectable hydrogels reflect tissue GAG content and that this methodology therefore has potential for in vivo monitoring of such constructs.
Introduction
An emerging approach to potential treatment of degenerative cartilage disease involves production and implantation of a biological tissue substitute that recapitulates the form and function of healthy native cartilage. A central issue in the successful development of such therapeutics is the ability to monitor and potentially modify implant properties on an ongoing basis. The application of magnetic resonance imaging (MRI) for visualizing cartilage clinically has been well established,1,2 while more recently, approaches have been developed to monitor cartilage matrix components in human subjects3–8 and in engineered cartilage constructs.9–14 These latter techniques may be particularly useful for tailoring therapeutic interventions after implantation into a defect.
While fibrous scaffolds have been used extensively toward the goal of tissue-based therapies for degenerative cartilage disease,15 approaches based on injectable materials offer distinct practical advantages.16 Injectable systems generally consist of cells suspended in a hydrogel that is still in its liquid monomer state, with tissue regeneration taking place after subsequent in vivo placement.
Collagen and proteoglycan (PG) constitute the two major solid components of cartilage. The negative charges of the glycosaminoglycan (GAG) side chains of PG define the tissue fixed charge density (FCD); the presence of these charges provides compressive resistance to the cartilage and is crucial for its mechanical function. Indeed, loss of FCD is seen early in the process of cartilage degeneration.17,18 An important development in noninvasive studies of cartilage has been the ability to estimate FCD using MRI19,20 through provision of gadolinium (Gd) to the tissue. The applicability of this technique has been demonstrated to extend to tissue-engineered cartilage constructs as well.9 However, the utility of the MRI-based noninvasive GAG measurement remains unvalidated in hydrogel systems suitable for implantation into cartilage defects. Accordingly, we assessed the ability of Gd-based MRI measurements to determine the FCD of cartilage developing within an injectable substrate for cartilage growth.
Materials and Methods
Chondrocyte isolation
Cartilage samples were obtained from the femoral grooves and condyles of the femorapatellar joint of 1–2-week-old bovines obtained from a research abattoir (Research 87, Boston, MA). The tissue was digested for 14 h in 0.2% (w/v) collagenase solution prepared from type II collagenase (Worthington, Freehold, NJ) dissolved in Dulbecco's modified Eagle's medium (DMEM, 4.5 g/L glucose) (Biofluids, Rockville, MD) supplemented with 5% (v/v) fetal bovine serum (FBS) (Hyclone, Logan, UT), and 32 nM L-ascorbic acid-2-phosphate (Sigma, St. Louis, MO). In addition, 1.85 mM of L-glutamine (Biofluids), 0.092 mM nonessential amino acids (NEAA; Biofluids), 92units/mL penicillin-G, 92 μg/mL streptomycin, 0.46 μg/mL amphotericin B (Fungizone; Biofluids), and 18 μg/mL gentamicin (Cambrex, Walkersville, MD) were added to each 500 mL of the medium. After digestion, the cells were filtered through an 80 μm nylon filter and washed with phosphate-buffered saline (PBS) (Biofluids).
Sample preparation and experimental design
A photopolymerizable hydrogel was used in all experiments and consisted of a 15% w/v nondegradable poly-(ethylene oxide) diacrylate (PEODA) polymer (Nektar, Huntsville, AL) in PBS. The cell seeding procedure using PEODA was based on methods previously reported.21,22 The photoinitiator, IrgaCure (Ciba Specialty Chemicals, Tarytown, NY), was mixed in 70% ethanol to a final concentration of 100 mg/mL and then added to the polymer solution (5 μL/mL) and mixed to achieve a final 0.05% (w/v) concentration. One hundred and twenty μL of this solution was transferred into a sterile cylindrical mold, with photo-polymerization initiated immediately upon addition of chondrocytes (20 × 106cells/mL; ∼2.4 million cells/construct). The solution was exposed to 365 nm light at 3–4mW/cm2 (Glowmark Systems, Upper Saddle River, NJ) for 7 min to achieve complete gelation. The cellular hydrogels were then removed from their molds and immersed in plates filled with the chondrocyte support medium for culturing. Chondrocyte culture medium was of the same formulation as used to dissolve collagenase for tissue digestion, except that for culture, the medium was fortified with 10% FBS. Samples were retained within an incubator throughout their growth period with medium changes performed two to three times per week. To confirm chondrocyte viability, a fluorescence-based Live-Dead assay was performed (Live/Dead Viability/Cytotoxicity Kit; Molecular Probes, Eugene, OR) on thin (<1 mm) axial sections of representative cellular hydrogel samples at 36 days of incubation.
A cellular hydrogels were prepared in an identical manner as the cellular hydrogels, without, however, the addition of chondrocytes prior to transfer into molds for photopolymerization. Experiments were performed with cellular hydrogels after 9, 16, 29, 36, 43, and 50 days of incubation (n = 5 at each time point). Control experiments were conducted with acellular hydrogels after 3, 24, and 37 days of incubation (n = 4 per time point).
MRI analysis
MRI experiments were performed using a Bruker DMX 400 NMR spectrometer equipped with a 9.4 T magnet and a 30 mm microimaging probe with 1000 milli-T/m shielded gradients (Bruker Biospin, Rheinstetten, Germany). A continuous stream of 95%:5% (volume/volume) humidified mixture of air and CO2 was supplied to the samples throughout the experiment. Temperature was maintained at 37°C. Samples were placed into custom-built holders fabricated from Ultem plastic material and inserted in NMR tubes in such a way as to permit circulation of DMEM from above and below each sample (Fig. 1A).
FIG. 1.
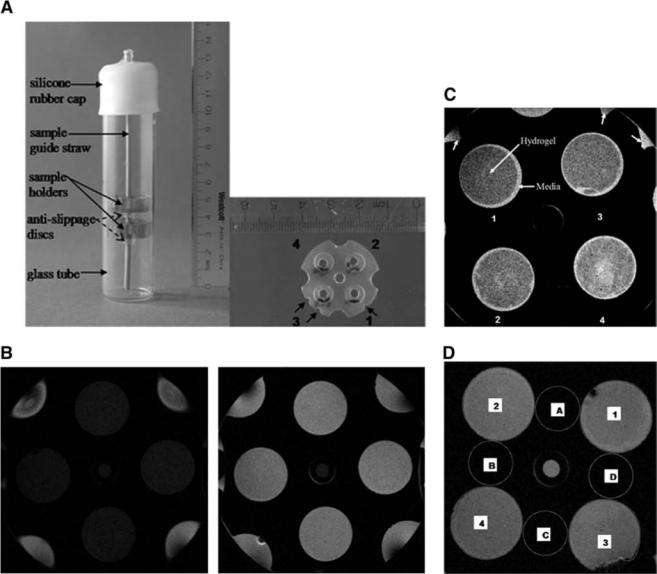
Positioning of samples in the NMR tube (left) and a detailed view of a single sample holder (right) in which the positioning notches have been indicated by arrows. (B) MRI image of cellular hydrogels before (left) and after (right) provision of Gd-DTPA; TR = 800 ms in both panels. (C) Contrast between hydrogel and surrounding cell culture medium after addition of Gd-DTPA is seen in heavily weighted T1 images (TR = 100 ms). Note that the media-filled notches have been indicated by the peripheral arrows. (D) Regions of interest used in signal-to-noise computations. Numbers on figure indicate regions of signal while letters refer to regions of noise.
Pilot scans were performed to permit placement of two axial imaging slices through the centers of the upper and lower sample holders, respectively. Images were obtained with field of view, 2.5 × 2.5 cm; matrix size, 256 × 256 pixels; slice thickness, 0.5 mm; and NEX, 2 signal averages.
Matrix FCD in the hydrogel constructs was measured using a modification of the Gd (Gd-DTPA2−, Magnevist; Berlex Laboratories, Wayne, NJ) exclusion method of Bashir et al.20 The longitudinal relaxation time, T1, was obtained using a spin-echo sequence with echo time TE = 8.2 ms, and a set of 12 repetition times, TR, varying from 360 ms to 15 s prior to provision of Gd (total scan time, 5.4 h) and from 50 ms to 1.6 s after equilibration in 2 mM Gd (total scan time, 40 min). To ensure full equilibration of Gd into and within the constructs prior to post-Gd data acquisition for calculation of FCD, T1's were acquired during the equilibration period; data were used only after stability of image contrast and of T1 was achieved. The mean time between provision of Gd and acquisition of the post-Gd data was 23 h.
Image quantification
T1 was measured by fitting the sum of the pixel intensities within the hydrogel regions as identified in the post-Gd images using ParaVision software (Bruker Biospin), with the same regions of interest then being used for the pre-Gd images. FCD was calculated from the pre- and post-Gd T1 values, assuming equal Gd-DTPA2− relaxivity in the hydro-gel and DMEM, and ideal Donnan equilibrium.20
GAG biochemical assay
After imaging, hydrogel constructs were digested in 1 mL of papainase solution (125 μg/mL papain and 10 mM L-cysteine [Sigma-Aldrich, St. Louis, MO] with 100 mM phosphate and 40% disodium salt [J.T. Baker, Phillipsburg, NJ], and 60% mono Na [Mallinckrodt, Paris, KY] and 10 mM EDTA [Sigma, Milwaukee, WI]) at a pH of 6.3 for 16 h at 60°C. GAG concentration was established using the dimethylene blue spectrophotometric assay for chondroitin sulfate.23
Histology
In separate samples, tissue was fixed in 10% formalin for 24 h and subsequently dehydrated, paraffin embedded, and sectioned into 5-μm-thick slices (Paragon Bioservices, Baltimore, MD) after 9, 16, 21, 28, 36, 43, and 50 days of growth. Sections were stained with Safranin-O to identify GAG in the extracellular matrix of the neocartilage. Acellular hydrogel samples were also sectioned for this histological staining protocol after 9 days.
Data and statistical analysis
Mean values of the FCD and GAG ± SD are reported. The correlation between GAG content and MRI-measured FCD in the cellular hydrogels was established using variance-weighted linear regression (SigmaPlot v10.0; Systat Software, Erkrath, Germany). The uncertainty in GAG was neglected because, in all cases, the range of the predictor variable was much greater than the variances of the individual points.24 A one-way ANOVA was used to assess the changes in FCD and GAG over time.
Results
Histological staining for PG
Red positive staining was absent in the case of acellular hydrogel samples (Fig. 2), as expected, since binding of the staining requires appropriate electrostatic interactions.25 Safranin-O is a cationic molecule and therefore binds to anionic carboxyl and sulfate groups in tissue macromolecules, such as GAGs. However, in acellular hydrogels, there are no ionized groups to which Safranin-O can bind.
FIG. 2.
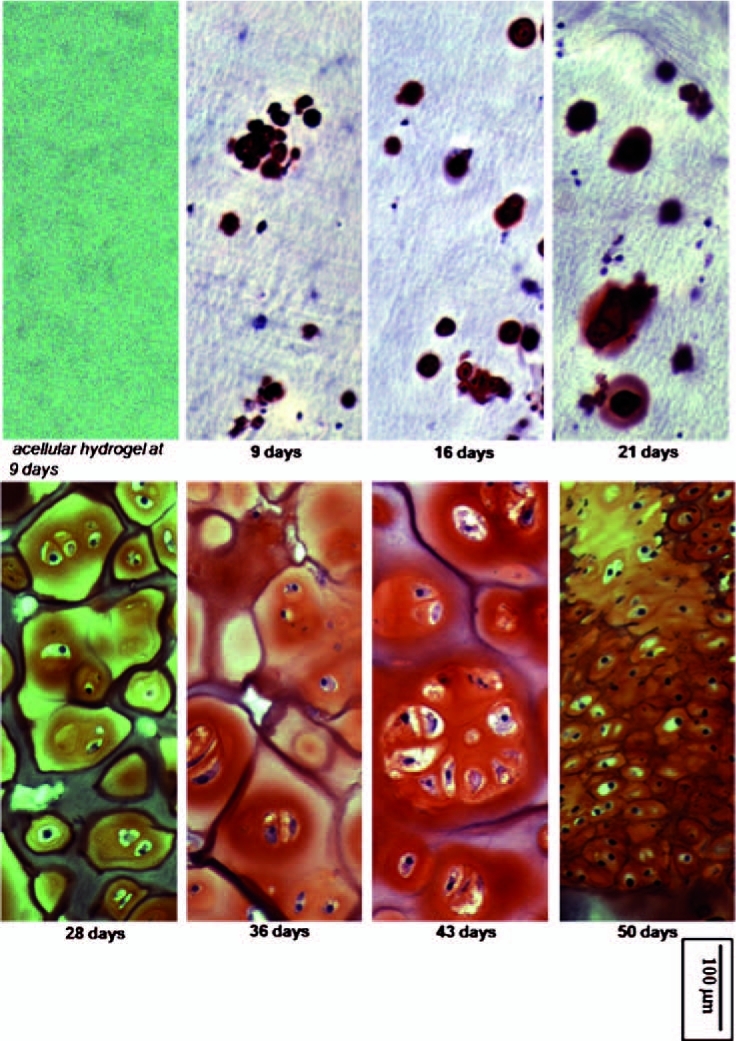
Safranin O staining for GAG at the indicated time points, with GAG-containing regions staining red. Hydrogel samples were sectioned axially (slice thickness of 5 μm), and photographed under a light microscope (magnification, 200×). Staining was positive for GAG in the extracellular matrix of chondrocyte-seeded PEODA hydrogels, with a general trend for increased staining with increasing incubation time. No staining was seen in acellular hydrogels. Color images available online at www.liebertonline.com/ten.
In contrast to the acellular hydrogels, chondrocyte-seeded hydrogels showed staining at the initial time point of 9 days. Staining increased progressively thereafter, with a maximum being reached at 43 days (Fig. 2). A particularly marked increase in PG was seen between 21 and 36 days. A decrease in staining was observed after 43 days. We note additionally that on day 50, the cell density of chondrocytes was greater than at days 36 or 43, and the cells appeared to have developed a multilayered appearance.
Biochemical assays for PG
A progressive increase in GAG content as determined by biochemical assay was observed up to the 36 days as shown in Figure 3A. The GAG content remained relatively constant from this time point onward. In addition, over 75% of the cells were found to be still viable in sections of chondrocyte-encapsulated hydrogel scaffolds at 36 days of incubation.
FIG. 3.
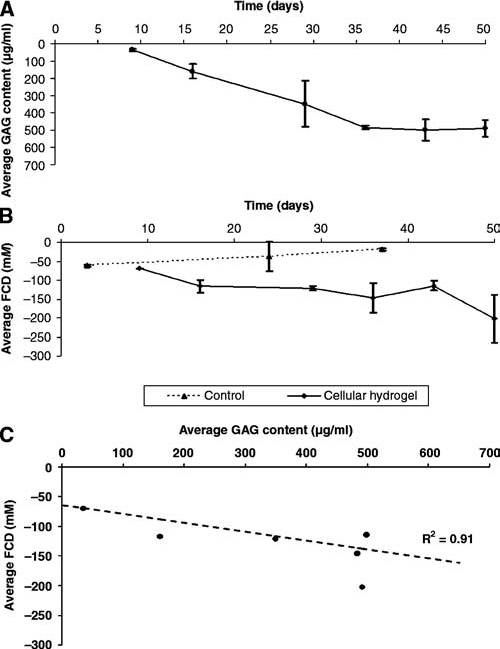
Average biochemically determined GAG content for cellular hydrogels (n = 5 at each time point). (B) Average MRI-determined FCD time course distribution for acellular (n = 4 at each time point) and cellular (n = 5 at each time point) hydrogels. (C) Correlation of FCD to GAG content in chondrocyte-seeded hydrogels over 50 days of incubation.
MRI measurement of PG
Samples were more readily visualized after addition of Gd (Fig. 1B). Further, in heavily T1-weighted post-Gd MR images (Fig. 1C, TR = 100 ms), there was visible contrast between the hydrogel constructs and the cell culture medium. Using segmented regions (Fig. 1D), the mean ± SD signal-to-noise ratios before and after Gd were found to be 4.25 ± 0.24 and 13.13 ± 1.39, respectively. PG content derived from the MRI Gd exclusion experiments did not show any trend toward development of FCD in the acellular hydrogel samples. In contrast, an increase in FCD was observed during culture for the samples that contained chondrocytes (Fig. 3B), in agreement with the biochemical results. The trend toward PG increase seen in both the MRI and the biochemical data was observed up to 36 days. In addition, as was the case in the GAG distribution (Fig. 3A), the FCD values also remained relatively unchanged beyond the 36-day time point. A correlation of R2 = 0.91 (p < 0.01) was found between MRI-derived FCD and GAG over the entire time course of 50 days (Fig. 3C). Finally, it was determined from the ANOVA that there was no significant change in FCD over time in the acellular hydrogels, while there was a statistically significant increase in FCD (p < 0.05) and GAG (p < 0.01) in the cellular hydrogels.
Discussion
MRI is emerging as a modality of choice for assessing articular cartilage repair, including the volume of fill,26–28 integration of repair tissue with the subchondral plate,29 and integration of engineered tissue with host tissue.14 MRI techniques that are more specific to cartilage matrix components, including FCD,19,20 permit characterization of the biochemical development of the tissue while concomitantly providing detailed spatial information.
Previous work has demonstrated the correlation of multiple MRI-derived endpoints with biochemically determined matrix components in a dynamic tissue-engineered system demonstrating ongoing cartilage matrix production.9,30 In the present study, we wished to focus on a tissue system with particular potential for clinical use, and on the GAG component of the matrix. The MRI-based approach used permits longitudinal and noninvasive FCD measurements, and has been applied to the assessment of cartilage integrity in vivo.3 It requires only routine measurement of the MRI spin-lattice relaxation time, T1, prior to and following the introduction of Gd-DTPA,2 a widely used ionic contrast agent.20 The dependence of T1 on local Gd-DTPA2− concentration permits this parameter to be interpreted as a specific indicator of local GAG content.
The tissue-engineered system evaluated here, a photo-polymerizable hydrogel, can in principle be implemented for tissue repair. It is clinically attractive primarily because it requires only injection into the defect site, eliminates the need for extensive ex vivo development of cartilage matrix from cells, and would readily conform to highly spatially irregular defects.16 In addition, these types of hydrogel scaffolds encapsulate cells, thereby allowing them to preserve a spherical morphology, which in turn promotes the retention of the chondrocyte phenotype.15 Nonetheless, such scaffolds remain to be fully optimized, including the requirement for extensive evaluation in vivo.
Toward this goal, we sought to determine whether MRI-derived FCD measurements would accurately reflect the GAG component of developing matrix as determined by gold-standard biochemical and histological assays. If successful, MRI-based monitoring of neocartilage development would provide an important outcome measure in the further development of injectable hydrogel tissue repair strategies. Indeed, our results demonstrate this potential through the correlation presented.
Similar to previous studies, it was observed that the FCD values evolve over time9,13,30 in cellular hydrogels, with a very strong correlation demonstrated between MRI-measured FCD and GAG concentration for culture times over 7 weeks. Qualitative histological staining for GAG further confirmed this trend. Importantly, acellular gels were found to have marginal observable changes in the apparent FCD with no defined temporal trends.
This study has certain limitations. It is important to note that while the biochemical GAG assay provides a bulk value that represents the entire sample, the MRI FCD measurement is slice specific. The development of heterogeneity in the sample would then limit the accuracy of the correlation between these modalities. In addition, the MRI studies were performed at a field strength of 9.4 T, which is substantially larger than the typical clinical field strengths of 1.5 or 3 T. We note that successful FCD measurements have nonetheless been carried out at these lower field strengths.3,31,32 Finally, while the present results provide strong evidence of the utility of MRI in monitoring the development of FCD in injectable hydrogel constructs, the effectiveness of this approach remains to be established in in vivo studies.33
We note that the present investigation was targeted to the specific hypothesis that noninvasive MRI measurements of FCD would correlate with biochemically determined GAG in developing neocartilage. This goal was distinct from any attempt to optimize tissue development and quality in these constructs. Indeed, the histologic phenotype of the cells in the PEODA scaffolds at 50 days appeared to differ somewhat from that seen at previous time points, so that interest would attach to more detailed phenotypic studies investigating, for example, expression of mRNA for aggrecan and collagen types I and II.
We conclude that GAG formation in injectable hydrogel scaffolds seeded with chondrocytes can be measured non-invasively by MRI. This capability should be useful in the development of cartilage repair protocols using such scaffolds.
Acknowledgment
The research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
References
- 1.Burgkart R. Glaser C. Hyhlik-Durr A. Englmeier K.H. Reiser M. Eckstein F. Magnetic resonance imaging-based assessment of cartilage loss in severe osteoarthritis: accuracy, precision, and diagnostic value. Arthritis Rheum. 2001;44:2072. doi: 10.1002/1529-0131(200109)44:9<2072::AID-ART357>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 2.McCauley T.R. Recht M.P. Disler D.G. Clinical imaging of articular cartilage in the knee. Semin Musculoskelet Radiol. 2001;5:293. doi: 10.1055/s-2001-19040. [DOI] [PubMed] [Google Scholar]
- 3.Bashir A. Gray M.L. Hartke J. Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41:857. doi: 10.1002/(sici)1522-2594(199905)41:5<857::aid-mrm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Duvvuri U. Charagundla S.R. Kudchodkar S.B. Kaufman J.H. Kneeland J.B. Rizi R. Leigh J.S. Reddy R. Human knee: in vivo T1(rho)-weighted MR imaging at 1.5 T—preliminary experience. Radiology. 2001;220:822. doi: 10.1148/radiol.2203001662. [DOI] [PubMed] [Google Scholar]
- 5.Gold G.E. Suh B. Sawyer-Glover A. Beaulieu C. Musculoskeletal MRI at 3.0 T: initial clinical experience. AJR Am J Roentgenol. 2004;183:1479. doi: 10.2214/ajr.183.5.1831479. [DOI] [PubMed] [Google Scholar]
- 6.Kurkijarvi J.E. Nissi M.J. Kiviranta I. Jurvelin J.S. Nieminen M.T. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and T2 characteristics of human knee articular cartilage: topographical variation and relationships to mechanical properties. Magn Reson Med. 2004;52:41. doi: 10.1002/mrm.20104. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe A. Boesch C. Obata T. Anderson S.E. Effect of multislice acquisition on T1 and T2 measurements of articular cartilage at 3T. J Magn Reson Imaging. 2007;26:109. doi: 10.1002/jmri.20962. [DOI] [PubMed] [Google Scholar]
- 8.Mori R. Ochi M. Sakai Y. Adachi N. Uchio Y. Clinical significance of magnetic resonance imaging (MRI) for focal chondral lesions. Magn Reson Imaging. 1999;17:1135. doi: 10.1016/s0730-725x(99)00033-8. [DOI] [PubMed] [Google Scholar]
- 9.Chen C.T. Fishbein K.W. Torzilli P.A. Hilger A. Spencer R.G. Horton W.E., Jr. Matrix fixed-charge density as determined by magnetic resonance microscopy of bioreactor-derived hyaline cartilage correlates with biochemical and biomechanical properties. Arthritis Rheum. 2003;48:1047. doi: 10.1002/art.10991. [DOI] [PubMed] [Google Scholar]
- 10.Miyata S. Numano T. Homma K. Tateishi T. Ushida T. Feasibility of noninvasive evaluation of biophysical properties of tissue-engineered cartilage by using quantitative MRI. J Biomech. 2007;40:2990. doi: 10.1016/j.jbiomech.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Neves A.A. Medcalf N. Brindle K. Functional assessment of tissue-engineered meniscal cartilage by magnetic resonance imaging and spectroscopy. Tissue Eng. 2003;9:51. doi: 10.1089/107632703762687537. [DOI] [PubMed] [Google Scholar]
- 12.Neves A.A. Medcalf N. Smith M. Brindle K.M. Evaluation of engineered meniscal cartilage constructs based on different scaffold geometries using magnetic resonance imaging and spectroscopy. Tissue Eng. 2006;12:53. doi: 10.1089/ten.2006.12.53. [DOI] [PubMed] [Google Scholar]
- 13.Potter K. Butler J.J. Adams C. Fishbein K.W. McFarland E.W. Horton W.E. Spencer R.G. Cartilage formation in a hollow fiber bioreactor studied by proton magnetic resonance microscopy. Matrix Biol. 1998;17:513. doi: 10.1016/s0945-053x(98)90099-3. [DOI] [PubMed] [Google Scholar]
- 14.Ramaswamy S. Wang D.A. Fishbein K.W. Elisseeff J.H. Spencer R.G. An analysis of the integration between articular cartilage and nondegradable hydrogel using magnetic resonance imaging. J Biomed Mater Res B Appl Biomater. 2006;77:144. doi: 10.1002/jbm.b.30404. [DOI] [PubMed] [Google Scholar]
- 15.Kuo C.K. Li W.J. Mauck R.L. Tuan R.S. Cartilage tissue engineering: its potential and uses. CurrOpin Rheumatol. 2006;18:64. doi: 10.1097/01.bor.0000198005.88568.df. [DOI] [PubMed] [Google Scholar]
- 16.Elisseeff J. Injectable cartilage tissue engineering. Expert Opin Biol Ther. 2004;4:1849. doi: 10.1517/14712598.4.12.1849. [DOI] [PubMed] [Google Scholar]
- 17.Poole A. Koopman W. A Textbook of Rheumatology. Baltimore, MD: Williams & Wilkins; 2000. Cartilage in health and disease; Arthritis and Allied Conditions; pp. 226–284. [Google Scholar]
- 18.Tiderius C.J. Olsson L.E. Leander P. Ekberg O. Dahlberg L. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med. 2003;49:488. doi: 10.1002/mrm.10389. [DOI] [PubMed] [Google Scholar]
- 19.Lesperance L.M. Gray M.L. Burstein D. Determination of fixed charge density in cartilage using nuclear magnetic resonance. J Orthop Res. 1992;10:1. doi: 10.1002/jor.1100100102. [DOI] [PubMed] [Google Scholar]
- 20.Bashir A. Gray M.L. Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36:665. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 21.Elisseeff J. McIntosh W. Anseth K. Riley S. Ragan P. Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 22.Varghese S. Theprungsirikul P. Sahani S. Hwang N. Yarema K.J. Elisseeff J.H. Glucosamine modulates chondrocyte proliferation, matrix synthesis, and gene expression. Osteoarthritis Cartilage. 2007;15:59. doi: 10.1016/j.joca.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 24.Draper N.R. Smith H. Applied Regression Analysis. New York: Wiley; 1981. [Google Scholar]
- 25.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg. 1971;53:69. [PubMed] [Google Scholar]
- 26.Chung C.B. Frank L.R. Resnick D. Cartilage imaging techniques: current clinical applications and state of the art imaging. Clin Orthop Relat Res. 2001;391(Suppl):S370. [PubMed] [Google Scholar]
- 27.Recht M. Bobic V. Burstein D. Disler D. Gold G. Gray M. Kramer J. Lang P. McCauley T. Winalski C. Magnetic resonance imaging of articular cartilage. Clin Orthop Relat Res. 2001;391(Suppl):S379. doi: 10.1097/00003086-200110001-00035. [DOI] [PubMed] [Google Scholar]
- 28.Ramaswamy S. Gurkan I. Sharma B. Cascio B. Fishbein K.W. Spencer R.G. Assessment of tissue repair in full thickness chondral defects in the rabbit using magnetic resonance imaging transverse relaxation measurements. J Biomed Mater Res B Appl Biomater. 2008;86B:375. doi: 10.1002/jbm.b.31030. [DOI] [PubMed] [Google Scholar]
- 29.Jackson D.W. Lalor P.A. Aberman H.M. Simon T.M. Spontaneous repair of full-thickness defects of articular cartilage in a goat model. A preliminary study. J Bone Joint Surg. 2001;83-A:53. doi: 10.2106/00004623-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Potter K. Butler J.J. Horton W.E. Spencer R.G. Response of engineered cartilage tissue to biochemical agents as studied by proton magnetic resonance microscopy. Arthritis Rheum. 2000;43:1580. doi: 10.1002/1529-0131(200007)43:7<1580::AID-ANR23>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 31.Gillis A. Bashir A. McKeon B. Scheller A. Gray M.L. Burstein D. Magnetic resonance imaging of relative glycosaminoglycan distribution in patients with autologous chondrocyte transplants. Invest Radiol. 2001;36:743. doi: 10.1097/00004424-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Tiderius C.J. Svensson J. Leander P. Ola T. Dahlberg L. dGEMRIC (delayed gadolinium-enhanced MRI of cartilage) indicates adaptive capacity of human knee cartilage. Magn Reson Med. 2004;51:286. doi: 10.1002/mrm.10714. [DOI] [PubMed] [Google Scholar]
- 33.Sharma B. Williams C.G. Khan M. Manson P. Elisseeff J.H. In vivo chondrogenesis of mesenchymal stem cells in a photopolymerized hydrogel. Plastic Reconstr Surg. 2007;119:112. doi: 10.1097/01.prs.0000236896.22479.52. [DOI] [PubMed] [Google Scholar]


