Abstract
Protein sorting through vesicular compartments is highly regulated to maintain the integrity and signaling of intracellular organelles in eukaryotic cells. Sorting Nexin-2 (SNX2) is involved in protein sorting in the trans-Golgi network, endosome, and/or lysosome compartments, with loss of function leading to defect in protein sorting and stress on organelles. To investigate the function of SNX2, we have identified the DEAD-box helicase Abstrakt (Abs) as an SNX2-interacting protein. The N-terminal domain of Abs interacts with the phox homology (PX) domain of SNX2 suggesting that PX domains may also participate in protein–protein interaction. Interestingly, both proteins undergo nucleocytoplasmic shuttling, and this process is responsive to serum withdrawal for Abs. Finally, expression of Abs reduced the cellular expression of SNX2 without altering its steady state mRNA levels. This unexpected interaction provides a novel mechanism whereby expression of proteins involved in membrane trafficking could be regulated by an RNA helicase.
Cells maintain complex regulatory mechanisms to ensure temporal and spatial specificity of vesicular trafficking and to coordinate events within the various intracellular compartments with that of other cellular processes. This requires sorting and targeting of proteins to the appropriate compartments. In yeast, the vacuolar protein sorting (VPS) family of genes are involved in various aspects of vacuole biogenesis and trafficking (Conibear and Stevens, 1998; Sato et al., 2001; Katzmann et al., 2002). One member of this family, VPS5, is required for proper localization of vacuolar proteins with loss of function resulting in misdirection of vacuolar proteins to the cell surface (Horazdovsky et al., 1997; Seaman et al., 1998). VPS5 belongs to a protein family collectively known as the sorting nexins (SNX), which in the human genome contains at least 16 homologues. Vps5p shows the highest similarity to mammalian SNX1, a protein initially identified in lysosomal degradation of the epidermal growth factor receptor (EGFR) (Kurten et al., 1996). Thus, by directing the intracellular trafficking of EGFR to the degradative lysosomal compartment, SNX1 could potentially alter the duration of EGFR signaling. This effect on signaling duration implies that the cellular levels of SNX must be regulated for optimal signal transduction.
The SNX family is characterized by the presence of a conserved phox homology (PX) domain, which binds various phosphatidylinositides (Xu et al., 2001; Cozier et al., 2002; Zhong et al., 2002). In contrast to their lipid-binding abilities, the role of PX domains in protein–protein interaction remains unclear. The C-terminal domain of SNX1 and SNX2 contains a Bin, amphiphysin, RVS (BAR) domain, which forms a crescent-shaped dimer and preferentially binds to highly curved membranes (Peter et al., 2003). The Yeast Vps5p forms a “retromer” complex consisting of four other proteins: Vps17p, Vps26p, Vps29p, and Vps35p (Seaman et al., 1998; Seaman, 2004). This complex appears to be conserved, as human orthologs of these proteins also exist as large complexes (Haft et al., 2000). Moreover, mammalian SNX1 and SNX2 are known to form a sub-complex (Haft et al., 1998, 2000), and genetic analysis of knockout mice indicates that SNX1 and SNX2 may have overlapping as well as unique functions (Schwarz et al., 2002). Their ability to form subcomplexes may provide a basis for their functional overlap while formation of heterogeneous complexes provides functional diversity at the various intracellular compartments. To identify proteins that might interact with and regulate the cellular functions of SNX2, we initiated a bacterial two-hybrid screen with SNX2 as bait. We have identified the DEAD-box helicase, Abstrakt (Abs), as an SNX2-interacting protein. We showed that the N-terminal domain of Abs interacts with the PX domain of SNX2. Interestingly, both proteins undergo nucleocytoplasmic shuttling, raising the possibility that interaction can occur in either compartment. Their cellular localization can be altered by serum withdrawal. Finally, co-expression of the wild type Abs reduced the cellular expression of SNX2. This unexpected interaction provides a novel mechanism whereby expression of proteins involved in protein trafficking such as SNX2 could be regulated by an RNA helicase such as Abs.
MATERIALS AND METHODS
Bacterial two-hybrid screen
The BacterioMatch® two-hybrid system (Stratagene, La Jolla, CA) was used to screen for potential SNX2-interacting proteins. Full length human SNX2 was subcloned into the EcoR1/Xho1 sites of the bait pBT plasmid, and used to screen a human fetal brain cDNA library. Positives were selected on LB-CTCK agar plates containing 34 μg/ml chloramphenicol, 15 μg/ml tetracycline, 500 μg/ml carbenicillin, and 50 μg/ml kanamycin. Potential positives were verified by re-transforming 10 ng each of the positive DNA and the original bait or the non-specific bait pBT/LGF2. Because of the high background, potential positives were selected based on increased number of transformants with the SNX2 bait relative to the non-specific LGF2 bait.
DNA constructs and in vitro pulldown assays
Full length human Abs was subcloned into the BglII/HindIII sites of a modified bacterial expression plasmid pQE9′ (Qiagen, Venlo, Netherlands) containing an N-terminal His6 followed by a hemagglutinin (HA) tag. The resulting fusion protein was used for in vitro binding assays with GST-tagged SNX2 expressed from the pGEX-2T* vector (Amersham Biosciences, Piscataway, NJ). Full length Abs was also cloned into pEGFP-C1 vector (Clontech, Palo Alto, CA) at BglII/HindIII sites, and HA-tagged Abs was cloned into pCMV5 vector (Invitrogen, Carlsbad, CA) at BglII/HindIII sites for mammalian expression. Similarly, FLAG-tagged SNX2 was cloned at NotI/EcoRI sites of pQE9′ vector to be used for in vitro binding assays. Myc-tagged SNX2 in pcDNA3.1 (+) vector was generously provided by Dr. C. Haft.
Escherichia coli fusion proteins were purified as described (Martincic et al., 1997; Gougeon et al., 2002). For His6-tagged proteins, 250 mM imidazole was used to elute the proteins from the Ni-NTA resin and used in GST pulldown assays. Alternatively, 50 mM EDTA was used to elute the proteins from Ni-NTA resin, which were then immobilized on CNBr-activated Sepharose 4B (Amersham Biosciences) as described by manufacturer. For GST pulldown assays, the GST-tagged proteins were retained on the glutathione beads after extensive washing and resuspended as a 50% slurry in binding buffer (25 mM Tris-HCl pH 7.5, 10% glycerol, 0.005% Triton X-100). A typical pulldown assay contained ~10 pmol of GST fusion protein and incubated with 10–30 pmol of His6-HA-tagged Abs in binding buffer. After incubation at 4°C for 2 h, the beads were washed three times with binding buffer, and processed for Western immunoblot. For pulldown with Abs, ~20 pmol of His6-HA-Abs cross-linked to CNBr-Sepharose 4B was incubated with ~30 pmol of FLAG-SNX2 in binding buffer at 4°C for 2 h. Beads were washed and treated for Western blot as indicated above.
Immunocytochemistry, cell fusion assay, and flow cytometry
Plasmid constructs were transfected into Chinese hamster ovary (CHO) using LipofectAMINE (Invitrogen). After 48 h, cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS, quenched with 0.1 M glycine in PBS, and stained with anti-Myc in blocking buffer (1% BSA, 2% normal goat serum, and 0.4% saponin in PBS). Alexa™-488 or -594 secondary antibodies (Molecular Probes, Eugene, OR) were used to detect the bound primary antibody. For Lepto-mycin B (LMB) treatment, transfected CHO cells were treated with fresh media containing 5 ng/ml of LMB (Calbiochem, La Jolla, CA) for 2 h at 37°C to inhibit nuclear export. Cells were then fixed and stained as described above.
For cell fusion assay, CHO cells were seeded onto 35 mm dishes and transfected with pEGFP-Abs. After 24 h, the cells were overlaid with 1 × 106 un-transfected CHO cells and incubated at 37°C for ~2 h with 10 μg/ml cycloheximide. Cells were then fused with 50% (w/v) polyethylene glycol 3350 (Sigma, St. Louis, MO) and diluted in incomplete MEMα media for 30 sec to form polykaryons. After extensive washing with PBS to remove the PEG3350, the cells were allowed to recover in fresh MEMα containing 5% FBS and cycloheximide for 1 h before the photobleaching experiment. Only one of the GFP-labeled nuclei in a multi-nucleated, GFP-Abs-containing cell was photo-bleached at maximum laser power for ~10 sec. Following the photobleach, images were taken every 5 or 10 min for a total of 90 min to assess recovery of the GFP-Abs signal.
For flow cytometry, control GFP or GFP-Abs transfected HeLa cells were sorted by their fluorescence signal through Cytomation MoFlo (Dako Cytomation, Fort Collins, CO). The GFP-positive cells were then incubated for 2–3 days before processing for quantitative Western analysis with anti-SNX2 (Pharmingen, San Diego, CA). Stably transfected HeLa were selected with and maintained in 625 μg/ml of G418. First strand cDNAs for quantitative RT-PCR analysis were produced with RETROscript kit (Ambion, Austin, TX) from mRNA isolated with MicroPoly(A)Pure (Ambion). GAPDH was used as an internal control and values for SNX2 were expressed as a ratio of GAPDH mRNA.
Nuclear and cytosolic fractionation
The NE-PER Kit (Pierce, Rockford, IL) was used to extract nuclear and cytoplasmic fractions from transfected CHO cells as per the manufacturer’s protocol. Samples were processed for Western blot with mouse anti-HA, mouse anti-Myc, or anti-SNX2. Goat anti-histone deacetylase-1 (HDAC-1) (Sigma) and rabbit anti-GDP-dissociation inhibitor (GDI) (Zymed, South San Francisco, CA) were used as a nuclear and cytosolic protein control, respectively. For serum withdrawal, the transfected cells were starved in serum free media for 4 h prior to NE-PER fractionation.
RESULTS
N-terminal region of Abs binds the PX domain of SNX2
We used the full length SNX2 as bait in a bacterial two-hybrid screen of a human fetal brain cDNA library. Although the screen produced many false positives, we were able to identify a potential interacting clone under stringent carbenicillin selection. Sequence analysis revealed it to be the full length human ortholog of Drosophila Abs, a DEAD-box RNA helicase with the characteristic DEAD sequence (Luking et al., 1998; Linder and Stutz, 2001). Abs was originally identified by its effect on axonal outgrowth of the Bolwig nerve and development of the adult visual system and CNS (Irion and Leptin, 1999; Schmucker et al., 2000). It is expressed at all developmental stages and is essential for survival.
To verify the interaction and to determine the domains within SNX2 and Abs responsible for the interaction, we performed in vitro pulldown experiments using partially purified bacterial recombinant proteins. Full length and fragments of SNX2 and Abs were expressed as GST, FLAG-tagged, and His6-HA-tagged proteins, respectively. The His6-HA-Abs was bound to GST-FLAG-SNX2 but not to the GST control (Fig. 1A). Deletion analysis of His6-HA-Abs indicated that the N-terminal region of Abs was sufficient for SNX2 binding (Fig. 1B). Similarly, deletion analysis of SNX2 showed that the PX domain only and that with the remaining C-terminal domain interacted strongly with His6-HA-Abs, whereas the N-terminus plus PX domain of SNX2 showed a weaker binding (Fig. 1C). The PX domain of SNX2 appeared to be the minimum required for binding to full length His6-HA-Abs. A weaker binding was observed with N-terminus plus PX domain compared to PX domain, which suggests that the N-terminal domain of SNX2 may influence accessibility to the PX domain. Therefore, we concluded that SNX2 and Abs likely interact through their PX and N-terminal domains, respectively.
Fig. 1.
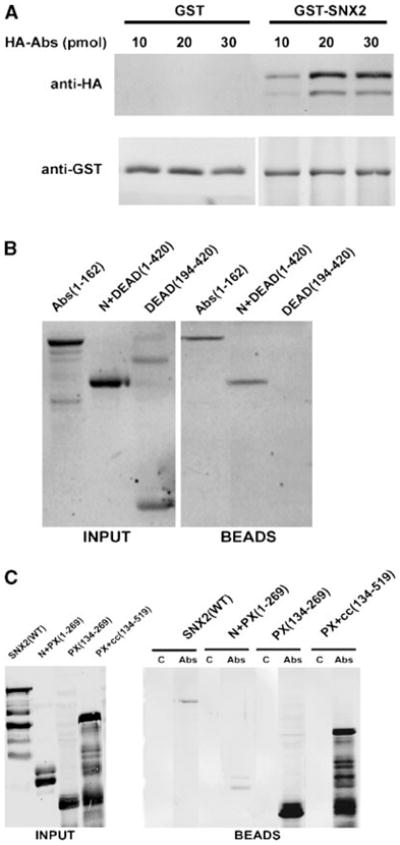
In vitro pulldown of Abstrakt (Abs) and sorting nexin-2 (SNX2). A: Increasing amounts of recombinant hemagglutinin (HA)-Abs was added to 5 pmol of GST-SNX2 or GST pre-bound to glutathione-agarose beads. Proteins bound to the beads were analyzed with anti-HA (top part) and anti-GST (bottom part) antibodies. B: Various HA-Abs deletion fragments (20 pmol each) were added to GST-SNX2 bound to glutathione-Sepharose beads. Numbers in parentheses indicate the inclusive range of amino acids in each Abs fragment. HA-Abs in the input (left part) and bound to the beads (right part) were analyzed with anti-HA. Fraction of the Abs DEAD(194-420) fragment often appear as a SDS-resistant dimer. C: Various FLAG-SNX2 fragments (labeled input, left part) were added to control Sepharose 4B beads (lanes labeled C, right part) or to equal volume of Abs immobilized on Sepharose 4B (lanes labeled Abs, right part). FLAG-SNX2 associated with the beads was detected with anti-FLAG antibodies.
Both Abs and SNX2 undergo nucleocytoplasmic shuttling
Abs is localized to both nucleus and cytoplasm in early stages of Drosophila embryonic development but restricted to the nucleus in older embryos (Irion and Leptin, 1999). To determine if this differential localization of Abs is conserved in mammalian cells, we transfected CHO cells with GFP-Abs and Myc-SNX2. Most GFP-Abs positive cells had a nuclear staining pattern (Fig. 2A). However, approximately 10% of the cells had a noticeable cytosolic as well as nuclear staining, with a stronger nuclear staining in all cases (Fig. 2B). Therefore, the results suggest that Abs might shuttle between the nucleus and cytoplasm with a bias towards the nucleus. CHO transfected with Myc-SNX2 showed a punctate endosomal staining pattern (Fig. 2C) in agreement with previous findings (Zhong et al., 2002). No significant shift in cellular localization of either protein was observed when co-expressed (data not shown). To address whether cytosolic pool of GFP-Abs might co-localize with SNX2, we analyzed the cytoplasmic distribution of the two proteins in co-transfected CHO cells by amplifying the cytoplasmic fluorescent GFP-Abs signal. Cytosolic GFP-Abs mostly showed a punctate staining pattern, which co-localizes with SNX2 (Fig. 2D–F). This co-localization raises the possibility that the two proteins might be present in the same endocytic compartment.
Fig. 2.
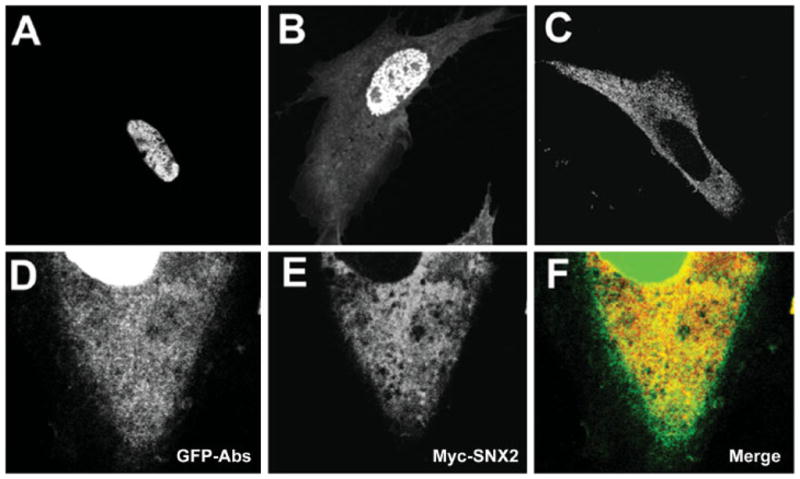
Cellular localization of GFP-Abs and Myc-SNX2. A, B: Representative images of Chinese hamster ovary (CHO) transfected with GFP-Abs. Most cells (~90%) exhibited a prominent nuclear signal (A) while some (~10%) exhibited both nuclear and cytosolic localization. C: CHO transfected with Myc-SNX2 showed a uniformly punctate distribution consistent with the endocytic compartment. D–F CHO co-transfected with GFP-Abs and Myc-SNX2 showing the cytosolic distribution of GFP-Abs (D), Myc-SNX2 (E), and merged (F) images.
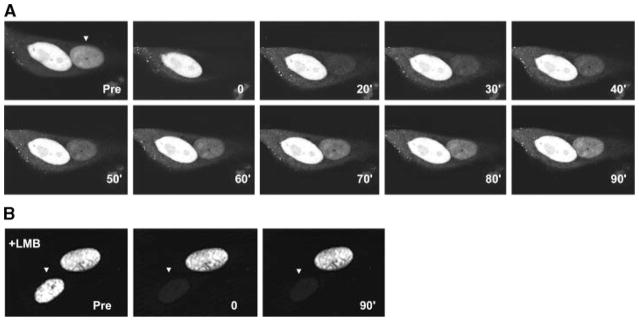
To demonstrate the likelihood that GFP-Abs might undergo nucleocytoplasmic shuttling, we performed a cell fusion assay (Kawamura et al., 2002) where the transfer of protein from one nucleus to another could be followed. When GFP-Abs transfected CHO cells were overlaid and fused with un-transfected cells, we observed green fluorescence in the nuclei of all multi-nucleated cells. This suggests transfer of GFP-Abs from the transfected to the un-transfected nucleus (Fig. 3A,B). To independently verify this, we performed fluorescence recovery after photobleaching (FRAP) on one of the nuclei within a polykaryon and monitored the green fluorescence recovery over time. Fluorescence recovery of the GFP-Abs signal in the photobleached nucleus was detectable by 30 min with near complete recovery in 60–90 min after photobleaching (Fig. 4A). Moreover, this recovery was completely abrogated in cells pre-treated with LMB, a drug that blocks CRM1-dependent nuclear export (Hamamoto et al., 1985) (Fig. 4B). Taken together, these results indicate that GFP-Abs is exported from the transfected nucleus in a CRM1-dependent pathway and then imported by the other.
Fig. 3.
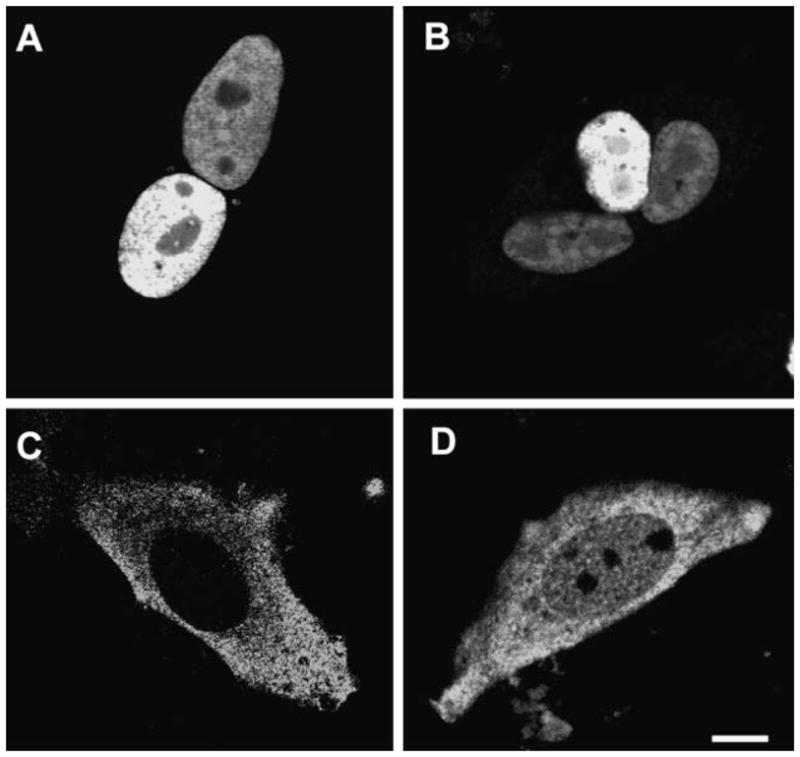
Cell fusion assay of GFP-Abs and nuclear localization of SNX2. CHO were transfected with GFP-Abs and fused with un-transfected CHO cells. All multi-nucleated cells exhibited a fluorescent nuclear signal (A, B). CHO transfected with Myc-SNX2 showed normal cytoplasmic punctate staining (C) but with significant nuclear localization when treated with the nuclear export blocker Leptomycin B (LMB) (D).
Fig. 4.
Fluorescence recovery after photobleaching (FRAP) analysis of GFP-Abs in fused, multi-nucleated CHO. CHO cells expressing GFP-Abs were fused with un-transfected cells. A: One of the nuclei was photobleached (arrowhead) and fluorescence recovery was monitored at 10 min intervals for 90 min as indicated on the lower right hand corner of each part. B: FRAP of a nucleus (arrowhead) in a multi-nucleated cell pre-treated with the nuclear export inhibitor LMB showing no fluorescence recovery (only the 90 min recovery time point is shown).
Certain endocytic proteins unexpectedly accumulate in the nucleus when nuclear export was inhibited by LMB (Duncan et al., 1997; Coda et al., 1998; Hyman et al., 2000; Vecchi et al., 2001; Poupon et al., 2002). To address whether SNX2 might also translocate to the nucleus, we blocked nuclear export with LMB to trap the nuclear translocated SNX2. Little, if any, SNX2 was detectable in the absence of LMB, but significant amount of the protein accumulated as speckles in the nucleus upon LMB treatment (Fig. 3C,D). Hence, both Abs and SNX2 can undergo nucleocytoplasmic shuttling, raising the possibility that the two proteins could interact in either compartment.
We next performed nuclear fractionation to verify that both Abs and SNX2 are present in the nucleus and cytoplasm. NE-PER (Pierce) fractionation of transfected CHO showed the presence of HA-tagged Abs and Myc-tagged SNX2 in cytosolic and nuclear fractions (Fig. 5). Probing the immunoblots with antibodies to HDAC-1 and GDI, a nuclear and cytosolic protein marker, respectively, confirmed the lack of significant contamination between the two fractions during NE-PER extraction. Interestingly, both SNX2 and Abs appeared as multiple bands, which would suggest possible post-translational modifications. Abs appeared as a doublet in the nucleus but as a triplet in the cytoplasmic fraction. SNX2 appeared as a doublet in both the cytoplasmic and nuclear fractions. Phosphatase treatment did not alter the mobility of the bands thereby eliminating post-translational phosphorylation as a primary cause (data not shown). Thus, other modifications are likely to be responsible for their mobility shift, and might possibly influence protein localization or interaction.
Fig. 5.
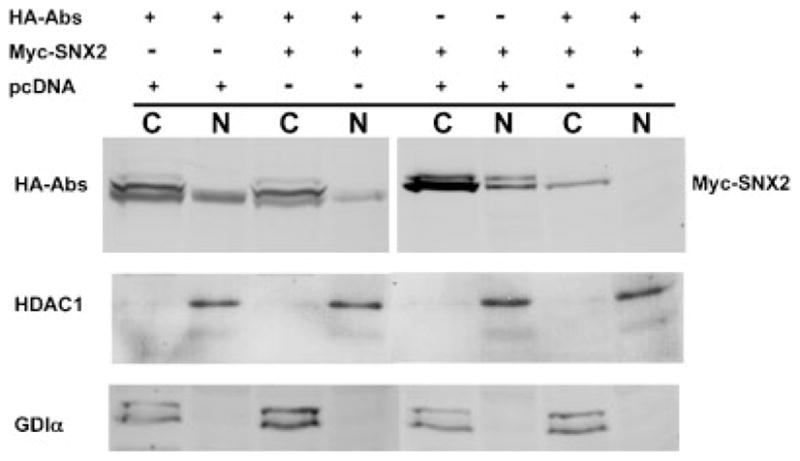
Nuclear and cytoplasmic fractionation of HA-Abs and Myc-SNX2. CHO were transfected with a combination of HA-Abs, Myc-SNX2, and empty pcDNA3.1(+) vector control and fractionated using the NE-PER kit. One quarter of each fractions were analyzed by Western immunoblot with anti-HA and anti-Myc antibodies. Histone deacetylase-1 (HDAC-1) and GDP-dissociation inhibitor (GDI)α were used as nuclear and cytosolic controls, respectively. Lanes indicated with C represents the cytosolic fraction and N the nuclear fraction.
N-terminal domain targets Abs to the nucleus
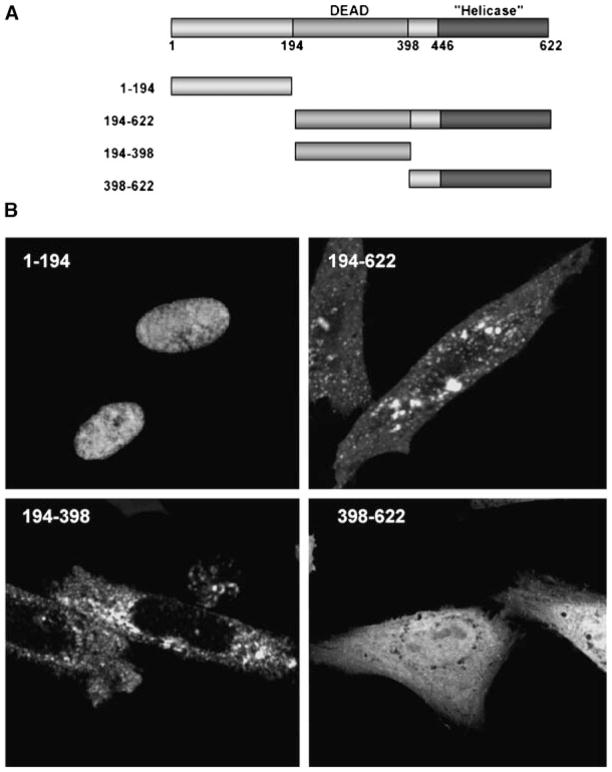
To determine the domain within Abs responsible for its nuclear localization, we generated GFP-fusions of Abs fragments (Fig. 6A) and monitored their cellular localization. GFP-fusion with the N-terminal domain showed the same nuclear localization as the full length Abs (Fig. 6B). Deletion of this domain in Abs(194-622) resulted in distinct punctate cytoplasmic distribution and loss of nuclear localization. The DEAD-box domain in Abs(194-398) also showed similar cytosolic staining pattern with no obvious nuclear localization. The identity of this punctate cytosolic compartment remains unclear as it shares limited co-localization with other known endosomal markers (data not shown). In contrast, GFP-fusion with the helicase domain in Abs(398-622) has a predominantly diffused cytosolic staining pattern with some nuclear localization. Although it is possible that the limited nuclear localization may result from co-transport with a protein that has one, we cannot rule out non-specific import as the size of the resulting fusion protein (~50 kDa) is clearly below the exclusion limit for passive diffusion through the nuclear pore complex (Paine et al., 1975; Gorlich and Mattaj, 1996). Taken together, we concluded that the N-terminal domain of Abs contains an NLS capable of targeting the protein to the nucleus.
Fig. 6.
Identification of the NLS within Abs. A: Schematic diagram of full length and truncated Abs with the DEAD-box, helicase domain, and residue number as indicated. The various Abs fragments were inserted into the C-terminus of GFP. B: Representative images of transfected CHO cells. The N-terminal 194 amino acids targeted GFP to the nucleus. Deletion of this domain in Abs(194-622) and Abs(194-398) resulted in punctate cytoplasmic staining and exclusion from the nucleus. Fusion with the helicase domain in Abs(398-622) resulted in uniform nuclear and cytoplasmic staining.
Serum withdrawal triggers cytoplasmic translocation of Abs
Abs is localized to cytoplasm during early stages of Drosophila development but translocates to the nucleus at later stages (Irion and Leptin, 1999). Conditions triggering this translocation process remain unknown. We decided to explore whether the presence or absence of fetal bovine serum (FBS) might alter the cellular localization of Abs. Serum was withdrawn from HA-Abs transfected HeLa cells for 4 h prior to NE-PER nuclear fractionation. Interestingly, serum withdrawal caused a shift in the cellular localization of Abs to the cytoplasmic compartment (Fig. 7). There was a twofold decrease in nuclear Abs upon serum withdrawal. In the presence of 5% FBS, 26% ± 4% (n = 4) of the total cellular HA-Abs fractionated with nuclear proteins. This decreased to approximately 13% ± 2% (n = 4) after serum withdrawal. Thus, the cellular localization of Abs is responsive to factors present in FBS.
Fig. 7.
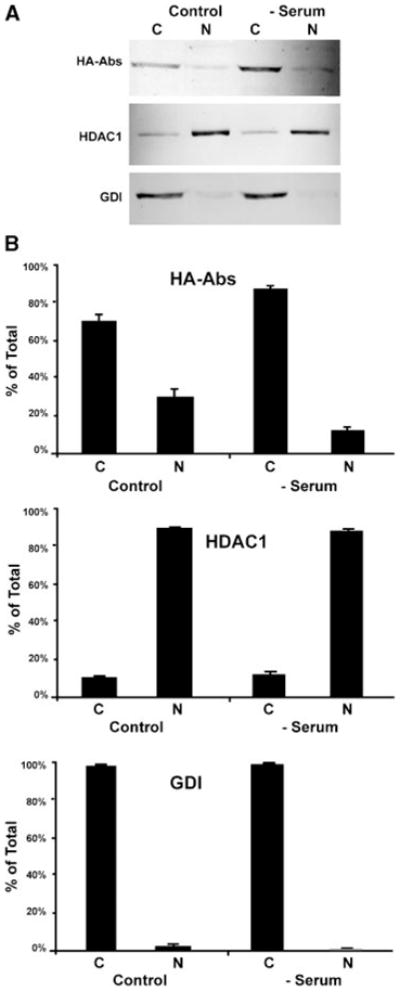
Serum withdrawal alters cellular localization of Abs and SNX2. HeLa cells were transfected with HA-Abs. After 24 h, fetal bovine serum (FBS) was withdrawn for 4 h (serum) prior to NE-PER nuclear fractionation while control cells were maintained in 5% FBS for the duration. A: Shows a representative Western immunoblot of the NE-PER fractions probed with anti-HA, HDAC, and GDI antibodies, as indicated. Each lane represents one quarter of the cytoplasmic (C) and nuclear (N) fractions. B: Quantitative analysis of the relative distribution of Abs, HDAC-1, and GDI in the NE-PER fractions indicate a shift in Abs from nuclear to cytoplasmic pool upon serum withdrawal (mean ± SEM; n = 4). The nuclear and cytoplasmic fractions were indicated by the nuclear and cytoplasmic localized HDAC1 and GDI, respectively.
Expression of Abs down regulates SNX2
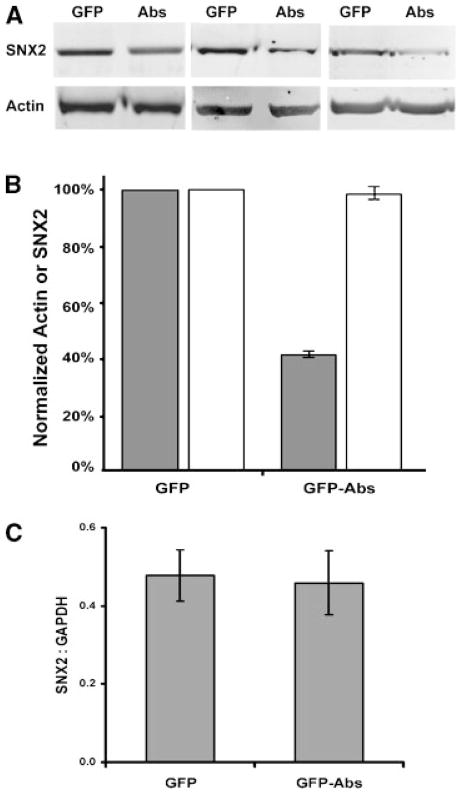
From the nuclear fractionation results (Fig. 5) and immunocytochemistry, we noticed a significant decrease in SNX2 expression upon co-expression of GFP-Abs. Because expression of Myc-tagged SNX2 can be easily influenced by transfection efficiency, we determined whether this decrease extended to endogenous SNX2 in cells transfected with Abs. We transfected HeLa cells with GFP control or GFP-Abs, and sorted the cells by fluorescence activated cell sorter (FACS) based on the GFP signal. The endogenous levels of SNX2 were then analyzed by quantitative Western immunoblot. A significant decrease (~41%) in endogenous SNX2 levels was observed in GFP-Abs expressing cells compared to GFP control (Fig. 8). No significant change in actin was observed in the GFP or GFP-Abs transfected cells. Since modulation of RNA metabolism is one of the known functions of DEAD-box helicases, we performed a quantitative RT-PCR analysis to determine whether this might be due to a decrease in steady state SNX2 mRNA. We established stable lines of HeLa cells expressing either GFP or GFP-Abs, and performed quantitative RT-PCR analysis of the levels of SNX2 mRNA normalized to that of GAPDH. No significant changes were observed on three independent cell lines of each. Therefore, we concluded that changes in transcription or steady state levels of SNX2 mRNA are unlikely to be the underlying cause of Abs-mediated down regulation.
Fig. 8.
Expression of GFP-Abs down regulates endogenous SNX2. HeLa transiently transfected with GFP control or GFP-Abs were enriched by fluorescence activated cell sorter (FACS). A: Crude lysate (15 μg) from three experiments were analyzed by Western immunoblot with anti-SNX2 antibodies. The blots were also probed for actin. B: Signal intensities were quantified using ImageQuant™ 5.2 Software (Amersham Biosciences, Piscataway, NJ). SNX2 (shaded bar) and actin (clear bar) values from GFP-Abs expressing cells were expressed as a percentage of the GFP controls. C: Quantitative RT-PCR analysis of HeLa cells stably transfected with GFP or GFP-Abs. SNX2 mRNA values normalized to that of GAPDH (mean ± SEM; n = 3) were from three independent stable lines.
DISCUSSION
We described here the unexpected interaction between the DEAD-box RNA helicase Abs and the endocytic vesicle protein SNX2. The interacting domain was confined to the PX domain of SNX2 (Fig. 1). PX domains are generally considered PI-binding modules (Worby and Dixon, 2002; Lemmon, 2003) with the PX domain of SNX2 preferentially binding to PI-3-P, PI-4-P, and PI-5-P in that order (Zhong et al., 2002). However, additions of the above PIs to the binding assay at a final concentration of 1 μM failed to significantly alter SNX2-Abs binding (data not shown), suggesting that the SNX2-Abs interacting site may not overlap with the PI-binding site or that access to the PX domain may require other factors as seen with the adaptor protein Fish (Abram et al., 2003). This may be the case for SNX2 as Abs bound strongly to the PX domain and much weaker when the N-terminal domain was present (Fig. 1C). SNX2 in turn interacted with the N-terminal domain of Abs. Interestingly, a bipartite NLS was detected at residues 42-58 of Abs in a search using PSORT (Nakai and Horton, 1999). This is likely to be the bona fide NLS as a GFP-fusion of the N-terminal domain containing this sequence directed nuclear localization of GFP fusion protein. The binding of SNX2 to this NLS-containing region raises the possibility that SNX2 might mask the NLS and alters nucleocytoplasmic shuttling of Abs.
Abs clearly undergoes nucleocytoplasmic shuttling. This conclusion was supported by four independent experimental observations. First, approximately 10% of GFP-Abs-expressing cells exhibited a noticeable cytosolic and nuclear distribution (Fig. 2B). This suggests that Abs can exit the nucleus under certain conditions. Second, all multi-nucleated cells in the cell fusion assay had a nuclear GFP-Abs signal (Fig. 3A,B), suggesting that the protein exited from one nucleus and imported by the other. Third, there was FRAP for one of the nuclei, with the recovery blocked by the nuclear export inhibitor LMB (Fig. 4A,B). This clearly indicates that recovery required CRM1-mediated export of GFP-Abs from the unbleached nucleus followed by entry into the bleached nucleus. Finally, NE-PER nuclear fractionation showed that Abs is present in both nuclear and cytosolic fractions (Fig. 5). We verified that this was not due to cross contamination during the NE-PER fractionation procedure as majority of the nuclear HDAC-1 and cytosolic GDIα markers remained at their expected cellular compartment. It is interesting to note that Abs appeared as triplet bands in the cytosolic fraction with only one or possibly two of these detectable in the nuclear fraction. Alkaline phosphatase treatment failed to alter mobility of these bands suggesting that phosphorylation state was not the primary cause (data not shown). Taken together, the data indicate that Abs shuttles between the nucleus and cytoplasm. Although it can potentially interact with SNX2 in the cytoplasm, the fact that SNX2 also undergoes nucleocytoplasmic shuttling in a CRM1-dependent manner (Fig. 3C,D) raises the possibility that the two proteins might also interact in the nucleus. Thus, SNX2 adds to the growing list of proteins in the endocytic pathway that shuttle between the nucleus and cytoplasm (Coda et al., 1998; Hyman et al., 2000; Poupon et al., 2002; Miaczynska et al., 2004). In this context, it behaves similarly to another sorting nexin, SNX6, that undergoes nucleocytoplasmic shuttling (Ishibashi et al., 2001). The broader functional role of nuclear translocation of endocytic proteins remains unclear.
We found that the level of Abs in the nucleus decreased while that in the cytoplasm increased upon serum withdrawal, suggesting translocation of Abs to the cytoplasm. This phenomenon may underlie the shift in cellular localization of Abs during Drosophila embryonic development. Abs is predominantly cytoplasmic during early developmental stages but translocates to the nucleus at later stages (Irion and Leptin, 1999). It is, therefore, likely that the lack of certain growth factor(s) during the early stages dictates the cytoplasmic localization of Abs while its subsequent nuclear translocation may coincide with expression of these factor(s). We also found that expression of Abs consistently reduced the expression of both endogenous and transfected SNX2. A change in transcription was ruled out as the primary cause since the endogenous and transfected SNX2, which were expressed under different promoters, were equally affected. Also, we did not observe any significant change in the steady state levels of SNX2 mRNA in Abs expressing cells. Taken together, Abs can either enhance the turnover of mRNA including SNX2 and GAPDH or exert a translational regulatory role. Both would be consistent with the broad effects of DEAD-box helicases on RNA metabolism and processes. This link with vesicle trafficking might be similar to that observed in yeast whereby disruption of the secretory pathway represses translation of ribosomal components, and induces a global change in protein expression (Deloche et al., 2004). This conceptual link between vesicle trafficking and gene expression is also strengthened by the recent discovery of APPL, another BAR-containing protein that undergoes nucleocytoplasmic shuttling, acting as a signaling link between endosomes and the nucleus (Miaczynska et al., 2004). Abs itself is known to regulate Inscuteable (Insc) protein levels (Irion et al., 2004). Although it physically interacts with Insc mRNA, Abs has no effect on Insc mRNA levels suggesting that it modulates Insc translation. This effect would be consistent with its influence on SNX2 expression. Thus, we favor the possibility that Abs also modulates translation of SNX2 and possibly other endocytic proteins. In this context, the SNX2-Abs interaction and subsequent recruitment of other factors might well provide the regulatory link to maintain the cellular level of SNX2. This linkage may be critical in transmitting membrane trafficking events to protein expression. Alternatively, interaction with SNX2 may simply mask the NLS of Abs causing it to remain in the cytoplasm and inhibits the translational machinery. In any case, this post-transcriptional control might serve to fine tune or safeguard against elevated levels of endocytic proteins that could potentially disrupt protein and organelle trafficking. Many interesting questions remain to be addressed regarding the role of Abs in, and whether this regulatory process extends to other proteins in the endocytic pathway.
Acknowledgments
We thank Beata Pekalska for her technical assistance. This work was supported by a Canadian Institutes of Health Research Operating Grant to J.K.N. and by a Natural Sciences and Engineering Research of Canada postgraduate scholarship to M.A.G.
Contract grant sponsor: Canadian Institutes of Health Research; Contract grant number: MOP-14642.
LITERATURE CITED
- Abram CL, Seals DF, Pass I, Salinsky D, Maurer L, Roth TM, Courtneidge SA. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J Biol Chem. 2003;278(19):16844–16851. doi: 10.1074/jbc.M300267200. [DOI] [PubMed] [Google Scholar]
- Coda L, Salcini AE, Confalonieri S, Pelicci G, Sorkina T, Sorkin A, Pelicci PG, Di Fiore PP. Eps15R is a tyrosine kinase substrate with characteristics of a docking protein possibly involved in coated pits-mediated internalization. J Biol Chem. 1998;273(5):3003–3012. doi: 10.1074/jbc.273.5.3003. [DOI] [PubMed] [Google Scholar]
- Conibear E, Stevens TH. Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim Biophys Acta. 1998;1404(1–2):211–230. doi: 10.1016/s0167-4889(98)00058-5. [DOI] [PubMed] [Google Scholar]
- Cozier GE, Carlton J, McGregor AH, Gleeson PA, Teasdale RD, Mellor H, Cullen PJ. The phox homolosgy (PX) domain-dependent, 3-phosphoinositide-mediated association of sorting Nexin-1 with an early sorting endosomal compartment is required for its ability to regulate epidermal growth factor receptor degradation. J Biol Chem. 2002;277(50):48730–48736. doi: 10.1074/jbc.M206986200. [DOI] [PubMed] [Google Scholar]
- Deloche O, de la Cruz J, Kressler D, Doere M, Linder P. A membrane transport defect leads to a rapid attenuation of translation initiation in Saccharomyces cerevisiae. Mol Cell. 2004;13(3):357–366. doi: 10.1016/s1097-2765(04)00008-5. [DOI] [PubMed] [Google Scholar]
- Duncan PI, Stojdl DF, Marius RM, Bell JC. In vivo regulation of alternative pre-mRNA splicing by the Clk1 protein kinase. Mol Cell Biol. 1997;17(10):5996–6001. doi: 10.1128/mcb.17.10.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlich D, Mattaj IW. Nucleocytoplasmic transport. Science. 1996;271(5255):1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- Gougeon PY, Prosser DC, Da-Silva LF, Ngsee JK. Disruption of Golgi morphology and trafficking in cells expressing mutant prenylated rab acceptor-1. J Biol Chem. 2002;277(39):36408–36414. doi: 10.1074/jbc.M205026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft CR, de la Luz Sierra M, Barr VA, Haft DH, Taylor SI. Identification of a family of sorting nexin molecules and characterization of their association with receptors. Mol Cell Biol. 1998;18(12):7278–7287. doi: 10.1128/mcb.18.12.7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft CR, de la Luz Sierra M, Bafford R, Lesniak MA, Barr VA, Taylor SI. Human orthologs of yeast vacuolar protein sorting proteins Vps26, 29, and 35: Assembly into multimeric complexes. Mol Biol Cell. 2000;11(12):4105–4116. doi: 10.1091/mbc.11.12.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto T, Uozumi T, Beppu T. Leptomycins A and B, new antifungal antibiotics. III. Mode of action of leptomycin B on Schizosaccharomyces pombe. J Antibiot (Tokyo) 1985;38(11):1573–1580. doi: 10.7164/antibiotics.38.1573. [DOI] [PubMed] [Google Scholar]
- Horazdovsky BF, Davies BA, Seaman MN, McLaughlin SA, Yoon S, Emr SD. A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol Biol Cell. 1997;8(8):1529–1541. doi: 10.1091/mbc.8.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman J, Chen H, Di Fiore PP, De Camilli P, Brunger AT. Epsin 1 undergoes nucleocytosolic shuttling and its eps15 interactor NH(2)-terminal homology (ENTH) domain, structurally similar to Armadillo and HEAT repeats, interacts with the transcription factor promyelocytic leukemia Zn(2)+ finger protein (PLZF) J Cell Biol. 2000;149(3):537–546. doi: 10.1083/jcb.149.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irion U, Leptin M. Developmental and cell biological functions of the Drosophila DEAD-box protein abstrakt. Curr Biol. 1999;9(23):1373–1381. doi: 10.1016/s0960-9822(00)80082-2. [DOI] [PubMed] [Google Scholar]
- Irion U, Leptin M, Siller K, Fuerstenberg S, Cai Y, Doe CQ, Chia W, Yang X. Abstrakt, a DEAD box protein, regulates Insc levels and asymmetric division of neural and mesodermal progenitors. Curr Biol. 2004;14(2):138–144. doi: 10.1016/j.cub.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Maita H, Yano M, Koike N, Tamai K, Ariga H, Iguchi-Ariga SM. Pim-1 translocates sorting nexin 6/TRAF4-associated factor 2 from cytoplasm to nucleus. FEBS Lett. 2001;506(1):33–38. doi: 10.1016/s0014-5793(01)02881-2. [DOI] [PubMed] [Google Scholar]
- Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multi-vesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3(12):893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Kawamura H, Tomozoe Y, Akagi T, Kamei D, Ochiai M, Yamada M. Identification of the nucleocytoplasmic shuttling sequence of heterogeneous nuclear ribonucleoprotein D-like protein JKTBP and its interaction with mRNA. J Biol Chem. 2002;277(4):2732–2739. doi: 10.1074/jbc.M108477200. [DOI] [PubMed] [Google Scholar]
- Kurten RC, Cadena DL, Gill GN. Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science. 1996;272(5264):1008–1010. doi: 10.1126/science.272.5264.1008. [DOI] [PubMed] [Google Scholar]
- Lemmon MA. Phosphoinositide recognition domains. Traffic. 2003;4(4):201–213. doi: 10.1034/j.1600-0854.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Linder P, Stutz F. mRNA export: Travelling with DEAD box proteins. Curr Biol. 2001;11(23):R961–963. doi: 10.1016/s0960-9822(01)00574-7. [DOI] [PubMed] [Google Scholar]
- Luking A, Stahl U, Schmidt U. The protein family of RNA helicases. Crit Rev Biochem Mol Biol. 1998;33(4):259–296. doi: 10.1080/10409239891204233. [DOI] [PubMed] [Google Scholar]
- Martincic I, Peralta ME, Ngsee JK. Isolation and characterization of a dual prenylated Rab and VAMP2 receptor. J Biol Chem. 1997;272(43):26991–26998. doi: 10.1074/jbc.272.43.26991. [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116(3):445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- Nakai K, Horton P. PSORT: A program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci. 1999;24(1):34–36. doi: 10.1016/s0968-0004(98)01336-x. [DOI] [PubMed] [Google Scholar]
- Paine PL, Moore LC, Horowitz SB. Nuclear envelope permeability. Nature. 1975;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: The amphiphysin BAR structure. Science. 2003;26:26. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- Poupon V, Polo S, Vecchi M, Martin G, Dautry-Varsat A, Cerf-Bensussan N, Di Fiore PP, Benmerah A. Differential nucleocytoplasmic trafficking between the related endocytic proteins Eps15 and Eps15R. J Biol Chem. 2002;277(11):8941–8948. doi: 10.1074/jbc.M108385200. [DOI] [PubMed] [Google Scholar]
- Sato TK, Overduin M, Emr SD. Location, location, location: Membrane targeting directed by PX domains. Science. 2001;294(5548):1881–1885. doi: 10.1126/science.1065763. [DOI] [PubMed] [Google Scholar]
- Schmucker D, Vorbruggen G, Yeghiayan P, Fan HQ, Jackle H, Gaul U. The Drosophila gene abstrakt, required for visual system development, encodes a putative RNA helicase of the DEAD box protein family. Mech Dev. 2000;91(1–2):189–196. doi: 10.1016/s0925-4773(99)00298-1. [DOI] [PubMed] [Google Scholar]
- Schwarz DG, Griffin CT, Schneider EA, Yee D, Magnuson T. Genetic analysis of sorting nexins 1 and 2 reveals a redundant and essential function in mice. Mol Biol Cell. 2002;13(10):3588–3600. doi: 10.1091/mbc.E02-03-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol. 2004;165(1):111–122. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142(3):665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchi M, Polo S, Poupon V, van de Loo JW, Benmerah A, Di Fiore PP. Nucleocytoplasmic shuttling of endocytic proteins. J Cell Biol. 2001;153(7):1511–1517. doi: 10.1083/jcb.153.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worby CA, Dixon JE. Sorting out the cellular functions of sorting nexins. Nat Rev Mol Cell Biol. 2002;3(12):919–931. doi: 10.1038/nrm974. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hortsman H, Seet L, Wong SH, Hong W. SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat Cell Biol. 2001;3(7):658–666. doi: 10.1038/35083051. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Lazar CS, Tronchere H, Sato T, Meerloo T, Yeo M, Songyang Z, Emr SD, Gill GN. Endosomal localization and function of sorting nexin 1. Proc Natl Acad Sci USA. 2002;99(10):6767–6772. doi: 10.1073/pnas.092142699. [DOI] [PMC free article] [PubMed] [Google Scholar]