Abstract
Variation in personality traits is 30% to 60% attributed to genetic influences. Attempts to unravel these genetic influences at the molecular level have, so far, been inconclusive. We performed the first genome-wide association study of Cloninger’s temperament scales in a sample of 5117 individuals, in order to identify common genetic variants underlying variation in personality. Participants’ scores on Harm Avoidance, Novelty Seeking, Reward Dependence, and Persistence were tested for association with 1,252,387 genetic markers. We also performed gene-based association tests and biological pathway analyses. No genetic variants that significantly contribute to personality variation were identified, while our sample provides over 90% power to detect variants that explain only 1% of the trait variance. This indicates that individual common genetic variants of this size or greater do not contribute to personality trait variation, which has important implications regarding the genetic architecture of personality and the evolutionary mechanisms by which heritable variation is maintained.
Keywords: genome-wide association, genes, personality, temperament, mutation, selection, maintenance of genetic variation, evolution
Introduction
Personality generally refers to those characteristics of the person that account for consistent patterns of feeling, thinking, and behaving (Pervin, Cervone, & John, 2005). Modern personality research focuses primarily on personality traits – dimensions of variation between individuals that are relatively stable over time and predict behaviour in various domains. The most prominent taxonomy of personality traits, the ‘Big Five’, is based on atheoretical factor analyses of self-descriptions. An alternative taxonomy, developed by Cloninger (Cloninger, 1986, 1987), aims to reflect the psychobiological etiology of personality. This model is purportedly based on empirical findings from genetic research, studies of longitudinal development, and psychometric studies of personality structure, as well as neuropharmacological and neuroanotomical studies of behaviour and learning (Cloninger, 1986). The model is widely utilised, although some studies have revealed psychometric limitations (Farmer & Goldberg, 2008) or failed to find support for the structure of the model at the biological or psychological level (Herbst, Zonderman, McCrae, & Costa, 2000).
Cloninger’s model originally consisted of three dimensions of personality (temperaments): Novelty Seeking, Harm Avoidance, and Reward Dependence, measured by the Tridimensional Personality Questionnaire (TPQ; Cloninger, 1986; Cloninger, Przybeck, & Svrakic, 1991). Reward Dependence originally included items measuring persistence, but the persistence items were later revealed to be uncorrelated with other Reward Dependence items; in a revised model, Persistence was designated as a fourth dimension of temperament (Cloninger, 1994). Although Cloninger also extended his model with three additional character (aspects of self concept) dimensions, measured by the Temperament and Character Inventory (TCI), we focus here on the four dimensions of temperament. Correlations between the different temperament scales are low and principal components analysis identifies each temperament as a separate factor (Cloninger, Svrakic, & Przybeck, 1993; Keller, Coventry, Heath, & Martin, 2005).
The four temperament dimensions of the psychobiological model are hypothesised to be associated with genetically independent neurobiological systems. Individual differences on these dimensions are thought to be the basis of individual variation in personality (Cloninger, 1986). Novelty Seeking reflects the tendency to respond strongly to novelty and cues for reward as well as relief from punishment, and is thought to play a role in the activation or initiation of behaviours. Harm Avoidance reflects the tendency to respond strongly to aversive stimuli, which leads to learned inhibition of behaviour, and is thought to play a role in the inhibition or ceasing of behaviours. Reward Dependence reflects the tendency to react strongly to rewards and to maintain behaviours previously associated with reward or relief of punishment, and is thought to play a role in the maintenance or continuation of behaviour (Cloninger, 1986, 1987). Persistence reflects the tendency to persevere despite frustration and fatigue (Cloninger, et al., 1993).
Based on evidence from physiopsychological and animal studies, variation in the temperament dimensions is thought to be influenced by activity in specific neurotransmitter pathways (Cloninger, 1986, 1987) – Novelty Seeking by dopaminergic activity, Harm Avoidance by serotonergic activity, and Reward Dependence by noradrenergic activity. Gerra et al. (2000) found that individual’s hormonal response to specific neurotransmitter agonists correlated with TPQ scale scores in accordance with Cloninger’s theory, but further evidence is needed to support the relationship between the different neurotransmitter pathways and Cloninger’s temperament scales.
As Cloninger (1987) predicted, scores on certain TPQ/TCI scales are associated with specific problem behaviours and psychological disorders, including depression, anxiety, bipolar disorder, obsessive compulsive disorder, conduct disorder, alcohol and drug dependence, criminal behaviour and antisocial personality disorder (Ettelt, et al., 2008; Howard, Kivlahan, & Walker, 1997; Khan, Jacobson, Gardner, Prescott, & Kendler, 2005; Mulder, Joyce, Sullivan, Bulik, & Carter, 1999; Nery, et al., 2008; Nery, et al., 2009; Ongur, Farabaugh, Iosifescu, Perlis, & Fava, 2005). Thus, the temperament scales are potential endophenotypes for these behaviours and disorders. An endophenotype is a more basic, heritable, underlying quantitative trait, which more directly reflects the influence of the genome (Gottesman & Gould, 2003).
Heritability estimates for the TPQ/TCI scales range from approximately 30% to 60% (Gillespie, Cloninger, Heath, & Martin, 2003; Gillespie, Johnstone, Boyce, Heath, & Martin, 2001; Heath, Cloninger, & Martin, 1994; Heiman, Stallings, Hofer, & Hewitt, 2003; Keller, et al., 2005), consistent with heritability estimates for other personality scales such as the Big Five (Jang, Livesley, & Vernon, 1996) and Eysenck’s personality dimensions (Keller, et al., 2005; Zietsch, Verweij, Bailey, Wright, & Martin, 2010). Despite these substantial genetic influences, identifying the specific genetic variants underlying individual differences on TPQ/TCI and other personality scales has proved difficult.
Genetic linkage and candidate gene association studies on personality have yielded mixed results. Linkage studies test for coinheritance of genetic markers and traits within families. There have been various linkage findings for the different personality scales, including Neuroticism (Kuo, et al., 2007; Neale, Sullivan, & Kendler, 2005; Wray, et al., 2008), Harm Avoidance (Cloninger, et al., 1998), Novelty Seeking (Curtis, 2004), Psychoticism and Extraversion (Gillespie, et al., 2008), but none have been consistently replicated. Candidate gene association studies test for a correlation in the population between scores on a personality scale and a specific genetic variant with a known function that could relate to personality. The two most extensively studied candidate genes are the dopamine D4 receptor gene DRD4 and serotonin transporter gene SLC6A4. Several studies have found association between a variant of the dopamine D4 receptor gene and Novelty Seeking (Benjamin, et al., 1996; Ebstein, Nemanov, Klotz, Gritsenko, & Belmaker, 1997; Ebstein, et al., 1996), and between a polymorphism (5-HTTLPR) in the promoter region of the serotonin transporter gene and anxiety-related traits like Harm Avoidance (Lesch, et al., 1996; Vormfelde, et al., 2006). However, other studies were unsuccessful in replicating these associations (i.e., Becker, El-Faddagh, Schmidt, & Laucht, 2007; Ebstein, Gritsenko, et al., 1997; Herbst, et al., 2000; Lang, et al., 2004; Malhotra, et al., 1996). A recent meta-analysis (Munafo, Yalcin, Willis-Owen, & Flint, 2008) concluded that the DRD4 gene (C-521T polymorphism) may be associated with Novelty Seeking and Impulsivity, explaining up to 3% of the phenotypic variance, but that publication bias may have distorted the findings. Another meta-analysis (Munafo, et al., 2009) found no significant association of the 5-HTTLPR genotype with Harm Avoidance or Eysenck’s Neuroticism scale, but they did report a significant association with the NEO Neuroticism scale. Two more recent large studies provided further mixed evidence, with one reporting no association of 5-HTTLPR with Neuroticism (Terracciano, et al., 2009), and the other finding a significant association with Neuroticism but not Harm Avoidance (Wray, et al., 2009).
Recent technological advances have enabled genome-wide association (GWA) studies. Here, single nucleotide polymorphisms (SNPs) across the entire genome are systematically tested for association with a given trait. The approach is considered “hypothesis-free” since no prior knowledge of gene function is considered. GWA studies have been successful in identifying some genetic variants underlying disease traits (Burton, et al., 2007; Visscher & Montgomery, 2009). They have also had some success in identifying genetic variants associated with smoking (e.g. LiuTozzi, et al., 2010), and with complex mental disorders including schizophrenia (Shi, et al., 2009; Stefansson, et al., 2009; The International Schizophrenia Consortium, 2009), bipolar disorder (The International Schizophrenia Consortium, 2009), and autism (Wang, et al., 2009). However, despite the high heritability of these disorders and traits, the identified genetic variants have been of very small effect (<1% of variance accounted for) and the aggregate effect of all the individual variants only accounts for a few percent of the trait variance, at most.
It is now thought that the genetic architecture of mental disorder is very complex, and may be difficult to resolve using standard GWA approaches (Manolio, et al., 2009; The Psychiatric GWAS Consortium Steering Committee, 2009). In particular, Keller and Miller (2006) argue, based on evolutionary genetic theory and empirical evidence, that mental disorder is likely to be due to the aggregate effect of many mildly harmful rare mutations, impossible to detect with standard GWA studies. However, using similar evolutionary genetic theory, Penke, Dennison and Miller (2007) argue that personality traits are likely to be under balancing selection, and therefore influenced by a limited number of common genetic variants of medium effect. If this is true, personality may be an ideal psychological trait to attack with the GWA approach.
Early evidence has been mixed. There have been three published GWA studies on personality traits - two on Neuroticism and one assessing all Big Five traits. Van den Oord et al. (2008) found potential association between variants in the MAMDC1 gene and Neuroticism, and Shifman et al. (2008) found suggestive association between the PDE4D gene and Neuroticism, which was replicated in one sample, but failed to replicate in two other samples. Terracciano et al. (2008) found potential association signals for all five scales, but the effect sizes were small and most of the associations failed to replicate in their follow-up samples. GWA has yet to be applied to Cloninger’s temperament scales, which could better reflect genetic influences given their purported basis in psychobiological experiments and theory.
In a sample of 5117 Australians of European ancestry from 2567 families, we perform the first GWA study of Cloninger’s temperament scales, in order to identify common genetic variants associated with individual differences in personality. Identification of genetic variants underlying personality traits might also broaden our understanding of behavioural and psychiatric disorders related to personality. On the other hand, if we do not detect any genetic variants that explain a substantial part of the variance in these traits, this would have strong implications regarding the genetic architecture of personality variation and its evolutionary basis.
Method
Participants
Health and Lifestyle Questionnaires were sent to two cohorts of Australian twins and their families (parents, children, spouses and siblings), the first in 1988 and the second in 1990. The total number of participants was over 27,000, with an age range of 17 to 96 (M = 39.7, SD = 15.3). Phenotypic data on the TPQ were available for 20,464 individuals, of which 5117 (1727 males and 3390 females) from 2567 independent families were genotyped. Phenotypic and genotypic data collection was approved by the Queensland Institute of Medical Research (QIMR) Ethics Committee and informed consent was obtained from all participants. More details about the phenotypic data collection can be found elsewhere (Heath, et al., 1994; Keller, et al., 2005).
Personality measures
A short version of the original TPQ was included as part of the Health and Lifestyle Questionnaire. Although the TPQ originally measured only three dimensions, subsequent revisions of the measure resulted in the addition of an extra dimension, Persistence. As such, we analysed five items that originally contributed to Reward Dependence as a separate Persistence scale. Additionally, after revision of the scale, one of the Reward Dependence items was assigned to Novelty Seeking. Our final personality measure included 18 Harm Avoidance, 19 Novelty Seeking, 12 Reward Dependence, and 5 Persistence items.
Each item could be answered with a true/false response and to avoid response set bias the items were phrased in such a way that for some items a true and for others a false answer adds to the subscale score. Scale scores were calculated by summing the item scores for each scale. For the complete sample (i.e. including the non-genotyped individuals), we then performed the following data preparation procedure. Missing items were replaced with the sample mean score on the specific item. Individuals with missing values on more than 25% of the scales’ items were treated as missing for that scale. Scale scores were then transformed by taking the arcsine of the square root, in order to minimize departures from normality (Eaves, Eysenck, & Martin, 1989; Freeman & Tukey, 1950). Finally, scores were corrected for age, age2, sex, sex*age, and sex*age2 effects and all scales were standardised separately for each sex to a mean of 0 and a standard deviation of 1.
Previous behaviour genetic analyses of the twins and siblings in our phenotypic sample indicated broad heritability estimates of 45% and 42% for Novelty Seeking, 40% and 40% for Harm Avoidance, 35% and 38% for Reward Dependence and 35% and 35% for Persistence, for males and females respectively, with remaining variance explained by unshared environmental influences (Keller, et al., 2005). Note that Keller et al. (2005) analysed one item as contributing to the Reward Dependence scale while we assigned it to the Novelty Seeking scale in accordance to the scales’ revision. For all temperament scales, reliability and internal consistency were determined to be satisfactory to good in an earlier study using subsamples of the complete phenotypic sample (Keller, et al., 2005). The 2.1 year test-retest correlations (as tested in 881 twins) was 0.79, 0.73, 0.68, and 0.64 for Harm Avoidance, Novelty Seeking, Reward Dependence, and Persistence, respectively; Cronbach’s α (as tested in 7834 to 7862 twins and siblings) was 0.61, 0.68, 0.75, and 0.84 for Harm Avoidance, Novelty Seeking, Reward Dependence, and Persistence, respectively. These reliability and internal consistency values are in accordance with those reported in other TPQ/TCI studies (Cloninger, 1994; Cloninger, et al., 1993).
In the same study on all twins and siblings in our phenotypic dataset evidence was found for sex differences in the source of genetic variation for Harm Avoidance and Reward Dependence (Keller, et al., 2005), implying that partly different genetic factors explain variance in these scales for males and females. Therefore, for these two scales we ran the GWA analysis separately for males and females, as well as for the sample as a whole.
Genotyping, quality control, and imputation procedures
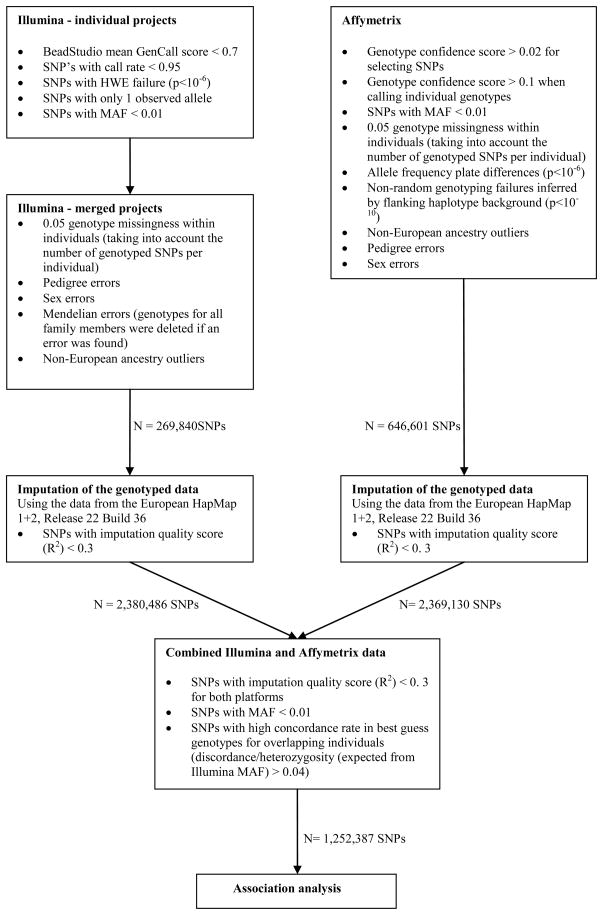
The QIMR Genetic Epidemiology Laboratory has collected a wide range of phenotypic variables and DNA samples as part of different projects. DNA samples were collected in accordance with standard protocols and submitted to different genotype centres using different SNP platforms (Illumina 317K, Illumina HumanCNV370-Quadv3, Illumina Human610-Quad, and Affymetrix 6.0). The quality control (QC) procedure we employed for the combined use of these Illumina and Affymetrix genotype data consisted of three steps (see Figure 1, Appendix I). Initial QC control - including checks for ancestry outliers, Hardy Weinberg Equilibrium, Mendelian errors, Minor Allele Frequency (MAF) - was applied separately to all different projects. Full details of these initial QC procedures for the Illumina and Affymetrix data are described in detail in Medland et al. (2009) and Wray et al. (submitted), respectively. As the individuals genotyped on the Affymetrix platform comprised a sample of major depressive disorder cases only, more stringent QC criteria were applied. We had 327 individuals genotyped on both the Illumina and the Affymetrix platform, allowing for cross project QC. These checks led us to a more stringent value (>0.02; using the Birdseed program) for the genotype confidence score for the Affymetrix data for selecting which SNPs to include in our dataset. After QC control of the individual projects, the data from the different Illumina projects were combined and additional QC was applied to this combined dataset.
Figure 1.
Summary information of the Quality Control procedure of the genotype data. Shown are the cut-offs for dropping SNPs or individuals from the dataset.
MAF=Minor allele frequency, HWE=Hardy Weinberg Equilibrium, SNPs=Single Nucleotide Polymorphism, * using the Birdseed v2 algorithm as implemented in the BirdSuite software (Korn, et al., 2008), **as implemented in PLINK mishap test (Purcell, et al., 2007).
The genotyped SNPs in common between the remaining Illumina (N=269,840) and Affymetrix (N=646,601) SNPs were relatively few (N=137,768). Therefore, genome-wide association analyses of the combined data could only be conducted using imputed genotypes. After initial QC checks, both datasets were imputed separately by MACH (Abecasis, unpublished) using the data from the European HapMap 1+2, Release 22 Build 36. Only SNPs with an imputation quality score (R2) greater than 0.3 were retained, which resulted in a total number of 2,380,486 imputed Illumina and 2,369,130 imputed Affymetrix SNPs.
SNPs were retained for analysis only if imputed successfully on both platforms, had a MAF > 0.01, and had high concordance in best-guess genotypes for the 327 individuals imputed twice (from Affymetrix and Illumina genotypes; the family-based association analysis requires use of best-guess genotypes). Specifically, high concordance was measured as discordance/heterozygosity > 0.04, where discordance is the proportion of individuals with discordant genotypes and heterozygosity = 2*MAF*(1-MAF), and MAF is the minor allele frequency estimated from the Illumina imputed set. The correction for heterozygosity removes the dependence of discordance rate on MAF. In total, 1,252,387 SNPs were available for association analyses, representing SNPs that are strongly validated for all samples. After QC, if only one individual from a monozygotic twin pair had been genotyped, the non-genotyped co-twin was assigned that genotype as well. The final genotyped sample in this study included 5117 individuals from 2567 families, including 797 monozygotic (MZ) twin pairs (1702 MZ twin individuals), of which 680 MZ individuals were assigned their cotwin’s genotype.
Statistical analyses
After imputation and quality control, the combined genotypic dataset consisted of 1,252,387 SNPs. The best guess genotype at each SNP was tested for association with the four TPQ scales using the family-based association test as implemented in Merlin (-fast-assoc, Chen & Abecasis, 2007), which accounts for family relationships including MZ twins. The additive genetic effect was calculated, in which the genotypic mean of the heterozygote (Aa) was modelled as the average of the two homozygotes (AA, aa). Because sex differences in the source of genetic variance have been found for Reward Dependence and Harm Avoidance, we also performed the association test for these variables for males and females separately. Association analyses of genotyped markers on the X-chromosome was conducted using Minx (as implemented in Merlin). Because the imputation software did not support sex chromosomes, SNPs at the X-chromosome are not imputed; the association analyses only included those SNPs that have been genotyped for at least 85% of the sample (N=7526). Association between a SNP and a phenotype is generally accepted to be genome-wide significant at α = 0.05 if the p value is 7.2*10−8 or smaller, as this corrects for the total number of independent tests (Dudbridge & Gusnanto, 2008). We performed eight separate association analyses, so declared significance level at 9.0*10−9 (7.2*10−8/8).
In order to determine if there are genes which harbour an excess of associated variants, we conducted a gene-based test (VEGAS) that can be used for GWAS with related individuals (Liu, McRae, et al., 2010). Genes are functional groups of nucleotides that code for proteins. The test summarises evidence for association on a per gene basis by considering the p-value of all SNPs within genes (including +/−50kb from the 5′ and 3′ UTR), while accounting for linkage disequilibrium (LD) and number of SNPs per gene1. The gene-based test identifies genes which show more signal of association than expected by chance given their length and LD between the SNPs. As such it tests for a different genetic architecture of genes than single SNP tests. The relevance of the gene-based test depends on the underlying genetic architecture of genes which is unknown and which is expected to differ between genes. Because we perform eight gene-based association tests each including 17,206 autosomal genes, we consider genes with a p-value below α = 3.6*10−7 (0.05/(8*17,206)) to be significant.
To detect underlying biological pathways of importance to personality, all genes with an empirical p value below p = 0.01 were included in a pathway analysis using the Ingenuity Pathway analysis program (Ingenuity Systems, release IPA 6.0). The Ingenuity database contains large amounts of up-to-date information (based on scientific publications) about the localisation, structure and biological function of proteins and their interactions. By means of pathway analysis it is possible to check whether the genes most associated with personality in our gene-based test are more prevalent in any known biological or canonical pathway than would be expected by chance. We set the alpha level at 0.01 and p-values for each pathway were corrected by the Benjamini-Hochberg multiple testing correction as implemented in Ingenuity. We used an alpha level of 0.01 rather than 0.05 to account for the multiple traits testing.
Statistical power
It is expected that many genes of very small effect size contribute to the genetic variance of complex behavioural phenotypes like personality. We estimated the empirical power our sample provides to detect genetic variants explaining 1% and 0.5% of the phenotypic variance by running association tests on simulated datasets in Merlin. The simulated datasets that are generated are similar to the original data in terms of marker informativeness, allele frequency, trait distribution, and missing data patterns, but original phenotypic values and individual’s genotypes for a selected SNP are replaced. The selected SNP is simulated such that it accounts for a specified proportion of the variance. The Merlin command we used is ‘--simulate --trait [variable name],[SNPname],0.01,0.39,0.60’, implying that the marker accounts for 0.01 of the phenotypic variance for a trait with a heritability of 0.40. The selected SNP we choose had a minor allele frequency of 0.25. For more information about the simulation procedure see http://www.sph.umich.edu/csg/abecasis/Merlin/reference/simulation.html.
Association analysis was conducted on 1000 data sets generated by the simulation procedure. The empirical power is estimated as that proportion of the 1000 association analyses in which a genome-wide significant association (α = 7.2*10−8) was detected. Results indicated that our sample provides 91.5% power to detect SNPs that explain 1% of the variance in the personality traits, and 26.2% power to detect SNPs that explain 0.5%.
Results
The average age of the genotyped sample is 34.7 years (SD=11.1) for males and 36.9 years (SD=12.5) for females. Older participants of both sexes scored lower on Novelty Seeking. Male and female means for the four temperament scales are shown in Table 1. In accordance to earlier findings (e.g., Stallings, Hewitt, Cloninger, Heath, & Eaves, 1996), females score higher on the Harm Avoidance and Reward Dependence scales, while males score slightly higher on Novelty Seeking and Persistence. The scales were therefore adjusted for sex and age effects and their interactions for the subsequent association analyses.
Table 1.
Descriptive statistics for Cloninger’s personality scales.
| Males | Females | |||||
|---|---|---|---|---|---|---|
| N | Range | Mean (SD) | N | Range | Mean (SD) | |
| Harm Avoidance | 1721 | 0–18 | 6.1 (4.2) | 3375 | 0–18 | 7.9 (4.3) |
| Novelty Seeking | 1716 | 0–19g | 8.5 (3.9) | 3371 | 0–19 | 8.2 (3.7) |
| Reward Dependence | 1721 | 0–12 | 6.7 (2.7) | 3375 | 0–12 | 8.4 (2.4) |
| Persistence | 1717 | 0–5 | 3.0 (1.5) | 3365 | 0–5 | 2.9 (1.5) |
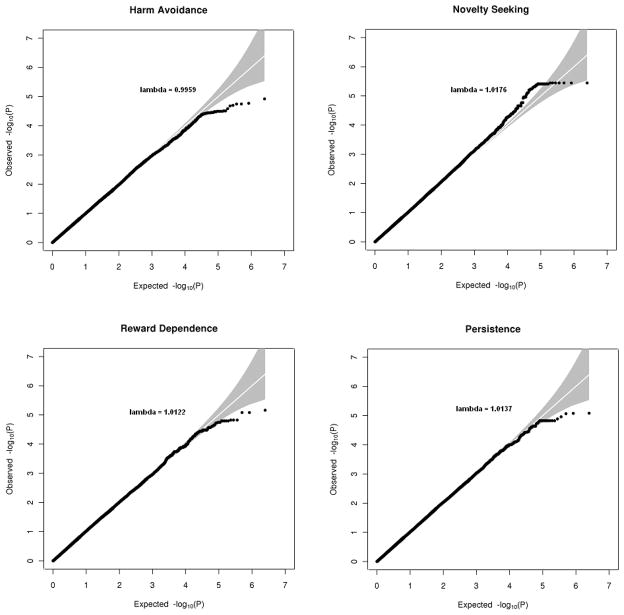
We tested 1,252,387 SNPs for association with four personality scales; Harm Avoidance, Novelty Seeking, Reward Dependence and Persistence. The Quantile-Quantile (QQ) plots for each scale, illustrating the observed p-values for the autosomal associations in relation to the expected p-values (based on the number of tests, under the null hypothesis of no association), are presented in Figure 2. Lambda (a measure for quantifying population stratification effects) for all variables is close to 1, indicating the residual population stratification effects are minimal. For three of the four scales we found fewer extremely low (at the very low end of the distribution) p-values than expected by chance. We checked whether this was due to the fact we use family data by running a GWAS with only independent individuals in Merlin as well as in PLINK (Purcell, et al., 2007). We also checked whether it was due to high LD between SNPs by testing only independent SNPs in Plink. The QQ-plots from these analyses did not differ markedly from the original analyses.
Figure 2.
Q-Q plots of observed and expected −log10(P) of the associations between SNPs and the four personality scales. Grey areas represent 95% confidence intervals.
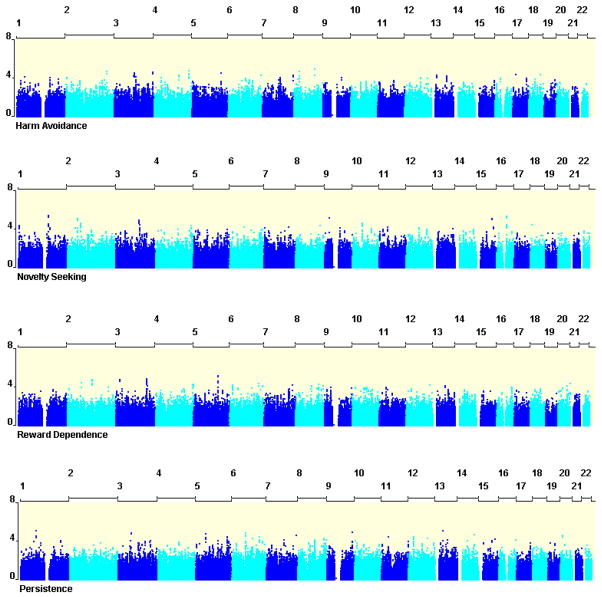
Results of our association analyses are shown in Figure 3. The lack of data points near the top of each panel (i.e. p ≈ 10−8) indicates that there were no strong association signals. SNPs in the top 50 with lowest p-values for each temperament scale are presented in Table 2 (excluding redundant SNPS that are in high LD (r2 > .70) with more significant SNPs).
Figure 3.
Results of the genome-wide association analyses for Cloninger’s personality scales. The x-axis shows the chromosome numbers and the y-axis the significance of the association signals (i.e. −log10(P) value).
Table 2.
Genetic markers showing strongest association with each of Cloninger’s personality scales (independent markers within top 50 SNPs).
| Chr | SNP | Base pair location | p-value | SNPs in LD | Minor allele | MAF | Heritability | Effect size | Closest gene | location |
|---|---|---|---|---|---|---|---|---|---|---|
| Harm avoidance | ||||||||||
| 8 | rs11780799 | 103048187 | 1.1 * 10−5 | 2 | A | .47 | .44% | .10 | NCALD | Intronic |
| 2 | rs10490747 | 207572396 | 1.7 * 10−5 | 1 | A | .29 | .41% | −.10 | DYTN | Intronic |
| 4 | rs11132986 | 174371781 | 2.0 * 10−5 | 9 | T | .23 | .41% | .11 | Upstream | |
| 8 | rs17057051 | 27227554 | 2.0 * 10−5 | G | .31 | .42% | .10 | PTK2B | Intronic | |
| 3 | rs12330727 | 100833748 | 2.5 * 10−5 | 15 | C | .15 | .43% | −.13 | Intergenic | |
| 3 | rs7625694 | 190117490 | 2.7 * 10−5 | 8 | T | .21 | .39% | .11 | CLDN16 | Intronic |
| 5 | rs11744339 | 145978081 | 3.3 * 10−5 | G | .12 | .38% | −.14 | PPP2R2B | Intronic | |
| 18 | rs7231234 | 59362518 | 3.5 * 10−5 | T | .21 | .38% | −.11 | Intergenic | ||
| 6 | rs1923380 | 165491551 | 3.80 * 10−5 | G | .25 | .39% | −.10 | Intergenic | ||
| 17 | rs971718 | 13125049 | 3.8 * 10−5 | 2 | C | .04 | .36% | −.23 | Intergenic | |
| 13 | rs885219 | 29246206 | 4.5 * 10−5 | G | .16 | .37% | .12 | POMP | Intronic | |
| 4 | rs17008522 | 125652667 | 4.9 * 10−5 | G | .32 | .38% | −.10 | ANKRD50 | Intergenic | |
| 13 | rs9544495 | 77997413 | 5.0 * 10−5 | A | .34 | .37% | −.09 | Intergenic | ||
| Novelty Seeking | ||||||||||
| 16 | rs4131099 | 51330531 | 3.8 * 10−6 | 4 | A | .22 | .50% | .11 | Intergenic | |
| 1 | rs3120665 | 152316590 | 4.0 * 10−6 | 21 | G | .16 | .48% | −.14 | Non-coding region | |
| 9 | rs961831 | 22362104 | 6.6 * 10−6 | 1 | G | .09 | .46% | −.17 | Intergenic | |
| 15 | rs1533665 | 78530940 | 7.3 * 10−6 | 3 | G | .36 | .45% | −.10 | ACSBG1 | Upstream |
| 2 | rs10176705 | 50744774 | 9.2 * 10−6 | 5 | T | .37 | .45% | .10 | NRXN1 | Intronic |
| 3 | rs1835856 | 116491672 | 1.1 * 10−5 | 8 | T | .16 | .43% | −.13 | LSAMP | Intronic |
| 2 | rs7588898 | 68041842 | 2.0 * 10−5 | 1 | G | .38 | .42% | .10 | Non-coding region | |
| Reward Dependence | ||||||||||
| 5 | rs1078425 | 122673060 | 9.8 * 10−6 | 2 | T | .23 | .43% | −.11 | CEP120 | Intergenic |
| 3 | rs601007 | 151866461 | 1.3 * 10−5 | 28 | C | .37 | .42% | −.10 | Non-coding region | |
| 2 | rs6751266 | 126613227 | 2.0 * 10−5 | 4 | T | .13 | .40% | −.14 | Intergenic | |
| 3 | rs9820712 | 20707225 | 2.1 * 10−5 | 2 | G | .40 | .40% | .09 | Intergenic | |
| 2 | rs6546442 | 69084118 | 3.2 * 10−5 | 6 | A | .07 | .39% | .17 | BMP10 | Intergenic |
| 5 | rs922433 | 122937801 | 3.4 * 10−5 | 1 | G | .24 | .36% | −.10 | CSNK1G3 | Intronic |
| 4 | rs13149354 | 187291107 | 3.6 * 10−5 | A | .24 | .38% | .09 | Intergenic | ||
| Persistence | ||||||||||
| 1 | rs12753569 | 76484014 | 7.6 * 10−6 | 5 | G | .49 | .45% | −.10 | Non-coding region | |
| 9 | rs7852296 | 126352218 | 8.6 * 10−6 | A | .09 | .44% | −.17 | DENND1A | Intronic | |
| 13 | rs532238 | 39953327 | 9.9 * 10−6 | A | .29 | .45% | .10 | LHFP | Intronic | |
| 3 | rs9839894 | 67516139 | 1.4 * 10−5 | 10 | C | .06 | .45% | .20 | SUCLG2 | Intronic |
| 6 | rs7775434 | 66241186 | 1.4 * 10−5 | 9 | T | .35 | .43% | .10 | EYS | Intronic |
| 5 | rs13154900 | 49636322 | 1.7 * 10−5 | 2 | C | .14 | .40% | .13 | Intergenic | |
| 14 | rs17650363 | 82171783 | 1.8 * 10−5 | A | .13 | .42% | −.14 | Intergenic | ||
| 7 | rs1860735 | 151354400 | 2.1 * 10−5 | T | .11 | .41% | −.14 | PRKAG2 | Intronic | |
| 12 | rs7955859 | 26339731 | 3.0 * 10−5 | 1 | T | .27 | .38% | −.10 | SSPN | Intergenic |
| 20 | rs4814041 | 11901222 | 3.0 * 10−5 | 4 | G | .16 | .38% | .12 | BTBD3 | Intronic |
| 11 | rs2038602 | 33886294 | 4.0 * 10−5 | G | .41 | .37% | −.09 | LMO2 | Synonymous-coding | |
| 5 | rs1121853 | 155685912 | 4.1 * 10−5 | 3 | G | .07 | .39% | .18 | Intergenic | |
| 6 | rs9385707 | 135168474 | 4.1 * 10−5 | 3 | C | .26 | .36% | .10 | Intergenic | |
Independent markers were those more than 500kb apart and in LD of r2 < 0.70. In nonindependent groups of markers, the most significant is shown, and SNPs_LD is the number of correlated SNPs that are in the top 50. Chr = Chromosome; MAF = Minor Allele Frequency;; Closest gene = name of gene if the SNP is located in a known gene or within 50kb distance from a gene. The base pair locations in this table were obtained from the HapMapI+II (b36r22) CEU legend files, the genes closest to the SNP were obtained from WGA Viewer using release 57.
No SNPs reached genome wide significance (α = 7.2*10−8) and the SNP with the lowest p-value for each personality scale explains less than 0.5% of the total variance. Also, the results for the sex-specific association tests for Harm Avoidance and Reward Dependence did not provide any genome-wide significant results (see Supplementary Table, S1), nor did the association tests on the genotyped SNPs on the X-chromosome. The top associated SNPs in the sex-specific analyses explained a higher percentage of the variance (up to almost 2% for males) than those in the full sample, a result that may support sex-specific effects. However, this result should be viewed with caution since smaller samples tend to overestimate the variance explained by the top SNPs (‘the winner’s curse’; Zhong & Prentice, 2010).
We examined whether any of the 50 SNPs with the lowest P-value for each scale were in or close to a gene of known relevant function. None of the top SNPs were previously related directly to personality. However, one of the top SNPs (rs10176705) for Novelty Seeking was located intronic to NRXN1, a gene previously found to play a role in neuropsychiatric disorders, including schizophrenia (Kirov, et al., 2009), autism (Glessner, et al., 2009), nicotine dependence (Nussbaum, et al., 2008), and with cognition (Need, et al., 2009). In our gene-based test the NRXN1 gene had a p-value of .01 (ranked 441). In the top 50 for Harm Avoidance in females, a SNP located intronic to ROBO-2 was previously suggestively associated with schizophrenia (Potkin, et al., 2009) and a SNP intronic to MCTP1 was suggestively associated with bipolar disorder (Scott, et al., 2009). Further, a SNP close to GABRG3 found for Reward Dependence in females showed suggestive association with alcohol dependence before (Dick, et al., 2004).
In the gene-based test, no genes reached significance (α = 3.6*10−7, see Table 3). The most notable result from the gene-based test was the top ranking of the axonal guidance gene SLIT2 for reward dependence (p = 2.8*10−5), a gene that has previously been associated with anger in suicide attempters (Sokolowski, Wasserman, & Wasserman, 2010).
Table 3.
Top five genes showing strongest association with each personality scale.
| Chromosome | Gene | Start position | End position | Number of SNPs in gene | P-value |
|---|---|---|---|---|---|
| Harm Avoidance | |||||
| 3 | SLC7A14 | 171660035 | 171786557 | 66 | 7.8 * 10−5 |
| 19 | TBC1D17 | 55072640 | 55083813 | 5 | 1.0 * 10−4 |
| 10 | C10orf128 | 50033896 | 50066413 | 88 | 1.1 * 10−4 |
| 2 | AAK1 | 69538630 | 69724481 | 89 | 1.3 * 10−4 |
| 21 | SON | 33837219 | 33871682 | 19 | 1.4 * 10−4 |
| Novelty Seeking | |||||
| 1 | FLG2a | 150587836 | 150599106 | 55 | 4.0 * 10−6 |
| 1 | FLGa | 150541274 | 150564303 | 33 | 4.0 * 10−6 |
| 12 | MSRB3 | 63958754 | 64146954 | 75 | 3.5 * 10−5 |
| 1 | CRNNa | 150648342 | 150653352 | 98 | 4.4 * 10−5 |
| 1 | RBM8A | 144218994 | 144222801 | 15 | 8.3 * 10−5 |
| Reward Dependence | |||||
| 4 | SLIT2 | 19864332 | 20229886 | 154 | 2.8 * 10−5 |
| 1 | FAM5B | 175407255 | 175518181 | 76 | 4.7 * 10−5 |
| 11 | TMPRSS13 | 117276569 | 117305325 | 43 | 7.9 * 10−5 |
| 13 | MYCBP2 | 76516792 | 76799178 | 133 | 9.0 * 10−5 |
| 11 | PRRG4 | 32808064 | 32832681 | 86 | 1.0 * 10−4 |
| Persistence | |||||
| 18 | KIAA0427 | 44319424 | 44643582 | 126 | 2.0*10−6 |
| 12 | BEST3 | 68333655 | 68379463 | 174 | 4.5 * 10−5 |
| 5 | AHRR | 357290 | 491405 | 80 | 5.2 * 10−5 |
| 3 | LOC730754 | 102777969 | 102778525 | 49 | 5.5 * 10−5 |
| 11 | CAT | 34417053 | 34450183 | 176 | 5.7 * 10−5 |
Gene boundaries of these genes are overlapping - SNPs can be allocated to multiple genes, so the same SNPs could be driving the signal in the different genes.
Next we tested whether genes with the strongest association signals were concentrated in known biological or canonical pathways. We performed biological pathway analyses including all genes with a p-value below .01 - this included 304 genes (1.8%) for Harm Avoidance, 351 genes (2.0%) for Novelty Seeking, 276 genes (1.6%) for Reward Dependence, and 279 genes (1.6%) for Persistence. Results indicated that our top genes were not significantly more prevalent in any known biological or canonical pathway.
Finally, we examined evidence for association in our sample for candidate genes and SNPs published in earlier association studies of personality, including the serotonin receptor gene (SLC6A4), the dopamine D4 receptor gene (DRD4), and SNPs and genes reported in previous GWA studies on personality (Shifman, et al., 2008; Terracciano, et al., 2008; van den Oord, et al., 2008). There have been no previous GWA studies on Cloninger’s scales, but there is substantial overlap between Cloninger’s scales and the Big Five/Eysenck’s scales. Based on inter-scale correlations reported in de Fruyt et al. (2000), we looked for overlapping signals in the following groups: Neuroticism (Harm Avoidance), Extraversion and Openness (Harm Avoidance, Novelty Seeking, and Reward Dependence), and Conscientiousness (Harm Avoidance, Novelty Seeking, and Persistence).
No SNPs (or if not available, proxies in high LD) with previously reported associations were even nominally significant (p < .05) in our data. Note that not all SNPs mentioned in earlier studies were available in our dataset. A SNP in the gene CDH23 was listed by Terraciano et al. (2008) in association with Extraversion (p = 1.1*10−5); this gene had a gene-based association p-value with Novelty Seeking of .002 in our study. However, the gene effect was not significant in their study, and absent in the replication samples in their own study. None of the other genes were nominally significant in our data - in particular the DRD4 (Novelty Seeking) and SLC6A4 (Harm Avoidance) genes had p-values of 0.32 and 0.65, respectively. We calculated that our SNPs captured 52% of the variance in the DRD4 gene and 80% of the variance in the SLC6A4 gene. We did this by identifying the overlap between our SNPs and all SNPs within 50kb of the genes (as per HapMap Genome Browser) and then calculating what proportion of the total variance of the gene our SNPs covered using the Tagger option in Haploview (selecting SNPs with a MAF > 0.05 only).
Discussion
We performed the first genome wide association analysis on the Cloninger temperament scales Harm Avoidance, Novelty Seeking, Reward Dependence, and Persistence in a population sample of 5117 people from 2567 families. Although we had over 90% power to detect SNPs accounting for 1% of the variance in the scales, we detected no genome-wide significant SNPs for any scale. The SNPs in our dataset (including imputed SNPs) account for the vast majority of the common genetic variation in the population (Frazer, et al., 2007). Moreover, although we only had 26% power to detect common variants that account for 0.5% of the variance, if such variants comprised only half of the genetic variation for each trait, 40 such variants would be implied for a trait with heritability of 40%, implying that ten (i.e. 0.26*40) such variants should have been detectable per trait, yet we detected none across all traits. Therefore, our results suggest that the genetic architecture of personality consists of either very many common variants of very small effect size or rare variants (not tagged in our SNP chips), or both.
These results are consistent with those from previous GWA studies on Eysenck’s Neuroticism scale (Shifman, et al., 2008) and the Big Five personality traits (Terracciano, et al., 2008), and prior genome-wide linkage studies on personality scales, which have failed to identify any consistently replicable genome-wide significant variants. However, the (lack of) results in genome-wide searches contrast with some earlier candidate gene studies that found association of DRD4 and SLC6A4 with Novelty Seeking/Extraversion and Harm Avoidance/Neuroticism, respectively (e.g., Benjamin, et al., 1996; Ebstein, 2006; Vormfelde, et al., 2006). Tellingly, these two genes showed no association at all with the corresponding scales in the present study despite the very large sample size, consistent with more recent evidence against a link between these genes and personality (Munafo, et al., 2009; Terracciano, et al., 2009).
Some of our top SNPs and genes were located in or near genes previously associated with other psychological traits, but none were directly related to personality. Our top genes did not overlap more than expected by chance with known functional molecular pathways. Furthermore, none of our top SNPs corresponded with the top SNPs in Terraciano et al. (2008), Shifman et al. (2008) and van den Oord et al. (2008) for overlapping Big Five/Eysenck scales (e.g., Harm Avoidance and Neuroticism, Novelty seeking and Extraversion); indeed, none of the top SNPs in those studies were even nominally significant (p < .05) in our data. This strongly reinforces the conclusion that, individually, common genetic variants do not contribute substantively to variation in personality.
This raises the question of ‘missing heritability’: if personality is heritable with 30–60% of the variance explained by genetic effects, why can we not find any specific genetic variants to account for that heritability? Missing heritability has been observed to a large extent in almost all complex traits (Maher, 2008). Proposed explanations focus on: many variants of very small effect that are yet to be found; rare variants that are poorly detected by available genotyping arrays that focus on variants present in at least 5% of the population; structural variants poorly captured by existing arrays, such as copy number variations; and low power to detect epistasis (interaction between genes) (Manolio, et al., 2009). Newer technologies (e.g. whole genome sequencing) and novel statistical approaches combined with larger samples and meta-analyses will contribute to our understanding of the genetic architecture of complex traits.
A robust theoretical framework could also help to gain a fuller understanding of the genetic basis of complex traits. In this vein, Penke, Denisson and Miller (2007) provided an evolutionary framework for relating the genetic architecture of personality traits to the selective pressures they have been under. Penke et al. argued that personality traits are most likely to have been under balancing selection by environmental heterogeneity (i.e. different selective pressures in different environments), often mediated by negative frequency-dependent selection (another form of balancing selection, where a phenotype is advantageous only when it is rare in the population). According to evolutionary genetic theory, traits under balancing selection should be influenced by a relatively limited number of common genetic variants with medium effect sizes (Barton & Keightley, 2002; Penke, et al., 2007; Roff, 1997). However, our findings falsify this prediction, since no individual common genetic variants account for more than half a percent of personality trait variation in our data. This suggests that personality variation is likely to be maintained by a mechanism other than balancing selection. One possibility is selective neutrality, where personality differences make virtually no difference at all to fitness in any environments. Penke et al. argue that this is implausible, given the pervasive importance of personality differences in social and romantic relationships among other things, but it ultimately depends on the correlation between the net effect of a specific genetic variant (across potentially multiple pleiotropic functions) and total fitness. The other possible mechanism for maintaining genetic variation is mutation-selection balance. In mutation-selection balance, the appearance of new mutations is balanced by purifying selection, which eliminates deleterious mutations. The time lag of purifying selection means all individuals carry a load of mildly deleterious mutations that have yet to be eliminated by selection2. Trait variation corresponds to variation in individuals’ mutation load. Traits under mutation-selection balance are expected to be influenced by very many rare genetic variants of small effect (Keller & Miller, 2006; Penke, et al., 2007; Zhang & Hill, 2005). This is not inconsistent with the present results, since GWA studies are unable to detect rare genetic variants of small effect (Manolio, et al., 2009).
In mutation-selection balance explanations, there is an optimal adaptive ‘design’ that is the product of selective processes that maximise fitness. Accumulated random mutations are likely to have pleiotropic downstream effects that disrupt this design, deteriorating fitness in various ways (Keller & Miller, 2006; Zhang & Hill, 2005). The deleterious effects of mutation load will be especially apparent in mental functioning, since the brain has such a large mutational target size (over half of the genome is probably expressed in the brain; Sandberg, et al., 2000). This lends itself well to explaining psychiatric disorders, where normal mental functioning is thought to be disrupted by mutation load to the point of drastic dysfunction (Keller & Miller, 2006). It is less clear how mutation load might manifest in traditional personality traits, since they have not generally been considered in the context of good scores (high fitness) or bad scores (low fitness) (Almagor, Tellegen, & Waller, 1995; Zietsch, 2009).
However, variation on Cloninger’s scales seems very likely to relate to fitness. For example, it is hard to imagine that individuals’ propensity to avoid harm (Harm Avoidance) would be unrelated to their survival and reproductive prospects. Very low levels of Harm Avoidance would lead to greater chance of injury or death, but very high levels would lead to excessive timidity that would likely impair survival and mate acquisition, especially in animals or in human hunter gatherer societies. Thus the ‘optimal adaptive design’ would be an intermediate level of Harm Avoidance, and the same could be argued for Novelty Seeking, Reward Dependence, and Persistence. In this scenario (stabilising selection), a mutation-selection balance explanation would involve high mutation loads disrupting the optimal design and being associated with maladaptive high and low extremes of each Cloninger personality scale. Purported indicators of mutation load such as fluctuating asymmetry and low intelligence (Gangestad & Yeo, 2006; Keller & Miller, 2006; Prokosch, Yeo, & Miller, 2005) would be expected to show a curvilinear (U-shaped) relationship with the scales. The offspring of relatives, being homozygous at more genetic loci and more likely to express the full effects of harmful recessive mutations, would be expected to have more extreme Cloninger scale scores (i.e. inbreeding depression should be associated with increased scale variance). Future research should test these predictions, and the research should include animal studies, especially given that Cloninger’s scales were developed in part using mouse models.
The present results, in combination with previous findings, indicate that variants of moderate or large effect do not play a role in variation in personality in the population - if they did, GWA studies should have found the common variants, and linkage studies should have found rare variants. This narrows the search to common and rare variants of small effect. Current GWA methods with increasingly large sample size will enable identification of common variants of ever-smaller effect size. However, current methods do not allow investigation of accumulated rare variants of small effect, which may play a substantial role in personality and other traits. A key challenge is to develop genotyping technologies and statistical approaches for quantifying mutation load across the genome (e.g. how many mutations (very rare alleles) an individual’s genome contains). In this regard, the dual problem with current GWA methods is that 1) rare variants are not included on SNP chips, and 2) the rarer a variant is, the less reliably the genotype can be determined. Whole genome sequencing, which will become feasible in large samples in the near future, has the potential to address these problems and greatly accelerate investigation of the effects of accumulated mutations (Morris & Zeggini, 2010), but will require large sample sizes.
In summary, the failure to find common genetic variants underlying Cloninger’s psychobiological temperament scales accords with previous studies that have failed to find common variants underlying Eysenck’s Neuroticism and the Big Five personality scales. That individual common genetic variants which explain 0.5% or more of the variance do not substantially affect personality has important implications for our understanding of its genetic architecture.
Supplementary Material
Acknowledgments
We would like to thank the twins and their families registered at the ATR for their participation. We also thank Dixie Statham (sample collection); Lisa Bowdler, Steven Crooks (DNA processing); David Smyth, Harry Beeby, and Daniel Park (IT support), and Jimmy Liu for his help with the gene-based test. Funding was provided by the Australian National Health and Medical Research Council (241944, 339462, 389927, 389875, 389891, 389892, 389938, 442915, 442981, 496739, 552485, 552498, 613608), the FP-5 GenomEUtwin Project (QLG2-CT-2002-01254), and the U.S. National Institutes of Health (NIH grants AA07535, AA10248, AA13320, AA13321, AA13326, AA14041, MH66206). A portion of the genotyping on which this study was based (Illumina 370K scans) was carried out at the Center for Inherited Disease Research, Baltimore (CIDR), through an access award to our late colleague Dr. Richard Todd (Psychiatry, Washington University School of Medicine, St Louis). Statistical analyses were partly conducted at the Genetic Cluster Computer (http://www.geneticcluster.org) which is financially supported by the Netherlands Scientific Organisation (NWO 480-05-003). K.J.H.V. is supported by an ANZ Trustees PhD scholarship in Medical Research. B.P.Z. is supported by a UQ Postdoctoral fellowship. S.E.M., B.B. and G.W.M. are supported by the National Health and Medical Research Council (NHMRC) Fellowship Scheme. N.R.W and D.R.N are supported by the Australian Research Council Future Fellowship Scheme.
Footnotes
The SNPs considered for each gene are those in a gene or within ±50kbs of a gene’s 5′ and 3′ UTRs. For a given gene with n SNPs, association p-values are first converted to chi-squared 1-df statistics. The gene-based test statistic is then the sum of all the chi-squared 1-df statistics within that gene. The test uses multivariate normal simulations to model the LD structure of SNPs within genes using the HapMap2 CEU genotypes, and therefore assumes that the LD structure in the European CEU sample is representative of our sample. To account for linkage disequilibrium, correlated chi-squared 1-df random variables can be generated for n SNPs by simulating an n-element multivariate normal vector with mean 0 and covariance matrix the n×n r SNP correlation matrix. The sum of all the squared elements will then have the same approximate distribution as the gene-based test statistic under the null hypothesis. Thus, an empirical gene-based p-value can be estimated by comparing the observed gene-based test statistic with those from a large number of multivariate normal simulated vectors.
Selection quickly eliminates mutations with the largest and most dominant harmful effects due to non-viability or infertility of the organism, so mutation loads consist largely of mildly harmful recessive mutations (Keller & Miller, 2006).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abecasis GR. (unpublished). MACH, from http://www.sph.umich.edu/csg/abecasis/MACH/index.html.
- Almagor M, Tellegen A, Waller NG. The Big-7 model - a cross-cultural replication and further exploration of the basic dimensions of natural-language trait descriptors. Journal of Personality and Social Psychology. 1995;69(2):300–307. [Google Scholar]
- Barton NH, Keightley PD. Understanding quantitative genetic variation. Nature Reviews Genetics. 2002;3(1):11–21. doi: 10.1038/nrg700. [DOI] [PubMed] [Google Scholar]
- Becker K, El-Faddagh M, Schmidt MH, Laucht M. Is the serotonin transporter polymorphism (5-HTTLPR) associated with harm avoidance and internalising problems in childhood and adolescence? Journal of Neural Transmission. 2007;114(3):395–402. doi: 10.1007/s00702-006-0577-4. [DOI] [PubMed] [Google Scholar]
- Benjamin J, Li L, Patterson C, Greenberg BD, Murphy DL, Hamer DH. Population and familial association between the D4 dopamine receptor gene and measures of novelty seeking. Nature Genetics. 1996;12(1):81–84. doi: 10.1038/ng0196-81. [DOI] [PubMed] [Google Scholar]
- Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, et al. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WM, Abecasis GR. Family-based association tests for genomewide association scans. American Journal of Human Genetics. 2007;81(5):913–926. doi: 10.1086/521580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety-states. Psychiatric Developments. 1986;4(3):167–226. [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants - a proposal. Archives of General Psychiatry. 1987;44(6):573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. The Temperament and Character Inventory (TCI): a guide to its development and use. Washington University, St Louis, Missouri: Centre for Psychobiology of Personality; 1994. [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM. The tridimensional personality questionnaire - United States normative data. Psychological Reports. 1991;69(3):1047–1057. doi: 10.2466/pr0.1991.69.3.1047. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Archives of General Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Van Eerdewegh P, Goate A, Edenberg HJ, Blangero J, Hesselbrock V, et al. Anxiety proneness linked to epistatic loci in genome scan of human personality traits. [Article] American Journal of Medical Genetics. 1998;81(4):313–317. doi: 10.1002/(sici)1096-8628(19980710)81:4<313::aid-ajmg7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Curtis D. Re-analysis of collaborative study on the genetics of alcoholism pedigrees suggests the presence of loci influencing novelty-seeking near D12S391 and D17S1299. [Article] Psychiatric Genetics. 2004;14(3):151–155. doi: 10.1097/00041444-200409000-00006. [DOI] [PubMed] [Google Scholar]
- De Fruyt F, Van de Wiele L, Van Heeringen C. Cloninger’s psychobiological model of temperament and character and the five-factor model of personality. Personality and Individual Differences. 2000;29(3):441–452. [Google Scholar]
- Dick DM, Edenberg HJ, Xuei XL, Goate A, Kuperman S, Schuckit M, et al. Association of GABRG3 with alcohol dependence. Alcoholism-Clinical and Experimental Research. 2004;28(1):4–9. doi: 10.1097/01.ALC.0000108645.54345.98. [DOI] [PubMed] [Google Scholar]
- Dudbridge F, Gusnanto A. Estimation of significance thresholds for genomewide association scans. Genetic Epidemiology. 2008;32(3):227–234. doi: 10.1002/gepi.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LJ, Eysenck HJ, Martin JM. Genes, culture and personality: an empirical approach. London: Academic Press; 1989. [Google Scholar]
- Ebstein RP. The molecular genetic architecture of human personality: beyond self-report questionnaires. Molecular Psychiatry. 2006;11(5):427–445. doi: 10.1038/sj.mp.4001814. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Gritsenko I, Nemanov L, Frisch A, Osher Y, Belmaker RH. No association between the serotonin transporter gene regulatory region polymorphism and the tridimensional personality questionnaire (TPQ) temperament of harm avoidance. Molecular Psychiatry. 1997;2(3):224–226. doi: 10.1038/sj.mp.4000275. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Nemanov L, Klotz I, Gritsenko I, Belmaker RH. Additional evidence for an association between the dopamine D4 receptor (D4DR) exon III repeat polymorphism and the human personality trait of Novelty Seeking. Molecular Psychiatry. 1997;2(6):472–477. doi: 10.1038/sj.mp.4000333. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, et al. Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of novelty seeking. Nature Genetics. 1996;12(1):78–80. doi: 10.1038/ng0196-78. [DOI] [PubMed] [Google Scholar]
- Ettelt S, Grabe HJ, Ruhrmann S, Buhtz F, Hochrein A, Kraft S, et al. Harm avoidance in subjects with obsessive-compulsive disorder and their families. Journal of Affective Disorders. 2008;107(1–3):265–269. doi: 10.1016/j.jad.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Farmer RF, Goldberg LR. A psychometric evaluation of the revised Temperament and Character Inventory (TCI-R) and the TCI-140. Psychological Assessment. 2008;20(3):281–291. doi: 10.1037/a0012934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449(7164):851–U853. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MF, Tukey JW. Transformations related to the angular and the square root. Annals of Mathematical Statistics. 1950;21(4):607–611. [Google Scholar]
- Gangestad SW, Yeo RA. Mutations, developmental instability, and the Red Queen. Behavioral and Brain Sciences. 2006;29(4):412. [Google Scholar]
- Gerra G, Zaimovic A, Timpano M, Zambelli U, Delsignore R, Brambilla F. Neuroendocrine correlates of temperamental traits in humans. Psychoneuroendocrinology. 2000;25(5):479–496. doi: 10.1016/s0306-4530(00)00004-4. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Cloninger CR, Heath AC, Martin NG. The genetic and environmental relationship between Cloninger’s dimensions of temperament and character. Personality and Individual Differences. 2003;35(8):1931–1946. doi: 10.1016/S0191-8869(03)00042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Johnstone SJ, Boyce P, Heath AC, Martin NG. The genetic and environmental relationship between the interpersonal sensitivity measure (IPSM) and the personality dimensions of Eysenck and Cloninger. Personality and Individual Differences. 2001;31(7):1039–1051. [Google Scholar]
- Gillespie NA, Zhu G, Evans DM, Medland SE, Wright MJ, Martin NG. A Genome-Wide Scan for Eysenckian Personality Dimensions in Adolescent Twin Sibships: Psychoticism, Extraversion, Neuroticism, and Lie. Journal of Personality. 2008;76(6):1415–1445. doi: 10.1111/j.1467-6494.2008.00527.x. [DOI] [PubMed] [Google Scholar]
- Glessner JT, Wang K, Cai GQ, Korvatska O, Kim CE, Wood S, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. [Article] Nature. 2009;459(7246):569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Heath AC, Cloninger CR, Martin NG. Testing a model for the genetic-structure of personality - A comparison of the personality systems of Cloninger and Eysenck. Journal of Personality and Social Psychology. 1994;66(4):762–775. doi: 10.1037//0022-3514.66.4.762. [DOI] [PubMed] [Google Scholar]
- Heiman N, Stallings MC, Hofer SM, Hewitt JK. Investigating age differences in the genetic and environmental structure of the tridimensional personality questionnaire in later adulthood. Behavior Genetics. 2003;33(2):171–180. doi: 10.1023/a:1022558002760. [DOI] [PubMed] [Google Scholar]
- Herbst JH, Zonderman AB, McCrae RR, Costa PT. Do the dimensions of the temperament and character inventory map a simple genetic architecture? Evidence from molecular genetics and factor analysis. American Journal of Psychiatry. 2000;157(8):1285–1290. doi: 10.1176/appi.ajp.157.8.1285. [DOI] [PubMed] [Google Scholar]
- Howard MO, Kivlahan D, Walker RD. Cloninger’s tridimensional theory of personality and psychopathology: Applications to substance use disorders. Journal of Studies on Alcohol. 1997;58(1):48–66. doi: 10.15288/jsa.1997.58.48. [DOI] [PubMed] [Google Scholar]
- Jang KL, Livesley WJ, Vernon PA. Heritability of the big five personality dimensions and their facets: A twin study. Journal of Personality. 1996;64(3):577–591. doi: 10.1111/j.1467-6494.1996.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Keller MC, Coventry WL, Heath AC, Martin NG. Widespread evidence for non-additive genetic variation in Cloninger’s and Eysenck’s personality dimensions using a twin plus sibling design. Behavior Genetics. 2005;35(6):707–721. doi: 10.1007/s10519-005-6041-7. [DOI] [PubMed] [Google Scholar]
- Keller MC, Miller G. Resolving the paradox of common, harmful, heritable mental disorders: Which evolutionary genetic models work best? Behavioral and Brain Sciences. 2006;29(4):385. doi: 10.1017/S0140525X06009095. [DOI] [PubMed] [Google Scholar]
- Khan AA, Jacobson KC, Gardner CO, Prescott CA, Kendler KS. Personality and comorbidity of common psychiatric disorders. British Journal of Psychiatry. 2005;186:190–196. doi: 10.1192/bjp.186.3.190. [DOI] [PubMed] [Google Scholar]
- Kirov G, Rujescu D, Ingason A, Collier DA, O’Donovan MC, Owen MJ. Neurexin 1 (NRXN1) deletions in schizophrenia. Schizophrenia Bulletin. 2009;35(5):851–854. doi: 10.1093/schbul/sbp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn JM, Kuruvilla FG, McCarroll SA, Wysoker A, Nemesh J, Cawley S, et al. Integrated genotype calling and association analysis of SNPs, common copy number polymorphisms and rare CNVs. Nature Genetics. 2008;40(10):1253–1260. doi: 10.1038/ng.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo PH, Neale MC, Riley BP, Patterson DG, Walsh D, Prescott CA, et al. A genome-wide linkage analysis for the personality trait neuroticism in the Irish affected sib-pair study of alcohol dependence. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2007;144B(4):463–468. doi: 10.1002/ajmg.b.30478. [DOI] [PubMed] [Google Scholar]
- Lang UE, Bajbouj M, Wernicke C, Rommelspacher H, Danker-Hopfe H, Gallinat J. No association of a functional polymorphism in the serotonin transporter gene promoter and anxiety-related personality traits. Neuropsychobiology. 2004;49(4):182–184. doi: 10.1159/000077363. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Liu JZ, McRae AF, Nyholt DR, Medland SE, Wray NR, Brown KM, et al. A versatile gene-based test for genome-wide association studies. American Journal of Human Genetics. 2010;87(1):139–145. doi: 10.1016/j.ajhg.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nature Genetics. 2010;42(5):366–368. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher B. Personal genomes: The case of the missing heritability. Nature. 2008;456(7218):18–21. doi: 10.1038/456018a. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Virkkunen M, Rooney W, Eggert M, Linnoila M, Goldman D. The association between the dopamine D-4 receptor (D4DR) 16 amino acid repeat polymorphism and Novelty Seeking. Molecular Psychiatry. 1996;1(5):388–391. [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medland SE, Nyholt DR, Painter JN, McEvoy BP, McRae AF, Zhu G, et al. Common variants in the trichohyalin gene are associated with straight hair in Europeans. American Journal of Human Genetics. 2009;85(5):750–755. doi: 10.1016/j.ajhg.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AP, Zeggini E. An Evaluation of Statistical Approaches to Rare Variant Analysis in Genetic Association Studies. Genetic Epidemiology. 2010;34(2):188–193. doi: 10.1002/gepi.20450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder RT, Joyce PR, Sullivan PF, Bulik CM, Carter FA. The relationship among three models of personality psychopathology: DSM-III-R personality disorder, TCI scores and DSQ defences. Psychological Medicine. 1999;29(4):943–951. doi: 10.1017/s0033291799008533. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Freimer NB, Ng W, Ophoff R, Veijola J, Miettunen J, et al. 5-HTTLPR Genotype and Anxiety-Related Personality Traits: A Meta-Analysis and New Data. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2009;150B(2):271–281. doi: 10.1002/ajmg.b.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Yalcin B, Willis-Owen SA, Flint J. Association of the dopamine D4 receptor (DRD4) gene and approach-related personality traits: Meta-analysis and new data. Biological Psychiatry. 2008;63(2):197–206. doi: 10.1016/j.biopsych.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Neale BM, Sullivan PF, Kendler KS. A genome scan of neuroticism in nicotine dependent smokers. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2005;132B(1):65–69. doi: 10.1002/ajmg.b.30095. [DOI] [PubMed] [Google Scholar]
- Need AC, Attix DK, McEvoy JM, Cirulli ET, Linney KL, Hunt P, et al. A genome-wide study of common SNPs and CNVs in cognitive performance in the CANTAB. Human Molecular Genetics. 2009;18(23):4650–4661. doi: 10.1093/hmg/ddp413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery FG, Hatch JP, Glahn DC, NiColetti MA, Monkul ES, Najt P, et al. Temperament and character traits in patients with bipolar disorder and associations with comorbid alcoholism or anxiety disorders. Journal of Psychiatric Research. 2008;42(7):569–577. doi: 10.1016/j.jpsychires.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery FG, Hatch JP, Nicoletti MA, Monkul ES, Najt P, Matsuo K, et al. Temperament and character traits in major depressive disorder: influence of mood state and recurrence of episodes. Depression and Anxiety. 2009;26(4):382–388. doi: 10.1002/da.20478. [DOI] [PubMed] [Google Scholar]
- Nussbaum J, Xu Q, Payne TJ, Ma JZ, Huang WH, Gelernter J, et al. Significant association of the neurexin-1 gene (NRXN1) with nicotine dependence in European- and African-American smokers. Human Molecular Genetics. 2008;17(11):1569–1577. doi: 10.1093/hmg/ddn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Farabaugh A, Iosifescu DV, Perlis R, Fava M. Tridimensional Personality Questionnaire factors in major depressive disorder: Relationship to anxiety disorder comorbidity and age of onset. Psychotherapy and Psychosomatics. 2005;74(3):173–178. doi: 10.1159/000084002. [DOI] [PubMed] [Google Scholar]
- Penke L, Denissen JJA, Miller GF. The evolutionary genetics of personality. European Journal of Personality. 2007;21(5):549–587. [Google Scholar]
- Pervin LA, Cervone D, John OP. Personality: theory and research. 9. Hoboken, NJ: Wiley; 2005. [Google Scholar]
- Potkin SG, Turner JA, Guffanti G, Lakatos A, Fallon JH, Nguyen DD, et al. A genome-wide association study of schizophrenia using brain activation as a quantitative phenotype. Schizophrenia Bulletin. 2009;35(1):96–108. doi: 10.1093/schbul/sbn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokosch MD, Yeo RA, Miller GF. Intelligence tests with higher g-loadings show higher correlations with body symmetry: Evidence for a general fitness factor mediated by developmental stability. Intelligence. 2005;33(2):203–213. [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff DA. Evolutionary quantitative genetics. New York: Chapman and Hall; 1997. [Google Scholar]
- Sandberg R, Yasuda R, Pankratz DG, Carter TA, Del Rio JA, Wodicka L, et al. Regional and strain-specific gene expression mapping in the adult mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11038–11043. doi: 10.1073/pnas.97.20.11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ, Muglia P, Kong XQ, Guan W, Flickinger M, Upmanyu R, et al. Genome-wide association and meta-analysis of bipolar disorder in individuals of European ancestry. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(18):7501–7506. doi: 10.1073/pnas.0813386106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460(7256):753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman S, Bhomra A, Smiley S, Wray NR, James MR, Martin NG, et al. A whole genome association study of neuroticism using DNA pooling. Molecular Psychiatry. 2008;13(3):302–312. doi: 10.1038/sj.mp.4002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski M, Wasserman J, Wasserman D. Association of polymorphisms in the SLIT2 axonal guidance gene with anger in suicide attempters. Molecular Psychiatry. 2010;15(1):10–11. doi: 10.1038/mp.2009.70. [DOI] [PubMed] [Google Scholar]
- Stallings MC, Hewitt JK, Cloninger CR, Heath AC, Eaves LJ. Genetic and environmental structure of the tridimensional personality questionnaire: Three or four temperament dimensions? Journal of Personality and Social Psychology. 1996;70(1):127–140. doi: 10.1037//0022-3514.70.1.127. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460(7256):744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Balaci L, Thayer J, Scally M, Kokinos S, Ferrucci L, et al. Variants of the serotonin transporter gene and NEO-PI-R Neuroticism: no association in the BLSA and SardiNIA samples. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2009;150B(8):1070–1077. doi: 10.1002/ajmg.b.30932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Sanna S, Uda M, Deiana B, Usala G, Busonero F, et al. Genome-wide association scan for five major dimensions of personality. Molecular Psychiatry. 2008 doi: 10.1038/mp.2008.113. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009 doi: 10.1038/nature08185. advanced online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Psychiatric GWAS Consortium Steering Committee. A framework for interpreting genome-wide association studies of psychiatric disorders. Molecular Psychiatry. 2009;14(1):10–17. doi: 10.1038/mp.2008.126. [DOI] [PubMed] [Google Scholar]
- van den Oord EJCG, Kuo PH, Hartmann AM, Webb BT, Moller HJ, Hettema JM, et al. Genomewide association analysis followed by a replication study implicates a novel candidate gene for neuroticism. Archives of General Psychiatry. 2008;65(9):1062–1071. doi: 10.1001/archpsyc.65.9.1062. [DOI] [PubMed] [Google Scholar]
- Visscher PM, Montgomery GW. Genome-wide Association Studies and Human Disease From Trickle to Flood. Jama-Journal of the American Medical Association. 2009;302(18):2028–2029. doi: 10.1001/jama.2009.1643. [DOI] [PubMed] [Google Scholar]
- Vormfelde SV, Hoell I, Tzvetkov M, Jamrozinski K, Sehrt D, Brockmuller J, et al. Anxiety- and novelty seeking-related personality traits and serotonin transporter gene polymorphisms. Journal of Psychiatric Research. 2006;40(6):568–576. doi: 10.1016/j.jpsychires.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Wang K, Zhang HT, Ma DQ, Bucan M, Glessner JT, Abrahams BS, et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature. 2009;459(7246):528–533. doi: 10.1038/nature07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, James MR, Gordon SD, Dumenil T, Ryan L, Coventry WL, et al. Accurate, large-scale genotyping of 5HTTLPR and flanking single nucleotide polymorphisms in an association study of depression, anxiety, and personality measures. Biological Psychiatry. 2009;66(5):468–476. doi: 10.1016/j.biopsych.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Middeldorp CM, Birley AJ, Gordon SD, Sullivan PF, Visscher PM, et al. Genome-wide linkage analysis of multiple measures of neuroticism of 2 large cohorts from Australia and the Netherlands. Archives of General Psychiatry. 2008;65(6):649–658. doi: 10.1001/archpsyc.65.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Pergadia ML, Blackwood DHR, Penninx BWJH, Gordon SD, Nyholt DR, et al. Genome-wide association study of major depressive disorder: New results, meta-analysis, and lessons learned. doi: 10.1038/mp.2010.109. (submitted) submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XS, Hill WG. Genetic variability under mutation selection balance. Trends in Ecology & Evolution. 2005;20(9):468–470. doi: 10.1016/j.tree.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Zhong H, Prentice RL. Correcting “‘Winner’s Curse” in odds ratios from genomewide association findings for major complex human diseases. Genetic Epidemiology. 2010;34(1):78–91. doi: 10.1002/gepi.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietsch BP. The genetic etiology of human sexuality. Brisbane: University of Queensland; 2009. [Google Scholar]
- Zietsch BP, Verweij KJH, Bailey JM, Wright MJ, Martin NG. Genetic and environmental influences on risky sexual behaviour and its relationship with personality. Behavior Genetics. 2010;40(1):12–21. doi: 10.1007/s10519-009-9300-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.