Abstract
Objective
To determine whether children with quadriplegic cerebral palsy (QCP) have a greater adipose tissue (AT) infiltration of skeletal muscle than typically developing children (12/group and 5–14 years).
Study design
Cross-sectional area (CSA) of AT and muscle in the midthigh were assessed with magnetic resonance imaging. Physical activity was assessed with an activity monitor.
Results
Children with QCP had 2.3-fold higher intermuscular AT CSA and 51% lower muscle CSA in the midthigh than control subjects. Midthigh intermuscular, subfascial, and subcutaneous AT CSA adjusted for midthigh muscle CSA were higher in children with QCP (all P < .05). Moreover, the proportion of intermuscular AT CSA and subfascial AT CSA relative to subcutaneous AT CSA in the midthigh were 2.5-fold and 1.8-fold higher in children with QCP than control subjects (all P < .05). Children with QCP also had 70% fewer physical activity counts, which was inversely related to intermuscular AT CSA (r = −0.76) and subfascial AT CSA (r = −0.63) adjusted for muscle CSA in the midthigh of children with QCP (both P < 0.05), but not in control subjects.
Conclusion
Children with QCP have a greater AT infiltration of skeletal muscle than typically developing children, which is related to their low level of physical activity.
Obesity is associated with an increased risk for chronic disease.1 Although obesity is characterized by excess adipose tissue (AT), the chronic diseases associated with obesity are often more strongly related to the degree of AT infiltration within and around skeletal muscle than total AT.2,3 Children with quadriplegic cerebral palsy (QCP) have decreased mobility, fat-free mass, and energy expenditure,4,5 all of which have been tied to excessive AT infiltration of skeletal muscle.3,6–8 Because cerebral palsy results from an injury to the brain before, during, or shortly after birth, the motor skills needed to adequately engage in physical activity are severely underdeveloped in children with QCP, and extreme sedentary living typically results.9 Consequently, if individuals with QCP have an increased AT infiltration of skeletal muscle, it may emerge during childhood. However, the presence of excessive AT infiltration remains uncertain, because in addition to their limited physical activity, fat-free mass, and energy expenditure, many children with QCP also show signs of undernutrition because of poor oral motor skills.10
After considering the paradox of undernutrition on one hand versus the decreased mobility, fat-free mass, and energy expenditure4,5 and the high risk of chronic disease associated with severe cerebral palsy11 on the other hand, it appears that an assessment of AT infiltration of skeletal muscle in individuals with QCP is needed. The primary purpose of this study was to determine whether children with QCP who are unable to ambulate independently have a greater AT infiltration of skeletal muscle in the midthigh than typically developing children of the same age, pubertal development, and sex. A secondary purpose was to determine whether the degree of AT infiltration of skeletal muscle was associated with physical activity assessed by using an accelerometer-based activity monitor.
METHODS
Fourteen Caucasian children with QCP who were unable to ambulate independently and 12 typically developing Caucasian children matched to the children with QCP for age, pubertal development, and sex, between the 10th and 90th percentiles for height, body mass, and body mass index (BMI) and not engaged in organized physical activity >3 hours/week participated in the study. The institutional review boards at the University of Delaware and the AI DuPont Hospital for Children approved the study. Subjects’ parents provided written consent and, when able, subjects provided written assent.
In children with QCP, forearm length was used to estimate height.12 In control subjects, height was measured to the nearest 0.1 cm by using a stadiometer. In all children, body mass was measured to the nearest 0.1 kg by using a digital wheelchair scale (Detecto 6550, Cardinal Scale, Webb City, Missouri). Age-based percentiles for height and body mass were determined by using published data from the Center for Disease Control.13
Pubertal development was assessed by a physician assistant using the Tanner staging technique.14 The technique is based on a 5-point scale, with 1 indicating no development and 5 indicating full development. Pubic hair and breast development were assessed in girls. Pubic hair and penis/testicular development were assessed in boys.
The degree of gross motor functional ability was assessed with the Gross Motor Functional Classification (GMFC) scale.9 On the 5-point scale, a GMFC 3 represents children who achieved independent sitting by 4 years of age and ambulate with assistive devices, the aid of an adult, or both; a GMFC 4 represents children who ambulate minimally, even with assistance, and have poor sitting trunk control; and a GMFC 5 represents children who lack independent motor function even for basic antigravity postural control.
The magnetic resonance imaging (MRI) procedure used was modified from a procedure developed for adults.15 Subjects were immobilized from the waist down with BodyFIX (Medical Intelligence, Schwabmunchen, Germany), as previously described. 16,17 Axial T1 weighted images (1-cm thick separated by 0.5 cm) were collected along the entire length of the non-dominant femur with a GE 1.5 Tesla magnetic resonance imager and a torso PA coil (TR = 750, TE = 14, FOV = 16, 1 NEX, 512 × 512 matrix).
Images at the level of the mid-third of the femur (ie, midthigh) were analyzed on a personal computer with custom software developed with Interactive Data Language (Research Systems, Boulder, Colorado). The software automatically identifies images in the midthigh on the basis of the number of images collected. AT was separated into intermuscular, subfascial, and subcutaneous subregions by using the raw images and the following semi-automated procedure. First, a line was traced directly over the deep fascia lata to separate subcutaneous AT from the intermuscular and subfascial AT. Second, a line was traced on the outside border of the femur to eliminate bone marrow from inclusion in the AT subregions. Third, a line was traced just inside the border of skeletal muscle to separate intermuscular AT from subfascial AT. In areas where adjacent muscles were separated, the line was drawn midway between the 2 muscle borders. The AT beneath the deep fascial layer was separated into intermuscular and subfascial subregions because the intermuscular subregion is more closely related to insulin sensitivity and has been shown to respond more strongly to intervention.2 Fourth, images were filtered, and image segmentation was performed with a fuzzy clustering algorithm,18 in which pixels are categorized as AT, muscle, and background (or bone) on the basis of their signal intensity.
The cross-sectional area (CSA) of muscle, total AT, intermuscular AT, subfascial AT, and subcutaneous AT were determined for each image in the midthigh. The average CSAs of all images in the midthigh are reported. Because the image at the top of the midthigh was partially shared by the upper thigh, and the image at the bottom of the midthigh was partially shared by the lower thigh, the contribution of the soft tissue in these images to the overall average was weighted appropriately. The percentage of soft tissue in the midthigh that was AT was calculated by using this equation:
Total AT mass in the midthigh was determined by multiplying AT volume by 0.923 g/cc, the assumed density of AT.19 Muscle mass in the midthigh was determined by multiplying muscle volume by 1.04 g/cc, the assumed density of muscle tissue.19 The volume of each tissue in the midthigh was determined by multiplying its cross-sectional area by 1.5 in the middle images to account for the thickness of the image (1 cm) and the space between images (0.5 cm) and an appropriately weighted value for the image at the top of the midthigh and the image at the bottom of the midthigh. The ratios of intermuscular AT CSA, subfascial AT CSA, and subcutaneous AT CSA to muscle CSA in the midthigh were created to account for differences in muscle size. To fully control for muscle CSA, the numerator was adjusted by subtracting the intercept of the regression of the numerator on the demoninator, as suggested by Allison et al.20 The ratios of intermuscular AT CSA and subfascial AT CSA to subcutaneous AT CSA in the midthigh were created to reflect the regional distribution of AT. Intermuscular AT CSA, subfascial AT CSA, ratios of intermuscular and subfascial AT CSA to muscle CSA, and ratios of intermuscular and subfascial AT CSA to subcutaneous AT CSA in the midthigh were viewed as markers of AT infiltration of skeletal muscle.
To assess the test-retest reliability of AT and muscle CSA in the midthigh, 8 children (2 children with QCP and 6 typically developing children) 5 to 12 years of age were tested on the same day after repositioning or on different days within 2 weeks apart. The 2 image sets were not different in total AT (62.4 ± 12.3 cm2 versus 61.8 ± 12.3 cm2), intermuscular AT (2.5 ± 1.6 cm2 versus 2.4 ± 1.5 cm2), subfascial AT (4.5 ± 2.2 cm2 versus 4.5 ± 2.3 cm2), subcutaneous AT (55.4 ± 9.3 cm2 versus 54.9 ± 9.3 cm2), or muscle (70.7 ± 16.9 cm2 versus 70.7 ± 16.2 cm2) CSA. The intraclass correlation coefficients were >0.99 for all repeat CSA measures.
Physical activity was estimated with an accelerometer-based activity monitor (Actical; MiniMitter, Sunriver, Oregon) worn around the waist on the non-dominant side in typically developing children and on the more affected side in children with QCP. The Actical activity monitor was used because it was validated with a broad range of gross and fine motor physical activities.21 Monitors were worn continuously for 4 days (3 days during the week and 1 day on the weekend). Physical activity was divided into sedentary, light, moderate, and vigorous levels on the basis of activity counts registered every 15 seconds (sedentary <.01; light = 0.01–0.04; moderate = 0.04–0.10; vigorous >0.10 kcal/min/kg).21 Four days of data from an activity monitor has good reliability and validity.22
Parents, with the aid of children (when able), recorded the child’s dietary intake for 2 weekdays and 1 weekend day. To facilitate accurate quantification of foods, each subject and their parent received a list of serving size estimates on the basis of comparisons to everyday objects (eg, 3 oz of meat or poultry is approximately the size of a deck of cards).23 Parents were contacted to clarify any uncertain foods or quantities. Total energy intake and the percent of energy intake from carbohydrate, fat, and protein were estimated from the diet records by using the USDA Food and Nutrient Database for Dietary Studies, 1.0.24
Data were analyzed with SPSS software (version 15.0; SPSS, Chicago, Illinois). Variables were checked for normality with skewness (>2.00), kurtosis (>2.00), and the Shapiro-Wilk test. Group differences were determined with independent t tests when they were normally distributed and the Mann-Whitney U tests when they were not normally distributed. All data are presented as means plus or minus SD. To assess whether the degree of AT infiltration of skeletal muscle in the midthigh was associated with physical activity, relationships between the ratios of AT CSA in the different subregions of the midthigh to muscle CSA in the midthigh and total physical activity counts were evaluated with Pearson correlation analysis. When outliers were detected with Cook’s D and standardized DfBeta scores >1 or when data were not normally distributed, data were transformed before correlations were assessed. The alpha level was set at 0.05. Sample size was determined using power charts from Cohen25 and data from Leroy-Willig et al,8 in which total body intramuscular fat was much higher in boys with Duchenne muscular dystrophy than control children (Cohen’s d = 3.0; n = 8/group). With a smaller effect size than observed in the Leroy-Willig et al study (Cohen’s d = 1.5) and setting power at 0.9, it was determined that at least 10 subjects per group were needed to adequately address the primary aim of this study.
RESULTS
One girl and 1 boy in the QCP group could not complete testing because of fear of the MRI testing prcedure. Characteristics of the subjects who completed the study are presented in Table I. There were 4 boys and 8 girls in each group. There were no group differences in age, Tanner stage, or BMI (P > .05). However, children with QCP had lower height (P < .01), height percentile (P < .001), femur length (P < .001), body mass (P = .04), and body mass percentile (P = .04) than control subjects. Height and body mass, but not BMI, were lower than the 50th percentiles for age in children with QCP (P < .05). Height, body mass, and BMI of control subjects were not different than the 50th percentiles for age (P > .05). Group comparisons of diet and physical activity are also presented in Table I. There were no group difference in the percentage of energy from carbohydrate, fat, and protein or total energy intake (all P > .05). There was no group difference in sedentary physical activity counts (P > .05); however, children with QCP had 47% fewer light (P = 0.02), 74% fewer moderate (P < .001), 87% fewer vigorous (P < .001), and 70% fewer total (P < .001) physical activity counts than control subjects.
Table I.
Physical characteristics, diet, and physical activity in children with quadriplegic cerebral palsy and typically developing control subjects
| QCP (n = 12) |
Controls (n = 12) |
|
|---|---|---|
| Age (years) | 10.2 ± 2.5 | 10.3 ± 1.4 |
| Tanner stage | ||
| Pubic hair | 1.3 ± 0.6 | 1.3 ± 0.6 |
| Breast/testicular | 1.3 ± 0.5 | 1.3 ± 0.6 |
| Height (m) | 1.26 ± 0.14* | 1.42 ± 0.07 |
| Height (%) | 15 ± 16* | 56 ± 20 |
| Femur length (m) | 0.33 ± 0.05* | 0.39 ± 0.03 |
| Body mass (kg) | 28.2 ± 12.3* | 35.3 ± 4.6 |
| Body mass (%) | 28 ± 37* | 55 ± 24 |
| BMI (kg/m2) | 17.0 ± 4.7 | 17.6 ± 2.0 |
| BMI (%) | 40 ± 38 | 52 ± 26 |
| GMFC (level 3/4/5) | 3/0/9 | – |
| Diet† | ||
| Carbohydrates (%) | 48 ± 17 | 54 ± 6 |
| Fat (%) | 38 ± 19 | 32 ± 6 |
| Protein (%) | 14 ± 4 | 14 ± 3 |
| Energy intake (kcal) | 1691 ± 460 | 1926 ± 294 |
| Physical activity | ||
| Sedentary (counts/day) | 4609 ± 1365 | 4773 ± 1010 |
| Light (counts/day) | 30 207 ± 19 544* | 56 888 ± 25 233 |
| Moderate (counts/day) | 79 330 ± 121 934* | 301 691 ± 87 564 |
| Vigorous (counts/day) | 4905 ± 12 033* | 37 607 ± 25 378 |
| Total (counts/day) | 119 052 ± 148 857* | 400 960 ± 101 564 |
Height (%), Height relative to age-based norms; body mass (%), body mass relative to age-based norms; BMI (%), BMI relative to age-based norms.
Values are means ± SD.
Group difference, P < .05.
n = 11 for children with QCP.
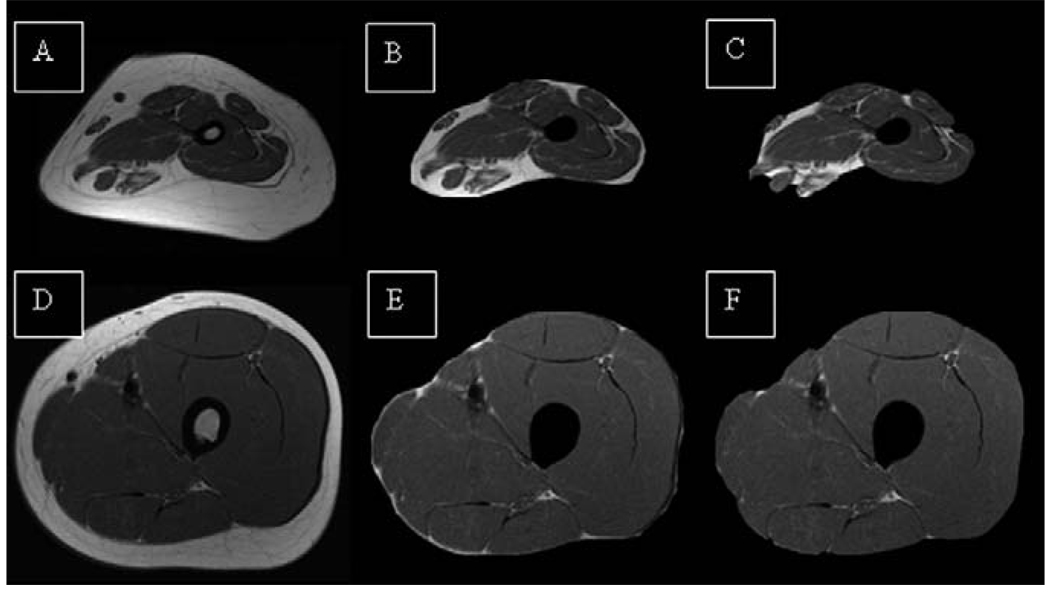
Midthigh muscle and AT CSAs are reported in Table II. Children with QCP had 51% lower midthigh muscle CSA than control subjects (P < .001). Although there was no group difference in total AT CSA in the midthigh, the percentage of soft tissue in the midthigh that was AT was 42% higher in children with QCP than in control subjects (P < .001). In addition, children with QCP had a higher intermuscular AT CSA (2.3-fold; P = .01) and showed a trend for a higher subfascial AT CSA (1.7-fold; P = .06) in the midthigh. Children with QCP also had a higher ratio of intermuscular AT CSA to muscle CSA (4.5-fold; P < .001), subfascial AT CSA to muscle CSA (3-fold; d = 2.10; P < .001), and subcutaneous AT CSA to muscle CSA (2-fold; P < .01) in the midthigh. Moreover, children with QCP had higher ratios of intermuscular AT CSA to subcutaneous AT CSA (2.5-fold; P < .01) and subfascial AT CSA to subcutaneous AT CSA (1.8-fold; P < .01) in the midthigh compared with control subjects. Representative images in Figure 1 (available at www.jpeds.com) show the greater AT infiltration of skeletal muscle in the midthigh of a prepubertal girl with QCP versus a typically developing prepubertal girl.
Table II.
Muscle and adipose tissue cross-sectional area in the midthigh of children with quadriplegic cerebral palsy and typically developing control subjects
| QCP (n = 12) |
Controls (n = 12) |
|
|---|---|---|
| Muscle (cm2) | 35.5 ± 13.5* | 72.0 ± 11.7 |
| Total AT (cm2) | 53.8 ± 29.1 | 50.2 ± 15.8 |
| % AT in soft tissue | 57.8 ± 11.5* | 40.6 ± 8.4 |
| Intermuscular AT (cm2) | 3.5 ± 2.4* | 1.5 ± 0.5 |
| Subfascial AT (cm2) | 4.8 ± 3.4† | 2.8 ± 0.8 |
| Subcutaneous AT (cm2) | 45.5 ± 24.6 | 45.9 ± 14.8 |
| Intermuscular AT/muscle | 0.09 ± 0.05* | 0.02 ± 0.01 |
| Subfascial AT/muscle | 0.12 ± 0.07* | 0.04 ± 0.01 |
| Subcutaneous AT/muscle | 1.16 ± 0.57* | 0.58 ± 0.23 |
| Intermuscular AT/subcutaneous AT | 0.08 ± 0.05* | 0.03 ± 0.01 |
| Subfascial AT/subcutaneous AT | 0.11 ± 0.04* | 0.06 ± 0.01 |
% AT in soft tissue, [Total AT mass /(AT mass + muscle mass)] × 100].
Values are means ± S.D.
P < .05.
P = .06.
Figure 1.
A-C, The progressive separation of AT from magnetic resonance images of the midthigh of a prepubertal girl with QCP and D–F, a typically developing prepubertal girl. A and D contain subcutaneous, subfascial, and intermuscular AT; B and E contain only subfascial and intermuscular AT; and C and F contain only intermuscular AT.
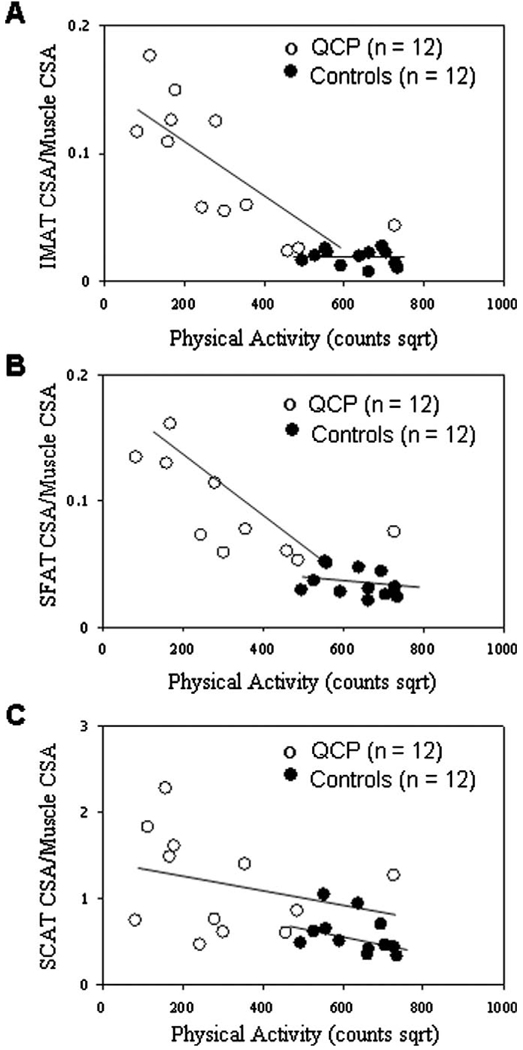
When the relationship between physical activity and measures of AT infiltration of skeletal muscle were examined, an outlier was detected in the QCP group; therefore, total physical activity counts were transformed by taking their square root. Transformed total physical activity counts were significantly and inversely related to the ratio of intermuscular AT CSA to muscle CSA (r = −0.76; P < .01) and the ratio of subfascial AT CSA to muscle CSA (r = −0.63, P = .03), but not to the ratio of subcutaneous AT CSA to muscle CSA (r = −0.28, P = .38) in the midthigh of children with QCP. None of the relationships were statistically significant in control subjects (r = −0.19, −0.37, and −0.39, respectively; P > .10). Scatter plots of transformed total physical activity counts versus the ratios of intermuscular AT CSA, subfascial AT CSA, and subcutaneous AT CSA to muscle CSA in the midthigh are presented in Figure 2.
Figure 2.
Scatter plots of transformed physical activity versus A, the ratio of intermuscular adipose tissue (IMAT) CSA to muscle CSA in the midthigh of children with QCP (r = −0.76; P < .01) and typically developing children (control subjects, r = −0.19; P = .56); B, the ratio of subfascial adipose tissue (SFAT) CSA to muscle CSA in the midthigh of children with QCP (r = −0.63; P = .01) and control subjects (r = −0.37; P = 0.24); and C, the ratio of subcutaneous adipose tissue (SCAT) CSA to muscle CSA in the midthigh of children with QCP (r = −0.28; P = .38) and control subjects (r = −0.39; P = .21).
DISCUSSION
We assessed AT infiltration of skeletal muscle in children with QCP. The high degree of AT infiltration observed in children with QCP in this study is consistent with findings in other groups with extremely limited physical activity and very low muscle mass.3,8 The finding of greater AT infiltration of skeletal muscle in children with QCP was not entirely expected, because in addition to their limited mobility, low muscle mass, and low energy expenditure, children with QCP also show signs of under-nutrition, such as reduced body size.10,26,27 Consistent with these earlier studies,10,26,27 children with QCP in this study were shorter and lighter than typically developing children. Only recently has concern about excessive energy intake in children with QCP emerged, especially in children who are fed using a gastrostomy tube.28 There are some indications that children with QCP in this study were consuming more energy than needed. For instance, despite a lower body mass in children with QCP than in control subjects, there was no group difference in energy intake. Furthermore, the 51% lower midthigh muscle CSA and 70% fewer activity counts in children with QCP than in control subjects suggests that children with QCP had much lower energy expenditure, which is consistent with earlier reports.4,27 However, because energy expenditure was not determined in this study and because there is evidence that parents of children with QCP overestimate their children’s energy intake,27 it cannot be determined whether excess energy intake contributed to the greater AT infiltration of skeletal muscle in children with QCP.
This study supports the notion that low physical activity is a major contributor to the high degree of AT infiltration of skeletal muscle in children with QCP. The inverse relationship between total physical activity counts and the ratios of intermuscular AT CSA and subfascial AT CSA to muscle CSA in the midthigh of children with QCP is consistent with a recent study by Manini et al7 in which adults who underwent lower limb suspension for 4 weeks experienced an increase in intermuscular AT in the thigh (14.5%) and calf (20%). The amount of subcutaneous AT in the calf and thigh did not change during the lower limb suspension period. Additional evidence suggesting physical activity drives the degree of AT infiltration of skeletal muscle is the recent finding that athletes with paraplegic spinal cord injury compared with non-athletes without spinal cord injury have higher intermuscular AT in their thighs, but similar amounts of intermuscular AT in their arms.29 It is worth noting that physical activity was not significantly related to the ratios of intermuscular AT CSA to muscle CSA or subfascial AT CSA to muscle CSA in the midthigh in typically developing children in this study. The nonsignificant relationship may be attributed to their limited range of physical activity counts. Typically developing children were excluded from the study if they participated in organized physical activity >3 hours/week. It is also plausible that the relationship between physical activity and markers of AT infiltration of skeletal muscle is more easily detected within the lowest range of physical activity.
There are study limitations that should be considered. One limitation is that intramyocellular lipid in skeletal muscle was not assessed. A high level of intramyocellular lipid in skeletal muscle, like high levels of intermuscular and subfascial AT,2 is associated with insulin resistance.30 Although there is evidence that intramyocellular lipid content is elevated in individuals with neurological disorders,31 whether children with QCP exhibit a high proportion of intramyocellular lipid is still unknown. A second study limitation is the absence of glucose tolerance and insulin sensitivity markers. Although higher proportions of intermuscular and subfascial AT are associated with poor glucose tolerance and insulin resistance in obese adults2 and other groups with limited mobility,3 studies are needed to determine whether the relationships exist in children with QCP. A third study limitation is the unknown accuracy of accelerometer-based activity monitors in the assessment of physical activity in children with QCP. Although the monitors used in this study have been validated in a wide range of physical activities,21 additional studies that focus on QCP are needed. A final study limitation is that magnetic resonance images could not be obtained from all children with QCP. However, most subjects (12 of 14 with QCP and 12 of 12 typically developing children) were able to complete all testing successfully, suggesting that the application of MRI in the assessment of AT infiltration of skeletal muscle in children is promising.
The findings suggest that children with QCP have a greater AT infiltration of skeletal muscle than typically developing children, which is related to their very low level of physical activity. Whether this increased AT infiltration during childhood is associated with poor glucose tolerance, insulin resistance, and the high rate of death from diseases of the circulatory system observed in individuals with severe cerebral palsy requires further investigation. Moreover, studies that assess whether increased physical activity would reduce the degree of AT infiltration of skeletal muscle in children with QCP are warranted.
Acknowledgments
This study was funded by the National Institutes of Health (HD50530) and the Cerebral Palsy International Research Foundation.
We express our deepest gratitude to all research participants and their families. We thank Malcolm Hughes, David Yost, and the staff in the MRI Suite at the AI duPont Hospital for Children for assistance with data collection and Sara Kanoff for assistance with MRI and dietary analysis.
Glossary
- AT
Adipose tissue
- BMI
Body mass index
- CSA
Cross-sectional area
- GMFC
Gross Motor Functional Classification
- MRI
Magnetic resonance imaging
- QCP
Quadriplegic cerebral palsy
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obes Res. 1995;3:73–95. doi: 10.1002/j.1550-8528.1995.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 2.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 3.Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury--a cross-sectional study. Spinal Cord. 2004;42:711–716. doi: 10.1038/sj.sc.3101652. [DOI] [PubMed] [Google Scholar]
- 4.Azcue MP, Zello GA, Levy LD, Pencharz PB. Energy expenditure and body composition in children with spastic quadriplegic cerebral palsy. J Pediatr. 1996;129:870–876. doi: 10.1016/s0022-3476(96)70031-8. [DOI] [PubMed] [Google Scholar]
- 5.Bandini LG, Schoeller DA, Fukagawa NK, Wykes LJ, Dietz WH. Body composition and energy expenditure in adolescents with cerebral palsy or myelodysplasia. Pediatr Res. 1991;29:70–77. doi: 10.1203/00006450-199101000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Ryan AS, Nicklas BJ, Berman DM, Dennis KE. Dietary restriction and walking reduce fat deposition in the midthigh in obese older women. Am J Clin Nutr. 2000;72:708–713. doi: 10.1093/ajcn/72.3.708. [DOI] [PubMed] [Google Scholar]
- 7.Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr. 2007;85:377–384. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- 8.Leroy-Willig A, Willig TN, Henry-Feugeas MC, Frouin V, Marinier E, Boulier A, et al. Body composition determined with MR in patients with Duchenne muscular dystrophy, spinal muscular atrophy, and normal subjects. Magn Reson Imaging. 1997;15:737–744. doi: 10.1016/s0730-725x(97)00046-5. [DOI] [PubMed] [Google Scholar]
- 9.Wood E, Rosenbaum P. The gross motor function classification system for cerebral palsy: a study of reliability and stability over time. Dev Med Child Neurol. 2000;42:292–296. doi: 10.1017/s0012162200000529. [DOI] [PubMed] [Google Scholar]
- 10.Henderson RC, Lark RK, Gurka MJ, Worley G, Fung EB, Conaway M, et al. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics. 2002;110:e5. doi: 10.1542/peds.110.1.e5. [DOI] [PubMed] [Google Scholar]
- 11.Strauss D, Cable W, Shavelle R. Causes of excess mortality in cerebral palsy. Dev Med Child Neurol. 1999;41:580–585. doi: 10.1017/s001216229900122x. [DOI] [PubMed] [Google Scholar]
- 12.Miller F, Koreska J. Height measurement of patients with neuromuscular disease and contractures. Dev Med Child Neurol. 1992;34:55–60. doi: 10.1111/j.1469-8749.1992.tb08563.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 14.Tanner J. Growth and adolescence. 2nd ed. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- 15.Modlesky CM, Bickel CS, Slade JM, Meyer RA, Cureton KJ, Dudley GA. Assessment of skeletal muscle mass in men with spinal cord injury using dual-energy X-ray absorptiometry and magnetic resonance imaging. J Appl Physiol. 2004;96:561–565. doi: 10.1152/japplphysiol.00207.2003. [DOI] [PubMed] [Google Scholar]
- 16.Modlesky CM, Subramanian P, Miller F. Underdeveloped trabecular bone microarchitecture is detected in children with cerebral palsy using high-resolution magnetic resonance imaging. Osteoporos Int. 2008;19:169–176. doi: 10.1007/s00198-007-0433-x. [DOI] [PubMed] [Google Scholar]
- 17.Modlesky CM, Kanoff SA, Johnson DL, Subramanian P, Miller F. Evaluation of the femoral midshaft in children with cerebral palsy using magnetic resonance imaging. Osteoporos Int. 2008 doi: 10.1007/s00198-008-0718-8. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suckling J, Sigmundsson T, Greenwood K, Bullmore ET. A modified fuzzy clustering algorithm for operator independent brain tissue classification of dual echo MR images. Magn Reson Imaging. 1999;17:1065–1076. doi: 10.1016/s0730-725x(99)00055-7. [DOI] [PubMed] [Google Scholar]
- 19.Snyder WS, Cook MJ, Nasset ES, Karhauserr LR, Howells GP, Tipton IH. International Commission on Radiological Protection. Oxford: 1975. Report of the task group on reference man. [Google Scholar]
- 20.Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB. Statistical considerations regarding the use of ratios to adjust data. Int J Obes. 1995;19:644–652. [PubMed] [Google Scholar]
- 21.Puyau MR, Adolph AL, Vohra FA, Zakeri I, Butte NF. Prediction of activity energy expenditure using accelerometers in children. Med Sci Sports Exerc. 2004;36:1625–1631. [PubMed] [Google Scholar]
- 22.Trost SG, Pate RR, Freedson PS, Sallis JF, Taylor WC. Using objective physical activity measures with youth: how many days of monitoring are needed? Med Sci Sports Exerc. 2000;32:426–431. doi: 10.1097/00005768-200002000-00025. [DOI] [PubMed] [Google Scholar]
- 23.National Institutes of Health (NIH) Publication No. 03-5287. 2006. Aug, Just enough for you. Updated. [Google Scholar]
- 24.USDA Food and Nutrient Database for Dietary Stuides, 1.0. Beltsville, MD: Agricultural Research Service, Food Surveys Research Group; 2004. [Google Scholar]
- 25.Cohen J. Statistical power for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 26.Stallings VA, Charney EB, Davies JC, Cronk LE. Nutrition-related growth failure of children with quadriplegic cerebral palsy. Dev Med Child Neurol. 1993;35:997–1006. doi: 10.1111/j.1469-8749.1993.tb11614.x. [DOI] [PubMed] [Google Scholar]
- 27.Stallings VA, Zemel BS, Davies JC, Cronk CE, Charney EB. Energy expenditure of children and adolescents with severe disabilities: a cerebral palsy model. Am J Clin Nutr. 1996;64:627–634. doi: 10.1093/ajcn/64.4.627. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan PB, Alder N, Bachlet AM, Grant H, Juszczak E, Henry J, et al. Gastrostomy feeding in cerebral palsy: too much of a good thing? Dev Med Child Neurol. 2006;48:877–882. doi: 10.1017/S0012162206001927. [DOI] [PubMed] [Google Scholar]
- 29.Mojtahedi MC, Valentine RJ, Arngrimsson SA, Wilund KR, Evans EM. The association between regional body composition and metabolic outcomes in athletes with spinal cord injury. Spinal Cord. 2008;46:192–197. doi: 10.1038/sj.sc.3102076. [DOI] [PubMed] [Google Scholar]
- 30.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 31.Barany M, Venkatasubramanian PN, Mok E, Siegel IM, Abraham E, Wycliffe ND, et al. Quantitative and qualitative fat analysis in human leg muscle of neuromuscular diseases by 1H MR spectroscopy in vivo. Magn Reson Med. 1989;10:210–226. doi: 10.1002/mrm.1910100206. [DOI] [PubMed] [Google Scholar]