Abstract
Heparan sulfate proteoglycans (HSPGs) play critical roles in the distribution and signaling of growth factors, but the molecular mechanisms regulating HSPG function are poorly understood. Here, we characterized Sulf1, which is a Drosophila member of the HS 6-O endosulfatase class of HS modifying enzymes. Our genetic and biochemical analyses show that Sulf1 acts as a novel regulator of the Wg morphogen gradient by modulating the sulfation status of HS on the cell surface in the developing wing. Sulf1 affects gradient formation by influencing the stability and distribution of Wg. We also demonstrate that expression of Sulf1 is induced by Wg signaling itself. Thus, Sulf1 participates in a feedback loop, potentially stabilizing the shape of the Wg gradient. Our study shows that the modification of HS fine structure provides a novel mechanism for the regulation of morphogen gradients.
Keywords: heparan sulfate proteoglycan, 6-O endosulfatase, morphogen gradient, Wingless, Drosophila
INTRODUCTION
Morphogens, such as Wingless/Wnt, Hedgehog, and BMPs, form concentration gradients within tissues and dictate cellular positional cues to organize tissue growth and patterning during animal development. The extracellular environment in which signaling molecules are secreted heavily influences the localization and stability of a morphogen (Wang et al., 2008). Among the molecules that have a major impact on morphogen gradients are heparan sulfate proteoglycans (HSPGs). HSPGs are carbohydrate-modified proteins that play important roles in a variety of biological processes, such as growth factor signaling and cell adhesion (reviewed in (Kirkpatrick and Selleck, 2007)). Genetic studies have shown that mutations affecting HSPG core-proteins or HS biosynthetic enzymes cause defects in morphogen signaling in Drosophila (reviewed in (Tabata and Takei, 2004)).
Wg protein is secreted by several rows of cells along the dorsoventral (DV) border of the Drosophila larval wing disc and forms a gradient that helps to orchestrate adult wing development. Wg signaling in this tissue is regulated by dally and dally-like (dlp), the Drosophila members of the Glypican family of HSPGs that are attached to the cell surface via a glycosylphosphatidylinositol (GPI)-anchor. dally is known to be a positive regulator of the Wg pathway (Franch-Marro et al., 2005; Fujise et al., 2001; Han et al., 2005), while mutations in dlp have differential effects on Wg signaling depending on the region of the wing disc. dlp down-regulates Wg signaling near the wing margin whereas it has a positive effect on it in at more peripheral regions (Kirkpatrick et al., 2004; Kreuger et al., 2004). It has been proposed that Dlp plays a role in long-range Wg diffusion by enhancing apical-basal trafficking of Wg (Gallet et al., 2008) or by acting as an exchange factor to regulate the amount of Wg available to receptor (Yan et al., 2009).
The biological function of HSPGs is not only dependent on their core protein structure but also relies on the heterogeneous fine structure of their sugar chains. During HS biosynthesis, HS chains are polymerized by EXT proteins in the Golgi. The nascent polysaccharide subsequently undergoes a series of modification events including O-sulfation at different positions. Since only a fraction of potential target units are modified in each biosynthetic step, the resulting HS chains have remarkable levels of structural heterogeneity. Several lines of evidence suggest that these HS fine structures regulate discrete signaling events at the cell surface (reviewed in (Gorsi and Stringer, 2007)). Recently, a novel family of HS modifying enzymes, the extracellular HS 6-O endosulfatases (Sulfs), were identified (Dhoot et al., 2001). Unlike other HS modifying enzymes that function in the Golgi during HS biosynthesis, Sulfs are believed to be secreted proteins that remove 6-O sulfate groups from internal sulfated domains of extracellular HS. The first identified Sulf molecule, QSulf1, was shown to increase Wnt signaling in avian embryonic somites (Dhoot et al., 2001). It was proposed that the activity of QSulf1 as well as other vertebrate homologues decreases the binding affinity between HS and the Wnt ligand, in turn promoting the access of Wnt to its receptor for signaling (Ai et al., 2003; Freeman et al., 2008; Nawroth et al., 2007; Tang and Rosen, 2009). In contrast, Sulfs have been shown to act as negative regulators of FGF signaling (Kamimura et al., 2006; Wang et al., 2004). Aberrant levels of Sulf expression are also associated with tumorigenesis (Li et al., 2005; Nawroth et al., 2007). Thus, post-synthetic remodeling of HS structures affects HSPG function and therefore provides a novel mechanism by which the activity and the distribution of growth factors can be regulated. However, the molecular basis for the in vivo function of Sulfs is poorly understood.
To elucidate the mechanism by which post-synthetic modulation of HS sulfation controls the activity and the distribution of secreted signaling molecules, we studied the role of Drosophila HS 6-O endosulfatase (Sulf1) in development. We show that Sulf1 affects the Wg gradient by controlling the stability and distribution of Wg protein. Structural analysis of HS from the Sulf1 mutants showed that the mutant HS bears abnormally high levels of tri-sulfated (tri-S) disaccharide units. Collectively, these results suggest that specific desulfation of HS by Sulf1 is required for Wg morphogen gradient formation. We also demonstrate that Sulf1 expression is under the control of Wg signaling, indicating that Sulf1 is involved in a feedback system of this pathway. Our findings add a novel layer to the molecular network regulating the formation and stabilization of morphogen gradients.
MATERIALS AND METHODS
Fly strains
Detailed information for the fly strains used is described in Flybase (http://flybase.bio.indiana.edu/) except where noted. The wild-type strain used was Oregon R. Other strains used were: P{GT1}GT000656, a P-element insertion line in the Sulf1 locus; Df(3R)sdb26 (breakpoints, 89B9;89C7), a chromosomal deficiency line; dallygem, a loss-of-function allele of dally; Hs6std770, a null allele of Hs6st; neuralizedA101; winglessen11 (wg-lacZ); engrailed (en)-Gal4; apterous (ap)-Gal4; hedgehog (hh)-Gal4; decapentaplegic (dpp)-Gal4; C96-Gal4; A9-Gal4; UAS-FLP; UAS-Sulf1 (Kamimura et al., 2006); UAS-dally (Takeo et al., 2005; Tsuda et al., 1999), UAS-dlp39.2 (Kirkpatrick et al., 2004), UAS-GPI-Dfz2 (Cadigan et al., 1998; Rulifson et al., 2000), and UAS-ArmS10 (Pai et al., 1997). Sulf1 homozygous mutant clones were generated by FLP-mediated mitotic recombination using FRT82B as previously described (Fujise et al., 2003). FLP was induced by heat-shock using hs-FLP (for mutant clones shown in Fig. 4D-D”) or by hh-Gal4 UAS-FLP (for mutant clones shown in Fig. 2E-E”). FLP-OUT clones overexpressing Sulf1 were induced as previously described (Struhl and Basler, 1993; Takeo et al., 2005) in wing disc bearing a Act5C>CD2>Gal4 transgene cassette, hsp70-flp, UAS-GFP, and UAS-Sulf1.
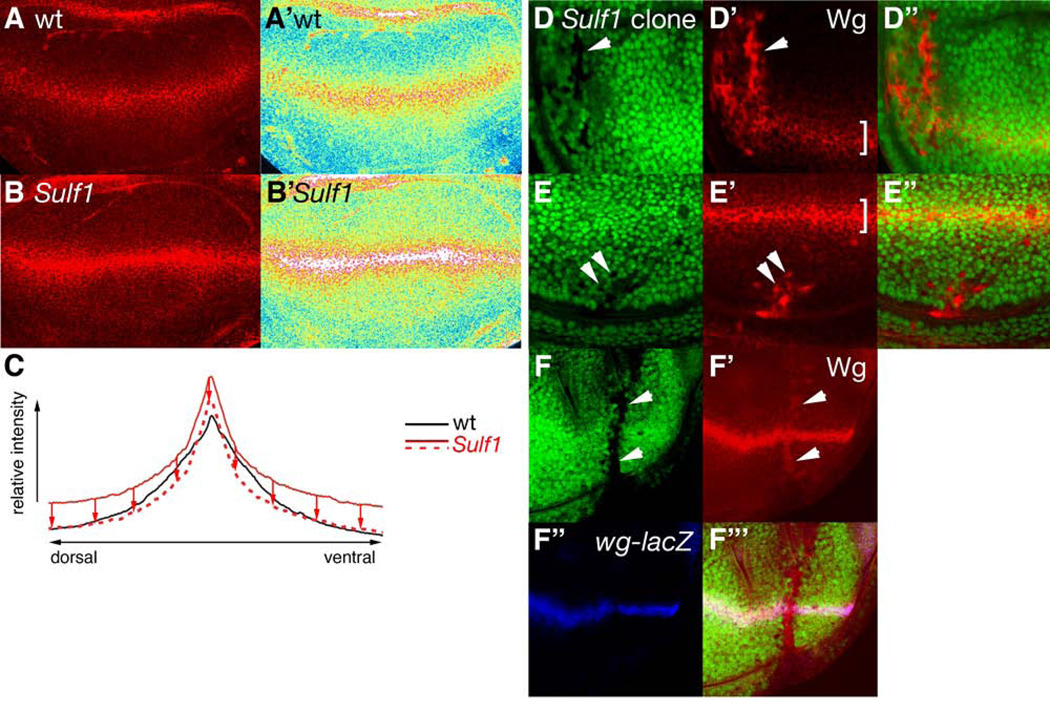
Figure 4. The level of extracellular Wg protein is elevated in Sulf1 mutant wing discs.
(A-B’) Immuno-staining for extracellular Wg protein in wild-type (A and A’) and Sulf1 (B and B’) discs. Signal intensity in A and B is shown in pseudocolor to compare intensity in A’ and B’, respectively. Pseudocolor scale ranges from white (highest signal intensity) to dark blue (lowest signal intensity). Note the higher intensity levels of Wg protein throughout the wing disc as a whole in the Sulf1 homozygous mutant. (C) Signal intensity of extracellular Wg was obtained from raw confocal images by Image J software. Intensity plots were generated by averaging intensity values for wild-type (continuous black line, n=29) and Sulf1 (continuous red line, n=18) wing discs. For a more direct comparison of gradient shape, a downward translation of the Sulf1 plot (red arrows/dashed red line) was transposed directly on top of wild-type. Sulf1 wing discs exhibited a steeper gradient of Wg signal intensity when compared to wild-type. (D-E”) Two examples are shown for the effect of small Sulf1 mutant clones on the distribution of extracellular Wg. GFP signal and extracellular staining of Wg are shown in green (D and E) and red (D’ and E’), respectively. D” and E” show merged images. Abnormally high levels of Wg protein accumulate in small Sulf1 clones (arrowheads). The brackets indicate the extracellular Wg signal located at the DV border. (F-F”’) A wing disc with small Sulf1 clones stained with anti-β-gal antibody to mark wg-lacZ expression. GFP signal, extracellular staining of Wg, and wg-lacZ are shown in green (F), red (F’), and blue (F”), respectively. F”’ shows a merged image of F, F’, and F”. wg-lacZ is not induced in small clones in which extracellular Wg protein accumulates (arrowheads).
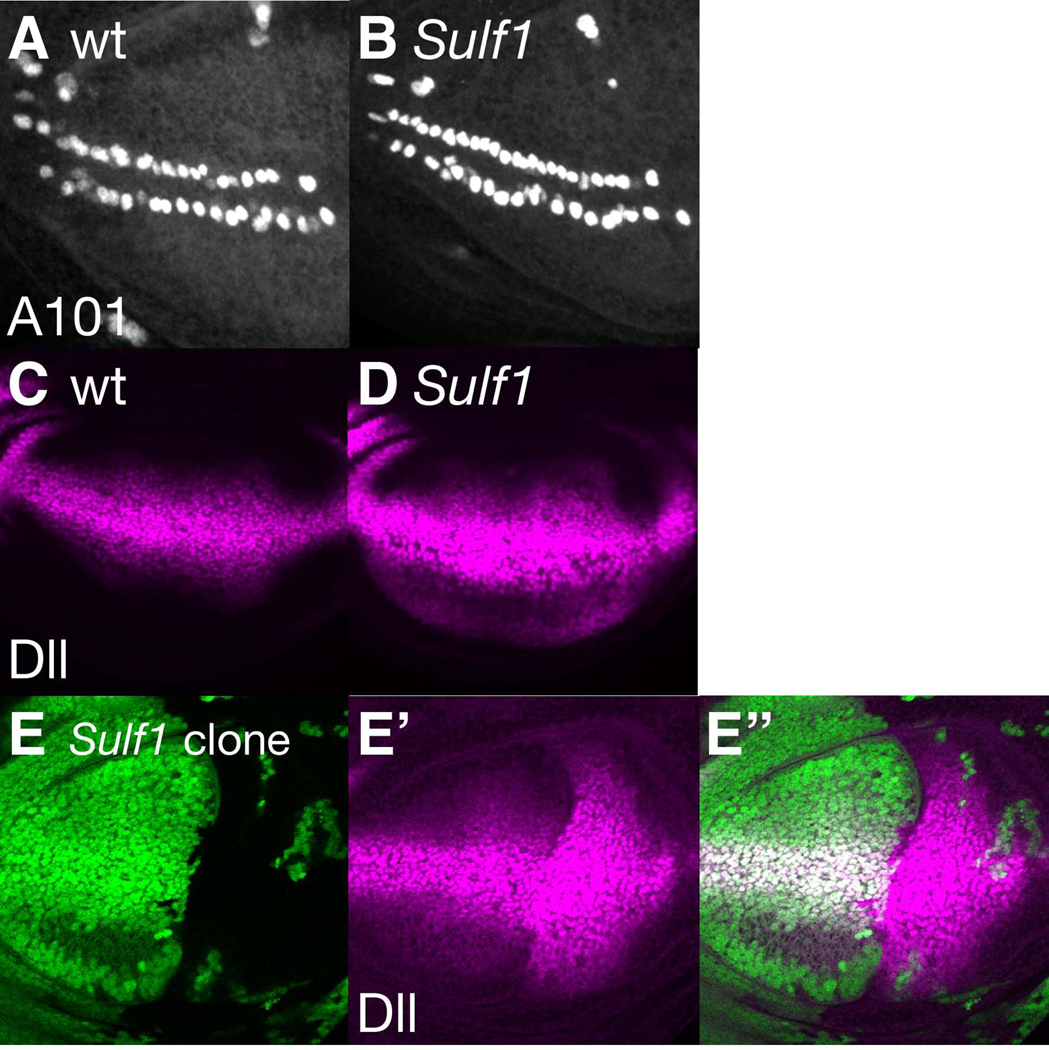
Figure 2. Wg signaling is up-regulated in Sulf1 mutants.
(A and B) neuA101 expression was visualized by anti-β-gal antibody staining of wild-type (A) and Sulf1 (B) mutant wing discs. For this and all other wing disc images, anterior is to the left and dorsal is to the top. (C and D) Anti-Dll antibody staining of wild-type (C) and Sulf1 (D) mutant wing discs. (E-E”) A wing disc baring a large Sulf1 mutant clone was stained with anti-Dll antibody. Posterior clones were induced by expression of UAS-FLP by hh-Gal4. Positions of Sulf1 mutant cells are shown by loss of GFP signal (green in E) and Dll staining is shown in magenta (E’). E” shows a merged image of E and E’.
To generate Sulf1 mutations, a P-element in P{GT1}GT000656 was excised by P-element transposase from P{ry+, Δ2–3} (99B). The resulting progeny were screened for loss of marker gene expression. Excision chromosomes were analyzed by PCR using flanking primers to identify deletions, and the extent of each deletion was determined by sequencing PCR products that spanned the junction.
Sulf1-ER was constructed by adding a KDEL coding sequence at the C-terminus of Sulf1 cDNA (Munro and Pelham, 1987). Sulf1-Golgi consists of amino acids 1–122 from GalNAc-T3 (GenBank accession number X92689, a gift from S. Cohen) cloned in-frame with amino acids 27–1115 of Sulf1. Wild-type Sulf1, Sulf1-ER, and Sulf1-Golgi were cloned into vector pUASg.attB (a gift from K. Basler), and transgenic strains bearing these constructs were made by BestGene Inc using ϕC31-mediated integration of respective plasmid DNA into Basler ZH line 68E (Bischof et al., 2007).
Immunostaining and in situ RNA hybridization
Antibody staining was performed according to standard procedures (Fujise et al., 2001). The following antibodies were used: Mouse anti-Distal-less (Dll) (1:500, a gift from D. Duncan), mouse anti-Achaete (Ac) (1:5, Developmental Studies Hybridoma Bank), guinea pig anti-Senseless (Sens) (1:1000, a gift from H. Bellen), rabbit anti-Spalt (Sal) (1:30, a gift from S. Selleck), rabbit anti-pSMAD3 [pS423/425] (1:1000, Epitomics), mouse anti-Wg (1:100, 4D4, DSHB), mouse anti-β-galactosidase (1:50, DSHB), guinea pig anti-β-galactosidase (1:2000, a gift from M. Kanai, (Kanai et al., 2005). Extracellular labeling of Wg protein was performed according to Strigini and Cohen (2000) (Strigini and Cohen, 2000) using the anti-Wg antibody (4D4) at 1:3 dilution. Secondary antibodies were from the AlexaFluor series (1:500; Molecular Probes).
In situ RNA hybridization was performed as described previously (Kamimura et al., 2001). Digoxigenin (Dig)-labelled Sulf1 RNA probes were synthesized using a DIG RNA Labelling Kit (Roche). For colorimetric staining, anti-Dig antibody conjugated with alkaline phosphatase was used as a secondary antibody. The signal was developed by a standard protocol using 3,3'-diaminobenzidine as a substrate. For fluorescent staining, wing discs from hh-Gal4 UAS-GFP/UAS-GPI-Dfz2 or dpp-GAL4 UAS-GFP/UAS-ArmS10 animals were incubated with the Sulf1 probe. The hybridized probes were reacted with anti-Dig-peroxidase (Roche), and the signal was detected using the TSA Biotin System (Perkin Elmer).
To confirm subcellular localization of Sulf1-ER and Sulf1-Golgi, we made HA-tagged forms of each respective Sulf1 construct. A single HA-tag was added on the C-terminal end of both wild-type Sulf1 and Sulf1-Golgi and between the C-terminus of Sulf1 and the KDEL sequence for Sulf1-ER. Subcellular localization of these modified forms of Sulf1 was examined using GFP-KDEL (a gift from K. Irvine) and Hs2st-Myc (Kamimura et al., 2006) as an ER and Golgi marker, respectively. Extracellular Sulf1 was detected by staining with rat anti-HA (1:100, Roche) using the extracellular staining methods described above (Strigini and Cohen, 2000).
Wg intensity plot analysis
Plot analyses of extracellular Wg staining was performed using ImageJ software and statistical analysis was completed with Microsoft Excel software. Raw data were obtained using a static rectangular box of arbitrary units in ImageJ placed in the central region of each wing disc. Extracted raw data from the plot profile menu in ImageJ were exported to an Excel spread sheet, where maximum peak intensity points were aligned for both wild-type and mutant discs. Averaged values for each genotype were then plotted in a Microsoft Excel graph together. For more direct comparison of gradient shape, wild-type and Sulf1 graphs were transposed directly on top of each other using Adobe Illustrator.
Preparation and HPLC analysis of HS disaccharides
HS isolation and disaccharide composition analysis were carried out as previously described (Kamimura et al., 2006; Toyoda et al., 2000). Briefly, approximately 200 mg of adult flies was homogenized to isolate HS. The HS sample was digested with a heparitinase mixture (Seikagaku), and the resulting disaccharides were separated using reversed-phase ion-pair chromatography. The effluent was monitored fluorometrically for post-column detection of HS disaccharides.
RESULTS
Sulf1 regulates sensory bristle formation on the anterior wing margin
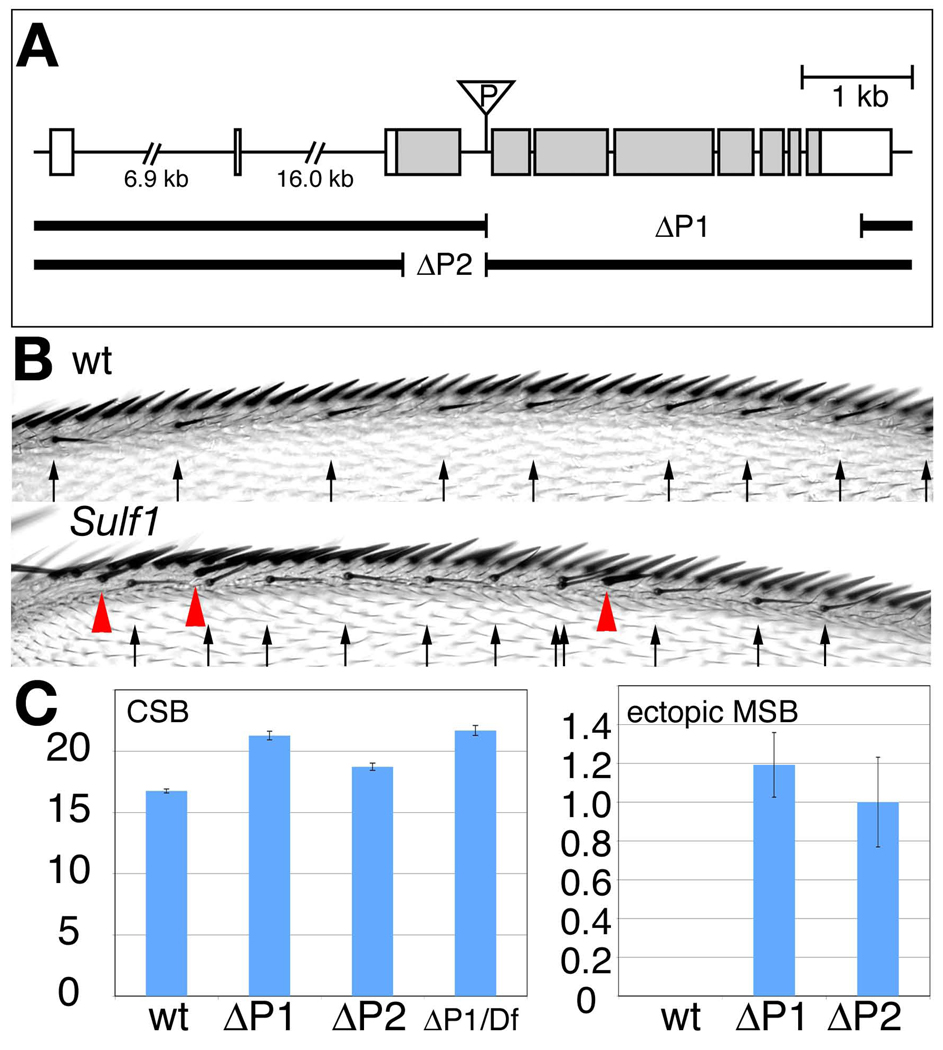
To study the roles of Sulf1 in development, we isolated mutations in the Sulf1 locus by imprecise excision of a P-element transposon. After inducing excision of P{GT1}GT000656, which is inserted in the third intron of the Sulf1 locus, we screened for deletions in the genomic DNA neighboring the original P-element insertion site. Two imprecise excision alleles were isolated by PCR-based screening and named Sulf1ΔP1 and Sulf1ΔP2. Breakpoints of both deletion alleles were mapped by sequencing. The molecular analysis of Sulf1ΔP1 showed that this mutant allele lacks exons 4–10, resulting in the loss of most of the protein coding sequence, including part of the catalytic domain of Sulf1 (Fig 1A). Sulf1ΔP2 allele bears a smaller deletion in exon 3, which causes a frame shift at the amino acid residue Ser59. This results in an early termination of translation at the amino acid 63. Residual nucleotides in the non-deleted region of Sulf1ΔP2 include sequences for the enzymatic active domain of the Sulf1 protein.
Figure 1. Phenotypes of Sulf1 mutants.
(A) Genomic organization of the Sulf1 locus. The shaded area indicates the protein coding region. Two sulf1 deletion alleles, Sulf1ΔP1 (ΔP1) and Sulf1ΔP2 (ΔP2), were generated by P-element imprecise excision. Sulf1ΔP1 lacks exon 4–10, which includes most of the protein coding region. Sulf1ΔP2 has a smaller deletion located in exon 3, which corresponds to the N-terminal domain of Sulf1 protein. This deletion causes a frame shift at amino acid residue Ser59. (B) Dorsal view of the anterior wing margin of wild-type (wt) and Sulf1 mutant adult wings. The arrows and red arrowheads indicate positions of chemosensory bristles and ectopic mechanosensory bristles, respectively. These phenotypes are consistent with an expanded region of high-level Wg signaling. (C) Bar graphs showing the number of chemosensory bristles (CSB; left) and ectopic mechanosensory bristles (MSB; right) in Sulf1 mutants. Values are shown for wild-type (wt), Sulf1ΔP1 (ΔP1), Sulf1ΔP2 (ΔP2), and Sulf1ΔP1/Df(3R)sdb26 (ΔP1/Df). Both Sulf1ΔP1 and Sulf1ΔP2 alleles had a significant increase in chemosensory (p<0.001), mechanosensory (p<0.001), and ectopic mechanosensory (p<0.001) bristles compared to wild-type. Sulf1ΔP1/Df(3R)sdb26 had a similar bristle number as Sulf1ΔP1 (p=0.2), which along with molecular data indicate that it is a null allele. Wing margin bristles were counted for more than 20 wings for each genotype. Error bars for each genotype were calculated using standard error (STDEV/SQRT(n)). P-values for the wing margin bristles were obtained using a one tailed, unpaired, Student’s t-test with equal variance in Microsoft Excel.
The isolated Sulf1 mutants were viable and fertile. However, we found that both mutants show several specific adult phenotypes pertaining to the wing margin bristles. Sulf1 mutations led to an increase in the number of chemosensory and mechanosensory bristles on the anterior wing margin (Fig. 1B and C). Sulf1 mutants also have ectopic mechanosensory bristles which have shifted more posteriorly than their normal position. All these phenotypes are consistent with abnormally elevated Wg signaling near the DV boundary of the mutant wing (Cadigan et al., 1998; Gerlitz and Basler, 2002; Giraldez et al., 2002; Kirkpatrick et al., 2004).
To further characterize the Sulf1 alleles, we used a deficiency line, Df(3R)sdb26, which lacks the cytological region 89B9-89C7 including the entire Sulf1 locus. The phenotypes of increased chemosensory and mechanosensory bristles, and ectopic mechanosensory bristles of Sulf1ΔP1 homozygous animals were as severe as those of transheterozygotes bearing Sulf1ΔP1 over Df(3R)sdb26 (Fig. 1C). These results, together with the molecular mapping of the deletion, defined Sulf1ΔP1 as a null allele of Sulf1. The phenotypes of Sulf1ΔP2 homozygous adults showed slightly less severe phenotypes compared to those of Sulf1ΔP2/Df(3R)sdb26 heterozygotes and Sulf1ΔP1 homozygotes, implying that this allele is hypomorphic. The Sulf1ΔP1 allele was thus used for all further analyses described below.
Sulf1 mutation upregulates Wg signaling in the developing wing
Since Sulf1 mutant alleles affect the formation of wing margin bristles, a Wg-dependent process, we assayed downstream markers of Wg signaling in the developing wing. First, we visualized sensory organ precursors (SOPs) using neuralizedA101 enhancer-trap. SOP formation is induced by high levels of Wg signaling in the anterior part of the DV boundary of the wing disc in the late third instar larval stage (Huang et al., 1991; Phillips and Whittle, 1993). Anti-β-gal antibody staining revealed a regular pattern of SOPs in wild-type discs (Fig. 2A). Sulf1 mutants exhibited an increase in SOP number (average number=20.5, n=27) when compared to wild-type (average number=18.4, n=21, p<0.005) (Fig. 2B). We often observed an irregular, broader distribution of the SOPs, suggesting that Wg signaling is enhanced at the presumptive wing margin. This phenotype is consistent with the increased number and ectopic distribution of wing margin bristles observed in Sulf1 mutant adults.
We next analyzed expression of Distal-less (Dll), a low-threshold target gene of Wg signaling. We found that Dll levels were consistently elevated in Sulf1 mutant wing discs compared to the wild-type counterparts (Fig. 2C and D). To confirm this result, Sulf1 mutant clones were generated by somatic recombination and their effects on Dll expression was examined. Fig. 2E-E” shows a wing disc that has a large Sulf1 mutant clone covering almost the entire posterior compartment. The area of cells positive for anti-Dll antibody staining was remarkably expanded in the mutant clone compared to wild-type control region. Thus, Wg signaling is up-regulated throughout Sulf1 mutant wing discs, indicating that Sulf1 is a novel negative regulator of Wg signaling.
Overexpression of Sulf1 compromises Wg signaling
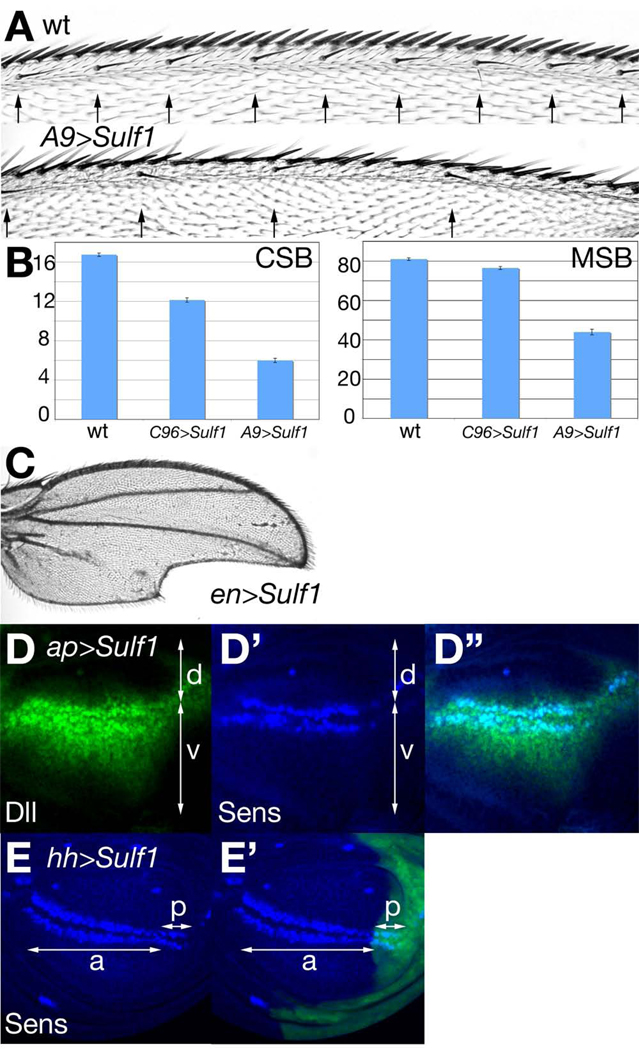
Based on the observation that Wg signaling is elevated in Sulf1 mutant discs, we hypothesized that Sulf1 overexpression would disrupt Wg signaling. To test this idea, a UAS-Sulf1 transgene was driven in the wing disc by various Gal4 drivers. We found that Sulf1-overexpressing adult wings exhibited a wide range of phenotypes. Sulf1 expression at a moderate level using C96-Gal4, a driver for the DV border of the wing disc, or A9-Gal4, a driver for the entire wing disc, resulted in a significantly reduced number of wing margin bristles (Fig. 3A and B). Sulf1 expression at higher levels, driven by en-Gal4 or hh-Gal4, led to notched wings (penetrance: 96%, n=27; Fig. 3C). These phenotypes are consistent with compromised Wg signaling (Phillips and Whittle, 1993). To determine if Sulf1 overexpression indeed affects Wg signaling, larval wing discs were stained for Dll. Sulf1 misexpression in the D compartment driven by ap-Gal4 abrogated Dll expression (Fig. 3D), confirming that Sulf1 inhibits Wg signaling. On the other hand, the effect of Sulf1 overexpression on Senseless (Sens), a high-threshold target for Wg signaling, was less evident (Fig. 3D’). Similarly, Sense expression was not obviously affected by Sulf1 overexpression by hh-Gal4 (Fig. 3E and E’). Thus, low- and high-threshold markers exhibited different sensitivities to alterations of Sulf1 activity. Collectively, these results are consistent with the idea that Sulf1 is a novel negative regulator of Wg signaling at the DV boundary of the developing wing.
Figure 3. Wg signaling is abrogated in Sulf1 overexpressing cells.
(A) Anterior wing margins of wild-type (upper) and A9>Sulf1 (lower) adult wings. Arrows indicate positions of chemosensory bristles. (B) Bar graph showing the number of chemosensory bristles (CSB; left) and mechanosensory bristles (MSB; right) in wild-type (wt), C96>Sulf1 and A9>Sulf1 animals. A significant decrease in chemosensory (C96>Sulf1 and A9>Sulf1: p<0.001) and mechanosensory (C96>Sulf1: p=0.03; A9>Sulf1: p<0.001) bristles was observed. Wing margin bristles were counted for more than 20 wings for each genotype. Error bars and p-values were calculated as described in Figure 1. (C) Wing notching phenotype observed in en>Sulf1 adult flies. (D-D”) Expression of Dll (green, D and D”) and Sens (blue, D’ and D”) in an ap>Sulf1 larval wing disc. Arrows mark the dorsal (d) and ventral (v) compartments. (E and E’) Expression of Sens in an hh>Sulf1 larval wing disc (blue). GFP in E’ marks the posterior compartment expressing Sulf1 (green). Arrows indicate the anterior (a) and posterior (p) compartments.
In addition to the Wg-dependent processes described above, Sulf1 overexpression also induced other phenotypes characteristic of reduced Dpp signaling. For example, regions overexpressing Sulf1 were substantially reduced in size, suggesting that cell proliferation is affected (Fig. 3D-E’). Since Dpp signaling is required for the normal proliferation of wing cells (Burke and Basler, 1996), this observation supports the idea that Sulf1 overexpression disrupts this pathway. The adult phenotypes of these animals are also consistent with this hypothesis: wings from a large portion of en>Sulf1 adult flies lacked crossveins or had incomplete anterior and posterior crossveins, and exhibited defects in longitudinal veins L4 and L5 (Fig. 3C). As would be expected for reduced Dpp signaling, we observed that ap>Sulf1 wing discs showed a reduced area expressing Spalt (Sal), a downstream Dpp transcriptional target (Fig. S1A and A’). In addition, phosphorylation of Mad protein, a direct readout of Dpp signaling, is also significantly decreased by Sulf1 overexpression (Fig. S1B and B’). The pMad staining was lost in the receiving cells, but was higher in the Dpp-expressing domain. Interestingly, this pattern in the dorsal compartment of ap>Sulf1 wing discs resembles that of dally mutant wing discs (Fujise et al., 2003), suggesting that Sulf1 overexpression may disrupt the co-receptor function of Dally. Thus, the adult phenotypes and analysis of Dpp targets indicated that excess Sulf1 activity compromises Dpp signaling as well as the Wg pathway.
Sulf1 affects extracellular levels of Wg protein
To analyze the mechanism for the regulation of Wg signaling by Sulf1, we examined the level of Wg protein in Sulf1 homozygotes. Wing discs were stained with anti-Wg antibody using a protocol designed to specifically detect Wg protein in the extracellular space (Strigini and Cohen, 2000). We found that overall levels of Wg protein in the mutant discs were higher than those of wild-type (Fig. 4A-B’). In addition to the change in the overall levels of the Wg gradient, we noticed a moderate but consistent difference in the staining pattern between wild-type and Sulf1 discs. In the mutant discs, the signal for extracellular Wg was more intense and concentrated at the DV boundary, when compared to wild-type discs, which show a broader distribution of Wg protein near the Wg-expressing cells. The differential distribution of Wg was further confirmed by a quantitative analysis on multiple discs. Average Wg signal intensity plots generated from both wild-type and Sulf1 homozygous discs confirmed elevated Wg protein levels throughout the mutant wing disc (Fig. 4C). Furthermore, the intensity plots indicated that the shape of the gradients is remarkably different. Directly overlaying the intensity plots for wild-type and Sulf1 mutants revealed that the extracellular level of Wg protein near the DV boundary forms a sharp peak in the mutants whereas the wild-type curve is more gradual (dashed red line in Fig. 4C), which suggests that Sulf1 affects Wg distribution near the DV boundary. These results suggest that Sulf1 has two effects on the Wg gradient: It reduces extracellular levels of Wg protein throughout the wing disc, and facilitates the lateral diffusion of Wg near the DV boundary.
To further confirm that Sulf1 affects Wg protein levels, wing discs bearing Sulf1 mutant clones were stained for extracellular Wg. In small mutant clones, typically one or a few cell diameter in size, we observed two separate phenotypic classes. The first class exhibited accumulation of extremely high levels of Wg protein in a cell autonomous fashion (penetrance: 60%, n=103, arrowhead, Fig. 4D-D”). The second class displayed more moderately elevated levels of Wg (penetrance: 40%, data not shown). The penetrance of either of the two Wg accumulation phenotypes was independent of clone position relative to the Wg-expressing cells. To determine if the accumulation of high levels of Wg protein in small Sulf1 clones is associated with the synthesis of Wg, wg expression was monitored by wgen11 (wg-lacZ). We observed that Wg accumulation occurs without the induction of wg transcription (Fig. 4F’-F”’). Interestingly, all larger clones showed the phenotypes of the second class, which is similar to homozygous null mutant discs. It is possible that during the growth of clones, cells with extremely high levels of Wg undergo apoptosis (Adachi-Yamada and O'Connor, 2002), allowing only more phenotypically mild clones to grow. Together, these results indicate that the up-regulation of Wg signaling in Sulf1 mutant tissue is due to the abnormal accumulation of Wg protein. Some HSPGs have been shown to regulate morphogen gradient formation by stabilizing ligand on the cell surface (Akiyama et al., 2008). Therefore, Sulf1-mediated modification of HS fine structure appears to modulate the activity of HSPGs to decrease extracellular levels of Wg protein.
Sulf1 overexpression reduces the extracellular levels of Wg protein
To further examine the role of Sulf1 in the Wg pathway, we directly visualized the distribution of Wg protein in wing discs overexpressing Sulf1. Staining for extracellular Wg protein revealed that its distribution was dramatically disrupted in the D compartment of ap>Sulf1 wing discs (Fig. 5A). However, because Sulf1 overexpression over a wide area decreases the compartment size, it was difficult to directly compare the shape of the Wg gradient between the D and V compartments. Therefore, we examined the effects of ectopically expressed Sulf1 in randomly induced clones using the FLP-OUT system (Struhl and Basler, 1993). Extracellular Wg protein levels were significantly reduced in the Sulf1 overexpressing FLP-OUT clones (arrowheads in Fig. 5B). Remarkably, the function of Sulf1 appears to be cell autonomous (Fig. 5C and C’).
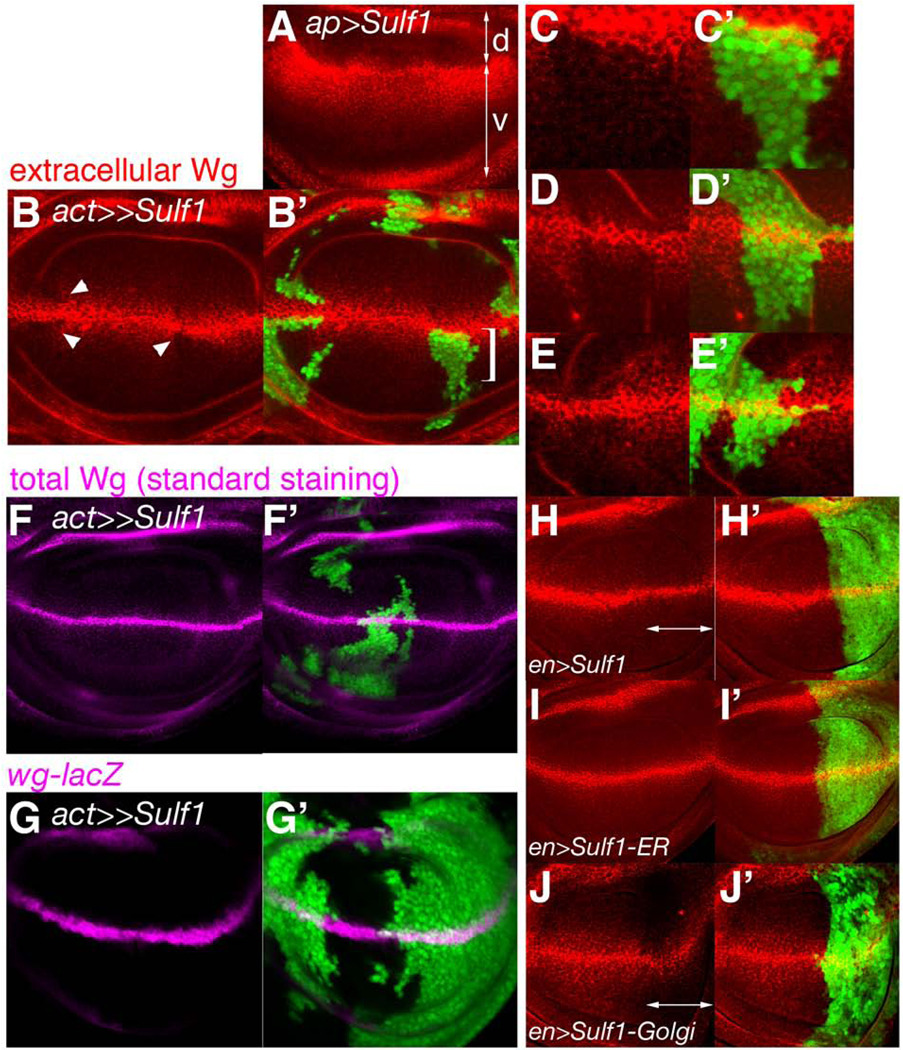
Figure 5. Effects of Sulf1 overexpression on the distribution of extracellular Wg protein.
(A) Extracellular Wg staining (red) of an ap>Sulf1 wing disc. Arrows indicate the dorsal (d) and ventral (v) compartments. (B-E’) Immuno-staining of extracellular Wg (red) in a wing disc bearing Sulf1-overexpressing FLP-OUT clones. (B and B’) Extracellular levels of Wg protein are reduced in the FLP-OUT clones (arrowheads in B) marked with GFP (B’). (C and C’) A high magnification view of the region marked by the bracket in B’, showing the cell autonomous nature of Sulf1’s effect on Wg levels. (D-E’) Two examples are shown for FLP-OUT clones located on the Wg expressing domain. (F and F’) A wing disc with Sulf1-overexpressing clones stained with anti-Wg antibody using the standard immuno-staining protocol. (G and G”) A wing disc with Sulf1 overexpressing clones stained with anti-β-gal antibody to mark wg-lacZ expression. (H-J’) Overexpression of modified forms of Sulf1. Extracellular levels of Wg protein (red) were examined in wing discs from en>Sulf1 (H and H’), en>Sulf1-ER (I and I’), and en>Sulf1-Golgi (J and J’). The posterior compartment is marked by GFP (green in H’, I’, and J’).
To determine whether Sulf1 affects wg expression and/or secretion, we detected Wg protein using a conventional immuno-staining protocol, which emphasizes intracellular Wg. Sulf1 overexpression did not show a detectable change in Wg staining by this staining method, indicating that expression or secretion of Wg protein is not significantly affected (Fig. 5D and D’). We also monitored wg expression using wg-lacZ. No significant change was observed in wg-lacZ signals between inside and outside of Sulf1 overexpressing clones (Fig. 5E and E’), also supporting that wg transcription was not altered. These results together suggest that Sulf1 modulates extracellular levels of Wg protein in a cell autonomous fashion without affecting wg expression.
The cell autonomous nature of Sulf1 activity raised the question of whether this enzyme can act in intracellular compartments. To clarify this point, we generated transgenic strains bearing UAS constructs to express an ER-tethered form (Sulf1-ER) and a Golgi-tethered form (Sulf1-Golgi) of Sulf1. Sulf1-ER and Sulf1-Golgi both localized to the expected compartments when expressed in S2 cells (Fig. S2). We expressed wild-type and the modified forms of Sulf1 in the posterior compartment of wing discs by en-Gal4, and assessed their ability to decrease extracelluler Wg in vivo (Fig. 5H-J’). A significant decrease in extracellular Wg levels was observed in the posterior compartment of en>Sulf1 but not en>Sulf1-ER discs (Fig. 5H-I’). Importantly, Sulf1-Golgi showed the activity to reduce extracellular Wg levels similar to wild-type form (Fig. 5J and J’). These results indicated that Sulf1 can function either in the Golgi or on the cell surface, or it may act in both locations. A similar observation was reported for QSulf1 (Ai et al., 2003).
Relationship between Hs6st and Sulf1
The discovery of Sulfs raises several questions. For example, what is the biological significance of having an enzyme that re-modifies HS structures? Why do only 6-O sulfate groups undergo the step of desulfation? To better understand the post-synthetic function and substrate specificity of Sulf1, we examined the genetic interactions between Sulf1 and Hs6st, which catalyzes 6-O sulfation of the glucosamine residues of HS in the Golgi. Sulf1 mutants display an increased number of wing margin bristles (Table 1). On the other hand, Hs6st mutants have a wild-type wing margin bristle phenotype. Our previous study on Hs2st and Hs6st showed that HS modifications can be adjusted in response to a defect in one type of sulfation: losses of 6-O sulfation can be compensated by increased 2-O sulfation, and vice versa, thus maintaining growth factor signaling essential for normal development in both Hs2st and Hs6st mutants (Kamimura et al., 2006).
Table 1. Hs6st is epistatic to Sulf1.
The number of chemosensory bristles (CSB) at the anterior dorsal wing margin of adult female flies for each genotype is shown. Hs6std770 was used as an Hs6st null mutation. Values represent mean ± standard error for bristle numbers counted for 20 or more wings for each genotype.
| Genotype | CSB |
|---|---|
| wild-type | 16.7±0.2 |
| Sulf1 | 21.3±0.3 |
| Hs6st | 16.3±0.2 |
| Sulf1 +/+ Hs6st | 17.7±0.2 |
| Sulf1 Hs6st/+ Hs6st | 16.4±0.7 |
| Sulf1 Hs6st/Sulf1 + | 20.9±0.6 |
| Sulf1 Hs6st | 16.9±0.2 |
If Sulf1 acts in a post-synthetic manner as has been proposed in vertebrate models (Ai et al., 2003; Dhoot et al., 2001; Morimoto-Tomita et al., 2002; Ohto et al., 2002; Wang et al., 2004), one can predict that Hs6st would be epistatic to Sulf1. Hs6st mutants have no detectable 6-O sulfation, so Sulf1 would be expected to have little or no effect on Hs6st mutant GAG chains or on the adult phenotype. Indeed, this proved to be the case: Hs6st Sulf1 double null animals showed wing margin phenotypes indistinguishable from those of Hs6st null animals (Table 1). In addition, the double mutants and Hs6st single mutants did not display the ectopic mechanosensory bristles that were observed in Sulf1 mutants (data not shown). These observations provide genetic evidence for the post-synthetic activity of Sulf1. They also demonstrate that the Sulf1 mutant phenotypes are dependent on 6-O sulfation, confirming the substrate specificity of this enzyme.
Genetic interactions between Sulf1 and glypican genes
We next asked which proteoglycan core protein(s) are regulated by Sulf1. Since both dlp and Sulf1 have a negative effect on Wg signaling at the DV border, we examined the genetic interactions between these two genes. As expected, each of these mutations increased the number of wing margin bristles. Sulf1 dlp double mutants had a moderately increased number of bristles (data not shown), but we did not observe an obvious synergistic effect in this experiment. We also tested the genetic relationship between Sulf1 and dally using the same assay system. We found that Sulf1 dally double mutants had a reduced number of both chemosensory and mechanosensory bristles, which mimics the phenotypes of dally single mutants (Table 2). In addition, dally heterozygosity partially suppressed the ectopic mechanosensory bristle phenotype of Sulf1 mutants. These results suggest that Sulf1 acts in the same pathway as dally and that dally is epistatic to Sulf1. Previous studies showed that dally is epistatic to dlp in bristle formation at the wing margin (Han et al., 2005). Therefore, our genetic interaction experiments support, but do not provide definitive evidence for, the idea that Sulf1 modifies HS chains of Dally and/or Dlp.
Table 2. Genetic interactions between Sulf1 and dally.
The numbers of chemosensory bristles (CSB), mechanosensory bristles (MSB), and ectopic mechanosensory bristles (ectopic MSB) at the anterior dorsal wing margin are shown for the indicated genotypes. dallygem was used as a dally loss-of-function mutant allele. Values represent mean ± standard error for bristle numbers counted for 20 or more wings for each genotype.
| Genotype | CSB | MSB | ectopic MSB |
|---|---|---|---|
| wild-type | 16.7±0.2 | 80.9±0.6 | 0 |
| Sulf1 | 21.3±0.3 | 93.2±0.7 | 1.2±0.2 |
| dally | 8.6±0.1 | 50.6±0.5 | 0 |
| dally Sulf1/dally + | 9.0±0.1 | 55.3±0.3 | 0 |
| dally Sulf1/+ Sulf1 | 20.6±0.5 | 93.5±1.6 | 0.5±0.2 |
| dally Sulf1 | 8.9±0.2 | 56.9±0.5 | 0 |
Effect of Sulf1 on glypican overexpression phenotypes
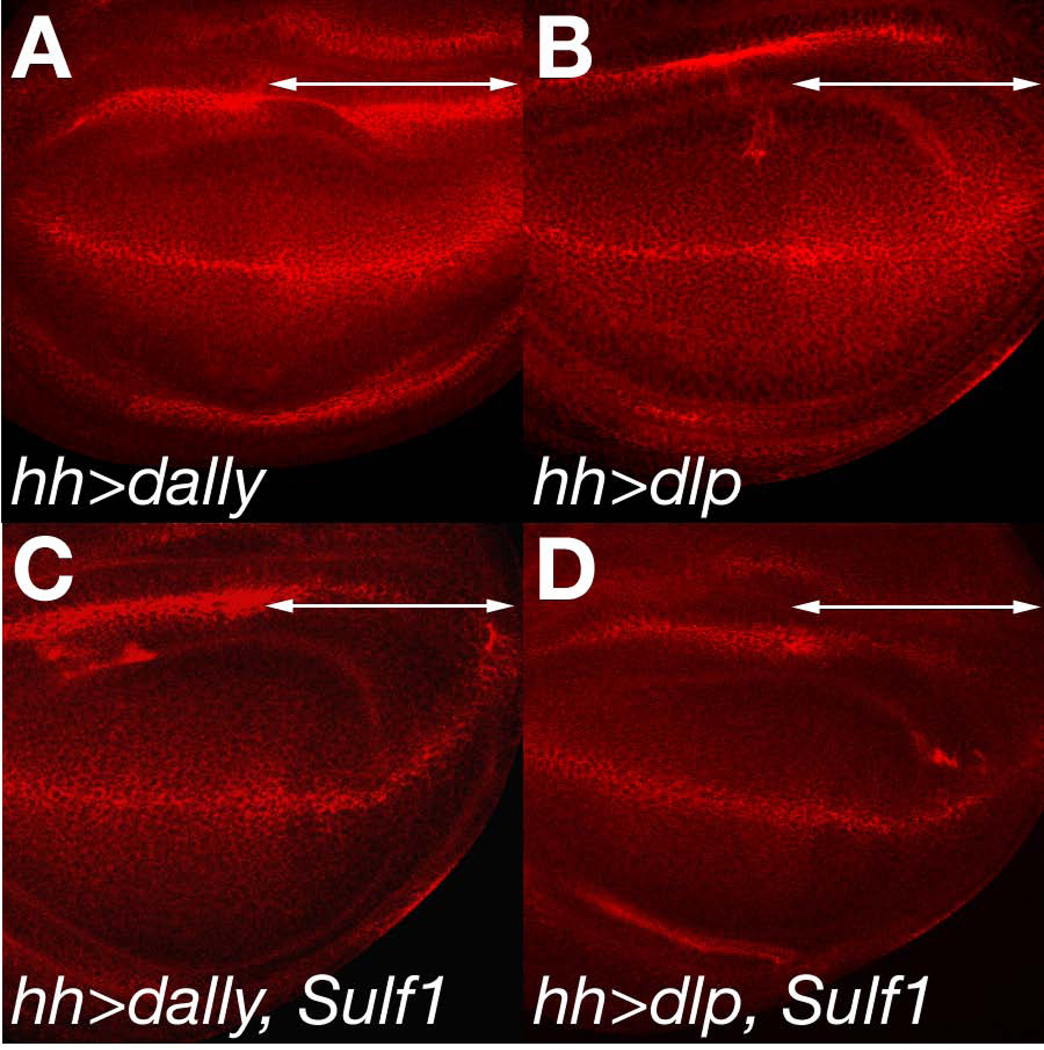
To further analyze the substrate specificity of Sulf1, we examined the effect of Sulf1 overexpression on the overexpession phenotypes of dlp and dally. Overexpression of dally in the posterior compartment using en-Gal4 or hh-Gal4 increases the levels of Wg protein due to stabilization (Fig. 6A;(Han et al., 2005)). dlp expression also results in a similar accumulation of Wg (Fig. 6B and B’;(Baeg et al., 2001; Han et al., 2005)). To determine if Sulf1 affects these phenotypes, we co-expressed Sulf1 with dally or dlp, and extracellular Wg levels were observed in each wing disc. As depicted in Fig. 6C and D, co-expression of Sulf1 with dally or dlp completely blocked the accumulation of Wg induced by glypican expression, leading to a dramatic loss of Wg protein. The signal intensity of extracellular Wg in the P compartment was lower than the A compartment (Fig. 6C and D), indicating that Sulf1 not only affected the overexpressed molecules but also endogenous HSPGs. Thus, Sulf1 suppressed the phenotype produced by overexpression of both glypicans, suggesting that Sulf1 can modulate HS structures of both Dlp and Dally in this artificial in vivo system. As discussed later, since expression patterns of Sulf1 is closely related to those of dally, but not dlp, Dally may be the major direct substrate of Sulf1 in the developing wing.
Figure 6. Sulf1 affects dlp- and dally-dependent Wg accumulation.
(A and B) Extracellular Wg staining of UAS-dally/+; hh-Gal4/+ (A) and UAS-dlp/+; hh-Gal4/+ (B) wing discs. Arrows show the posterior compartment where UAS transgenes were driven by hh-Gal4. (C and D) Effect of Sulf1 co-expression on the dally and dlp overexpression phenotypes. UAS-dally/+; hh-Gal4/UAS-Sulf1 (C) and UAS-dlp/+; hh-Gal4/UAS-Sulf1 (D) wing discs were stained for extracellular Wg. Co-expression of Sulf1 with dally or dlp blocked the overexpression phenotypes of both glypicans.
HS disaccharide profiles of Sulf1 mutants
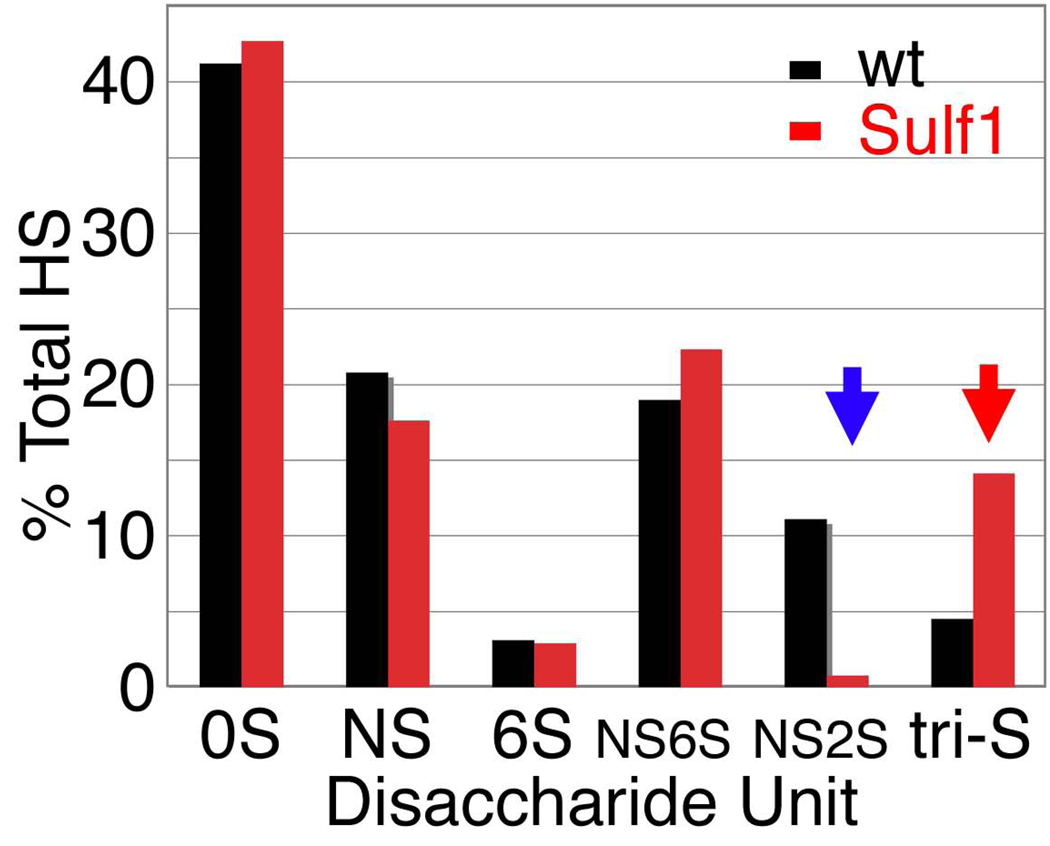
To characterize the enzymatic activity of Sulf1, we studied the disaccharide structures of HS isolated from Sulf1 mutants. HS was purified from adult flies and completely digested into disaccharide units by heparin lyases. Differently modified disaccharides were separated by reverse-phase HPLC. The disaccharide profile of Sulf1 mutant HS revealed abnormally high levels of the tri-S disaccharide unit (ΔUA2S-GlcNS6S) (Fig. 7). Instead, the levels of the NS2S unit (ΔUA2S-GlcNS) were significantly reduced in the Sulf1 mutants. These observations confirmed that HS is normally synthesized in a hypersulfated form and excess 6-O sulfate groups are later trimmed by Sulf1. Notably, there was no significant increase in the amount of 6S (ΔUA-GlcNAc6S) or NS6S (ΔUA-GlcNS6S) disaccharide units, indicating a high substrate specificity for Sulf1. This enzyme selectively removes 6-O sulfate groups from tri-S ΔUA2S-GlcNS6S disaccharide units of HS. This substrate specificity of Sulf1 is similar to that of vertebrate homologues reported previously (Ai et al., 2006; Ai et al., 2003).
Figure 7. HS disaccharide profiling of Sulf1 mutants.
Graphical depiction of disaccharide composition of HS from wild-type (black) and Sulf1 mutants (red), represented as percent of total HS. The Sulf1 mutant has a decrease in NS2S groups (blue arrow) and a concomitant increase in NS2S6S groups (red arrow). Abbreviations for disaccharides are: 0S, ΔUA-GlcNAc; NS, ΔUA-GlcNS; 6S, ΔUA-GlcNAc6S; NS6S, ΔUA-GlcNS6S; NS2S, ΔUA2S-GlcNS; tri-S, ΔUA2S-GlcNS6S.
Expression of the Sulf1 gene is controlled by Wg signaling
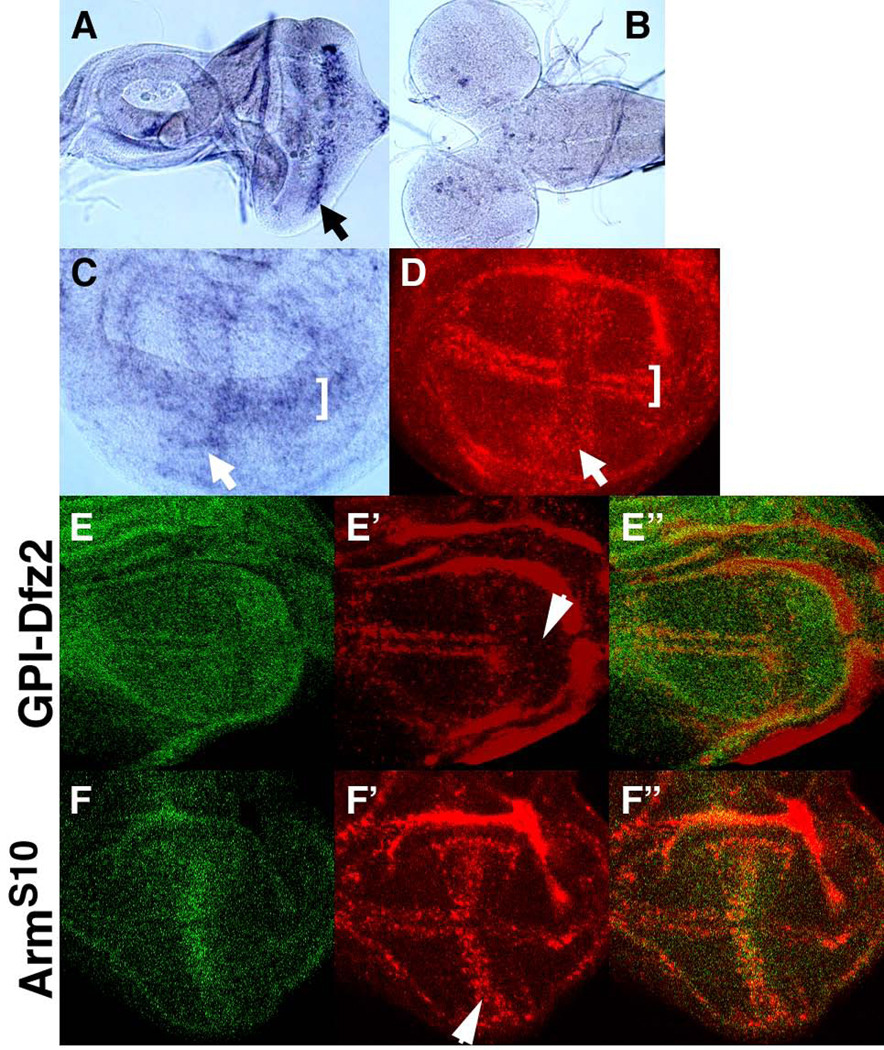
Previous studies have shown that some HS modifying enzymes are controlled at the transcriptional level, thus providing a mechanism for temporal and spatial regulation of HS fine structure (reviewed in (Gorsi and Stringer, 2007)). In situ hybridization revealed that Sulf1 expression exhibits specific patterns in different larval tissues. Sulf1 mRNA is expressed at high levels in the morphogenetic furrow of the eye disc (Fig. 8A) and in selective sets of cells in the central brain region of the larval CNS (Fig. 8B). Sulf1 is also expressed in tracheal precursor cells (data not shown). This is interesting given that these cells also express high levels of Hs6st, which transfers 6-O sulfate groups onto HS (Kamimura et al., 2001).
Figure 8. Regulation of Sulf1 expression by Wg signaling.
(A–D) Sulf1 mRNA expression in the developing imaginal tissues was analyzed by in situ hybridization. High levels of Sulf1 expression were detected in the morphogenetic furrow in the eye disc (arrow in A) and in specific sets of cells in the central brain (B). In the wing discs, hybridized probe was detected with a colorimetric reaction (C) or fluorescent dye (D). Sulf1 is expressed at high levels near the AP compartment boundary (arrows) and the DV border (brackets) of the wing disc. (E-F”) Sulf1 mRNA expression was examined in hh-Gal4 UAS-GFP/UAS-GPI-Dfz2 (E-E”) and dpp-GAL4 UAS-GFP/UAS-ArmS10 (F-F”) wing discs. GFP signal from UAS-GFP is shown in green (E, E”, F and F”). (E-E”) A dominant negative form of the Wg receptor, GPI-Dfz2, was expressed in the posterior compartment by hh-Gal4. Sulf1 expression was reduced by GPI-Dfz2 (red in E’). (F-F”) Expression of a constitutively active form of Arm, ArmS10, by dpp-Gal4, up-regulated Sulf1 expression (red in E’).
One intriguing pattern of Sulf1 transcripts is the high level of expression near the AP and DV boundaries of the wing disc (Fig. 8C and D). Since Wg signaling regulates expression of a number of genes, including components of the Wg pathway at the DV boundary (Cadigan et al., 1998; Gerlitz and Basler, 2002; Giraldez et al., 2002), the Sulf1 expression pattern prompted us to determine if Sulf1 expression is regulated by Wg signaling. To address this question, we modulated Wg signaling using the Gal4/UAS system and monitored the effect on Sulf1 expression by in situ hybridization with fluorescent probes. Expression of UAS-GPI-Dfz2, a dominant negative form of the Wg receptor Dfrizzled2 (Dfz2), partially compromises Wg signaling (Cadigan et al., 1998; Rulifson et al., 2000). We observed that GPI-Dfz2 expression by hh-Gal4 eliminated the DV border expression of Sulf1 mRNA (Fig. 8E-E”). Expression of a constitutively active form of Armadillo (Arm), UAS-ArmS10, can induce ectopic activation of Wg signaling (Pai et al., 1997). ArmS10 is highly lethal when expressed in large domains, but we found that its expression at the AP compartment boundary driven by dpp-Gal4 is partially penetrant, allowing us to obtain wing discs from surviving animals. Fluorescent in situ staining of Sulf1 transcripts in dpp>ArmS10 displayed a massive accumulation of Sulf1 mRNA in the dpp-expressing cells, where Sulf1 expression is normally low (Fig. 8F-F”). Together, these results indicate that Wg signaling at the DV boundary induces expression of Sulf1, which acts as a negative regulator of Wg signaling.
DISCUSSION
Although roles for HSPGs in morphogen signaling and distribution have been well established, the molecular basis of these activities remains to be elucidated. A number of genetic and in vitro analyses have demonstrated the critical importance of HS moieties for HSPG function during developmental patterning (Bornemann et al., 2004; Han et al., 2004; Nakato and Kimata, 2002; Takei et al., 2004). Recent reports using mutant forms of Dally and Dlp that lack all HS attachment sites have revealed the essential contribution of the core protein to regulating growth factor binding and signaling activity (Kirkpatrick et al., 2006; Yan et al., 2009). Thus, the regulatory function of HSPGs is likely to be affected by a combination of both HS and core protein structures.
HS biosynthesis is a complex, multi-step process catalyzed by Golgi enzymes in a highly organized fashion (reviewed in (Gallagher, 2001)). Recent studies have demonstrated that extracellular Sulfs further modify the HS fine structures in a post-synthetic manner (reviewed in (Gorsi and Stringer, 2007)). Thus, Sulfs may contribute to generating structural diversity and modify the number of ligand binding sites on HS at the cell surface.
Models for the regulation of the Wg gradient by Sulf1
To better understand the importance of regulating HS sulfation during development, we investigated the role of Drosophila Sulf1 in patterning and morphogenesis. Sulf1 mutant wings show specific phenotypes characteristic of abnormally high levels of Wg signaling near the Wg-expressing cells. We demonstrated that extracellular levels of Wg protein were elevated throughout the Sulf1 mutant wing discs, and decreased in cells overexpressing Sulf1. In addition, a Sulf1 mutation caused Wg to accumulate near its source, altering the shape of the gradient. Thus, Sulf1 is a novel regulator of Wg gradient formation. Disaccharide analysis of Sulf1 mutant HS showed abnormally high levels of tri-S disaccharide units, indicating that Sulf1 regulates Wg signaling by modulating HS fine structure. Given that Sulf1 decreases local levels of Wg protein in the extracellular space (Fig. 4 and 5), it is likely that the domain structure of HS to which the Wg ligand preferentially binds includes tri-S disaccharide unit(s) as a major component. Thus, Sulf1 may redefine the shape of the Wg gradient by removing some of the Wg-binding sites from HS on the cell surface.
It has been shown in Drosophila embryos that a significant fraction of Wg protein is retained on the expressing cells in a HSPG-dependent manner (Pfeiffer et al., 2002). High levels of dally expression near the DV boundary of the wing disc (Fujise et al., 2001) suggest that Wg may be also trapped by HS in the developing wing. In such a situation, Sulf1 activity could reduce the trapping of Wg by cell surface HSPGs near the expressing cells. Wg protein, thus released from HS, could have two possible fates. First, Wg ligand could be quickly internalized by nearby cells for degradation. Second, released Wg ligand could escape degradation and migrate away from the trapped site. Therefore, theoretically, Sulf1 can affect the Wg gradient through two differential activities: (1) destabilization of Wg and (2) enhancement of Wg re-distribution by facilitating Wg release from the HSPGs. We showed that Sulf1 reduces extracellular levels of Wg protein without affecting wg expression (Fig. 5). In addition, Wg signal intensity plots for wild-type and Sulf1 mutant discs suggested that Sulf1 affects Wg distribution near the DV boundary (Fig. 4C). Thus, our observations are consistent with the idea that Sulf1 indeed modulates the Wg gradient by influencing both Wg stability and distribution.
How can Sulf1 contribute to lateral distribution of Wg? Gallet et al. (2008) has proposed that Dlp mediates apicobasal trafficking of Wg, which is required for its long-range gradient formation (Gallet et al., 2008). A more recent study has shown that Dlp can act in a biphasic manner to potentiate Wg long-range signaling (Yan et al., 2009). In this model, Dlp either competes with the receptor or provides ligand to the receptor, dependent on its ratio to Wg and the receptor. In both models, however, since dlp expression is repressed at the DV compartment border (Kirkpatrick et al., 2004), an additional mechanism by which Wg reaches the dlp-expressing cells appears to be required. Wg secreted from cells at the DV boundary is likely to be first trapped by Dally, a glypican expressed at high levels in this region (Fujise et al., 2001). One possible function of Sulf1 is to facilitate the short-range movement of Wg from the expressing cells to Dlp (Fig. 9). In this model, Sulf1, which is also abundant near the source of Wg, removes 6-O sulfate groups from Dally HS chains. This enzymatic cleavage would lower the efficiency of Wg trapping by Dally, allowing it to migrate away from the DV boundary. Released Wg would now have a better chance to reach Dlp, which recaptures and facilitates further diffusion of Wg (Gallet et al., 2008; Yan et al., 2009). Thus, our study demonstrates that modification of HS fine structure provides a novel mechanism to shape morphogen gradients.
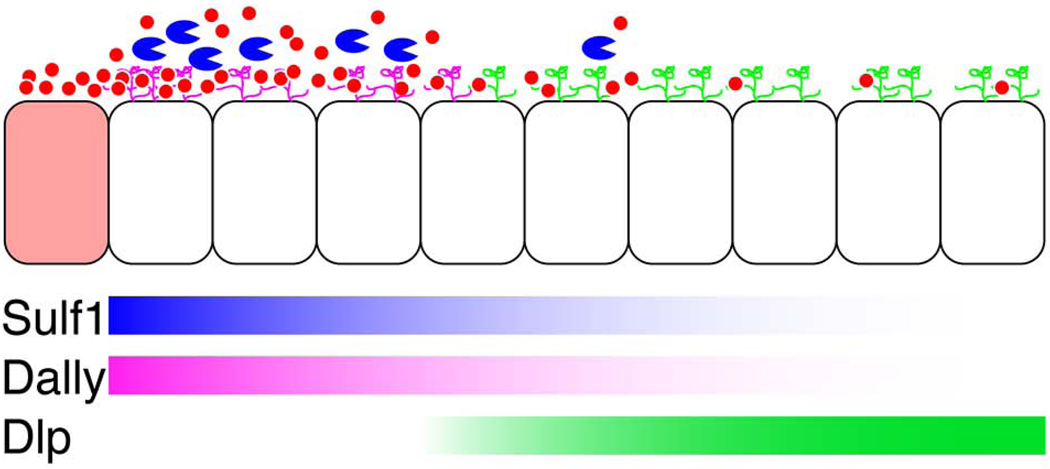
Figure 9. A model of the role of Sulf1 in Wg gradient formation.
Sulf1 (blue) functions by enzymatically modifying the number of 6-O sulfate groups on glucosamine residues of HS tri-sulfated disaccharides. This post-synthetic reduction of 6-O sulfate groups would release Wg (red circle) from HSPGs. Released Wg can undergo either degradation or re-distribution to the periphery of the wing pouch. Since Dally (magenta) is expressed at high levels near the Wg-expressing domain, Wg protein is likely to be first trapped by Dally. Sulf1, which is expressed at high levels in the same region as Dally, may function to release Wg from Dally. Wg released from Dally by Sulf1 can be degraded or re-captured by Dlp (green), of which expression domain is high in the peripheral cells of the wing disc. Wg secreting cells (pink) at the DV border are located on the left side of the figure opposite of Wg non-secreting cells (white) which expand into the periphery of the wing pouch. Expression domains of Sulf1, Dally, and Dlp are represented by blue, magenta, and green graded bars respectively.
Given that vertebrate Sulfs are known to positively regulate Wnt signaling (Ai et al., 2003), it is surprising that Drosophila Sulf1 has an opposite effect on the Wg pathway. Our results suggest that Drosophila Sulf1 has a similar biochemical activity and we expect that a direct consequence of the function of Sulf enzymes on Wnt/Wg protein is also similar between vertebrate and invertebrate models: Sulfs release Wnt/Wg ligands from HSPGs. We propose that the fate of the released Wnt/Wg could be different dependent on extracellular environment. In vertebrate systems where Sulfs enhance Wnt signaling, released Wnt appears to have better chance to bind and activate receptors (Ai et al., 2003). On the other hand, a major fraction of Wg protein detached from HSPGs may be degraded in the Drosophila wing disc.
Although Sulfs are believed to function post-synthetically in the extracellular space, the effects of Sulf1 function were observed cell autonomously. In addition, our experiments using Sulf1-Golgi showed that this modified form retains the ability to decrease extracellular levels of Wg protein, indicating that Sulf1 does not have to be secreted into the extracellular space to function. Thus, Sulf1 may act in the Golgi and/or on the cell surface. If Sulf1 acts extracellularly, Sulf1 is likely to adhere to the surface of the secreting cells as has been shown in vertebrate models: previous studies reported that Sulf enzymes associate with the cell fraction and not the medium fraction of transfected cultured cells (Ai et al., 2003; Dhoot et al., 2001; Lai et al., 2003; Morimoto-Tomita et al., 2002; Ohto et al., 2002). The binding of QSulf1 to the cell fraction in CHO cells has shown to be dependent on a large hydrophilic domain (Dhoot et al., 2001). Since a similar conserved hydrophilic domain is found in Drosophila Sulf1, we hypothesize that Sulf1 may bind to a constituent of the ECM in close proximity to the expressing cells.
We found that small Sulf1 clones show more severe phenotypes than large clones and Sulf1 homozygous mutant discs (Fig. 4D-E”). This observation suggests that morphogen gradients are more severely disrupted in a developmental field with discontinuity of cell surface HS structures (e.g. discs with Sulf1 small clones) compared to one where HS sulfation is uniformly altered (e.g. Sulf1 homozygous mutant discs). However, the molecular mechanism behind this difference remains to be elucidated.
Sulf1 forms a feedback loop of Wg signaling
In situ hybridization showed that Sulf1 mRNA is expressed at high levels near both the AP and DV borders of the wing disc (Fig. 8). Interestingly, this feature is similar to the expression pattern of dally in the wing disc. The DV boundary expression of dally is induced by Wg signaling (Fujise et al., 2001). We showed that expression of Sulf1, like that of dally, is induced by Wg signaling. Thus, Sulf1, a negative regulator of the Wg pathway, participates in a negative feedback loop within this morphogen system. We previously showed that Dally is a component of the negative feedback loop for the Dpp signaling pathway, potentially stabilizing the shape of the Dpp gradient (Fujise et al., 2003). In addition, Wg also induces Notum, which is a secreted antagonist of Wg and functions through the posttranslational cleavage of glypicans at the wing margin (Gerlitz and Basler, 2002; Giraldez et al., 2002; Kreuger et al., 2004). Collectively, these results implicate HSPGs and HS biosynthetic machinery components as general constituents of morphogen feedback systems, supporting the stability and the robustness of morphogen signaling gradients.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to D. Duncan, K. Basler, H. Bellen, S. Selleck, M. Kanai, K. Irvine, S. Cohen, the Developmental Studies Hybridoma Bank, and the Bloomington Stock Center for fly stocks and reagents. We thank M. Masu and C. Kirkpatrick for helpful discussions and critical reading of the manuscript. This work was supported by research grants from NIH (R01 HD042769).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adachi-Yamada T, O'Connor MB. Morphogenetic apoptosis: a mechanism for correcting discontinuities in morphogen gradients. Dev Biol. 2002;251:74–90. doi: 10.1006/dbio.2002.0821. [DOI] [PubMed] [Google Scholar]
- Ai X, Do AT, Kusche-Gullberg M, Lindahl U, Lu K, Emerson CP., Jr Substrate specificity and domain functions of extracellular heparan sulfate 6-O-endosulfatases, QSulf1 and QSulf2. J Biol Chem. 2006;281:4969–4976. doi: 10.1074/jbc.M511902200. [DOI] [PubMed] [Google Scholar]
- Ai X, Do AT, Lozynska O, Kusche-Gullberg M, Lindahl U, Emerson CP., Jr QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J Cell Biol. 2003;162:341–351. doi: 10.1083/jcb.200212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Kamimura K, Firkus C, Takeo S, Shimmi O, Nakato H. Dally regulates Dpp morphogen gradient formation by stabilizing Dpp on the cell surface. Dev Biol. 2008;313:408–419. doi: 10.1016/j.ydbio.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornemann DJ, Duncan JE, Staatz W, Selleck S, Warrior R. Abrogation of heparan sulfate synthesis in Drosophila disrupts the Wingless, Hedgehog and Decapentaplegic signaling pathways. Development. 2004;131:1927–1938. doi: 10.1242/dev.01061. [DOI] [PubMed] [Google Scholar]
- Burke R, Basler K. Dpp receptors are autonomously required for cell proliferation in the entire developing Drosophila wing. Development. 1996;122:2261–2269. doi: 10.1242/dev.122.7.2261. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Fish MP, Rulifson EJ, Nusse R. Wingless repression of Drosophila frizzled 2 expression shapes the Wingless morphogen gradient in the wing. Cell. 1998;93:767–777. doi: 10.1016/s0092-8674(00)81438-5. [DOI] [PubMed] [Google Scholar]
- Dhoot GK, Gustafsson MK, Ai X, Sun W, Standiford DM, Emerson CP., Jr Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science. 2001;293:1663–1666. doi: 10.1126/science.293.5535.1663. [DOI] [PubMed] [Google Scholar]
- Franch-Marro X, Marchand O, Piddini E, Ricardo S, Alexandre C, Vincent JP. Glypicans shunt the Wingless signal between local signalling and further transport. Development. 2005;132:659–666. doi: 10.1242/dev.01639. [DOI] [PubMed] [Google Scholar]
- Freeman SD, Moore WM, Guiral EC, Holme AD, Turnbull JE, Pownall ME. Extracellular regulation of developmental cell signaling by XtSulf1. Dev Biol. 2008;320:436–445. doi: 10.1016/j.ydbio.2008.05.554. [DOI] [PubMed] [Google Scholar]
- Fujise M, Izumi S, Selleck SB, Nakato H. Regulation of dally, an integral membrane proteoglycan, and its function during adult sensory organ formation of Drosophila. Dev Biol. 2001;235:433–448. doi: 10.1006/dbio.2001.0290. [DOI] [PubMed] [Google Scholar]
- Fujise M, Takeo S, Kamimura K, Matsuo T, Aigaki T, Izumi S, Nakato H. Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Development. 2003;130:1515–1522. doi: 10.1242/dev.00379. [DOI] [PubMed] [Google Scholar]
- Gallagher JT. Heparan sulfate: growth control with a restricted sequence menu. J Clin Invest. 2001;108:357–361. doi: 10.1172/JCI13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet A, Staccini-Lavenant L, Therond PP. Cellular trafficking of the glypican Dally-like is required for full-strength Hedgehog signaling and wingless transcytosis. Dev Cell. 2008;14:712–725. doi: 10.1016/j.devcel.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Gerlitz O, Basler K. Wingful, an extracellular feedback inhibitor of Wingless. Genes Dev. 2002;16:1055–1059. doi: 10.1101/gad.991802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez AJ, Copley RR, Cohen SM. HSPG modification by the secreted enzyme Notum shapes the Wingless morphogen gradient. Dev Cell. 2002;2:667–676. doi: 10.1016/s1534-5807(02)00180-6. [DOI] [PubMed] [Google Scholar]
- Gorsi B, Stringer SE. Tinkering with heparan sulfate sulfation to steer development. Trends Cell Biol. 2007;17:173–177. doi: 10.1016/j.tcb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Han C, Belenkaya TY, Khodoun M, Tauchi M, Lin X. Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signalling and gradient formation. Development. 2004;131:1563–1575. doi: 10.1242/dev.01051. [DOI] [PubMed] [Google Scholar]
- Han C, Yan D, Belenkaya TY, Lin X. Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development. 2005;132:667–679. doi: 10.1242/dev.01636. [DOI] [PubMed] [Google Scholar]
- Huang F, Dambly-Chaudiere C, Ghysen A. The emergence of sense organs in the wing disc of Drosophila. Development. 1991;111:1087–1095. doi: 10.1242/dev.111.4.1087. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Fujise M, Villa F, Izumi S, Habuchi H, Kimata K, Nakato H. Drosophila heparan sulfate 6-O-sulfotransferase (dHS6ST) gene. Structure, expression, and function in the formation of the tracheal system. J Biol Chem. 2001;276:17014–17021. doi: 10.1074/jbc.M011354200. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Koyama T, Habuchi H, Ueda R, Masu M, Kimata K, Nakato H. Specific and flexible roles of heparan sulfate modifications in Drosophila FGF signaling. J Cell Biol. 2006;174:773–778. doi: 10.1083/jcb.200603129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai MI, Okabe M, Hiromi Y. seven-up Controls switching of transcription factors that specify temporal identities of Drosophila neuroblasts. Dev Cell. 2005;8:203–213. doi: 10.1016/j.devcel.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CA, Dimitroff BD, Rawson JM, Selleck SB. Spatial regulation of Wingless morphogen distribution and signaling by Dally-like protein. Dev Cell. 2004;7:513–523. doi: 10.1016/j.devcel.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CA, Knox SM, Staatz WD, Fox B, Lercher DM, Selleck SB. The function of a Drosophila glypican does not depend entirely on heparan sulfate modification. Dev Biol. 2006;300:570–582. doi: 10.1016/j.ydbio.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CA, Selleck SB. Heparan sulfate proteoglycans at a glance. J Cell Sci. 2007;120:1829–1832. doi: 10.1242/jcs.03432. [DOI] [PubMed] [Google Scholar]
- Kreuger J, Perez L, Giraldez AJ, Cohen SM. Opposing activities of Dally-like glypican at high and low levels of Wingless morphogen activity. Dev Cell. 2004;7:503–512. doi: 10.1016/j.devcel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Lai J, Chien J, Staub J, Avula R, Greene EL, Matthews TA, Smith DI, Kaufmann SH, Roberts LR, Shridhar V. Loss of HSulf-1 up-regulates heparin-binding growth factor signaling in cancer. J Biol Chem. 2003;278:23107–23117. doi: 10.1074/jbc.M302203200. [DOI] [PubMed] [Google Scholar]
- Li J, Kleeff J, Abiatari I, Kayed H, Giese NA, Felix K, Giese T, Buchler MW, Friess H. Enhanced levels of Hsulf-1 interfere with heparin-binding growth factor signaling in pancreatic cancer. Mol Cancer. 2005;4:14. doi: 10.1186/1476-4598-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J Biol Chem. 2002;277:49175–49185. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Nakato H, Kimata K. Heparan sulfate fine structure and specificity of proteoglycan functions. Biochim Biophys Acta. 2002;1573:312–318. doi: 10.1016/s0304-4165(02)00398-7. [DOI] [PubMed] [Google Scholar]
- Nawroth R, van Zante A, Cervantes S, McManus M, Hebrok M, Rosen SD. Extracellular sulfatases, elements of the Wnt signaling pathway, positively regulate growth and tumorigenicity of human pancreatic cancer cells. PLoS ONE. 2007;2:e392. doi: 10.1371/journal.pone.0000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto T, Uchida H, Yamazaki H, Keino-Masu K, Matsui A, Masu M. Identification of a novel nonlysosomal sulphatase expressed in the floor plate, choroid plexus and cartilage. Genes Cells. 2002;7:173–185. doi: 10.1046/j.1356-9597.2001.00502.x. [DOI] [PubMed] [Google Scholar]
- Pai LM, Orsulic S, Bejsovec A, Peifer M. Negative regulation of Armadillo, a Wingless effector in Drosophila. Development. 1997;124:2255–2266. doi: 10.1242/dev.124.11.2255. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S, Ricardo S, Manneville JB, Alexandre C, Vincent JP. Producing cells retain and recycle Wingless in Drosophila embryos. Curr Biol. 2002;12:957–962. doi: 10.1016/s0960-9822(02)00867-9. [DOI] [PubMed] [Google Scholar]
- Phillips RG, Whittle JR. wingless expression mediates determination of peripheral nervous system elements in late stages of Drosophila wing disc development. Development. 1993;118:427–438. doi: 10.1242/dev.118.2.427. [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Wu CH, Nusse R. Pathway specificity by the bifunctional receptor frizzled is determined by affinity for wingless. Mol Cell. 2000;6:117–126. [PubMed] [Google Scholar]
- Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Curr Biol. 2000;10:293–300. doi: 10.1016/s0960-9822(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Tabata T, Takei Y. Morphogens, their identification and regulation. Development. 2004;131:703–712. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]
- Takei Y, Ozawa Y, Sato M, Watanabe A, Tabata T. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development. 2004;131:73–82. doi: 10.1242/dev.00913. [DOI] [PubMed] [Google Scholar]
- Takeo S, Akiyama T, Firkus C, Aigaki T, Nakato H. Expression of a secreted form of Dally, a Drosophila glypican, induces overgrowth phenotype by affecting action range of Hedgehog. Dev Biol. 2005;284:204–218. doi: 10.1016/j.ydbio.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Tang R, Rosen SD. Functional consequences of the subdomain organization of the sulfs. J Biol Chem. 2009;284:21505–21514. doi: 10.1074/jbc.M109.028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H, Kinoshita-Toyoda A, Selleck SB. Structural analysis of glycosaminoglycans in Drosophila and Caenorhabditis elegans and demonstration that tout-velu, a Drosophila gene related to EXT tumor suppressors, affects heparan sulfate in vivo. J Biol Chem. 2000;275:2269–2275. doi: 10.1074/jbc.275.4.2269. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Kamimura K, Nakato H, Archer M, Staatz W, Fox B, Humphrey M, Olson S, Futch T, Kaluza V, Siegfried E, Stam L, Selleck SB. The cell-surface proteoglycan Dally regulates Wingless signalling in Drosophila. Nature. 1999;400:276–280. doi: 10.1038/22336. [DOI] [PubMed] [Google Scholar]
- Wang S, Ai X, Freeman SD, Pownall ME, Lu Q, Kessler DS, Emerson CP., Jr QSulf1, a heparan sulfate 6-O-endosulfatase, inhibits fibroblast growth factor signaling in mesoderm induction and angiogenesis. Proc Natl Acad Sci U S A. 2004;101:4833–4838. doi: 10.1073/pnas.0401028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- Yan D, Wu Y, Feng Y, Lin SC, Lin X. The core protein of glypican Dally-like determines its biphasic activity in wingless morphogen signaling. Dev Cell. 2009;17:470–481. doi: 10.1016/j.devcel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.