SUMMARY
Conflict procedures can be used to study the receptor mechanisms underlying the anxiolytic effects of benzodiazepines and other GABAA receptor modulators. In the present study, we first determined the efficacy and binding affinity of the benzodiazepine diazepam and recently synthesized GABAA receptor modulators JY-XHe-053, XHe-II-053, HZ-166, SH-053-2'F-S-CH3 and SH-053-2'F-R-CH3 at GABAA receptors containing α1, α2, α3 and α5 subunits. Results from these studies suggest that each compound displayed lower efficacy at GABAA receptors containing α1 subunits and varying degrees of efficacy and affinity at GABAA receptors containing α2, α3 and α5 subunits. Next, we assessed their anxiolytic effects using a rhesus monkey conflict procedure in which behavior was maintained under a fixed-ratio schedule of food delivery in the absence (non-suppressed responding) and presence (suppressed responding) of response-contingent electric shock. Relatively non-selective compounds, such as diazepam and JY-XHe-053 produced characteristic increases in rates of suppressed responding at low to intermediate doses and decreased the average rates of non-suppressed responding at higher doses. XHe-II-053 and HZ-166 also produced increases in suppressed responding at low to intermediate doses, but were ineffective at decreasing rates of non-suppressed responding, consistent with their relatively low efficacy at GABAA receptors containing α1 and α5 subunits. In contrast, SH-053-2’F-S-CH3 and SH-053-2’F-R-CH3 produced only partial increases in suppressed responding and were ineffective on non-suppressed responding, consistent with their profiles as partial agonists at GABAA receptors containing α2, α3 and α5 subunits. These behavioral effects suggest that the anxiolytic and rate-reducing effects of GABAA receptor positive modulators are dependent on their relative efficacy and affinity at different GABAA receptor subtypes.
INTRODUCTION
Benzodiazepine-type drugs act as positive allosteric modulators of γ-aminobutyric acid type A (GABAA) receptors, and are highly efficacious agents for the treatment of anxiety-related disorders. The therapeutic use of benzodiazepine-type drugs is constrained, however, by other characteristic effects such as daytime drowsiness, impairment of motor coordination, memory deficits, and reinforcing effects that may contribute to their abuse (Griffiths and Weerts, 1997; Nutt, 2005). Research during the past two decades has revealed the existence of multiple subtypes of the GABAA receptor (e.g., Pritchett et al., 1989; Rudolph et al., 2001; Olsen and Sieghart, 2008). Subsequent reports have postulated that the diverse behavioral effects of benzodiazepine-type drugs may reflect actions at different subtypes of GABAA receptors (Rudolph et al., 1999; McKernan et al., 2000; Löw et al., 2000; Rowlett et al., 2005). Therefore, it may be possible to dissociate the clinically advantageous and unwanted side-effects of these compounds.
GABAA receptors are pentameric proteins composed of several subunits that form a GABA-gated chloride channel. The majority of GABAA receptors are composed of α, β , and γ subunits and benzodiazepines bind predominantly to a site on the native GABAA receptor that is located at the interface of the γ 2 subunit and one of the α1, α2, α3, or α5 subunits. Benzodiazepines generally are inactive at corresponding α4- and α6-subunit containing receptors. Approximately 90% of the GABAA receptors in the brain that possess a benzodiazepine binding site contain α1, α2, and α3 subunits (McKernan and Whiting, 1996), and GABAA receptors containing α1 subunits (α1GABAA receptors) have been implicated in the sedative effects of benzodiazepines, whereas GABAA receptors containing α2 and α3 subunits (α2GABAA and α3GABAA receptors) have been implicated in the anxiolytic effects of benzodiazepines (McKernan et al., 2000; Löw et al., 2000; Rowlett et al., 2005; Dixon et al., 2008). GABAA receptors containing α5 subunits (α5GABAA receptors), in contrast, are a relatively minor population that may play a role in memory processes, but not anxiolysis or motor effects (Collinson et al., 2002; Crestani et al., 2002; Atack et al., 2006, but see Savic et al., 2008). Therefore, novel benzodiazepine-like drugs that have pharmacological selectivity for α2GABAA and/or α3GABAA receptors and low receptor activity at α1GABAA and α5GABAA receptors may be particularly useful as anxiolytics lacking sedative and amnestic side effects.
The preclinical assessment of the anxiolytic effects of drugs can be accomplished objectively and quantitatively with operant-based conflict procedures. In these procedures, positively reinforced behavior is suppressed by response-contingent administration of a noxious stimulus (e.g., mild electric shock; for review, see Millan 2003). Drugs with anxiolytic effects produce characteristic increases in the rates of responding that are suppressed by response-contingent delivery of shock (e.g., Geller and Seifter 1960; Cook and Davidson 1973; Kleven and Koek 1999; Rowlett et al., 2006), and a particular strength of conflict procedures is their predictive validity with respect to therapeutic effects in humans. In this regard, strong positive correlations between the potency of benzodiazepines to engender anti-conflict effects and to be clinically effective in humans have been demonstrated in rats and pigeons (Cook and Davidson 1973; Kleven and Koek 1999), and more recently in rhesus monkeys (Rowlett et al., 2006). A distinct advantage in using rhesus monkeys in a conflict procedure arises from their close genetic similarity to humans.
In the present study, we first assessed the efficacy and binding affinity of the conventional benzodiazepine diazepam and the recently synthesized and structurally related positive GABAA receptor modulators JY-XHe-053, XHe-II-053 and HZ-166. The efficacy and binding affinity of the 4-methyl-JY-XHe-053 stereoisomers SH-053-2'F-S-CH3 and SH-053-2'F-R-CH3 was also assessed. Each of the novel GABAA receptor modulators demonstrated lower efficacy at α1GABAA receptors relative to diazepam at the drug concentrations that can be reached under our experimental conditions. Further, each compound demonstrated varying degrees of either binding selectivity or relative efficacy for α2GABAA, α3GABAA and α5GABAA receptor subtypes. The primary aim of the present study was to help elucidate the role of these receptor subtypes in the anxiolytic effects of benzodiazepines. Therefore, we used a rhesus monkey conflict procedure to assess the anti-conflict and rate-reducing effects of these drugs. Based on studies suggesting that benzodiazepine action at α1GABAA receptors contributes to their sedative effects, whereas α2GABAA and α3GABAA receptors are important for benzodiazepine-induced anxiolysis, our hypothesis was that the novel GABAA receptor modulators would have anti-conflict effects similar to diazepam but would not be effective in disrupting rates of non-suppressed responding.
MATERIALS AND METHODS
Drugs
JY-XHe-053 (8-ethynyl-6-(2-fluorophenyl)-4H-2,5,10b-triaza-benzo[e]azulene-3-carboxylic acid ethyl ester), XHe-II-053 (8-ethynyl-6-phenyl-4H-2,5,10b-triaza-benzo[e]azulene-3-carboxylic acid ethyl ester), HZ-166 (8-ethynyl-6-(2’-pyridine)-4H-2,5,10b-triaza-benzo[e]azulene-3-carboxylic acid ethyl ester), SH-053-S-CH3-2’F (the S-enantiomer of 4-methyl-JY-XHe-053; (S)-8-ethynyl-6-(2-fluoro-phenyl)-4-methyl-4H-2,5,10b-triaza-benzo[e]azulene-3-carboxylic acid ethyl ester) and SH-053-R-CH3-2’F (the R-enantiomer of 4-methyl-JY-XHe-053; (R)-8-ethynyl-6-(2-fluoro-phenyl)-4-methyl-4H-2,5,10b-triaza-benzo[e]azulene-3-carboxylic acid ethyl ester) (Cook et al., 2009) were synthesized at the Department of Chemistry and Biochemistry, University of Wisconsin-Milwaukee. JY-XHe-053, XHe-II-053, HZ-166, SH-053-2'F-S-CH3 and SH-053-2'F-R-CH3 have a similar pharmacokinetic profile and duration of action (Rivas et al., 2009; Cook et al., 2010). Diazepam was purchased from Tocris-Cookson (Ellisville, MO, USA). All drugs were dissolved in 20% ethanol, 60% propylene glycol, and 20% sterile water. If necessary, the pH of a solution was adjusted to 7.0 with 1N HCl.
Competition Binding Assays
Competition binding assays were performed in a total volume of 0.5 mL at 4 ° C for 1 hour using [3H]flunitrazepam as the radiolabelled ligand. A total of 6 μg of cloned human GABAA receptor DNA containing desired α subtype along with β2 and γ2 subunits were used for transfecting HEK 293T cell line using Fugene 6 (Roche Diagnostic) transfecting reagent. Cells were harvested 48 hrs after transfection, washed with Tris-HCl buffer (pH 7.0) and Tris Acetate buffer (pH 7.4) and resulting pellets were stored at −80 C until assayed. On the day of the assay, pellets containing 20–50 μg of GABAA receptor protein were re-suspended in (50 mM Tris-acetate pH 7.4 at 4 degree) and incubated with the radiolabel as previously described (Choudhary et al., 1992). Nonspecific binding was defined as radioactivity bound in the presence of 100 μM diazepam and represented less than 20% of total binding. Membranes were harvested with a Brandel cell harvester followed by three ice-cold washes onto polyethyleneimine-pretreated (0.3%) Whatman GF/C filters. Filters were dried overnight and then soaked in Ecoscint A liquid scintillation cocktail (National Diagnostics; Atlanta, GA). Bound radioactivity was quantified by liquid scintillation counting. Membrane protein concentrations were determined using an assay kit from Bio-Rad (Hercules, CA) with bovine serum albumin as the standard.
Electrophysiological experiments
cDNAs of rat GABAA receptor subunits were used for generating the respective mRNA′s that were then injected into Xenopus laevis oocytes (Nasco, WI) as described previously (Savic et al., 2008). For electrophysiological recordings, oocytes were placed on a nylon-grid in a bath of Xenopus Ringer solution (XR, containing 90 mM NaCl, 5 mM HEPES-NaOH (pH 7.4), 1 mM MgCl2, 1 mM KCl and 1 mM CaCl2). The oocytes were constantly washed by a flow of 6 ml/min XR which could be switched to XR containing GABA and/or drugs. Drugs were diluted into XR from DMSO-solutions resulting in a final concentration of 0.1 % DMSO perfusing the oocytes. Drugs were pre-applied for 30 sec before the addition of GABA, which was co-applied with the drugs until a peak response was observed. Between two applications, oocytes were washed in XR for up to 15 min to ensure full recovery from desensitization. For current measurements the oocytes were impaled with two microelectrodes (2–3 m?Ω) which were filled with 2 mM KCl. All recordings were performed at room temperature at a holding potential of −60 mV using a Warner OC-725C two-electrode voltage clamp (Warner Instruments, Hamden, CT). Data were digitised, recorded and measured using a Digidata 1322A data acquisition system (Axon Instruments, Union City, CA).
Animals
Behavioral subjects were individually housed adult rhesus monkeys (Macaca mulatta) maintained at 90–95% of their free-feeding weights. Aside from experimental procedures, monkeys were maintained on a 12-hr lights-on/12-hr lights-off cycle (lights on at 7:00 AM) and water was available continuously. All testing procedures were conducted prior to 12 noon. Monkeys were prepared with chronic indwelling venous catheters according to the procedures described by Platt et al. (2005). Throughout all testing the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) was adhered to, and the experimental procedures were approved by the Harvard Medical School Institutional Animal Care and Use Committee (Standing Committee on Animals). All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available.
Conflict Procedure
Four male and two female rhesus monkeys were trained on a multiple schedule of reinforcement as described in detail by Rowlett et al. (2006). Monkeys had various durations of exposure to this procedure, and these times ranged from 1–8 years. A daily session consisted of 4 cycles, each preceded by a 10 min time out period in which all lights in the chamber were off and responding had no programmed consequences. Each cycle consisted of two components. The first component was signaled by red stimulus lights and consisted of a fixed ratio 18 (FR18) schedule of food pellet delivery (Bioserve, Frenchtown, NJ) followed by a 10 s time out. The second component, signaled by green stimulus lights, consisted of the FR 18 schedule of food delivery combined with a FR 20 schedule of foot shock delivery (1.5–3.0 mA, adjusted for each monkey based on individual performance, 0.25 s duration). Delivery of a food pellet was followed by a 10 s time out in which responding had no scheduled consequences. Both components were 5 min in duration, or ended after the monkey obtained 5 food pellets or received 3 foot shocks, whichever occurred first.
Test sessions were conducted once or twice per week when monkeys reached stable performance, defined as the average rates of responding for component 1 and component 2 not varying by ± 20% over five consecutive sessions, with no upward or downward trends. During test sessions, i.v. injections of vehicle or drug were administered in the 5th minute of each time out (i.e., 5 min prior to the beginning of each cycle). In successive cycles, increasing doses of the test drug were administered using a cumulative dosing procedure. The dependent measure was the average rates of responding (responses/s), calculated by dividing responses by time during components 1 and 2, excluding responding during time outs or reinforcer delivery.
Data analysis
Effects of doses of compounds were evaluated by conducting a priori Bonferroni t-tests, comparing individual doses to vehicle injection with an alpha level set at p ≤ 0.05. Potency values (dose engendering 50% maximum effect, ED50) were calculated in individual monkeys by log-linear regression when at least three data points were available on the linear portion of the dose-effect curve or by interpolation when only two data points (one above and one below 50%) were available. Individual ED50 values were converted to their log values for calculation of means and SEM and then converted back to linear values for presentation.
RESULTS
In Vitro Profiles
Table 1 shows the in vitro binding affinity of diazepam, JY-XHe-053, XHe-II-053 and HZ-166 and the 4-methyl-JY-XHe-053 stereoisomers SH-053-2’F-S-CH3 and SH-053-2’F-R-CH3 at GABAA receptor subtypes. The six compounds produced a relatively broad range of affinities across the receptor subtypes, with no compound demonstrating substantial selectivity for a particular receptor subtype. JY-XHe-053 and SH-053-2’F-R-CH3 were the most selective compounds across GABAA receptor subtypes, displaying 18- and 8- fold selectivity for α5GABAA receptors, respectively.
Table 1.
Binding affinity at αxβ 3γ 2 GABAA/benzodiazepine site subtypes. Measurements were made in duplicate. Ki values are reported in nM.
| Compound | α1 | α2 | α3 | α4 | α5 | α6 |
|---|---|---|---|---|---|---|
| Diazepam | 14.0 | 7.8 | 13.9 | NDa | 13.4 | NDa |
| JY-XHe-053 | 22.0 | 12.3 | 34.9 | NDb | 0.7 | NDb |
| XHe-II-053 | 247.0 | 40.0 | 90.0 | >1000 | 13.0 | >1000 |
| HZ-166 | 300.0 | 160.0 | 527.0 | NDb | 82.0 | >5000 |
| SH-053-2'F-S-CH3 | 468.2 | 33.3 | 291.5 | NDb | 19.2 | >5000 |
| SH-053-2'F-R-CH3 | 759.1 | 948.2 | 768.8 | NDb | 95.2 | NDb |
ND, not determined
Binding at α4GABAA and α4GABAA receptors have not been determined, but since the 6-phenyl group is present, the ligand will not bind to these receptors.
Table 2 shows the in vitro efficacy data at presumed physiologically relevant concentrations of 100 nM and 1μM for diazepam, JY-XHe-053, XHe-II-053, HZ-166, SH-053-2’F-S-CH3 and SH-053-2’F-R-CH3 as the percentage of control current. Relative to diazepam, the efficacy values were reduced at α1GABAA across all five compounds, with JY-XHe-053 exerting the greatest positive modulation and SH-053-2’F-R-CH3 exerting the least positive modulation. The efficacy values for each compound were also reduced at α2GABAA and α3GABAA receptors relative to diazepam, but were larger than their respective values at α1GABAA receptors. JY-XHe-053 and SH-053-2’F-R-CH3 exerted the greatest and least positive modulation at α2GABAA and α3GABAA receptors, respectively. Relative to diazepam, the efficacy values for JY-XHe-053, XHe-II-053 and HZ-166 were reduced at α5GABAA receptors, while the efficacy values for SH-053-2’F-S-CH3 and SH-053-2’F-R-CH3 were either decreased or increased, depending on the concentration considered. HZ-166 exerted the least positive modulation while SH-053-2'F-S-CH3 exerted the greatest positive modulation at α5GABAA receptors.
Table 2.
Efficacy at αxβ 3γ 2 GABAA receptor subtypes as % of control current at 100 nM and 1 μM concentrations. Data are presented as 100 nM/1 μM.
| Compound | α1 | α2 | α3 | α5 |
|---|---|---|---|---|
| Diazepam | 239/314 | 426/536 | 437/752 | 274/342 |
| JY-XHe-053 | 169/248 | 307/410 | 365/596 | 220/246 |
| XHe-II-053 | 130/194 | 209/329 | 265/513 | 150/186 |
| HZ-166 | 113/167 | 165/313 | 149/346 | 130/174 |
| SH-053-2'F-S-CH3 | 116/164 | 170/348 | 138/301 | 218/389 |
| SH-053-2'F-R-CH3 | 111/154 | 124/185 | 125/220 | 183/387 |
Anti-Conflict Effects of 8-acetylene imidazobenzodiazepines
Figure 2 shows the effects of diazepam, JY-XHe-053, XHe-II-053, HZ-166 on the fixed-ratio schedule of food pellet delivery (non-suppressed responding) and the concurrent schedule of food delivery and electric shock presentation (suppressed responding). Following vehicle administration, rates of responding during both components were similar to those observed during training sessions (i.e. between 2.0–3.0 responses/s during the non-suppressed component, and less that 0.1 responses/s during the suppressed component). Diazepam and JY-XHe-053 increased the mean rates of suppressed responding and was significantly different compared to vehicle at doses of 0.3- 1.0 mg/kg, resulting in mean ED50 values (±SEM) of 0.18 (0.14–0.24) and 0.15 (0.09–0.25) mg/kg for diazepam and JY-XHe-053, respectively. In addition, both diazepam and JY-XHe-053 attenuated the rates of non-suppressed responding at a dose of 3.0 mg/kg, resulting in ED50 values of 1.1 (0.88–1.3) and 2.2 (1.7–2.9) mg/kg for diazepam and JY-XHe-053, respectively.
Fig. 2.
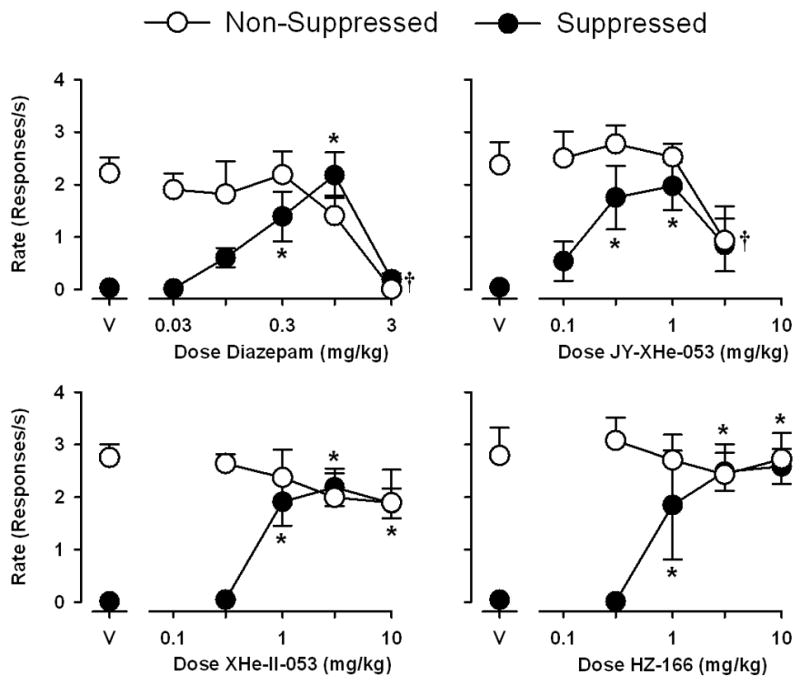
Anti-conflict effects of diazepam, JY-XHe-053, XHe-II-053 and HZ-166 in rhesus monkeys trained under a multiple schedule of food presentation (non-suppressed responding) and food + shock presentation (suppressed responding). Abscissae, cumulative intravenous dose of drug in mg/kg. Ordinates, response rate as responses per second. Each data point represents the mean (± S.E.M.) from four monkeys. Points above “V” represent data after vehicle administration. Asterisks represent significant differences relative to vehicle for suppressed responding and daggers represent significant differences relative to vehicle for non-suppressed responding (Bonferroni t-tests, p<0.05).
XHe-II-053 and HZ-166 produced similar increases in the rates of suppressed responding at doses of 1.0–10.0 mg/kg, resulting in an ED50 value of 0.71 (0.56–0.90) mg/kg for XHe-II-053 and an ED50 value of 0.80 (0.57–1.1) mg/kg for HZ-166. Across a 30-fold dose range, neither XHe-II-053 nor HZ-166 affected response rates during the non-suppressed component.
Anti-conflict effects of 4-methyl-JY-XHe-053 stereoisomers
Figure 3 shows the anti-conflict effects of the 4-methyl-JY-XHe-053 stereoisomers SH-053-2’F-S-CH3 and SH-053-2’F-R-CH3 on non-suppressed and suppressed responding. Following vehicle administration, rates of responding during both components were similar to those observed during training sessions (i.e. between 2.0–3.0 responses/s during the non-suppressed component, and less that 0.1 responses/s during the suppressed component). SH-053-2’F-S-CH3 produced significant increases in suppressed responding [F(5,23)=3.134, p<0.05], resulting in an ED50 value of 0.82 (0.58–1.2) mg/kg, however Bonferroni tests suggested that there was no significant differences between any dose of SH-053-2’F-S-CH3 and vehicle. SH-053-2’F-R-CH3 produced partial increases in the mean rates of suppressed responding, resulting in an ED50 value of 3.4 (2.3–5.2) mg/kg, however this effect did not reach statistical significance [F(3,15)=2.750, not significant (n.s.)]. Across the dose range tested, neither SH-053-2’F-S-CH3 nor SH-053-2’F-R-CH3 affected response rates during the non-suppressed component across the dose range tested.
Fig. 3.
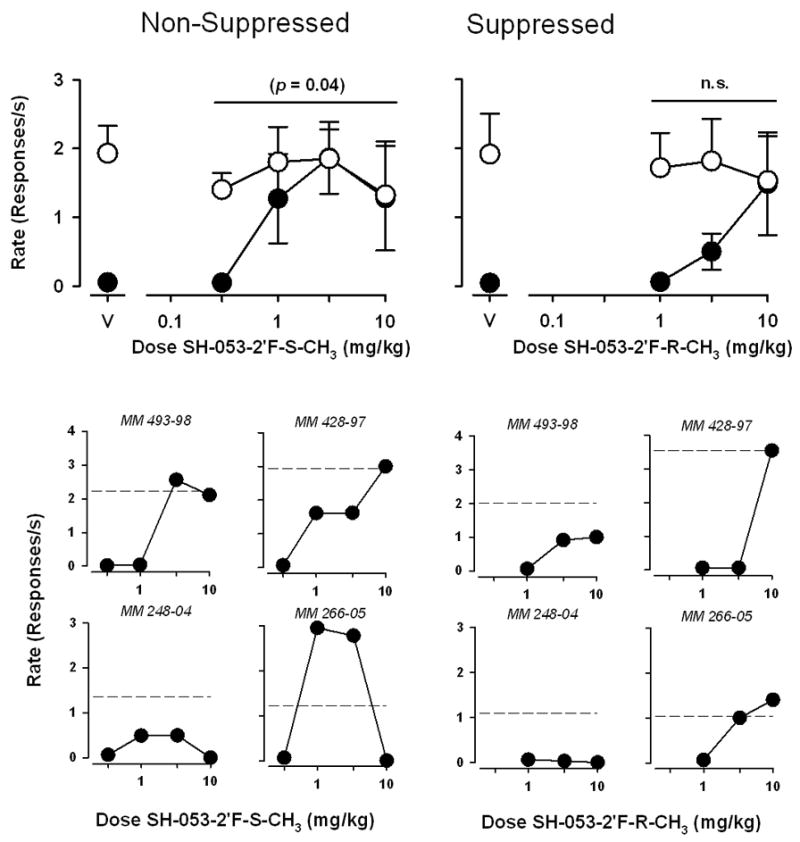
Anti-conflict effects of SH-053-2’F-S-CH3 and SH-053-2’F-R-CH3 in rhesus monkeys trained under a multiple schedule of food presentation (non-suppressed responding) and food + shock presentation (suppressed responding). Abscissae, cumulative intravenous dose of drug in mg/kg. Ordinates, response rate as responses per second. Top panels, each data point represents the mean (± S.E.M.) from four monkeys. Points above “V” represent data after vehicle administration. Bottom panels, data points represent the rate of suppressed responding in individual monkeys. Dashed lines represent rates of non-suppressed responding after vehicle administration.
As evident from the results of the statistical analyses described above, the findings with SH-053-2’F-S-CH3 and SH-053-2’F-R-CH3 were associated with a relatively high degree of variance. To assess the source of variability more closely, we analyzed suppressed responding across individual monkeys (Figure 3). SH-053-2’F-S-CH3 produced increases in suppressed responding that reached or exceeded non-suppressed control levels in three of the monkeys tested, and produced partial (~50%) increases in suppressed responding in the fourth monkey. SH-053-2’F-R-CH3 produced increases in suppressed responding up to levels of non-suppressed responding in two monkeys tested, and either a partial increase or no effect in the other two monkeys.
DISCUSSION
The purpose of the present study was to characterize the potential anxiolytic-like effects of five novel 8-acetylene imidazobenzodiazepines: JY-XHe-053, XHe-II-053, HZ-166, SH-053-2’F-S-CH3 and SH-053-2’F-R-CH3. Each of the compounds demonstrated a reduced positive modulation at α1GABAA receptors relative to the prototypical benzodiazepine diazepam at the drug concentrations that can be reached under our experimental conditions. Potentiation of GABA at α2GABAA and α3GABAA receptors was also lower relative to diazepam; however the efficacy value at these receptor subtypes was greater than the α1GABAA efficacy value across each of the compounds. Additionally, each compound produced varying degrees of potentiation at α5GABAA receptors, with SH-053-2’F-S-CH3 and SH-053-2’F-R-CH3, the active isomers of 4-methyl-JY-XHe-053, exceeding those produced by diazepam.
Behavioral procedures that assess the effects of drugs on experimentally-induced conflict are used often to assess the potential anxiolytic effects of these drugs in humans (Geller and Seifter, 1962; Spealman, 1979; Kleven and Koek, 1999; Rowlett et al., 2006). The anti-conflict effects of a series of conventional benzodiazepines with nonselective efficacy across α1GABAA, α2GABAA, α3GABAA, and α5GABAA receptors subtypes has been described (Rowlett et al., 2006). In the present study, diazepam produced behavioral effects that were consistent with those of previously reported findings: it engendered a robust anti-conflict effect at low to intermediate doses that was both graded and dose-dependent. Further, this effect occurred at doses similar to those that would be predicted based on relative potencies for benzodiazepines that are effective in the clinic (Rowlett et al., 2006). Together, these observations provide further support for the use of the rhesus monkey conflict procedure in predicting the anxiolytic effects of drugs in humans.
The main finding from these experiments is that, similar to diazepam, the novel compounds JY-XHe-053, XHe-II-053 and HZ-166 produced increases in positively reinforced behavior that was suppressed by response-contingent electric shock. In contrast, SH-053-2’F-S-CH3 and SH-053-2’F-R-CH3 produced increases in suppressed responding in some monkeys and not others, with the average data for SH-053-2’F-R-CH3 not reaching statistical significance. Among the novel compounds studied, only JY-XHe-053 produced diazepam-like reductions in rates of non-suppressed responding. These findings suggest that the anxiolytic and rate-reducing effects of benzodiazepines and other GABAA receptor positive modulators are dependent on their relative efficacy and affinity at GABAA receptor subtypes.
Previous studies from our laboratory also have used the rhesus monkey conflict procedure to assess the anxiolytic effects of other GABAA receptor positive modulators with either selective affinity or selective efficacy for GABAA receptor subtypes (Licata et al., 2005; Rowlett et al., 2005; Rowlett et al., 2006). Results from these studies provide evidence for a differential role of GABAA receptors in the anxiolytic effects of benzodiazepines. As an example, L-838,417, a drug with functional selectivity and partial agonist activity at α2GABAA, α3GABAA, and α5GABAA receptors, produced an anti-conflict effect similar to conventional non-selective benzodiazepines (Rowlett et al, 2005). A similar result was observed when SL651498, a drug with high intrinsic efficacy at α2GABAA and α3GABAA receptors, was assessed in the conflict procedure (Licata et al., 2005). Together with data suggesting that drugs selective for α1GABAA receptors (e.g. zolpidem, zaleplon) are only marginally effective in this procedure (Rowlett et al., 2005; Rowlett et al., 2006), these experiments have supported a key role for α2GABAA and α3GABAA receptors, but not α1GABAA receptors, in benzodiazepine-induced anxiolysis. Subsequent studies with TPA023 (a drug with partial agonist properties at α2GABAA and α3GABAA receptors) in other rodent and primate models of anxiety have supported this hypothesis (Atack et al., 2006).
In the present study, the in vitro electrophysiology experiments suggest that XHe-II-053 and HZ-166 have high intrinsic efficacy at α2GABAA and α3GABAA receptor subtypes relative to their efficacy at α1GABAA and α5GABAA receptor subtypes. When XHe-II-053 and HZ-166 were assessed in the conflict procedure, each produced an anti-conflict effect that was quantitatively similar to diazepam. Together, these data provide further evidence that compounds with selective efficacy at α2GABAA and α3GABAA receptors can produce anxiolytic effects in primates. Over the dose range tested, XHe-II-053 and HZ-166 produced an anti-conflict effect without producing diazepam-like alterations in non-suppressed responding. The lack of response rate-suppressing effects of HZ-166 and XHe-II-053 is similar to our previous results with L-838,417 and SL651498 (Rowlett et al., 2005; Licata et al., 2005), and it is noteworthy that each compound (XHe-II-053, HZ-166, L-838,417 and SL651498) has reduced efficacy at α1GABAA receptors relative to diazepam. These findings support the idea that the α1GABAA receptor subtype may be involved in the response rate-reducing effects of benzodiazepines at doses greater than those that produce anxiolysis.
Similar to the pharmacological profile of XHe-II-053 and HZ-166, the electrophysiological experiments suggest that JY-XHe-053 has preferential activity at α2GABAA and α3GABAA receptor subtypes. In agreement with the hypothesis that α2GABAA and/or α3GABAA receptors mediate the anxiolytic effects of benzodiazepines, JY-XHe-053 also produced a robust anti-conflict effect. Unlike XHe-II-053 and HZ-166 however, JY-XHe-053 also produced significant reductions in response rates at the highest dose tested. This finding was unexpected, considering the relatively low intrinsic efficacy of JY-XHe-053 at α1GABAA receptors. However, it is interesting to note that, relative to XHe-II-053 and HZ-166, JY-XHe-053 has both greater efficacy at and greater affinity for α1GABAA receptors. These observations support the hypothesis that α2GABAA and α3GABAA receptors mediate the anxiolytic effects of benzodiazepines, and also raise the possibility that the subtle differences at α1GABAA receptors between JY-XHe-053 and the other compounds may be sufficient for rate-reducing effects.
In contrast to the diazepam-like anti-conflict and rate-reducing effects observed with JY-XHe-053, the two isomers of 4-methyl-JY-XHe-053, SH-053-2’F-S-CH3 and SH-053-2’F-R-CH3 produced relatively weak increases in suppressed responding and failed to produce significant decreases in non-suppressed responding. The reasons for these differences are unclear. Based on the in vitro data, the primary differences between the two isomers and the parent compound were that (1) the affinities of the isomers were reduced considerably relative to JY-XHe-053, (2) the efficacies of the isomers at the α5GABAA receptor were increased relative to that of the parent compound and (3) the efficacy of SH-053-2’F-R-CH3 at α2GABAA and α3GABAA receptors at the achievable drug concentrations was markedly less than that of the parent compound. The relatively low potencies of the isomers may have necessitated very high levels of compound for consistent effects, raising the possibility that high enough concentrations were not sufficient in brain to for consistent effects to occur. Additionally, it is interesting to note that among the compounds tested, both SH-053-2’F-S-CH3 and SH-053-2’F-R-CH3 have substantial efficacy and high affinity for α5GABAA receptors. In fact, in contrast to the other compounds and receptor subtypes, SH-053-2’F-S-CH3 and SH-053-2’F-R-CH3 both exceed diazepam in efficacy at this subtype when assessed at the physiologically relevant concentration of 1 μM. Therefore, the unexpected finding that SH-053-2’F-R-CH3 lacked significant anti-conflict effects may result from its pharmacological action at α5GABAA receptors coupled with its low efficacy at α2GABAA and α3GABAA receptors. Further, the statistically significant but weak anti-conflict effects of SH-053-2’F-S-CH3 may also be a consequence of its unique pharmacological profile at α2GABAA, α3GABAA, and α5GABAA receptors. Support for these hypotheses comes from a recent report implicating α5GABAA receptors in benzodiazepine-induced psychomotor effects (Savic et al., 2008), which the authors suggest may serve to mask anxiolysis as measured in preclinical procedures.
The experiments described here provide further evidence that the anti-conflict effects of benzodiazepines in non-human primates are likely mediated by different GABAA receptors that contain distinct α subunits. Additionally, the results from this study suggest that it is possible to separate anxiolytic-like effects from effects indicative of a general disruption of behavior. Our findings provide further evidence that novel benzodiazepine-like drugs that have pharmacological selectivity for α2GABAA and/or α3GABAA receptors and low receptor activity at α1GABAA and α5GABAA receptors may be particularly useful as non-sedating anxiolytics. Also, the results from our studies suggest that subtle differences in α1GABAA receptor activation may be sufficient for the rate-reducing effects of benzodiazepine-like drugs. Finally, our findings raise the possibility that exceptional activity at α5GABAA receptors may blunt the anxiolytic-like effects of benzodiazepines, regardless of their pharmacology at other receptor subtypes. Together, these observations should provide an important framework for studying the role of different GABAA receptor subtypes in the behavioral effects of benzodiazepine-type drugs, which in turn should help guide both the current clinical use of benzodiazepines as well as the development of improved therapeutic agents for treating anxiety disorders.
Fig. 1.
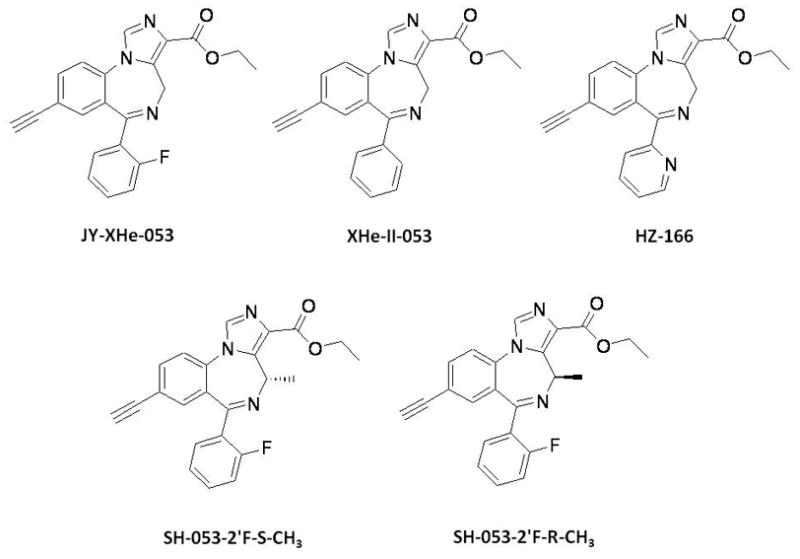
Chemical structures of diazepam, JY-XHe-053, XHe-II-053, HZ-166, SH-053-2’F-S-CH3 and SH-053-2’F-R-CH3.
Acknowledgments
This work was supported by USPHS grants DA11792, RR00168, and MH046851. The authors acknowledge the technical assistance of Kristen Bano and Annemarie Duggan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atack JR, Wafford KA, Tye SJ, Cook SM, Sohal B, Pike A, Sur C, Melillo D, Bristow L, Bromidge F, Ragan I, Kerby J, Street L, Carling R, Castro JL, Whiting P, Dawson GR, McKernan RM. TPA023 [7-(1,1-Dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-30ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for α2 and α3-containing GABAA receptors, is a nonsedating anxiolytic in rodents and primates. J Pharmacol Exp Ther. 2006;316:410–422. doi: 10.1124/jpet.105.089920. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook L, Davidson AB. Effects of behaviorally active drugs in a conflict-punishment procedure in rats. In: Garattini S, Mussini E, Randall LO, editors. The benzodiazepines. Raven; New York: 1973. pp. 327–345. [Google Scholar]
- Cook JM, Zhou H, Huang S, Srirama Sarma PVV, Zhang C. Stereospecific anxiolytic and anticonvulsant agents with reduced muscle-relaxant, sedative-hypnotic and ataxic effects. 7,618,958 B2. US Patent. 2009 Issued November 17,2009.
- Cook JM, Edwankar R, Edwankar C, Huang S, Jain H, Rivas F, Yang J, Zhou H. Selective anticonvulsant agents and their uses. 2010. T08025US Utility, Serial # 12/725,763, Filed March 17, 2010. [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CI, Rosahl TW, Stephens DN. Targeted deletion of the GABRA2 gene encoding alpha2-subunits of GABA(A) receptors facilitates performance of a conditioned emotional response, and abolishes anxiolytic effects of benzodiazepines and barbiturates. Pharmacol Biochem Behav. 2008;90:1–8. doi: 10.1016/j.pbb.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Geller I, Seifter J. The effects of meprobamate, barbiturate, damphetamine and promazine on experimentally-induced conflict in the rat. Psychopharmacologia. 1960;1:482–492. [Google Scholar]
- Griffiths RR, Weerts EM. Benzodiazepine self-administration in humans and laboratory animals--implications for problems of long-term use and abuse. Psychopharmacology (Berl) 1997;134:1–37. doi: 10.1007/s002130050422. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. Effects of different classes of partial benzodiazepine agonists on punished and unpunished responding in pigeons. Psychopharmacology (Berl) 1999;144:405–410. doi: 10.1007/s002130051024. [DOI] [PubMed] [Google Scholar]
- Licata SC, Platt DM, Cook JM, Sarma PV, Griebel G, Rowlett JK. Contribution of GABAA receptor subtypes to the anxiolytic-like, motor, and discriminative stimulus effects of benzodiazepines: studies with the functionally selective ligand SL651498 [6-fluoro-9-methyl-2-phenyl-4-(pyrrolidin-1-yl-carbonyl)-2,9-dihydro-1H-pyridol[3,4-b]indol-1-one] J Pharmacol Exp Ther. 2005;313:1118–1125. doi: 10.1124/jpet.104.081612. [DOI] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy JM, Rülicke T, Bluethmann H, Möhler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. Overview of diagnosis and drug treatments of anxiety disorders. CNS Spectr. 2005;10:49–56. doi: 10.1017/s1092852900009901. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt DM, Carey GJ, Spealman RD. Intravenous self-administration techniques in monkeys. In: Enna S, Williams M, Ferkany J, Kenakin T, Porsolt R, Sullivam J, editors. Current Protocols in Neuroscience. Unit 9.21. Wiley; New York: 2005. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Lüddens H, Seeburg PH. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Rivas FM, Stables JP, Murphree L, Edwankar RV, Edwankar CR, Huang S, Jain HD, Zhou H, Majumder S, Sankar S, Roth BL, Ramerstorfer J, Furtmüller R, Sieghart W, Cook JM. Antiseizure activity of novel gamma-aminobutyric acid (A) receptor subtype-selective benzodiazepine analogues in mice and rat models. J Med Chem. 2009;52:1795–1798. doi: 10.1021/jm801652d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci U S A. 2005;102:915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Lelas S, Tornatzky W, Licata SC. Anti-conflict effects of benzodiazepines in rhesus monkeys: relationship with therapeutic doses in humans and role of GABAA receptors. Psychopharmacology (Berl) 2006;184:201–211. doi: 10.1007/s00213-005-0228-8. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Möhler H. GABA(A) receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22:188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- Savić MM, Clayton T, Furtmüller R, Gavrilović I, Samardzić J, Savić S, Huck S, Sieghart W, Cook JM. PWZ-029, a compound with moderate inverse agonist functional selectivity at GABA(A) receptors containing alpha5 subunits, improves passive, but not active, avoidance learning in rats. Brain Res. 2008;1208:150–159. doi: 10.1016/j.brainres.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spealman RD. Comparison of drug effects on responding punished by pressurized air or electric shock delivery in squirrel monkeys: pentobarbital, chlordiazepoxide, d-amphetamine and cocaine. J Pharmacol Exp Ther. 1979;209:309–315. [PubMed] [Google Scholar]


