Abstract
The efficacy of antibody immunity against Streptococcus pneumoniae stems from the ability of opsonic, serotype (ST)-specific antibodies to pneumococcal capsular polysaccharide (PPS) to facilitate killing of the homologous ST by host phagocytes. However, PPS-specific antibodies have been identified that are protective in mice, but do not promote opsonic killing in vitro, raising the question of how they mediate protection in vivo. To probe this question, we investigated the dependence of antibody efficacy against lethal systemic (intraperitoneal, i.p.) infection with Streptococcus pneumoniae serotype 3 (ST3) on macrophages and neutrophils for the following PPS3-specific monoclonal antibodies (MAbs) in survival experiments in mice using a non-opsonic human IgM (A7), a non-opsonic mouse IgG1 (1E2) and an opsonic mouse IgG1 (5F6). The survival of A7- and PPS3-specific and isotype control-MAb-treated neutrophil-depleted and neutrophil-sufficient and macrophage-depleted and macrophage-sufficient mice were determined after i.p. challenge with ST3 strains 6303 and WU2. Neutrophils were dispensable for A7 and the mouse MAbs to mediate protection in this model, but macrophages were required for the efficacy of A7 and optimal mouse MAb-mediated protection. For A7-treated mice, macrophage-depleted mice had higher blood CFU, cytokines and peripheral neutrophil levels than macrophage-sufficient mice, and macrophage-sufficient mice had lower tissue bacterial burdens than control MAb-treated mice. These findings demonstrate that macrophages contribute to opsonic and non-opsonic PPS3-specific MAb-mediated protection against ST3 infection by enhancing bacterial clearance and suggest that neutrophils do not compensate for the absence of macrophages in the model used in this study.
Keywords: Pneumococcal capsular polysaccharide, Pneumococcal capsular polysaccharide antibodies, Opsonic antibodies, Non-opsonic antibodies, Serotype 3 pneumococcus, Pneumococcal sepsis, Mouse models of pneumococcal disease, Macrophages, Neutrophils, Phagocytes
Introduction
The ability of pneumococcal capsular polysaccharide (PPS) serotype (ST)-specific antibody to prevent invasive pneumococcal disease has been linked to PPS-specific antibody-mediated opsonic killing of pneumococcus by host phagocytes (opsonophagocytosis) [1;2]. A large body of work demonstrating that primary neutrophils and cell lines differentiated to neutrophils promote opsonic killing of pneumococcus by PPS-specific antibody has led to the use of opsonophagocytic antibody titers as surrogate markers of PPS vaccine immunogenicity/efficacy [2–4]. Nonetheless, mouse and human PPS-specific MAbs have been identified that are not opsonic in vitro, but are highly protective against lethal pneumococcal challenge in mice [5–7].
Antibody-dependent opsonophagocytosis is a cooperative antibody function that requires effector phagocytes. Neutrophils, the major effector cell type that is employed in opsonophagocytosis assays with pneumococcus [3;4;8], were found to be required for resistance to lethal intranasal pneumococcal infection in naïve mice in some models [9;10]. However, their importance in resistance to experimental pneumococcal infection appears to differ in innate and acquired immune responses and as a function of serotype (ST) and infection model [6;9;11;12]. For example, neutrophils increased the lethality of pulmonary challenge with ST8 pneumococcus in naïve mice [11] and had no effect on survival in one model of ST3 infection [12], but increased resistance to a ST3 strain in another model [9]. In studies of antibody immunity in an intranasal challenge model with ST3, neutrophils were required for two opsonic PPS3-specific IgG1s to mediate protection, but dispensable for the efficacy of a non-opsonic IgG1 [6]. The role of neutrophils in IgM-mediated protection against ST3 has not been evaluated previously.
Macrophages are also important effector phagocytes. Their role in host defense against pneumococcus has been predominantly studied in intranasal and colonization models in naïve mice [13;14]. Macrophages were required for resistance to the lethality of intranasal challenge with ST3, with their benefit stemming from modulation of the inflammatory response and clearance of apoptotic neutrophils, rather than bacterial clearance [14]. On the other hand, macrophages were required for clearance of primary pneumococcal infection of the nasopharynx with ST23F, whereas neutrophils were required for clearance in previously infected mice [13]. For Cryptococcus neoformans, a beneficial role for macrophages depended on the animal model, whereby pulmonary macrophages were essential for resistance to lethal infection in rats, but detrimental and disease-enhancing in mice [15]. Similar to mice with C. neoformans, depletion of alveolar macrophages enhanced resistance to murine M. tuberculosis [16]. In contrast, depletion of macrophages decreased survival in mice challenged with Bacillus anthracis, whereas increasing the number of macrophages conferred protection [17]. In contrast to these studies in pulmonary infection models, data on the effect of macrophages on systemic infection is limited. Macrophages were required for protection against Enterococcus faecium peritonitis in an intraperitoneal infection model [18]. However, to our knowledge, the requirement for macrophages in antibody-mediated protection against systemic infection in mice and/or dissemination has not been investigated.
Previous studies have shown that a human IgM MAb (A7) to the capsular polysaccharide of ST3 (PPS3) does not induce phagocytic killing in vitro, although it is protective in mice against lethal intraperitoneal challenge [7;19]. In this model, A7-mediated protection required C3 [7], was a function of C3 deposition on the ST3 surface [20], and was associated with bacterial clearance and a reduction in blood and tissue IL-6 and KC levels [19]. In addition, A7-mediated protection did not require B cells, CD4 cells or CD8 T cells [19]. Hence, we wondered whether phagocytes are required for A7-mediated protection in mice. In this study, we determined the requirement for neutrophils and macrophages in A7-mediated protection against intraperitoneal challenge with two strains of ST3 that have been studied extensively in mouse models of pneumococcal disease, WU2 and 6303 [6;7;21–25]. Our results show that neutrophils were dispensable, but macrophages were essential for A7 to mediate protection against lethal challenge.
Materials and Methods
Bacteria
S. pneumoniae ST3 strain 6303 (American Type Culture Collection, Manassas, VA) and strain WU2 (provided by Susan Hollingshead, University of Alabama at Birmingham, Birmingham, AL) were used. Strains 6303 and WU2 have been used extensively in mouse models of pneumococcal disease [6;7;21–25]. WU2 was used in landmark studies that established the efficacy of MAbs against pneumococcus [21;26] and 6303 has been used in studies of antimicrobial agents and complement against pneumococcus [24;25]. The ST3 strains were grown in tryptic soy broth (TSB; Difco Laboratories, Sparks, MD) to mid-log phase in 5% CO2 at 37°C, frozen in TSB in 10% glycerol, and stored at −80°C until it was used as described previously [5–7;19]. Prior to use, pneumococci were rapidly thawed, placed on ice, and diluted in TSB to the desired amount. To confirm the amount of ST3 administered, diluted pneumococci were plated onto a Trypticase agar plate containing 5% sheep's blood (Becton Dickinson, Franklin Lakes, NJ), incubated overnight at 5% CO2 at 37°C and counted the following day.
Monoclonal antibodies
A7 [IgM(κ)] is a human PPS3-specific MAb, derived from XenoMouse™ mice, that protects mice from death after intraperitoneal (i.p.) challenge with ST3 [7;19]. A7 was purified by affinity chromatography using anti-human IgM-coated beads (Sigma-Aldrich, St. Louis, MO). A human myeloma IgM (Calbiochem, San Diego, CA) was used as a negative control. MAbs 1E2 and 5F6 [(IgG1(κ)] are previously described mouse IgG1s [6]. 1E2 is non-opsonic and 5F6 is opsonic in vitro, whereas both MAbs protect against intranasal (i.n.) ST3 (WU2) infection in mice [6]. 31B12 is an [IgG1(κ)] to PPS8 (GenBank accession numbers for VH and VL are FJ972829 and FJ972830) that was used as an isotype control for the mouse MAbs.
Mice
Male wild-type C57BL/6 (6–8 weeks old) mice were obtained from Jackson Laboratory (Bar Harbor, ME). TLR2−/− mice on the C57Bl/6 background [27] were obtained from Shizuo Akira (Osaka University, Japan). Mice were maintained by the Institute for Animal Studies at the Albert Einstein College of Medicine (AECOM), Bronx, NY, in accordance with the rules and regulations of animal welfare at the Albert Einstein College of Medicine.
Pneumococcal challenge in phagocyte-depleted PPS3-specific MAb-treated and control mice
Challenge strains
Intraperitoneal (i.p.) challenge experiments were done with both ST3 strains, WU2 and 6303 to ensure that our results were not strain-specific. Given that 6303 is more virulent than WU2 [6;7;19;28], a higher inoculum of WU2 was used. In most experiments, 30 CFU of 6303 and 50 CFU of WU2 were used, because these inocula led to a similarly lethal phenotype at approximately the same time after infection.
Macrophage depletion
To deplete peritoneal macrophages, mice were injected with 100µl of clodronate liposomes, intraperitoneally (i.p.), for three consecutive days as described [29]. Cl2MDP (or clodronate) was a gift of Roche Diagnostics (GmbH, Mannheim, Germany) and encapsulated in liposomes as previously described [30]. PBS-encapsulated liposomes, which do not deplete macrophages, were used as a control for the liposomes. Macrophage depletion was verified by microscopy in blood smears stained with Turk’s solution and by FACS analysis of peritoneal lavage fluid in naïve mice 18 hr post-depletion.
Neutrophil depletion
To deplete neutrophils, mice were injected with 25µg/100µl i.p. with rat MAb RB6-8C5 (RB6) as described [11;31]. RB6 (provided by Dr. Marta Feldmesser, Albert Einstein College of Medicine) was purified from ascites. Rat IgG (rIgG, Sigma-Aldrich, St. Louis, MO) was used as an isotype control.
MAb administration and pneumococcal challenge
After the cellular depletions, 24 hrs for macrophage depletion and 18 hrs for neutrophil depletion, mice were injected i.p. with 10µg of (a treatment or control) MAb one hr prior to i.p. challenge with the ST3 (6303 - A7 and human IgM control; and WU2 – A7, 1E2, 5F6 and mouse IgG1 controls). As a control for pneumococcal killing in the 6303 model, 10mg/kg penicillin (PCN) was administered subcutaneously (s.c.) in the neck region one hr prior to i.p. administration, followed by two more injections six and 12 hrs after the first, as previously described [19].
Survival of MAb-treated and control TLR2−/− mice
The survival of A7-treated TLR2−/− mice was determined using the same MAb-administration and i.p. infection protocol described above, without cellular depletion.
Determination of bacterial burden in A7-treated and control mice
To gain further insight into the mechanism of efficacy of the human MAb A7, the bacterial burden in blood and tissues was determined. In experiments separate from the survival studies, clodronate liposomes, PBS liposomes or PBS alone were administered to groups of mice prior to MAb administration and infection as described above. Then, mice were bled from the retro-orbital sinus 18 hr post-infection and killed by cervical dislocation. For CFU determinations in the peritoneal cavity, mice were killed by cervical dislocation, the peritoneal cavity was injected with ~5–8ml Veronal buffered saline (Fisher Scientific, Pittsburgh, PA) and the wash was withdrawn. Peritoneal washes were then centrifuged and resuspended in 1ml of Veronal buffer. Blood and peritoneal washes were serially diluted in TSB or Veronal buffer, plated onto TSB plates with 5% sheep’s blood (Becton Dickinson), incubated for 24 hr at 5% CO2 at 37°C and counted the following day.
In separate experiments, in separate groups of mice, blood, lung, liver and peritoneal cavity CFU were determined in tissues from macrophage-sufficient mice 18hr after MAb administration and infection as described above. Tissues were collected and prepared as previously described [19].
Determination of tissue cytokine concentrations in A7-treated and control mice
Serum, splenic and liver keratinocyte-derived chemokine (KC) and interleukin (IL)-6 levels were determined by ELISA 18 hr after infection, as previously described [19]. These mediators were measured because they had been previously found to be modulated in blood and spleen in the setting of A7-mediated protection [19], and because they are produced by macrophages as well as neutrophils and other cell types in the setting of systemic inflammation [18]. Blood and tissues samples for the ELISA were obtained from separate groups of mice that received liposomes and were infected with ST3, bled from the retro-orbital sinus and killed by cervical dislocation, after which the spleens were aseptically removed as described [19]. Blood was allowed to clot on ice for one hour after which sera were separated by centrifugation for 30 min at 3000 × g at 4°C and stored at −20°C until use. Spleens were homogenized in 1 ml of Hanks’ balanced salt solution (Mediatech, Herndon, VA) and centrifuged for 30 min at 2000 × g at 4°C. Supernatants were collected and stored at −20°C until use in the ELISAs. Care was exercised to avoid endotoxin contamination by using autoclaved materials and thoroughly rinsing instruments with 70% ethanol between each dissection. ELISA kits (R&D Systems, Minneapolis, MN) were used according to the manufacturer’s protocol as described [19]. In separate experiments in macrophage sufficient mice, liver IL-6 and KC were determined 18 hrs after infection.
White blood cell counts in A7-treated and control mice
Whole blood was diluted 1:20 in Turk’s solution (1% glacial acetic acid and 0.01% gentian violet in distilled H2O) as described in [19;31]. Cells were then counted in a hemocytometer to determine the total white blood cell count. For the differential count, whole blood was diluted in 10% EDTA and smeared onto a slide as described [19]. Cells were stained with a Hema 3 stain set (Fisher Scientific, Pittsburgh, PA). Monocytes, lymphocytes, and neutrophils were scored by light microscopy, based on morphology, for a total count of 100 cells as described in [19;31].
Statistical Analysis
The number of CFUs in blood and peritoneal washes, cytokine levels and white blood cell counts were compared with the unpaired t test or Mann-Whitney if a test of normality was not passed. Grubbs’ test was used to detect outliers. Mouse survival data were analyzed with the Kaplan-Meier log rank survival test. All statistical analyses were performed using Prism (v.4.02 for Windows; GraphPad Software, San Diego, CA). A P value of <0.05 was used for statistical significance.
Results
Pneumococcal challenge experiments
Macrophage-depleted mice
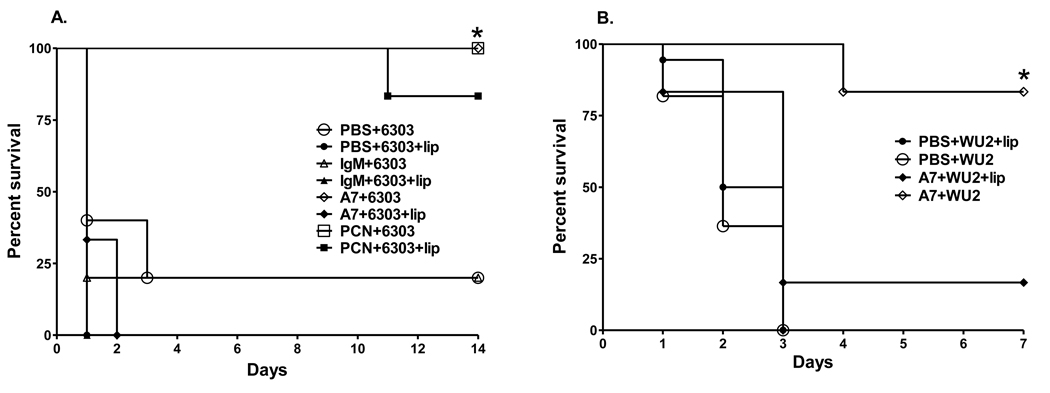
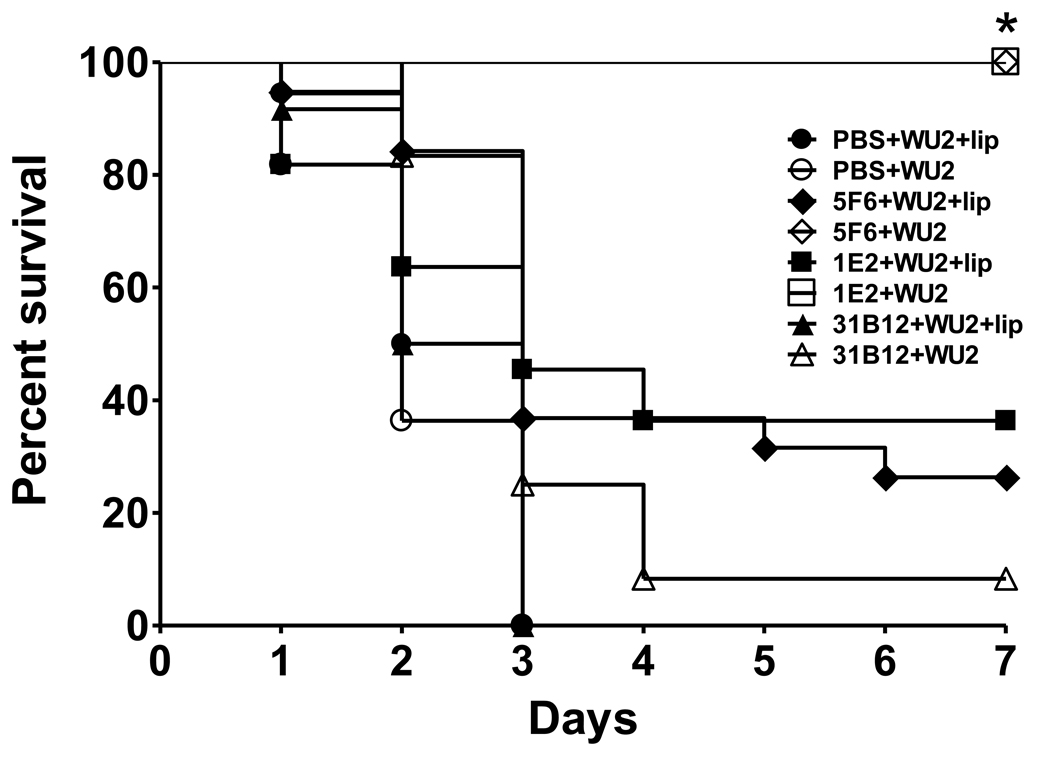
The survival of A7-treated macrophage-sufficient mice was significantly prolonged compared to A7-treated macrophage-depleted mice after challenge with both ST3 strains Figure 1 (6303, P=0.001, Figure 1A; WU2, P=; 0.014, Figure 1B, Kaplan-Meier log rank survival test). The survival of A7-treated macrophage-depleted mice was not significantly different than that of macrophage-depleted and macrophage-sufficient controls; and the survival of PBS- and control IgM-treated mice was not significantly different in macrophage-sufficient and macrophage-depleted mice (Figure 1A and 1B). PCN prolonged survival compared to PBS- and IgM-treated controls in macrophage-sufficient and macrophage-deficient mice (Figure 1A). PBS-encapsulated liposomes, which were used as a control for the liposomes in which the macrophage-depleting compound, chlodronate, was encapsulated, did not affect A7-mediated protection or the lethality of ST3 (Supplementary Fig. S1). The survival of macrophage-sufficient mice that were treated with the PPS3-specific mouse MAbs 5F6 and 1E2 and challenged with WU2 was significantly prolonged compared to macrophage-depleted mice (Figure 2, P<0.0001; macrophage-depleted v macrophage-sufficient mice, for each MAb; Kaplan-Meier log rank survival test); however, the survival of 1E2 macrophage-depleted mice was prolonged compared to PBS control mice (Figure 2, 1E2+WU2+lip v PBS+WU2+lip, P=0.049; 1E2+WU2+lip v PBS+WU2, P=0.043) and that of 5F6-treated macrophage-depleted mice was prolonged compared to PBS control and isotype control-treated macrophage-depleted mice (Figure 2, 5F6+WU2+lip v PBS+WU2+lip, P=0.003; 5F6+WU2+lip v PBS+WU2, P=0.002; 5F6+WU2+lip v 31B12+WU2+lip, P=0.007).
Figure 1. Survival after i.p. infection with (A) 30 CFU ST3 (6303) and (B) 50 CFU ST3 (WU2), comparing macrophage-depleted and macrophage-sufficient mice.
The percent of PBS-, isotype control IgM-, PCN- and A7-treated mice surviving after i.p. infection at the times designated on the x-axis is depicted. Open symbols represent macrophage-sufficient mice; closed symbols represent macrophage-depleted mice. *p<0.05 between groups for the designated treatments; Kaplan-Meier log rank survival test. N=6 mice per group.
Figure 2. Survival after i.p. infection with 50 CFU ST3 (WU2), comparing macrophage-depleted and macrophage-sufficient mice.
The percent of PBS, 31B12-, 5F6- and 1E2-treated mice surviving after i.p. infection at the times designated on the x-axis is depicted. Open symbols represent macrophage-sufficient mice; closed symbols represent macrophage-depleted mice. *p<0.05 between groups for the designated treatments; Kaplan-Meier log rank survival test. N=6–22 mice per group.
Neutrophil depletion survival study
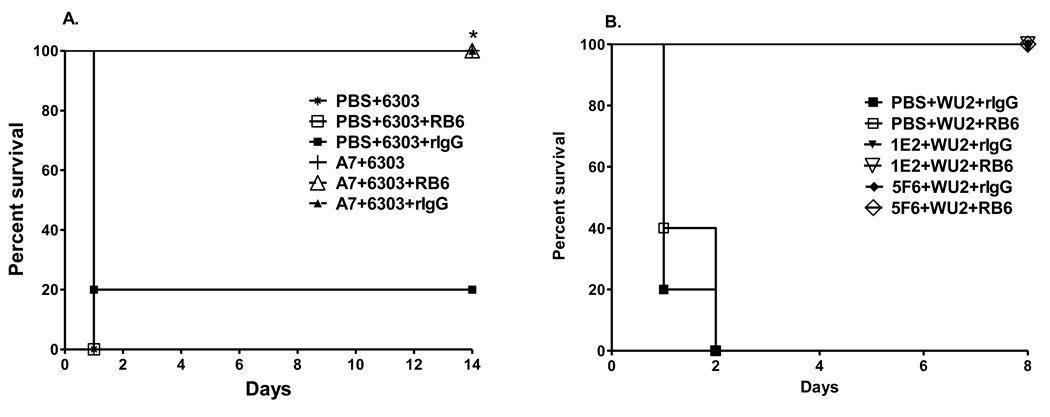
The survival of A7-treated mice after infection with 30 CFU 6303 was significantly prolonged compared to PBS-, RB6- and rIgG-treated mice (Figure 4A, A7 v PBS, P=0.003; A7-rIgG-treated v PBS-rIgG-treated, P= 0.014; A7-RB6-treated v PBS-RB6-treated, P= 0.003; Kaplan-Meier log rank survival test).The survival of 1E2- and 5F6-RB6-treated mice and rIgG-treated mice after infection with 100 CFU WU2 was identical, 100% (Figure 4B, P=1.00) and significantly greater than that of rIgG- and RB6-treated mice (Figure 4B, 1E2-rIgG v PBS-rIgG, P=0.002; 1E2-RB6 v PBS-RB6, P=0.002; 5F6-rIgG v PBS-rIgG, P=0.002; 5F6-RB6 v PBS-RB6, P=0.002; Kaplan-Meier log rank survival test).
Figure 4. Survival after i.p. infection with (A) 30 CFU ST3 (6303) and (B) 100 CFU ST3 (WU2), comparing neutrophil- depleted and neutrophil-sufficient mice.
The percent of PBS-, A7, 5F6 and 1E2-treated mice surviving after i.p. infection at the times designated on the x-axis is depicted. Closed symbols represent rIgG-treated mice; open symbols represent RB6-treated mice. *p<0.05 between groups for the designated treatments; Kaplan-Meier log rank survival test. N=5 mice per group.
TLR deficient mice
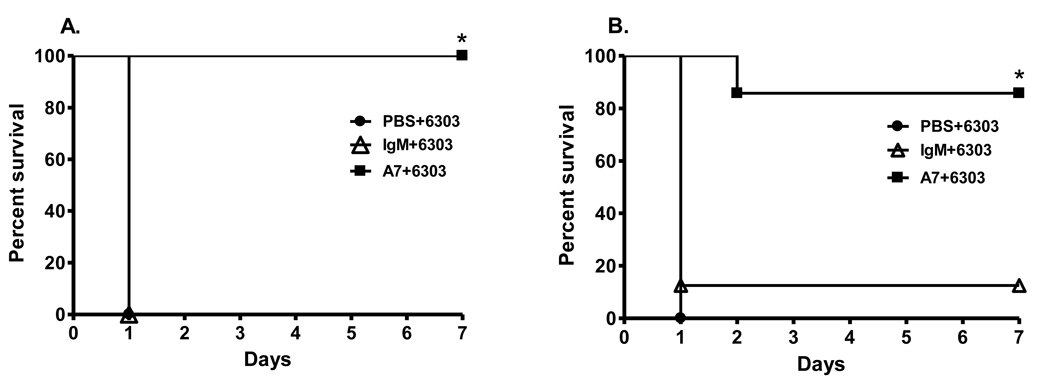
A7 significantly prolonged survival in TLR2−/− mice and there was no significant difference in the survival of A7-treated Wt and TLR2−/− mice (Figure 3A and 3B).
Figure 3. Survival after i.p. infection with 30 CFU ST3 (6303) in (A) wild-type mice and (B) TLR2−/− mice.
The percent survival of PBS-, control IgM-, and A7-treated mice surviving after i.p. infection at the times designated on the x-axis is depicted. *P<0.05 for comparison to A7 treated groups; Kaplan-Meier log rank survival test. N=7–8 mice per group.
Bacterial burden
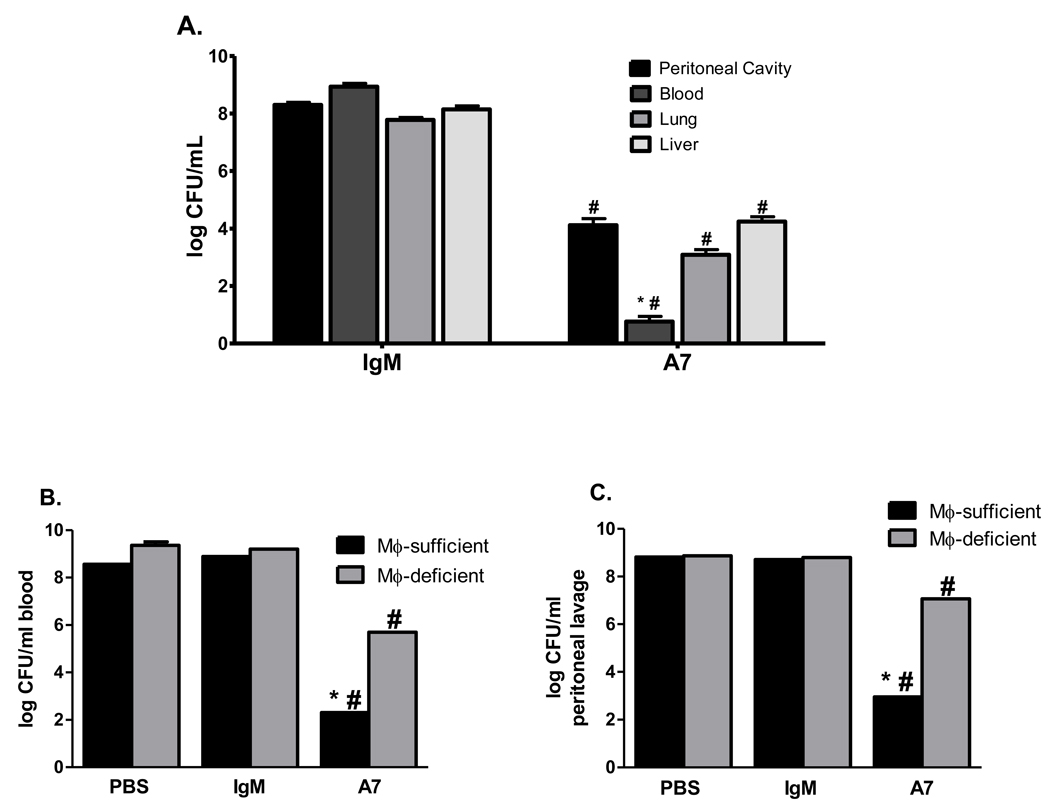
To delve further into the mechanism of protection of the human IgM MAb A7, CFU were determined in organ tissues from macrophage-depleted and macrophage-sufficient mice 18 hrs after infection. Given that a newly described complement receptor on Kupffer cells in the liver has been shown to mediate intravascular clearance of other bacteria [32], we determined liver CFU in addition to blood, lung, and peritoneal cavity CFU. Liver, lung, blood and peritoneal CFU were significantly higher in control IgM than A7-treated macrophage sufficient mice (Figure 5A, liver- A7 v control IgM, P=0.001; lung- A7 v control IgM, P<0.0001; blood- A7 v control IgM, P=0.0003; peritoneal cavity- A7 v control IgM, P<0.0001). For A7-treated mice, blood CFU were significantly lower than peritoneal cavity, lung and liver CFU among macrophage-sufficient mice (Figure 5A, blood CFU v peritoneal cavity CFU, P=0.004; blood CFU v lung CFU, P=0.03; blood CFU v liver CFU, P=0.002), whereas for control IgM-treated mice, blood, peritoneal cavity, lung and liver CFU were comparable. For A7-treated mice, macrophage-sufficient mice had significantly fewer blood (Figure 5B) and peritoneal cavity (Figure 5C) CFU than PBS- and control IgM-treated mice (blood - A7 v PBS, P=0.004; A7 v control IgM, P=0.003; peritoneal cavity - A7 v PBS, P=0.01; A7 v control IgM, P=0.02) and significantly fewer blood and peritoneal CFU than macrophage-depleted mice (blood - P=0.04; peritoneal cavity - P=0.02); however, A7-treated macrophage-depleted mice had significantly fewer blood CFU than PBS- and control IgM-treated macrophage depleted mice (blood - A7 v PBS, P=0.02; peritoneal cavity - A7 v control IgM, P=0.04). Hence, A7 treatment was associated with a reduction in CFU compared to controls (PBS and control IgM), irrespective of macrophage status, but the reduction in CFU was larger among macrophage-sufficient mice. No differences were observed in blood or peritoneal cavity CFU in PBS- or control IgM-treated mice, regardless of macrophage depletion.
Figure 5. Bacterial burden after i.p. infection with 30 CFU ST3 (6303).
CFU in the peritoneal cavity, blood, liver and lung in macrophage-sufficient mice (A) and blood (B) and peritoneal cavity (C) in macrophage-depleted mice 18 hr after i.p. infection. Black bars represent macrophage-sufficient mice; gray bars represent macrophage-depleted mice (B and C). Each bar represents the mean + SEM (A) or median (B and C) of the designated group. * p<0.05 comparing A7 treatment groups; # p< 0.05 comparing A7 treatment groups to PBS and control IgM treatment groups; unpaired t test (A), Mann-Whitney U test (B and C). N=5–15 mice per group. The lowest limit of detection for this assay is 20 CFU.
Serum and splenic cytokine concentrations
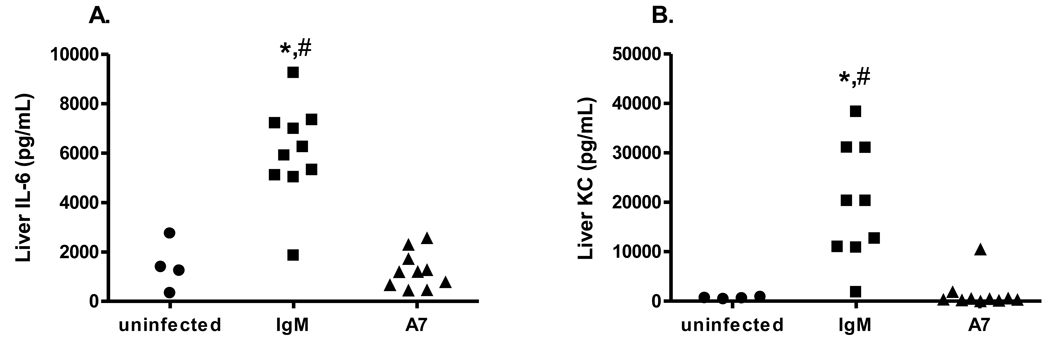
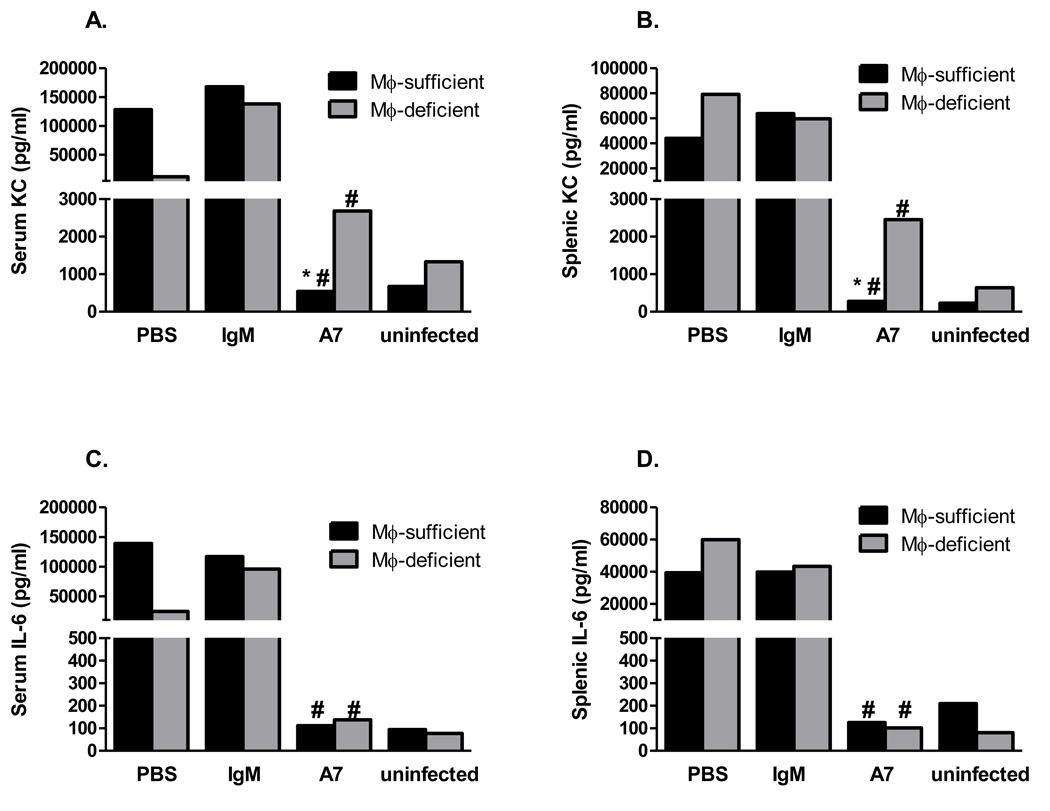
IL-6 and KC were previously shown to be significantly higher in sera and spleens from control and PCN-treated than A7-treated mice [19]. In this study, IL-6 and KC levels were determined in livers from A7-, control IgM- and PBS-treated macrophage-sufficient and in sera and spleens from A7-, control IgM- and PBS-treated macrophage-sufficient and macrophage-depleted mice 18 hr post-infection. Liver IL-6 and KC levels were significantly higher in IgM-treated than A7-treated macrophage-sufficient and PBS-treated, macrophage-sufficient mice (Figure 6A, control IgM v A7, P<0.0001; control IgM v PBS, P=0.004; Figure 6B, control IgM vs A7, P=0.0005; control IgM v PBS, P=0.007). Serum KC was higher among A7-treated macrophage-depleted than macrophage-sufficient mice (Figure 7A, P=0.01), but a similar trend for IL-6 did not reach statistical significance (Figure 7C, P=0.67). Spleen and serum IL-6 were significantly lower among macrophage-depleted A7-treated than PBS and control IgM-treated mice and serum KC was lower among macrophage-depleted A7-treated than control IgM-treated mice (Figure 7A, A7 v PBS, P=0.04; A7 v control IgM, P=0.006; Figure 7B, A7 v PBS, P=0.02; A7 v control IgM, P=0.01; Figure 7C, P=0.006). As in Fabrizio et al (13), A7-treated macrophage-sufficient mice had significantly lower levels of serum and spleen IL-6 and KC than PBS- and control IgM-treated mice.
Figure 6. Liver IL-6 (A) and KC (B) concentrations 18 hours after infection with 30 CFU ST3 (6303).
Levels of liver IL-6 and KC are shown on the y-axis for uninfected, control IgM- and A7-treated mice. * p<0.05 comparing A7 to PBS treatment groups; # P<0.001 comparing A7 to IgM treatment group, Mann-Whitney U test. N=4–10 mice per group.
Figure 7. Serum and spleen KC and IL-6 concentrations 18 hr after infection with 30 CFU ST3 (6303).
Black bars represent macrophage-sufficient mice; gray bars represent macrophage-depleted mice. Each bar represents the median of the designated group. * p<0.05 comparing A7 treatment groups; # p< 0.05 comparing A7 treatment groups to PBS and control IgM treatment groups, Mann-Whitney U test. N=4–7 mice per group. The lowest limit of detection for both KC and IL-6 is 7.8 pg/ml.
Peripheral leukocyte count
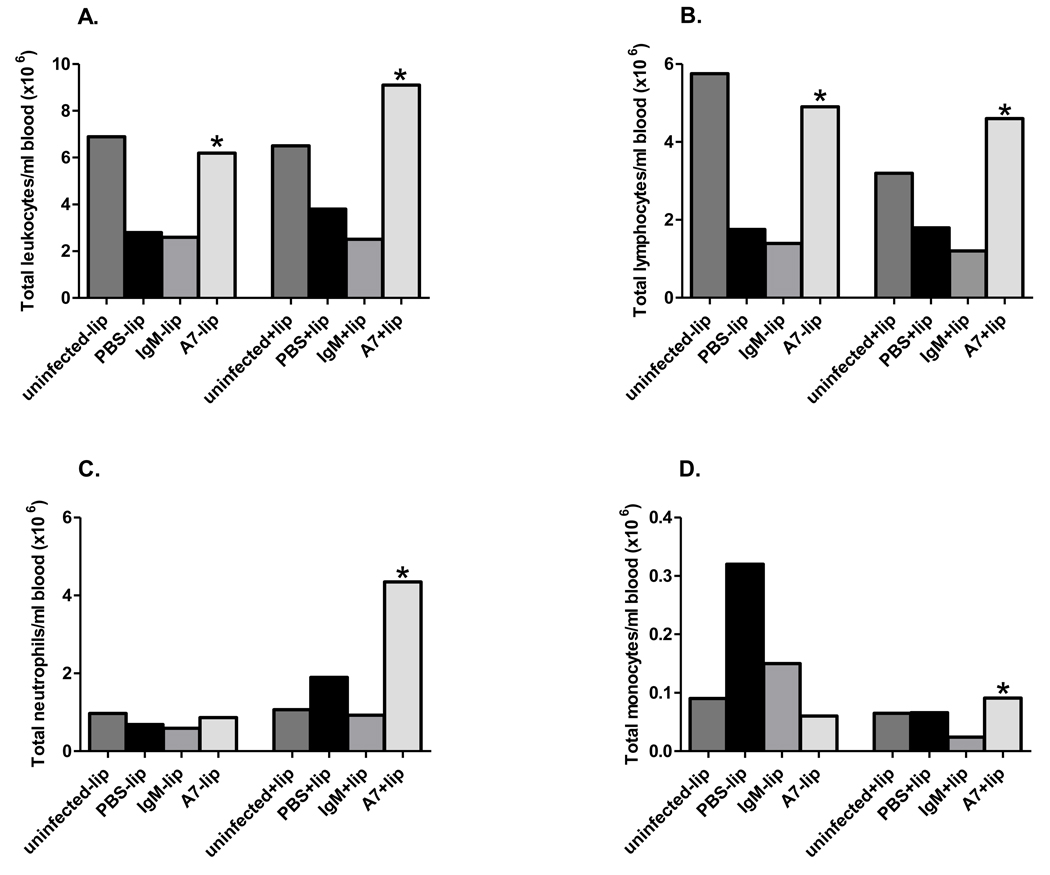
Total and differential white blood counts were determined 18 hr post-infection (Figure 8). A7-treated mice had significantly higher levels of total leukocytes and lymphocytes than PBS- and control IgM-treated mice, regardless of macrophage depletion (Figure 8A and 8B, P <0.05). Macrophage-depleted A7-treated mice had significantly higher neutrophil counts than PBS-, control IgM- and naïve macrophage-depleted and A7-treated macrophage-sufficient mice (Figure 8C, A7 vs PBS, P=0.02; A7 vs control IgM, P=0.01; A7 vs naïve, P=0.03; A7 vs A7-treated macrophage-sufficient mice, P=0.02) and higher monocyte counts than control IgM-treated mice (Figure 8D, P=0.02). Hence, A7 treatment was associated with higher levels of lymphocytes in macrophage-sufficient and macrophage-depleted mice, and macrophage depletion was associated with higher levels of neutrophils and monocytes in A7-treated mice.
Figure 8. Total white blood cell count and differential after i.p. infection with 30 CFU ST3 (6303).
Total leukocytes (A), lymphocytes (B), neutrophils (C) and monocytes (D) enumerated 18 hr after i.p infection. Each bar represents the median of the designated group. * p<0.05 between groups for the designated treatments, Mann Whitney U test. N=4–9 mice per group.
Discussion
The experiments reported herein were undertaken to investigate whether the mechanism by which a non-opsonic human IgM (A7) mediates protection against systemic pneumococcal disease in mice depends on host phagocytes. To exclude the possibility that a species and/or isotype difference could have confounded our findings, we also investigated the phagocyte dependence of the efficacy of an opsonic (5F6) and a non-opsonic (1E2) mouse IgG1 PPS3-specific MAb. We performed these studies in an i.p. model of systemic pneumococcal disease, because bacteremia and sepsis are major causes of morbidity and mortality in human pneumococcal disease, and death in mouse models is generally attributable to bacteremia and sepsis, irrespective of the route of infection [5;6;11;33–35]. Macrophage depletion did not increase the virulence of ST3 in this model. Hence, we were able to assess the dependence of MAb-mediated protection on macrophages and neutrophils without an independent effect of macrophages on virulence.
Our data show that macrophage depletion abrogated the efficacy of the human IgM MAb A7 against systemic (i.p.) infection with ST3-6303 and ST3-WU2 and significantly reduced the efficacy of the mouse IgG1 MAbs 1E2 and 5F6 against ST3-WU2 in the same infection model. Hence, macrophages enhanced antibody-mediated protection in vivo irrespective of the antibody species (mouse or human), isotype (IgM or IgG) or opsonic activity in vitro. In contrast, neutrophils were dispensable for A7- and mouse MAb-mediated protection. Neutrophils were previously shown to be required for the efficacy of the opsonic (5F6), but not the non-opsonic (1E2) IgG1 MAb, against i.n. challenge with ST3 [6]. Neutrophils were required for clearance of Enterococcus faecium from the peritoneal cavity in naïve mice [36], with their role linked to macrophage recruitment, which was indispensable for bacterial clearance [18]. However, neutrophils were not required for early clearance of ST23F in a mouse nasopharyngeal colonization model in which macrophages mediated early bacterial clearance [37]. Our data show that macrophage depletion was associated with a marked reduction in A7-mediated bacterial clearance from blood and peritoneal cavity. Macrophage depletion resulted in a lethal increase in inflammation in the lungs in a ST3 i.n. infection model without affecting bacterial clearance [14], but it reduced clearance of ST23F in a colonization model [13] and E. faecium in an i.p. infection model [18]. Macrophage-mediated clearance, responsiveness to pneumococcus and immunodulation were TLR2-dependent in the foregoing models [13;38;39]. Given that TLR2 was dispensable for A7 to mediate protection in our model, it must interact with a different macrophage pattern recognition molecule or receptor. Fc receptors were previously shown to be required for the mouse IgG1s to mediate protection against i.n. challenge with WU2 [6]. Although an Fcα/μ receptor that binds mouse IgM has been identified, its role in host defense has not been established [40–42].
PPS3-specific IgG was shown to promote immune transfer of ST3 from human red blood cells (RBC) to macrophages in vitro [22]. This RBC CR1-dependent mechanism might contribute to pneumococcal clearance in humans, but would be unlikely in mice, because mice lack RBC CR1 [43;44]. Classical papers describe hepatic sequestration as a hallmark of complement-dependent intravascular clearance of pneumococci, which was enhanced by immunization [45;46]. Given that A7-mediated protection requires macrophages (this study) and C3 [7] and correlates with the amount of A7-dependent C3 deposition on the ST3 capsule [28], C3-opsonized ST3 could bind macrophage complement receptors. An interesting such receptor is CrIg, a relatively newly described C3-binding receptor that is highly and preferentially expressed by resident tissue macrophages, including Kupffer cells in the liver [32;47;48]. CrIg was found to mediate intravascular clearance of Listeria monocytogenes and Staphylococcus aureus, a hallmark of which was a higher liver to blood CFU ratio [32]. Similarly, our data show that A7-treated macrophage-sufficient mice had a markedly higher ratio of liver to blood CFU than control IgM-treated mice, suggesting that A7 might promote intravascular clearance of ST3 in the liver by Kupffer cells, and/or in the peritoneum by resident peritoneal macrophages. Studies to address this hypothesis are under investigation in our laboratory but were beyond the scope of this study.
Our data show that serum and splenic levels of IL-6 and KC were higher in A7-treated macrophage-depleted than macrophage-sufficient mice, but lower than in control IgM- and PBS-treated controls. The latter is consistent with previous work which established that, compared to IgM and PBS controls, A7 treatment was associated with lower levels of IL-6 and KC [19]. Similar to an i.p. model of E. faecium infection in macrophage-depleted mice [18], we found that IL-6 and KC levels were increased and paralleled CFU in macrophage-depleted mice. Compared to macrophage-sufficient mice, A7-treated macrophage-depleted mice exhibited significantly increased levels of KC, a neutrophil chemoattractant, and peripheral neutrophils. Although the increase in lymphocytes in A7-treated macrophage-sufficient mice could reflect immunomodulation (see [14;19]), the increase in neutrophils, lymphocytes and monocytes in A7-treated, macrophage-depleted mice could reflect the cellular response to inflammation that has been reported in macrophage-depleted mice [18;29]. However, given that A7 did not protect macrophage-depleted mice despite increased neutrophil recruitment, our data suggest that neutrophils cannot substitute for macrophages in A7-mediated protection, and neutrophils and macrophages do not play functionally redundant roles in antibody immunity in our model.
The data reported herein show that under the conditions used in this study, the PPS3-specific mouse IgGs prolonged survival compared to a control MAb in macrophage-depleted mice, although their efficacy was significantly less than that observed in macrophage-sufficient mice. Given that peritoneal inflammation has been shown to induce macrophage recruitment in macrophage-depleted mice [29], this could reflect the activity of newly recruited macrophages in macrophage-depleted mice. Although newly recruited macrophages were impaired in their ability to induce clearance of E. faecium in macrophage-depleted mice [18], Fc receptor binding of specific IgG might enhance/induce macrophage activation. Alternatively, PPS3-specific IgGs could mediate protection via effector cells other than neutrophils and macrophages. Further work is required to determine the precise mechanism by which A7 and the mouse MAbs mediate protection. Nonetheless, our data establish that macrophages facilitate A7-mediated MAb-mediated bacterial clearance and possibly, immunomodulation, as evidenced by the relative decrease in tissue and blood cytokines in A7-treated mice. These findings suggest that antibody therapy could hold promise for diminishing the inflammatory response to pneumococcal infection, which is a major cause of morbidity and mortality, and that its effect/s could depend on resident macrophages. Interestingly, CRIg has immunomodulatory functions [49–51], supporting the idea that if PPS3-specific antibodies mediate protection via this receptor, they could also function as immunomodulators.
Supplementary Material
The percent of PBS- and A7-treated mice surviving after i.p. infection at the times designated on the x-axis is depicted. Open symbols represent PBS encapsulated liposomes-treated mice; closed symbols represent untreated mice. N=6 mice per group.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01AI045459 and R01AI044374) to L.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Martinez JE, Romero-Steiner S, Pilishvili T, et al. A flow cytometric opsonophagocytic assay for measurement of functional antibodies elicited after vaccination with the 23-valent pneumococcal polysaccharide vaccine. Clin Diag Lab Immunol. 1999;6:581–586. doi: 10.1128/cdli.6.4.581-586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol. 2006 Feb;13(2):165–169. doi: 10.1128/CVI.13.2.165-169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez JE, Clutterbuck EA, Li H, Romero-Steiner S, Carlone GM. Evaluation of multiplex flow cytometric opsonophagocytic assays for determination of functional anticapsular antibodies to Streptococcus pneumoniae. Clin Vaccine Immunol. 2006 Apr;13(4):459–466. doi: 10.1128/CVI.13.4.459-466.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero-Steiner S, Libutti D, Pais LB, et al. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns T, Abadi M, Pirofski L. Modulation of the lung inflammatory response to serotype 8 pneumococcus infection by a human monoclonal IgM to serotype 8 capsular polysaccharide. Infect Immun. 2005;73:4530–4538. doi: 10.1128/IAI.73.8.4530-4538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian H, Weber S, Thorkildson P, Kozel TR, Pirofski LA. Efficacy of opsonic and nonopsonic serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibodies against intranasal challenge with Streptococcus pneumoniae in mice. Infect Immun. 2009 Apr;77(4):1502–1513. doi: 10.1128/IAI.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang Q, Zhong Z, Lees A, Pekna M, Pirofski L. Structure-function relationships for human antibodies to pneumococcal capsular polysaccharide from transgenic mice with human immunoglobulin Loci. Infect Immun. 2002 Sep;70(9):4977–4986. doi: 10.1128/IAI.70.9.4977-4986.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guy B, Testart C, Gimenez S, et al. Comparison of polymorphonuclear cells from healthy donors and differentiated HL-60 cells as phagocytes in an opsonophagocytic assay using antigen-coated fluorescent beads. Clin Diagn Lab Immunol. 2000 Mar;7(2):314–317. doi: 10.1128/cdli.7.2.314-317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun K, Salmon SL, Lotz SA, Metzger DW. Interleukin-12 promotes gamma interferon-dependent neutrophil recruitment in the lung and improves protection against respiratory Streptococcus pneumoniae infection. Infect Immun. 2007 Mar;75(3):1196–1202. doi: 10.1128/IAI.01403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadioglu A, Andrew PW. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol. 2004 Mar;25(3):143–149. doi: 10.1016/j.it.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Marks M, Burns T, Abadi M, et al. Influence of neutropenia on the course of serotype 8 pneumococcal pneumonia in mice. Infect Immun. 2007 Apr;75(4):1586–1597. doi: 10.1128/IAI.01579-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang E, Simard M, Ouellet N, Bergeron Y, Beauchamp D, Bergeron MG. Pathogenesis of pneumococcal pneumonia in cyclophosphamide-induced leukopenia in mice. Infect Immun. 2002 Aug;70(8):4226–4238. doi: 10.1128/IAI.70.8.4226-4238.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009 Jul;119(7):1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knapp S, Leemans JC, Florquin S, et al. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2003 Jan 15;167(2):171–179. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- 15.Shao X, Mednick A, Alvarez M, van RN, Casadevall A, Goldman DL. An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. J Immunol. 2005 Sep 1;175(5):3244–3251. doi: 10.4049/jimmunol.175.5.3244. [DOI] [PubMed] [Google Scholar]
- 16.Leemans JC, Juffermans NP, Florquin S, et al. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J Immunol. 2001 Apr 1;166(7):4604–4611. doi: 10.4049/jimmunol.166.7.4604. [DOI] [PubMed] [Google Scholar]
- 17.Cote CK, Rea KM, Norris SL, van RN, Welkos SL. The use of a model of in vivo macrophage depletion to study the role of macrophages during infection with Bacillus anthracis spores. Microb Pathog. 2004 Oct;37(4):169–175. doi: 10.1016/j.micpath.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Leendertse M, Willems RJ, Giebelen IA, et al. Peritoneal macrophages are important for the early containment of Enterococcus faecium peritonitis in mice. Innate Immun. 2009 Feb;15(1):3–12. doi: 10.1177/1753425908100238. [DOI] [PubMed] [Google Scholar]
- 19.Fabrizio K, Groner A, Boes M, Pirofski LA. A human monoclonal immunoglobulin M reduces bacteremia and inflammation in a mouse model of systemic pneumococcal infection. Clin Vaccine Immunol. 2007 Apr;14(4):382–390. doi: 10.1128/CVI.00374-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabrizio K, Manix C, Guimaraes A, Nosanchuk JD, Pirofski LA. Aggregation of Streptococcus pneumoniae by a pneumococcal capsular polysaccharide-specific human monoclonal IgM correlates with antibody efficacy in vivo. Clin Vaccine Immunol. 2010 Mar 3; doi: 10.1128/CVI.00410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briles DE, Nahm M, Schroer K, et al. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Szalai AJ, Hollingshead SK, Nahm MH, Briles DE. Antibody to the type 3 capsule facilitates immune adherence of pneumococci to erythrocytes and augments their transfer to macrophages. Infect Immun. 2009 Jan;77(1):464–471. doi: 10.1128/IAI.00892-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira DM, Darrieux M, Silva DA, et al. Characterization of protective mucosal and systemic immune responses elicited by pneumococcal surface protein PspA and PspC nasal vaccines against a respiratory pneumococcal challenge in mice. Clin Vaccine Immunol. 2009 May;16(5):636–645. doi: 10.1128/CVI.00395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neeleman C, Geelen SP, Aerts PC, et al. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect Immun. 1999 Sep;67(9):4517–4524. doi: 10.1128/iai.67.9.4517-4524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan MC, Cooper BW, Nightingale CH, Quintiliani R, Lawlor MT. Evaluation of the efficacy of ciprofloxacin against Streptococcus pneumoniae by using a mouse protection model. Antimicrob Agents Chemother. 1993 Feb;37(2):234–239. doi: 10.1128/aac.37.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briles DE, Claflin JL, Schroer K, Forman C. Mouse IgG3 antibodies are highly protective against infection with Streptococcus pneumoniae. Nature. 1981;294:88–90. doi: 10.1038/294088a0. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999 Oct;11(4):443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 28.Fabrizio K, Manix C, Guimaraes AJ, Nosanchuk JD, Pirofski LA. Aggregation of Streptococcus pneumoniae by a pneumococcal capsular polysaccharide-specific human monoclonal IgM correlates with antibody efficacy in vivo. Clin Vaccine Immunol. 2010 May;17(5):713–721. doi: 10.1128/CVI.00410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ajuebor MN, Das AM, Virag L, Flower RJ, Szabo C, Perretti M. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J Immunol. 1999 Feb 1;162(3):1685–1691. [PubMed] [Google Scholar]
- 30.van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994 Sep 14;174(1–2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 31.Stephens-Romero SD, Mednick AJ, Feldmesser M. The pathogenesis of fatal outcome in murine pulmonary aspergillosis depends on the neutrophil depletion strategy. Infect Immun. 2005 Jan;73(1):114–125. doi: 10.1128/IAI.73.1.114-125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helmy KY, Katschke KJ, Jr, Gorgani NN, et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006 Mar 10;124(5):915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 33.Tian H, Groner A, Boes M, Pirofski L. Pneumococcal capsular polysaccharide vaccine-mediated protection of immunodeficient mice against serotype 3 Streptococcus pneumoniae. Infect Immun. 2007;75:1643–1650. doi: 10.1128/IAI.01371-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orihuela CJ, Gao G, McGee M, Yu J, Francis KP, Tuomanen E. Organ-specific models of Streptococcus pneumoniae disease. Scand J Infect Dis. 2003;35(9):647–652. doi: 10.1080/00365540310015854. [DOI] [PubMed] [Google Scholar]
- 35.Clatworthy MR, Smith KG. FcgammaRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J Exp Med. 2004 Mar 1;199(5):717–723. doi: 10.1084/jem.20032197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leendertse M, Willems RJ, Giebelen IA, Roelofs JJ, Bonten MJ, van der Poll T. Neutrophils are essential for rapid clearance of Enterococcus faecium in mice. Infect Immun. 2009 Jan;77(1):485–491. doi: 10.1128/IAI.00863-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthias KA, Roche AM, Standish AJ, Shchepetov M, Weiser JN. Neutrophil-toxin interactions promote antigen delivery and mucosal clearance of Streptococcus pneumoniae. J Immunol. 2008 May 1;180(9):6246–6254. doi: 10.4049/jimmunol.180.9.6246. [DOI] [PubMed] [Google Scholar]
- 38.Leendertse M, Willems RJ, Giebelen IA, et al. TLR2-dependent MyD88 signaling contributes to early host defense in murine Enterococcus faecium peritonitis. J Immunol. 2008 Apr 1;180(7):4865–4874. doi: 10.4049/jimmunol.180.7.4865. [DOI] [PubMed] [Google Scholar]
- 39.Knapp S, Wieland CW, van ' V, et al. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J Immunol. 2004 Mar 1;172(5):3132–3138. doi: 10.4049/jimmunol.172.5.3132. [DOI] [PubMed] [Google Scholar]
- 40.Ghumra A, Shi J, Mcintosh RS, et al. Structural requirements for the interaction of human IgM and IgA with the human Fcalpha/mu receptor. Eur J Immunol. 2009 Apr;39(4):1147–1156. doi: 10.1002/eji.200839184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kikuno K, Kang DW, Tahara K, et al. Unusual biochemical features and follicular dendritic cell expression of human Fcalpha/mu receptor. Eur J Immunol. 2007 Dec;37(12):3540–3550. doi: 10.1002/eji.200737655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibuya A, Sakamoto N, Shimizu Y, et al. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat Immunol. 2000 Nov;1(5):441–446. doi: 10.1038/80886. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Wang JP, Ghiran I, et al. CR1 expression on mouse erythrocytes mediates clearance of Streptococcus pneumoniae by immune adherence. Infect Immun. 2010 May 3; doi: 10.1128/IAI.01263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Repik A, Pincus SE, Ghiran I, et al. A transgenic mouse model for studying the clearance of blood-borne pathogens via human complement receptor 1 (CR1) Clin Exp Immunol. 2005 May;140(2):230–240. doi: 10.1111/j.1365-2249.2005.02764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown EJ, Hosea SW, Frank MM. The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev Infect Dis. 1983 Sep;5 Suppl 4:S797–S805. doi: 10.1093/clinids/5.supplement_4.s797. [DOI] [PubMed] [Google Scholar]
- 46.Hosea SW, Brown EJ, Frank MM. The critical role of complement in experimental pneumococcal sepsis. J Infect Dis. 1980;142:903–909. doi: 10.1093/infdis/142.6.903. [DOI] [PubMed] [Google Scholar]
- 47.Wiesmann C, Katschke KJ, Yin J, et al. Structure of C3b in complex with CRIg gives insights into regulation of complement activation. Nature. 2006 Nov 9;444(7116):217–220. doi: 10.1038/nature05263. [DOI] [PubMed] [Google Scholar]
- 48.He JQ, Wiesmann C, van Lookeren CM. A role of macrophage complement receptor CRIg in immune clearance and inflammation. Mol Immunol. 2008 Oct;45(16):4041–4047. doi: 10.1016/j.molimm.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Crispin JC, Dalle LJ, Tsokos GC. A Novel Inhibitor of the Alternative Pathway of Complement Attenuates Intestinal Ischemia/Reperfusion-Induced Injury. J Surg Res. 2009 Jun 25; doi: 10.1016/j.jss.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 50.Vogt L, Schmitz N, Kurrer MO, et al. VSIG4, a B7 family-related protein, is a negative regulator of T cell activation. J Clin Invest. 2006 Oct;116(10):2817–2826. doi: 10.1172/JCI25673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katschke KJ, Jr, Helmy KY, Steffek M, et al. A novel inhibitor of the alternative pathway of complement reverses inflammation and bone destruction in experimental arthritis. J Exp Med. 2007 Jun 11;204(6):1319–1325. doi: 10.1084/jem.20070432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The percent of PBS- and A7-treated mice surviving after i.p. infection at the times designated on the x-axis is depicted. Open symbols represent PBS encapsulated liposomes-treated mice; closed symbols represent untreated mice. N=6 mice per group.