Summary
Hfq is a global regulatory RNA-binding protein. We have identified and characterized an atypical Hfq required for gene regulation and infectivity in the Lyme disease spirochete Borrelia burgdorferi. Sequence analyses of the putative B. burgdorferi Hfq protein revealed only a modest level of similarity with the Hfq from Escherichia coli, although a few key residues are retained and the predicted tertiary structure is similar. Several lines of evidence suggest that the B. burgdorferi bb0268 gene encodes a functional Hfq homolog. First, the hfqBb gene (bb0268) restores the efficient translation of an rpoS::lacZ fusion in an E. coli hfq null mutant. Second, the Hfq from B. burgdorferi binds to the small RNA DsrABb and the rpoS mRNA. Third, a B. burgdorferi hfq null mutant was generated and has a pleiotropic phenotype that includes increased cell length and decreased growth rate, as found in hfq mutants in other bacteria. The hfqBb mutant phenotype is complemented in trans with the hfq gene from either B. burgdorferi or, surprisingly, E. coli. This is the first example of a heterologous bacterial gene complementing a B. burgdorferi mutant. The alternative sigma factor RpoS and the outer membrane lipoprotein OspC, which are induced by increased temperature and required for mammalian infection, are not upregulated in the hfq mutant. Consequently, the hfq mutant is not infectious by needle inoculation in the murine model. These data suggest that Hfq plays a key role in the regulation of pathogenicity factors in B. burgdorferi and we hypothesize that the spirochete has a complex Hfq-dependent sRNA network.
Keywords: Hfq, RpoS, DsrA, OspC, sRNA
Introduction
Borrelia burgdorferi is the causative agent of Lyme disease (Burgdorfer et al., 1982; Benach et al., 1983; Steere et al., 1983; Radolf et al., 2010). The spirochete cycles between a tick vector and a vertebrate host (Lane et al., 1991; Spielman, 1994; Piesman and Schwan, 2010), altering its gene expression to transition between and survive within these two vastly different environments (Singh and Girschick, 2004; Samuels and Radolf, 2009; Skare et al., 2010). The increase in temperature associated with transmission from the tick to the mammal is one signal known to increase synthesis of outer surface lipoprotein C (OspC) and other virulence factors (Schwan et al., 1995; Stevenson et al., 1995; Fingerle et al., 2000; Yang et al., 2000; Revel et al., 2002; Alverson et al., 2003; Ojaimi et al., 2003). OspC synthesis depends on the alternative sigma factor RpoS (σS or σ38), which has emerged as a global regulator of the enzootic cycle (Hübner et al., 2001; Caimano et al., 2004; Burtnick et al., 2007; Caimano et al., 2007) and is required for infection in the murine model of Lyme disease (Caimano et al., 2004; Blevins et al., 2009). The response regulator Rrp2 and the alternative sigma factor RpoN regulate the transcription of rpoS in response to an unknown environmental signal(s) (Hübner et al., 2001; Yang et al., 2003), while post-transcriptional temperature-dependent RpoS synthesis is regulated by the small RNA (sRNA) DsrABb (Lybecker and Samuels, 2007), the only sRNA identified to date in B. burgdorferi.
Recently, sRNAs have emerged as major regulators in the expression of many bacterial genes, including those involved in virulence and the stress response (Lease and Belfort, 2000; Hengge-Aronis, 2002; Repoila et al., 2003; Gottesman, 2004; Storz et al., 2004; Majdalani et al., 2005; Narberhaus et al., 2006; Serganov and Patel, 2007; Fröhlich and Vogel, 2009; Klinkert and Narberhaus, 2009). Many sRNAs post-transcriptionally regulate gene expression by base pairing with target trans-encoded mRNAs, affecting either their translation or stability (Repoila et al., 2003; Gottesman, 2004; Majdalani et al., 2005). In Escherichia coli, the sRNA DsrA base pairs with the rpoS mRNA upstream region, releasing the Shine-Dalgarno sequence and start site from a stem-loop that inhibits translation. In B. burgdorferi, DsrABb and the upstream region of the rpoS transcript have extensive complementarity, suggesting a similar mechanism of translational regulation (Lybecker and Samuels, 2007). Most trans-acting antisense sRNAs require the RNA chaperone Hfq (Massé et al., 2003b; Gottesman, 2004; Storz et al., 2004; Valentin-Hansen et al., 2004; Majdalani et al., 2005; Romby et al., 2006; Brennan and Link, 2007; Waters and Storz, 2009). Hfq is a highly conserved RNA-binding protein that was first identified in E. coli as a host factor required for Qβ bacteriophage replication (Franze de Fernandez et al., 1968). Hfq is the bacterial homolog of the eukaryotic and archaeal Sm and LSm proteins (Arluison et al., 2002; Möller et al., 2002; Schumacher et al., 2002; Sun et al., 2002; Zhang et al., 2002; Sauter et al., 2003). Sm and LSm proteins are involved in various aspects of RNA metabolism, including processing of nuclear RNA, RNA localization, and mRNA decay. The Sm and LSm proteins are characterized by two highly conserved motifs, Sm1 and Sm2, and form cyclic ring-shaped oligomers (Achsel et al., 1999; Kambach et al., 1999; Achsel et al., 2001). The Hfq protein is an oligomeric torus, like Sm and LSm (Törö et al., 2001; Törö et al., 2002). Whereas Sm and LSm proteins form heptameric structures that are homomeric in archaea and heteromeric in eukaryotes, most Hfq homologs are found as homohexamers (reviewed in Brennan and Link, 2007).
Hfq hexamers have two independent RNA-binding surfaces that have different specificities. The proximal surface was defined based on the Staphylococcus aureus crystal structure and includes the Sm2 motif (Schumacher et al., 2002). RNAs that bind this surface typically have AU-rich single-stranded sequences either just 3′ or just 5′ of a stem-loop (Brescia et al., 2003; Moll et al., 2003b; Geissmann and Touati, 2004; Večerek et al., 2005). These RNAs bind by circumnavigating the proximal face of the central cavity. The distal face of Hfq, on the opposite side of the torus, also binds RNAs (Mikulecky et al., 2004). This surface, which has been characterized in a recent X-ray crystal structure, is typically associated with poly(A) and A-rich structures (Link et al., 2009). The crystal structure predicts that the distal surface has specificity for purine-rich repeats with the sequence (ARN)2–5. However, several other secondary structural regions have been identified as Hfq-binding sites, including hairpin motifs, a loop region connecting two stems in a pseudoknot structure, and the T- and D-stems of tRNA (Antal et al., 2005; Lee and Feig, 2008).
Hfq functions as an RNA chaperone by facilitating the interaction of an sRNA with its target mRNA via colocalizing the RNAs and/or altering the structure of the RNAs (Sledjeski et al., 2001; Massé and Gottesman, 2002; Møller et al., 2002; Zhang et al., 2002; Kawamoto et al., 2006; Večerek et al., 2007). Hfq protects sRNAs from degradation likely by blocking the RNase E binding site on the sRNA (Massé et al., 2003a; Moll et al., 2003a). Hfq also functions in mRNA turnover by stabilizing an mRNA, stimulating turnover of the mRNA by promoting polyadenylation, or facilitating base pairing between an sRNA and its target RNA resulting in cleavage of both RNAs by RNase E (Tsui et al., 1997; Vytvytska et al., 2000; Folichon et al., 2003).
An E. coli hfq null mutant has a pleiotropic phenotype that includes decreased growth rate, increased cell length, and increased sensitivity to stress conditions (Tsui et al., 1994; Muffler et al., 1997). Hfq is required for post-transcriptional regulation of rpoS and, therefore, the syntheses of more than 50 proteins are affected by hfq mutations (Muffler et al., 1996; Brown and Elliott, 1997). Moreover, Hfq plays an important role in several pathogenic bacteria: hfq mutations either attenuate or abolish virulence (reviewed in Chao and Vogel, 2010). Hfq is largely conserved in bacteria (Sun et al., 2002); however, B. burgdorferi lacks an annotated hfq gene in its genome (Fraser et al., 1997) and BLAST searches using sequences of conserved hfq genes do not reveal a homolog. We now identify and characterize an unusual Hfq in B. burgdorferi that regulates RpoS and is required for murine infection by needle inoculation.
Results
Identification of Hfq in B. burgdorferi
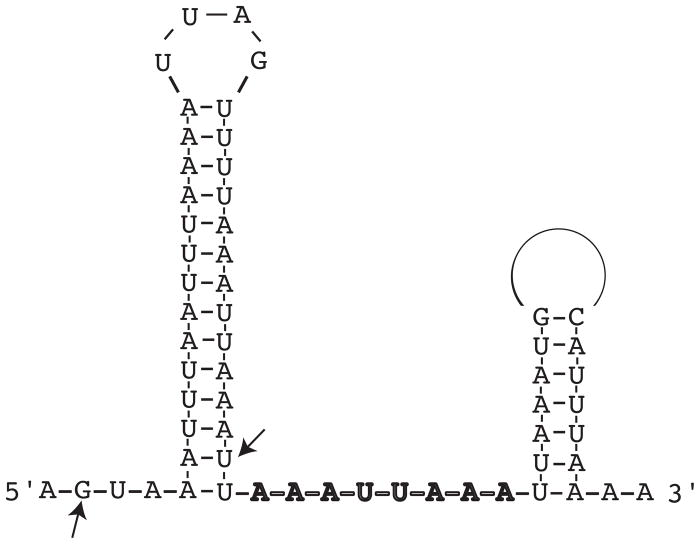
The RNA chaperone Hfq is typically required for RNA-RNA interactions between small regulatory RNAs and their cognate target mRNAs. After identifying and characterizing the first sRNA, DsrABb, in B. burgdorferi, we hypothesized that the spirochete has an Hfq despite the lack of an annotated, or readily identifiable, homolog in its genome. This was supported by an MFOLD (Zuker, 2003) secondary structure prediction of DsrABb that revealed a putative Hfq-binding site, an AU stretch of residues situated between two stable stem loops (Fig. 1). BLAST searches of the B. burdorferi genome were performed using as queries the Hfq sequences from E. coli and S. aureus, as well as just the highly conserved N-terminal 68 amino acids of E. coli Hfq. No homologous sequences were found in B. burgdorferi (data not shown). We next searched the genome for small ORFs that had the potential to form Sm1 and Sm2 motifs, and we identified a conserved hypothetical ORF encoded by the bb0268 gene, which was predicted to have 33% similarity and 12% identity with E. coli Hfq. A ClustalW alignment revealed that several important amino acids are conserved and a Phyre prediction suggested that this ORF could fold into an Hfq-like structure (Fig. 2). The residues comprising the nucleotide-binding pocket of Hfq, in the S. aureus Hfq-RNA complex (Schumacher et al., 2002), are conserved or similar in the predicted BB0268 protein. Other Hfq motifs (Sm1 NG and Sm2 F/YKHA) are partially conserved in BB0268. However, the BB0268 protein (159 amino acids) is considerably larger than most Hfq proteins (typically 77 to 110 amino acids). The N-terminal 68 amino acids of known Hfq homologs are highly conserved and include the Sm motifs, while the C-terminal portions of the proteins vary in length and sequence (Sauter et al., 2003). The ClustalW alignment demonstrated that the BB0268 sequence is most similar to the N-terminal domain of the canonical E. coli Hfq protein, but BB0268 has a longer C-terminal tail with a divergent sequence (Fig. 2A).
Fig. 1.
Predicted secondary structure of DsrABb. The arrows flank the rpoS complementary region (34 nucleotides). The sequence predicted to be the Hfq-binding site is in bold. The open circle represents a loop of 32 nucleotides.
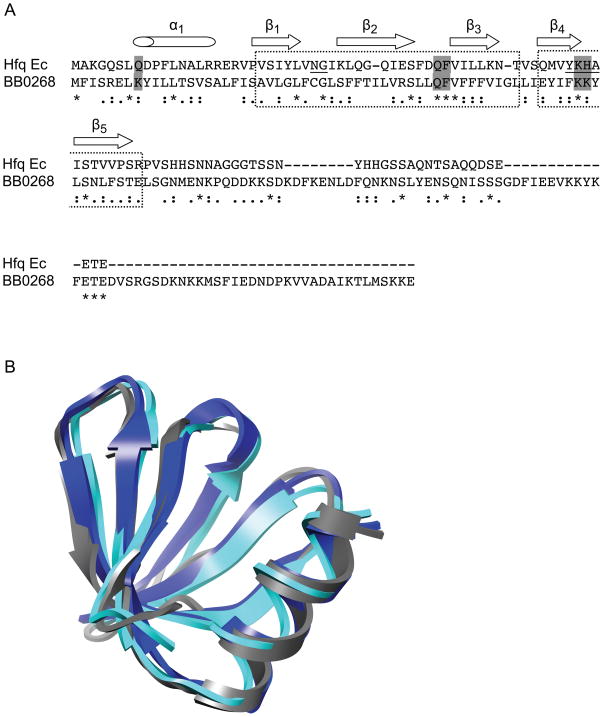
Fig. 2.
Hfq structural homology. (A) ClustalW alignment of the BB0268 conserved hypothetical protein with the E. coli (Ec) Hfq protein. Amino acids denoted by asterisks (*) are identical, colons (:) strongly similar and dots (.) weakly similar. The dashed boxes indicate the Sm1 and Sm2 motifs and the secondary structure of the E. coli Hfq is indicated above the sequence. Two of the signature motifs of Hfq, the Sm1 NG and the Sm2 F(Y)KHA, are underlined. The residues that are important in RNA binding by the S. aureus Hfq are shown in gray boxes. This figure panel is modified from Nielsen et al. (2007). (B) Modeling of Hfq structures. The structure of HfqBb (gray) was predicted using Phyre and superimposed onto the experimentally determined S. aureus Hfq (blue) and E. coli Hfq (cyan) structures using Chimera. Hfq homologs from many organisms have a variable C-terminal extension that has not been structurally characterized; therefore, we have no information on the folded structure of the C-terminal region of HfqBb and it is not shown.
B. burgdorferi bb0268 complements an E. coli hfq mutant
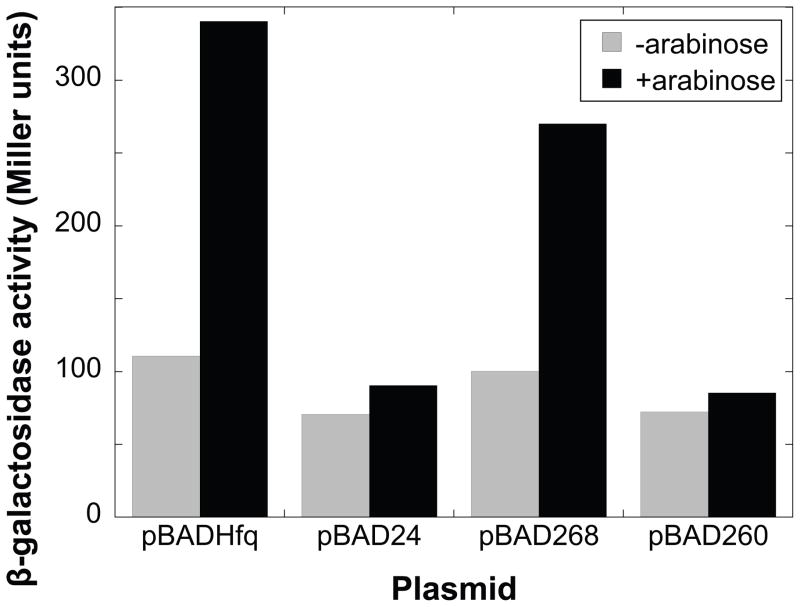
In E. coli, Hfq is required for DsrA to stimulate translation of rpoS in response to a decrease in temperature and entrance into stationary phase (Sledjeski et al., 2001). Evidence suggests that Hfq colocalizes rpoS and DsrA, presenting the two RNAs in favorable positions for complex formation (Brescia et al., 2003; Lease and Woodson, 2004). To determine if BB0268 functions as an RNA chaperone, similar to Hfq in E. coli, we assayed its ability to trans-complement an hfq null mutant of E. coli (Sledjeski et al., 2001). The bb0268 gene was fused to an arabinose-inducible promoter in the vector pBAD24. The empty pBAD24 vector and conserved hypothetical ORF bb0260, which shares sequence similarity with bb0268, were used as negative controls; E. coli hfq was used as a positive control. Hfq is needed for growth-phase related regulation of rpoS::lacZ at 30°C in E. coli. The efficient translation of an rpoS::lacZ fusion, in response to changes in cell density, was measured with a β-galactosidase assay. bb0268 partially restored the efficient translation of rpoS::lacZ at 30°C (Fig. 3), suggesting that it has an Hfq-like RNA chaperone activity in E. coli. In contrast, neither bb0260 nor the empty vector complemented the hfq null mutant in E. coli. Five independent experiments with bb0268 yielded similar results. Therefore, we now refer to BB0268 as HfqBb.
Fig. 3.
BB0268 has Hfq-like activity in E. coli. hfq-1 mutant strains containing an rpoS::lacZ translational fusion and carrying the indicated plasmids were assayed for total β-galactosidase activity. pBADHfq expresses E. coli Hfq under control of the araBAD promoter. pBAD24 is the empty araBAD vector. pBAD268 and pBAD260 have the B. burgdorferi bb0268 and bb0260 ORFs under the control of the araBAD promoter. Cultures were grown at 30 C either with (black bars) or without (gray bars) 150 μM arabinose and samples were taken at various times during logarithmic growth. Total β-galactosidase activity was determined as described by Miller (1972). Three independent experiments with BB0260 and five independent experiments with BB0268 were performed and yielded similar results.
Recombinant HfqBb binds rpoS mRNA and DsrA
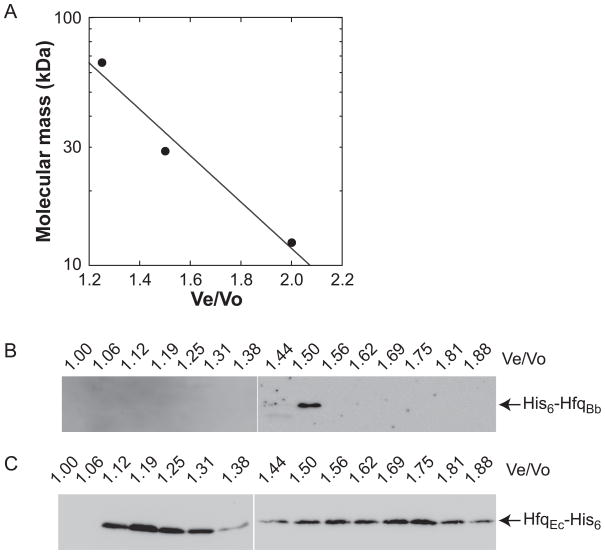
The Hfq and Sm/Lsm family of proteins form homohexameric and heteroheptameric oligomers (Tsui et al., 1994; Sun et al., 2002; Valentin-Hansen et al., 2004). We purified recombinant HfqBb using an N-terminal His-tag. The predicted molecular mass of HfqBb is ~20 kDa (with the His-tag) and gel filtration chromatography indicated that it has a molecular mass of ~34 kDa in solution (Fig. 4), suggesting that HfqBb forms a dimer or a monomer, rather than the characteristic hexamer, under these in vitro conditions. In contrast, E. coli Hfq was primarily a hexamer as analyzed by gel filtration chromatography under the same conditions, although monomer was also observed in this experiment (Fig. 4C).
Fig. 4.
HfqBb is not a stable hexamer in solution. (A) Standard curve of the Sigma gel filtration molecular weight markers resolved on a Superdex 75 HR 10/30 column. Regression analysis yielded the equation: y = 869.58 × e(−2.1536x), which was used to calculate molecular mass. Recombinant HfqBb (B) and E. coli Hfq (C) were resolved on a Superdex 75 HR 10/30 column and fractions analyzed using a HisProbe-HRP.
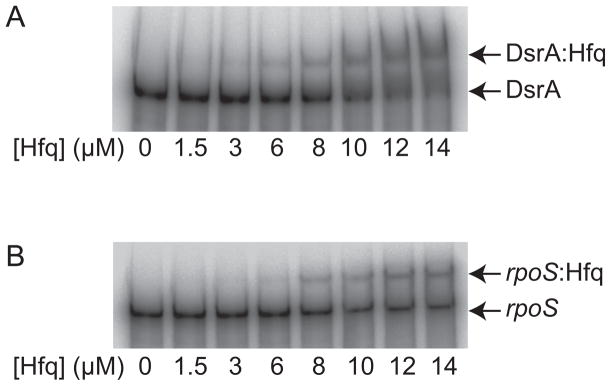
Electrophoretic mobility shift assays (EMSAs) were performed using recombinant HfqBb protein and in vitro-transcribed rpoS and DsrABb RNAs (Fig. 5). The RNAs were heated to 65°C for 10 min and slow-cooled to room temperature. The RNAs were then incubated with increasing amounts of HfqBb protein. The HfqBb protein bound both rpoS and DsrABb as seen by the decreased migration of the RNAs in the EMSA (Fig. 5).
Fig. 5.
The HfqBb protein binds RNA. Gel mobility shift analyses of HfqBb binding to DsrABb (A) and rpoS (B). The RNAs were labeled with [α-32P]UTP during in vitro transcription, gel-purified, and incubated with increasing concentrations of purified recombinant HfqBb. The binding reactions were fractionated on a native Novex® Pre-Cast 6% DNA retardation gel.
Construction and complementation of the hfqBb mutant
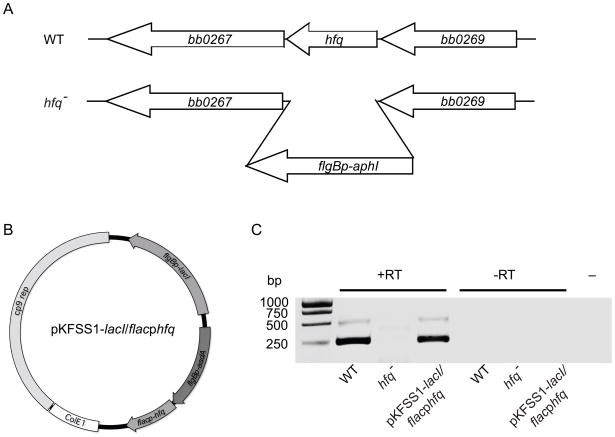
To determine the function of HfqBb in B. burgdorferi, we deleted the hfqBb gene in a low-passage infectious clone of strain 297 (Fig. 6A). Two different plasmids were constructed to complement the hfqBb mutation. The hfqBb (bb0268) and the E. coli hfq ORFs were fused to the inducible flac promoter and inserted into the shuttle vector pKFSS1-lacI, which carries the lacI gene fused to the constitutive flgB promoter (Fig. 6B). The two complementing plasmids and the parental shuttle vector were transformed into the hfqBb mutant strain. RT-PCR analysis demonstrated that the wild-type and hfqBb-complemented strains, but not the hfqBb mutant strain, expressed hfqBb transcript (Fig. 6C).
Fig. 6.
Mutation and complementation of hfqBb. (A) Strategy for disrupting the hfqBb gene (bb0268). The hfqBb gene was replaced with the kanamycin resistant cassette (flgBp-aphI). Transformation into wild-type (WT) B. burgdorferi and homologous recombination generated an hfqBb mutant strain (hfq−). (B) Plasmid for trans-complementing the hfqBb mutant strain. The B. burgdorferi or E. coli hfq genes were fused to the inducible promoter flacp and inserted into the modified shuttle vector pKFSS1-lacI, which carries the lacI repressor gene fused to the constitutive flgB promoter in the multiple cloning site. The plasmid also carries both a B. burgdorferi replication origin (cp9 rep) from the 9-kb circular plasmid and an E. coli replication origin (ColE1) from pCR®-XL-TOPO. (C) RT-PCR of hfq mRNA in the wild type (WT), the hfqBb mutant (hfq−), and the hfqBb mutant complemented with hfqBb (hfq− pKFSS1-lacI flacphfqBb). The RT-PCR (+RT), the PCR on samples that were not treated with reverse transcriptase (−RT), and the PCR without template (−) were fractionated on a 2% agarose gel and stained with ethidium bromide.
The hfqBb mutant has a decreased growth rate and increased cell length
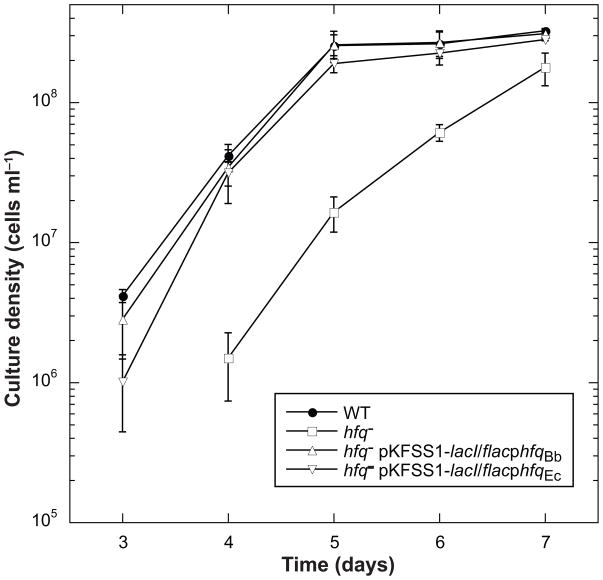
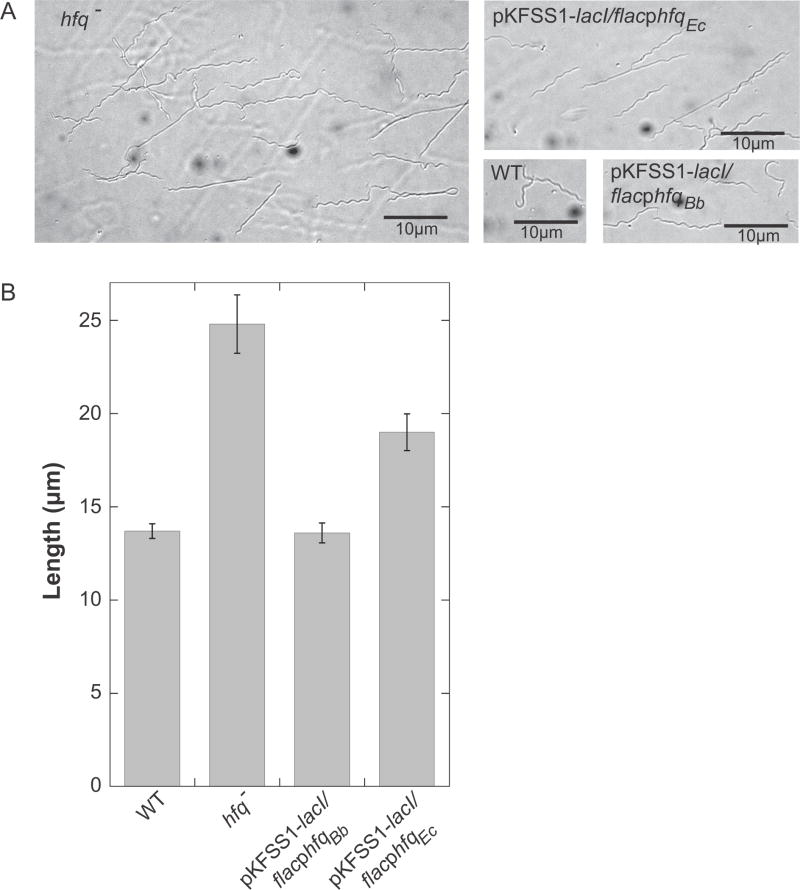
We assayed growth rate using a wild-type low-passage infectious clone of 297, the hfqBb mutant, and the two complemented strains. B. burgdorferi cultures were grown at 23°C to a low cell density (1–3 × 107 cells ml−1) and then inoculated into a culture at 37°C at a cell density of 1 × 103 cells ml−1. Cell densities were determined daily until cultures reached stationary phase. The hfqBb mutant grew slower than the wild type; both the B. burgdorferi and E. coli hfq genes complemented the growth phenotype of the hfqBb mutant (Fig. 7). The hfqBb mutant strain also was significantly longer than wild type. The cell length phenotype was fully complemented by the hfqBb gene expressed in trans and, somewhat unexpectedly, partially complemented by the E. coli hfq gene (Fig. 8).
Fig. 7.
Growth phenotype of the hfqBb mutant. Growth curve of the wild type (WT), hfqBb mutant (hfq−), and hfqBb mutant trans-complemented with either hfq from B. burgdorferi (hfq−pKFSS1-lacI flacphfqBb) or hfq from E. coli (hfq− pKFSS1-lacI flacphfqEc) following a temperature shift to 37°C. The means of the cell densities from three independent experiments are graphed with error bars representing the standard error of the means.
Fig. 8.
Cell morphology phenotype of the hfqBb mutant. Cultures were grown at 37°C to mid-log phase and the cells were visualized using Nomarski microscopy and analyzed using ImageJ. (A) Microscopic images of the wild-type (WT), hfqBb mutant (hfq−), and complementing strains (pKFSS1-lacI/flacphfqBb or Ec). (B) ImageJ was used to measure the length of fifty spirochetes per strain and the means of the lengths are graphed with error bars representing the standard error of the means. Differences between wild-type and hfqBb mutant strains and between hfqBb mutant and pKFSS1-lacI/flacphfqBb-complemented strains are statistically significant (P < 0.01) by both a one-way ANOVA with a Tukey’s post-hoc test and a Kruskal–Wallis test; differences between hfqBb mutant and pKFSS1-lacI/flacphfqEc-complemented strains (P < 0.01) and between wild-type and pKFSS1-lacI/flacphfqEc-complemented strains (P < 0.001) are significant by both a two-tailed unpaired t-test and a Mann Whitney U test.
RpoS and OspC are regulated by HfqBb
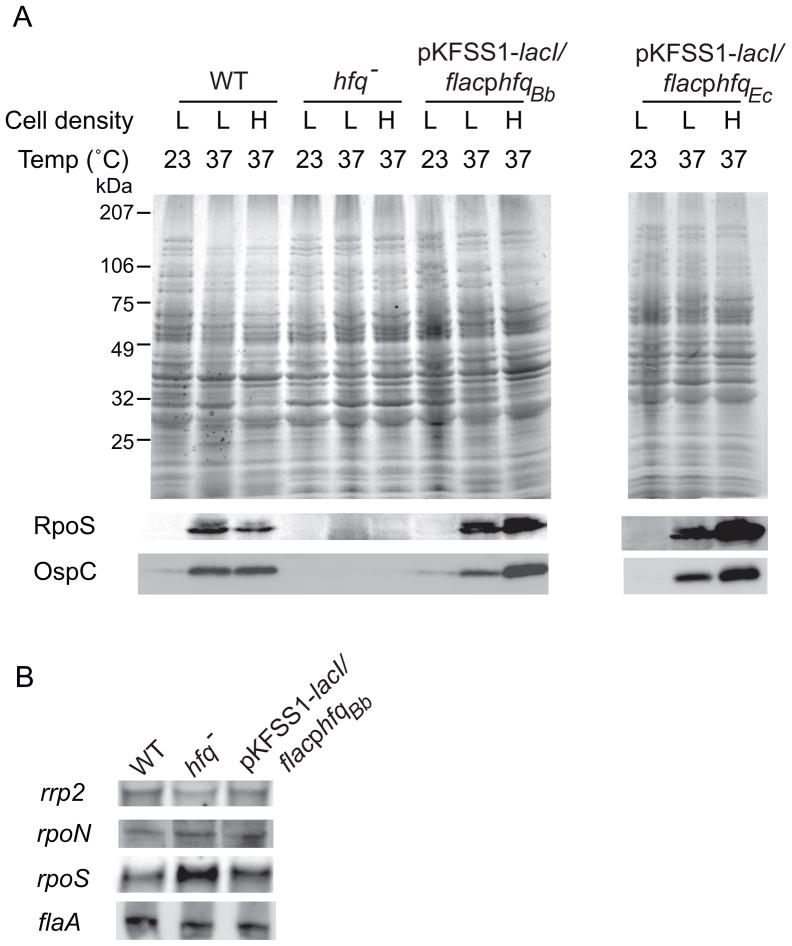
DsrABb post-transcriptionally regulates RpoS levels in response to increased temperature at low cell density (Lybecker and Samuels, 2007). This sRNA has extensive complementarity to the upstream region of the rpoS mRNA and our data suggest that DsrA stimulates efficient translation of the rpoS mRNA through base pairing with the upstream region of the mRNA. Hfq in other bacteria is required for regulating trans-encoded sRNAs and often post-transcriptionally regulates RpoS levels, so we expected that the hfqBb mutant strain would show reduced levels of RpoS and OspC proteins in response to a temperature shift at a low cell density, as seen in the dsrABb mutant strains. B. burgdorferi low-passage wild-type, hfqBb mutant, and complemented strains were temperature-shifted to 37°C and grown to low and high cell densities (1–3 × 107 and 1× 108 cells ml−1, respectively). Western blot analyses of whole cell lysates demonstrated that the wild-type and complemented strains displayed an increase in RpoS and OspC levels after a temperature shift, while the hfqBb mutant strain showed reduced levels of RpoS and OspC (Fig. 9A). Surprisingly, the E. coli hfq also restored the temperature-dependent increase in RpoS and OspC. The inducible promoter fusions are leaky in these trans-acting configurations (Fig. 6C and data not shown), so IPTG was not required for transcription of the complementing hfq genes. Adding high levels of IPTG caused decreases in RpoS and OspC levels, presumably due to the overabundance of Hfq inhibiting translation (data not shown).
Fig. 9.
HfqBb regulates RpoS levels post-transcriptionally in response to a temperature shift. Immunoblot analyses of whole-cell lysates of wild-type, hfqBb mutant, and trans-complemented strains at 23°C and after a temperature shift to 37°C at low (L) and high (H) cell densities (1–3 × 107 and 1 × 108 cells ml−1, respectively(A). The top panel is a Coomassie brilliant blue-stained SDS-PAGE gel and the bottom panels are immunoblots probed with either anti-RpoS or anti-OspC antibodies. Three independent experiments were performed and representative data are shown. Northern blot analyses of total RNA (15 μg) fractionated on a 6% polyacrylamide 7 M urea gel, blotted to a nylon membrane, and hybridized with a rpoS, rpoN, rrp2, or flaA single-stranded RNA probe (B). Two independent experiments were performed and representative data are shown; the signal was quantified on a FujiFilm LAS-3000.
Transcription of rpoS requires RpoN and Rrp2 (Hübner et al., 2001; Yang et al., 2003). To further understand the mechanism by which HfqBb regulates RpoS, the steady-state levels of rpoS, rpoN, and rrp2 mRNA were analyzed by Northern blot analyses (Fig. 9B). The constitutively expressed flaA transcript was used as a gel loading control. The amounts of the rpoN and rrp2 transcripts were similar between the wild-type, mutant and complemented strains demonstrating that Hfq does not regulate the levels of rpoN or rrp2 mRNA. However, the levels of rpoS transcript were 1.5-fold higher in the hfqBb mutant strain compared to the wild-type and complemented strains; this pattern of regulation was also observed in the dsrABb mutant strain (Lybecker and Samuels, 2007). These data, taken together, suggest that HfqBb regulates RpoS synthesis post-transcriptionally and does not stabilize the rpoS mRNA.
As previously seen with the dsrABb mutant (Lybecker and Samuels, 2007), serial passage of the hfqBb mutant results in a reversal of the phenotype. B. burgdorferi cultures were serially passaged at 23°C and then temperature-shifted to 37°C and grown to a low cell density. Western blot analyses demonstrated that both RpoS and OspC syntheses were increased, rather than decreased, in the mutant compared to the wild type after as few as six passages (data not shown). The mechanism by which the phenotype switches is not yet understood.
HfqBb is required for mouse infection by needle inoculation
Several bacterial species require Hfq for virulence (Chao and Vogel, 2010). We hypothesized that the hfqBb mutant strain would not be infectious in mice due to the absence of RpoS and OspC. We assayed the infectivity of the hfqBb mutant in the mouse model of Lyme disease by needle inoculation (Barthold et al., 2010). B. burgdorferi cultures were temperature-shifted to 37°C and grown to a low cell density (1–3 × 107 cells ml−1); C3H-HeJ female mice were needle inoculated with 5 × 103 cells i.p. Tissue samples were cultured and assayed for the presence of B. burgdorferi by dark-field microscopy; cultures that were negative by this method were also screened by PCR (Wang et al., 2010). The hfqBb mutant was unable to infect mice and complementation with hfq from B. burgdorferi, but not E. coli, restored infectivity (Table 1). Thus, hfqBb is essential for B. burgdorferi infection of mice by needle inoculation.
Table 1.
HfqBb is required for mouse infection by needle inoculation
| # of positive mice by culture | |||
|---|---|---|---|
| B. burgdorferi strain | Ear | Joint | Bladder |
| 297 (BbAH130) WT | 6/6 | 6/6 | 6/6 |
| 297 hfq− | 0/6 | 0/6 | 0/6 |
| 297 hfq− high passage | 0/3 | 0/3 | 0/3 |
| 297 hfq−/flacp-hfqBb | 3/3 | 3/3 | 3/3 |
| 297 hfq−/flacp-hfqEc | 0/3 | 0/3 | 0/3 |
The high-passage hfqBb mutant retained the plasmids essential for infectivity and was used to needle inoculate mice. RpoS and OspC are highly expressed in the high-passage hfqBb mutant, so we expected it to be infectious, but the strain did not infect the mice (Table 1). These data and the finding that the hfqBb mutant complemented with hfq from E. coli was avirulent, even though it expressed wild-type levels of RpoS, suggest HfqBb may play additional roles in mammalian infection besides regulation of RpoS.
Discussion
The RNA chaperone Hfq has emerged as an important global post-transcriptional regulator in mRNA translational control by sRNAs (Valentin-Hansen et al., 2004). In this study, we identified and characterized an unusual Hfq in B. burgdorferi. The HfqBb primary sequence does not have a high degree of similarity or identity to annotated Hfq homologs, but does retain a few key residues and was predicted to have a superimposable tertiary structure (Fig. 2). A BLAST search using full-length HfqBb, or the more highly conserved N-terminal region of HfqBb, as the query only identified hypothetical open reading frames within the spirochete phylum (data not shown). Hfq is characterized as a part of the Sm/Lsm protein family and these proteins form ring-shaped oligomeric structures that are homohexameric in bacteria and heteroheptameric in eukaryotes. Despite these differences, the Hfq and Sm/Lsm monomers fold into a similar structure, consisting of an N-terminal α-helix followed by a curved five-stranded β-sheet (Schumacher et al., 2002; Sauter et al., 2003). Our gel filtration data suggested that recombinant HfqBb does not form a stable hexamer in solution (Fig. 4), which is unlike most other characterized Hfq homologs. However, we hypothesize that the functional quaternary structure of HfqBb in vivo is a homohexamer, which is unstable under our experimental conditions. Possibly, our inability to observe a hexamer in vitro is due to the presence of a lysine residue rather than the conserved histidine at position 57 (in the E. coli sequence), which is known to be involved in stabilizing the hexameric structure (Moskaleva et al., 2010). Despite the differences in primary and quaternary structure between HfqBb and the canonical Hfq, our results clearly establish that HfqBb functions as an RNA chaperone as demonstrated by hfqBb complementing an rpoS translational defect in an hfq null mutant of E. coli (Fig. 3). Moreover, E. coli hfq complemented the hfqBb mutant phenotype with regard to growth rate (Fig. 7), cell length (Fig. 8), and RpoS and OspC induction (Fig. 9). This is the first time, to our knowledge, that a heterologous bacterial gene has complemented a B. burgdorferi mutant.
The hfqBb mutant has a phenotype that includes decreased growth rate, increased cell length, altered RpoS regulation, and loss of infectivity. The B. burgdorferi and E. coli hfq genes complemented the in vitro mutant phenotype demonstrating that the phenotype is due to the loss of hfqBb; these phenotypes have been observed in several hfq mutants of other bacteria (Brennan and Link, 2007; Chao and Vogel, 2010). RpoS is required for mammalian infection by B. burgdorferi, and, therefore, the hfqBb mutant was not expected to be infectious in the mouse model. Our findings established that HfqBb is required for mammalian infectivity, but that the mechanism is not solely due to the loss of RpoS induction. A high-passage hfqBb mutant strain that produces RpoS and OspC was still not infectious in mice (Table 1). These data demonstrated that HfqBb is required for infectivity in mice independent of RpoS and OspC expression. We hypothesize that there are other Hfq-dependent sRNAs in B. burgdorferi that regulate expression of virulence factors. In E. coli and Salmonella typhimurium, Hfq has other regulatory functions independent of its effects on RpoS expression (Muffler et al., 1997; Sittka et al., 2007). Hfq in the pathogenic bacteria S. typhimurium, Vibrio cholerae, and Brucella abortus is required for full virulence, but the mechanism is independent of the requirement of Hfq for RpoS expression (Robertson and Roop, 1999; Ding et al., 2004; Sittka et al., 2007).
We hypothesized that HfqBb regulates RpoS synthesis via the sRNA DsrABb and, thus, we expected the RpoS defect would be similar in the dsrABb mutant and hfqBb mutant strains. Our data demonstrated that HfqBb post-transcriptionally regulates the temperature-dependent increase in RpoS. Surprisingly, unlike the dsrABb mutant strain, which only shows reduced levels of RpoS at a low cell density (log-phase growth), the hfqBb mutant strain had reduced levels of RpoS at both low and high cell density (log and stationary growth phase, respectively), suggesting that HfqBb also plays a DsrABb-independent role in RpoS regulation.
In conclusion, we have identified and characterized an unusual Hfq protein in the Lyme disease spirochete B. burgdorferi that is essential for infectivity. Although only one sRNA has been identified to date in B. burgdorferi, the pleiotropic phenotype of the hfqBb mutant suggests other sRNAs exist and likely play a role in the enzootic life cycle.
Experimental procedures
B. burgdorferi strains and culture conditions
B. burgdorferi low-passage virulent strain 297 (clone BbAH130) (Hübner et al., 2001) and all derivatives used in this study were cultivated in Barbour-Stoenner-Kelly II (BSK-II) complete medium (Barbour, 1984) at either 23°C or 37°C. Cultures were passaged to 1 × 105 cells ml−1 from 23°C to 37°C and grown to either 1–3 × 107 cells ml−1 (low density), which corresponds to mid-log phase, or about 1 × 108 cells ml−1 (high density), which corresponds to stationary phase. For growth curves, cultures were grown at 23°C to 1–3 × 107 cells ml−1 and passaged to 1 × 103 cells ml−1 at 37°C. Cell density was determined using a Petroff Hausser counting chamber (Hausser Scientific Partnership) as previously described (Samuels, 1995). All cultures were counted in triplicate and at least three independent experiments were performed.
Sequence analysis and structural modeling
The ClustalW sequence alignment was performed with NPS@: Network Protein Sequence Analysis (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_clustalw.html) (Combet et al., 2000). A computational prediction of the HfqBb fold was performed using Protein Homology/analogY Recognition Engine (Phyre) (http://www.sbg.bio.ic.ac.uk/~phyre/) (Kelley and Sternberg, 2009). The resulting PDB coordinate file covering residues 6–71 was then visualized and overlaid with the structures of Hfq from E. coli (PDB 1HK9) (Sauter et al., 2003) and Hfq from S. aureus (PDB 1KQ2) (Schumacher et al., 2002) using Chimera (http://www.cgl.ucsf.edu/chimera/) (Pettersen et al., 2004).
E. coli strains and plasmid construction for hfq complementation
E. coli strain DDS1631 has an hfq null mutation and an rpoS::lacZ translational fusion (Sledjeski et al., 2001). The pBADHfq plasmid (pDDS400) carries the E. coli hfq gene cloned into the EcoRI and PstI sites of the arabinose-inducible vector pBAD24 (Sledjeski et al., 2001). The bb0268 and bb0260 genes were PCR-amplified using the proofreading enzyme KOD with engineered MfeI and PstI sites at the 5′ and 3′ ends, respectively (Table 2). After poly(A) tailing with Taq, the PCR products were cloned into pCR®2.1-TOPO. Positive transformants were screened by PCR and confirmed by DNA sequencing. The pBAD260 and pBAD268 plasmids were generated by directionally cloning the MfeI-PstI excised bb0268 and bb0260 fragments into the EcoRI and PstI sites of pBAD24.
Table 2.
Oligonucleotides used.
| Name | Sequence (5′-3′) | Function |
|---|---|---|
| BB268 1F+MfeI | ACAATTGACCATGTTTATAAGCAGGGAATTG | Complementation in E. coli |
| BB268 480R+PstI | ACTGCAGTTATTCCTTCTTGCTCATTAAAG | |
| BB260 1F+MfeI | ACAATTGACCATGAAAGGGTTTTTAGCG | |
| BB260 1014R+PstI | ACTGCAGTTAAACATATTGATCTTTTTC | |
| BB268 1F+NdeI | CATATGTTTATAAGCAGGGAATTG | Complementation, expression, and RT-PCR |
| BB268 480R+AatII | GACGTCTTATTCCTTCTTGCTCATTAAAG | |
| BB268 249R+AatII | GACGTCTTAACTCTTCTTATCATCTTG | RT-PCR |
| Echfq 1F+NdeI | CATATGGCTAAGGGGCAATCTTTAC | Complementation in B. burgdorferi |
| Echfq 310R+AatII | GACGTCTTATTCGGTTTCTTCGCTGTC | |
| BB268 U1048F | TTTCTTATGTTACGGATGGGC | Mutant construction |
| BB268 U4R+Aat+Age | ACCGGTCATGACGTCTTATTCCACACCAAAAAATC | |
| BB268 474F+AatII | GACGTCGGAATAAATATGGATGACAGGGC | |
| BB268 D1578+AgeI | ACCGGTTACTATATCTCCAGTAGCAGGTCC | |
| dsrA L1F+T7 | TAATACGACTCACTATAGGGAAAGCTAAATTAGAAGAGTTTATTG | Template for in vitro transcription |
| dsrA L275/352R | CATAAAAACTTTTTTTTGAATAG | |
| rpoS U449F+T7 | TAATACGACTCACTATAGGGTCACAAAATCTAAAATTTAAAAATC | |
| rpoS 128R | GTTTTTTGCTTTTGCATTGC | |
| rpoS 705R+T7 | TAATACGACTCACTATAGGCGGTGCTTTTTTTGGGACTATTG | Northern blot probes |
| rpoS 443F | GATTCAACCTATCTCCTGCTCAG | |
| rrp2 185F | GAGAAAAATTGCTCAAAATA | |
| rrp2 394R+T7 | TAATACGACTCACTATAGGCTTTTGAGTTTTTTCAAGAAG | |
| rpoN 285F | GCAATTAAGAATTCAAAGAA | |
| rpoN 572R+T7 | TAATACGACTCACTATAGGCTTTTGAGTTTTTTCAAGAAG | |
| flaA 64F | GCTCAAGAGACTGATGGATTAGC | |
| flaA 284R+T7 | TAATACGACTCACTATAGGCGCAGAAGGAGTAAGTAAAACGCTC |
E. coli Hfq complementation
β-galactosidase units were assayed using the Zhang and Bremer (1995) modification of the Miller assay (1972). Briefly, cells were grown with shaking at 30°C in lysogeny broth medium supplied with kanamycin and carbomycin in the presence or absence of 150 μM arabinose. The cultures were inoculated 1:100 from overnight cultures and samples were taken at an OD600 ranging from 0.4 to 1.0 (approximately log phase). The OD600 of each culture was measured and 30μl aliquots were removed and mixed with 70 μl of permeabilization solution [100 mM Na2HPO4, 20 mM KCl, 2 mM MgSO4, 0.8 mg ml−1 CTAB (hexadecyltrimethylammonium), 0.4 mg ml−1 sodium deoxycholate and 5.4 μl ml−1 2- mercaptoethanol] and stored at room temperature. The samples were incubated at 30°C for 30 min. The substrate solution [60 mM Na2HPO4, 40 mM NaH2PO4, 1 mg ml−1 o-nitrophenyl-β-D-galactoside (ONPG) and 2.7 μl ml−1 2-mercaptoethanol] was also incubated at 30°C for 30 min and 600 μl of substrate solution was added to each sample. After sufficient color developed, 700 μl of stop solution (1 M Na2CO3) was added to each sample and the time was recorded. The samples were centrifuged at 14,000 rpm in an Eppendorf 5417R for 5 min to remove cell particulates. The OD420 (between 0.05 and 1.0) was measured for each sample and the total β-galactosidase units were calculated from the formula:
The volumes of permeabilization solution and cell culture varied between experiments and were appropriately changed in the calculation of total β-galactosidase units.
Cloning and protein purification of Hfq
The hfqBb gene was amplified by PCR with the proofreading enzyme KOD, poly(A) tailed, and cloned into pCR®2.1-TOPO (Table 2). The hfqBb gene was directionally cloned into a derivative of pET28b with an N-terminal, tobacco etch virus protease-cleavable His6 tag (Corbett et al., 2004) using an engineered NdeI site at the 5′ end of the gene and the pCR®2. 1-TOPO vector BamHI site. The resulting plasmid pET28b-TEV::Hfq was sequenced and transformed into E. coli Rosetta (DE3) cells (Novagen). Cell cultures were grown at 37°C to an OD600 of 0.4–0.6, induced with 1 mM of IPTG and grown overnight at 11°C and again overnight at 18°C. Cells were lysed using Bugbuster (Novagen), filtered through a 0.45 μm filter and loaded directly onto 1 ml Ni2+ column (HisTrap HP, Amersham Biosciences). The resin was washed with ten column volumes of 20 mM NaH2PO4, 500 mM NaCl and 5 mM imidazole, pH 7.4, five column volumes of 1M NH4Cl, and five column volumes of 1M urea, and finally eluted with a 10-ml linear gradient of 10 to 500 mM imidazole, pH 7.4, in 20 mM NaH2PO4 and 500 mM NaCl. The fractions containing Hfq were dialyzed into a storage buffer (20 mM NaH2PO4 and 125 mM NaCl, pH 7.4) and concentrated in Amicon Ultra-4 spin columns. The concentration of the purified recombinant protein was determined with the BCA protein assay (Pierce). The E. coli Hfq protein was purified as previously described (Mikulecky et al., 2004).
Gel filtration
Recombinant purified HfqBb and E. coli Hfq were dialyzed overnight in 50 mM Tris-HCl, pH 7.5 and 100 mM KCl. Sigma gel filtration molecular weight markers were run on a Superdex 75 HR 10/30 column and a standard curve was plotted. The Hfq proteins were run on the column and the molecular mass was calculated using the standard curve.
Template preparation for in vitro transcription
B. burgdorferi strain 297 total DNA was used as a template in PCR with the T7 bacteriophage promoter added to the 5′ end of either the forward primer to generate sense RNAs for the EMSA or the reverse primer to generate antisense RNAs for probes in Northern blots (Table 2). The PCR products were visualized on an agarose gel stained with ethidium bromide. If a single product was visualized, the PCR product was precipitated with ammonium acetate and resuspended in DEPC-treated water. If more than one product was visible, the appropriate band was purified with the Qiagen gel purification kit following the manufacturer’s protocol and resuspended in DEPC-treated water.
In vitro synthesized RNA
DsrABb and rpoS RNAs for EMSAs were synthesized and radioactively labeled by incorporation of [α-32P]UTP during in vitro transcription using the T7-Megascript In Vitro Transcription kit (Ambion) according to the protocol suggested by the manufacturer. PCR products (described above) were used as templates for the in vitro transcription reactions. Reaction time was increased to overnight at 37°C to increase the yield of short products. Following in vitro transcription, the full-length RNA transcripts were resolved on a 6% polyacrylamide-7M urea gel and eluted from gel slices by incubating in elution buffer (20 mM Tris-HCL pH 7.5, 0.5 M ammonium acetate, 10 mM EDTA, 0.1% SDS) at 37°C overnight or 65°C for 2 h. The RNAs were purified and concentrated by an ammonium acetate precipitation and resuspended in 60 μl of DEPC-treated water. OD260 was used to determine the concentration of RNAs.
Electrophoretic mobility shift assay (EMSA)
The purified in vitro synthesized DsrABb and rpoS RNAs were incubated with increasing amounts of purified recombinant HfqBb in binding buffer (10 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 50 mM NaCl, and 50 mM KCl). 0.17 pmoles of DsrABb or rpoS RNA was mixed with 7 μl of the binding buffer, heated to 65°C for 10 min, and slowly cooled to room temperature. Two μl of serially diluted HfqBb was added to the RNA and incubated for 5 min at room temperature. The samples were mixed with 10 μl gel loading buffer II (Ambion), fractionated on a Novex® Pre-Cast 6% DNA retardation gel (Invitrogen) in 1X TBE at 100V for 2 h. The gel was dried, exposed to a phosphorimager screen, and visualized using a Fujifilm FLA-3000G PhosphorImager.
Gene disruption and complementation of hfqBb
The hfqBb (bb0268) gene was deleted and replaced with a kanamycin resistance cassette (Bono et al., 2000). Regions flanking the hfqBb ORF were amplified by PCR (Table 2). The 3′ end of the upstream flanking sequence and the 5′ end of the downstream flanking sequence were engineered with AatII sites for insertion of the kanamycin resistance cassette. The two flanking regions were cloned into pCR®2. 1-TOPO and ligated together to form a 2.1 kb target DNA fragment. The antibiotic resistance cassette was cloned into the synthetic AatII site. The plasmid was linearized with AhdI and electroporated into competent B. burgdorferi as previously described (Samuels, 1995). B. burgdorferi transformants were cloned in liquid BSK-II medium (Yang et al., 2004) containing kanamycin (200 μg ml−1) at 34°C and a 1.5% CO2 atmosphere. Transformants were screened by PCR with primers that flank the insertion site.
Two different plasmids were constructed to complement the hfqBb mutant strain. The E. coli or B. burgdorferi hfq genes were fused to the inducible flac promoter (Gilbert et al., 2007) and inserted into a modified pKFSS1 vector (Frank et al., 2003) carrying the lacI repressor gene fused to the constitutive flgB promoter in the multiple cloning site. The recombinant plasmids were electroporated into the kanamycin-resistant hfqBb mutant and selected in both streptomycin and kanamycin. The resulting transformants were assayed for the hfqBb expression plasmid by both PCR and xenodiagnosis (transformation of plasmid DNA from B. burgdorferi into E. coli).
RT-PCR
Total RNA was isolated from 100-ml cultures using TRIzol™ (Gibco BRL) as previously described (Lybecker and Samuels, 2007). RNA samples were treated with TurboDNase (Ambion) and RT-PCR was performed using the RETROscript™ kit (Ambion) according to manufacturer’s instructions, except a gene-specific primer was used in the reverse transcription reaction instead of random decamers (Table 2).
Microscopic analysis of B. burgdorferi
Cell cultures were grown at 37°C to a low cell density (1–3× 107 cells ml−1) and centrifuged at 5800 × g. Cell pellets were resuspended in 100 μl of water. Samples were visualized by differential interference contrast (DIC) microscopy using a Nikon E800 microscope at 100× . B. burgdorferi length was measured using ImageJ software. The length of hfqBb mutant cells do not display a normal distribution, so significance was evaluated using both parametric (one-way ANOVA with a Tukey’s post-hoc test and a two-tailed unpaired t-test) and non-parametric (Kruskal–Wallis one-way ANOVA and a Mann Whitney U test) methods.
SDS-PAGE and immunoblotting
B. burgdorferi protein extracts were prepared as previously described (Lybecker and Samuels, 2007). Protein extracts were fractionated in Novex® Pre-Cast 4–20% Tris-Glycine gels (Invitrogen) and transferred to Immobilon-P PVDF membranes (Millipore) by electroblotting. Proteins were detected with either an anti-RpoS antiserum (Yang et al., 2000) or anti-OspC antiserum (Yang et al., 2005). The membranes were developed by chemiluminescence using the ECL Plus Western Blotting detection system (Amersham Biosciences) on a Fujifilm LAS-3000 (Yang et al., 2005; Gilbert et al., 2007).
Northern blot analysis
Total RNA was isolated from 100-ml cultures using TRIzol™ (Gibco BRL) as previously described (Lybecker and Samuels, 2007). RNA samples were treated with TurboDNase (Ambion) and fractionated in a Novex® Pre-Cast 6% polyacrylamide 7 M urea gel (Invitrogen) according to the manufacturers’ instructions. The RNA was transferred to a BrightStar-Plus™ membrane by electroblotting using the Xcell SureLock™ Mini-Cell (Invitrogen) following the manufacturer’s specifications. Northern blot hybridizations were performed using the NorthernMax™ kit (Ambion) according to the manufacturer’s instructions. Non-isotopic RNA probes were generated from in vitro transcription of PCR-generated DNA templates using the MAXIscript® in vitro transcription kit (Ambion). Biotin-16-UTP (Roche) was used in the in vitro transcription reaction. Northern blot membranes were developed by chemiluminescence using the BrightStar® Biodetect™ nonisotopic detection kit on a Fujifilm LAS-3000.
Mouse infectivity studies
C3H-HeJ female mice were needle inoculated intraperitoneally (i.p) with 5 × 103 cells of wild-type 297 (BbAh130), hfqBb mutant, or complemented strains. All strains used in mouse infectivity studies were screened for the presence of plasmids essential for infectivity (lp28-1, lp25, and lp54) (Purser and Norris, 2000; Labandeira-Rey and Skare, 2001). Three weeks post injection, ear punches were taken and cultured in BSK-II. Five weeks post injection, mice were sacrificed and ear punches, tibiotarsal joints and bladders were collected and cultivated in BSK-II. Cultures were screened for the presence and growth of B. burgdorferi by darkfield microscopy. In addition, PCR was used to screen the cultures that appeared negative by dark-field microscopy. The University of Montana Institutional Animal Care and Use Committee approved all experimental procedures involving mice (protocol number 038-08SSDBS-081208).
Acknowledgments
We thank Dan Drecktrah, Steve Lodmell, and Jörg Vogel for thoughtful and critical reading of the manuscript; Dan Drecktrah for help with image analysis; Jesse Hay for the use of his microscope; James Berger, Kevin Corbett, Mike Norgard, Craig Sampson (and the CDC Bacterial Zoonoses Branch), Darren Sledjeski, Philip Stewart, and Frank Yang for strains, plasmids, and/or antibodies; Dan Drecktrah, Jon Graham, Jean-Marc Lanchy, Steve Lodmell, Michele McGuirl, and Jörg Vogel for useful discussions; the LAR staff for assistance with animal experiments; Nilshad Salim and Tessa Samuels for logistics; Patty McIntire (Murdock DNA Sequencing Facility) for DNA sequencing; and Laura Hall and Kelsey Lau for excellent technical assistance. C.A.A. was supported by a Watkins Scholarship from The University of Montana, an Undergraduate Research Award from the Davidson Honors College, a MILES Honors Fellowship through a grant from the Howard Hughes Medical Institute, and funding from the NSF EPSCoR Undergraduate Research Program. This work was supported by grants from the National Institutes of Health (AI051486 to D.S.S. and GM075068 to A.L.F.).
References
- Achsel T, Brahms H, Kastner B, Bachi A, Wilm M, Lührmann R. A doughnut-shaped heteromer of human Sm-like proteins binds to the 3′-end of U6 snRNA, thereby facilitating U4/U6 duplex formation in vitro. EMBO J. 1999;18:5789–5802. doi: 10.1093/emboj/18.20.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achsel T, Stark H, Lührmann R. The Sm domain is an ancient RNA-binding motif with oligo(U) specificity. Proc Natl Acad Sci USA. 2001;98:3685–3689. doi: 10.1073/pnas.071033998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alverson J, Bundle SF, Sohaskey CD, Lybecker MC, Samuels DS. Transcriptional regulation of the ospAB and ospC promoters from Borrelia burgdorferi. Mol Microbiol. 2003;48:1665–1677. doi: 10.1046/j.1365-2958.2003.03537.x. [DOI] [PubMed] [Google Scholar]
- Antal M, Bordeau V, Douchin V, Felden B. A small bacterial RNA regulates a putative ABC transporter. J Biol Chem. 2005;280:7901–7908. doi: 10.1074/jbc.M413071200. [DOI] [PubMed] [Google Scholar]
- Arluison V, Derreumaux P, Allemand F, Folichon M, Hajnsdorf E, Régnier P. Structural modelling of the Sm-like protein Hfq from Escherichia coli. J Mol Biol. 2002;320:705–712. doi: 10.1016/s0022-2836(02)00548-x. [DOI] [PubMed] [Google Scholar]
- Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, Cadavid D, Philipp MT. Animal models of borreliosis. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 359–411. [Google Scholar]
- Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Bast TF, Habicht GS, et al. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Blevins JS, Xu H, He M, Norgard MV, Reitzer L, Yang XF. Rrp2, a σ54-dependent transcriptional activator of Borrelia burgdorferi, activates rpoS in an enhancer-independent manner. J Bacteriol. 2009;191:2902–2905. doi: 10.1128/JB.01721-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono JL, Elias AF, Kupko JJ, III, Stevenson B, Tilly K, Rosa P. Efficient targeted mutagenesis in Borrelia burgdorferi. J Bacteriol. 2000;182:2445–2452. doi: 10.1128/jb.182.9.2445-2452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan RG, Link TM. Hfq structure, function and ligand binding. Curr Opin Microbiol. 2007;10:125–133. doi: 10.1016/j.mib.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Brescia CC, Mikulecky PJ, Feig AL, Sledjeski DD. Identification of the Hfq-binding site on DsrA RNA: Hfq binds without altering DsrA secondary structure. RNA. 2003;9:33–43. doi: 10.1261/rna.2570803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L, Elliott T. Mutations that increase expression of the rpoS gene and decrease its dependence on hfq function in Salmonella typhimurium. J Bacteriol. 1997;179:656–662. doi: 10.1128/jb.179.3.656-662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease–a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Burtnick MN, Downey JS, Brett PJ, Boylan JA, Frye JG, Hoover TR, Gherardini FC. Insights into the complex regulation of rpoS in Borrelia burgdorferi. Mol Microbiol. 2007;65:277–293. doi: 10.1111/j.1365-2958.2007.05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Eggers CH, Hazlett KR, Radolf JD. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect Immun. 2004;72:6433–6445. doi: 10.1128/IAI.72.11.6433-6445.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Eggers CH, Morton EA, Gilbert MA, Schwartz I, Radolf JD. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol. 2007;65:1193–1217. doi: 10.1111/j.1365-2958.2007.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y, Vogel J. The role of Hfq in bacterial pathogens. Curr Opin Microbiol. 2010;13:24–33. doi: 10.1016/j.mib.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Combet C, Blanchet C, Geourjon C, Deléage G. NPS@: network protein sequence analysis. Trends Biochem Sci. 2000;25:147–150. doi: 10.1016/s0968-0004(99)01540-6. [DOI] [PubMed] [Google Scholar]
- Corbett KD, Shultzaberger RK, Berger JM. The C-terminal domain of DNA gyrase A adopts a DNA-bending β-pinwheel fold. Proc Natl Acad Sci USA. 2004;101:7293–7298. doi: 10.1073/pnas.0401595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Davis BM, Waldor MK. Hfq is essential for Vibrio cholerae virulence and downregulates σE expression. Mol Microbiol. 2004;53:345–354. doi: 10.1111/j.1365-2958.2004.04142.x. [DOI] [PubMed] [Google Scholar]
- Fingerle V, Laux H, Munderloh UG, Schulte-Spechtel U, Wilske B. Differential expression of outer surface proteins A and C by individual Borrelia burgdorferi in different genospecies. Med Microbiol Immunol. 2000;189:59–66. doi: 10.1007/pl00008257. [DOI] [PubMed] [Google Scholar]
- Folichon M, Arluison V, Pellegrini O, Huntzinger E, Régnier P, Hajnsdorf E. The poly(A) binding protein Hfq protects RNA from RNase E and exoribonucleolytic degradation. Nucleic Acids Res. 2003;31:7302–7310. doi: 10.1093/nar/gkg915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank KL, Bundle SF, Kresge ME, Eggers CH, Samuels DS. aadA confers streptomycin resistance in Borrelia burgdorferi. J Bacteriol. 2003;185:6723–6727. doi: 10.1128/JB.185.22.6723-6727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franze de Fernandez MT, Eoyang L, August JT. Factor fraction required for the synthesis of bacteriophage Qβ-RNA. Nature. 1968;219:588–590. doi: 10.1038/219588a0. [DOI] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, et al. Genomic sequence of a Lyme disease spirochete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- Fröhlich KS, Vogel J. Activation of gene expression by small RNA. Curr Opin Microbiol. 2009;12:674–682. doi: 10.1016/j.mib.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Geissmann TA, Touati D. Hfq, a new chaperoning role: binding to messenger RNA determines access for small RNA regulator. EMBO J. 2004;23:396–405. doi: 10.1038/sj.emboj.7600058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MA, Morton EA, Bundle SF, Samuels DS. Artificial regulation of ospC expression in Borrelia burgdorferi. Mol Microbiol. 2007;63:1259–1273. doi: 10.1111/j.1365-2958.2007.05593.x. [DOI] [PubMed] [Google Scholar]
- Gottesman S. The small RNA regulators of Escherichia coli: roles and mechanisms. Annu Rev Microbiol. 2004;58:303–328. doi: 10.1146/annurev.micro.58.030603.123841. [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R. Signal transduction and regulatory mechanisms involved in control of the σS (RpoS) subunit of RNA polymerase. Microbiol Mol Biol Rev. 2002;66:373–395. doi: 10.1128/MMBR.66.3.373-395.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc Natl Acad Sci USA. 2001;98:12724–12729. doi: 10.1073/pnas.231442498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambach C, Walke S, Young R, Avis JM, de la Fortelle E, Raker VA, et al. Crystal structures of two Sm protein complexes and their implications for the assembly of the spliceosomal snRNPs. Cell. 1999;96:375–387. doi: 10.1016/s0092-8674(00)80550-4. [DOI] [PubMed] [Google Scholar]
- Kawamoto H, Koide Y, Morita T, Aiba H. Base-pairing requirement for RNA silencing by a bacterial small RNA and acceleration of duplex formation by Hfq. Mol Microbiol. 2006;61:1013–1022. doi: 10.1111/j.1365-2958.2006.05288.x. [DOI] [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- Klinkert B, Narberhaus F. Microbial thermosensors. Cell Mol Life Sci. 2009;66:2661–2676. doi: 10.1007/s00018-009-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labandeira-Rey M, Skare JT. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28–1. Infect Immun. 2001;69:446–455. doi: 10.1128/IAI.69.1.446-455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RS, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- Lease RA, Belfort M. Riboregulation by DsrA RNA: trans-actions for global economy. Mol Microbiol. 2000;38:667–672. doi: 10.1046/j.1365-2958.2000.02162.x. [DOI] [PubMed] [Google Scholar]
- Lease RA, Woodson SA. Cycling of the Sm-like protein Hfq on the DsrA small regulatory RNA. J Mol Biol. 2004;344:1211–1223. doi: 10.1016/j.jmb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Lee T, Feig AL. The RNA binding protein Hfq interacts specifically with tRNAs. RNA. 2008;14:514–523. doi: 10.1261/rna.531408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link TM, Valentin-Hansen P, Brennan RG. Structure of Escherichia coli Hfq bound to polyriboadenylate RNA. Proc Natl Acad Sci USA. 2009;106:19292–19297. doi: 10.1073/pnas.0908744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lybecker MC, Samuels DS. Temperature-induced regulation of RpoS by a small RNA in Borrelia burgdorferi. Mol Microbiol. 2007;64:1075–1089. doi: 10.1111/j.1365-2958.2007.05716.x. [DOI] [PubMed] [Google Scholar]
- Majdalani N, Vanderpool CK, Gottesman S. Bacterial small RNA regulators. Crit Rev Biochem Mol Biol. 2005;40:93–113. doi: 10.1080/10409230590918702. [DOI] [PubMed] [Google Scholar]
- Massé E, Escorcia FE, Gottesman S. Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev. 2003a;17:2374–2383. doi: 10.1101/gad.1127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci USA. 2002;99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé E, Majdalani N, Gottesman S. Regulatory roles for small RNAs in bacteria. Curr Opin Microbiol. 2003b;6:120–124. doi: 10.1016/s1369-5274(03)00027-4. [DOI] [PubMed] [Google Scholar]
- Mikulecky PJ, Kaw MK, Brescia CC, Takach JC, Sledjeski DD, Feig AL. Escherichia coli Hfq has distinct interaction surfaces for DsrA, rpoS and poly(A) RNAs. Nat Struct Mol Biol. 2004;11:1206–1214. doi: 10.1038/nsmb858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor; New York: 1972. [Google Scholar]
- Moll I, Afonyushkin T, Vytvytska O, Kaberdin VR, Bläsi U. Coincident Hfq binding and RNase E cleavage sites on mRNA and small regulatory RNAs. RNA. 2003a;9:1308–1314. doi: 10.1261/rna.5850703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll I, Leitsch D, Steinhauser T, Bläsi U. RNA chaperone activity of the Sm-like Hfq protein. EMBO Rep. 2003b;4:284–289. doi: 10.1038/sj.embor.embor772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller T, Franch T, Højrup P, Keene DR, Bächinger HP, Brennan RG, Valentin-Hansen P. Hfq: a bacterial Sm-like protein that mediates RNA-RNA interaction. Mol Cell. 2002;9:23–30. doi: 10.1016/s1097-2765(01)00436-1. [DOI] [PubMed] [Google Scholar]
- Moskaleva O, Melnik B, Gabdulkhakov A, Garber M, Nikonov S, Stolboushkina E, Nikulin A. The structures of mutant forms of Hfq from Pseudomonas aeruginosa reveal the importance of the conserved His57 for the protein hexamer organization. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66:760–764. doi: 10.1107/S1744309110017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muffler A, Traulsen DD, Fischer D, Lange R, Hengge-Aronis R. The RNA-binding protein HF-I plays a global regulatory role which is largely, but not exclusively, due to its role in expression of the σS subunit of RNA polymerase in Escherichia coli. J Bacteriol. 1997;179:297–300. doi: 10.1128/jb.179.1.297-300.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muffler A, Traulsen DD, Lange R, Hengge-Aronis R. Posttranscriptional osmotic regulation of the σS subunit of RNA polymerase in Escherichia coli. J Bacteriol. 1996;178:1607–1613. doi: 10.1128/jb.178.6.1607-1613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narberhaus F, Waldminghaus T, Chowdhury S. RNA thermometers. FEMS Microbiol Rev. 2006;30:3–16. doi: 10.1111/j.1574-6976.2005.004.x. [DOI] [PubMed] [Google Scholar]
- Nielsen JS, Bøggild A, Andersen CBF, Nielsen G, Boysen A, Brodersen DE, Valentin-Hansen P. An Hfq-like protein in archaea: crystal structure and functional characterization of the Sm protein from Methanococcus jannaschii. RNA. 2007;13:2213–2223. doi: 10.1261/rna.689007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojaimi C, Brooks C, Casjens S, Rosa P, Elias A, Barbour A, et al. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect Immun. 2003;71:1689–1705. doi: 10.1128/IAI.71.4.1689-1705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Piesman J, Schwan TG. Ecology of borreliae and their arthropod vectors. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 251–278. [Google Scholar]
- Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci USA. 2000;97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Salazar JC, Dattwyler RJ. Lyme disease in humans. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 487–533. [Google Scholar]
- Repoila F, Majdalani N, Gottesman S. Small non-coding RNAs, co-ordinators of adaptation processes in Escherichia coli: the RpoS paradigm. Mol Microbiol. 2003;48:855–861. doi: 10.1046/j.1365-2958.2003.03454.x. [DOI] [PubMed] [Google Scholar]
- Revel AT, Talaat AM, Norgard MV. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc Natl Acad Sci USA. 2002;99:1562–1567. doi: 10.1073/pnas.032667699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson GT, Roop RM., Jr The Brucella abortus host factor I (HF-I) protein contributes to stress resistance during stationary phase and is a major determinant of virulence in mice. Mol Microbiol. 1999;34:690–700. doi: 10.1046/j.1365-2958.1999.01629.x. [DOI] [PubMed] [Google Scholar]
- Romby P, Vandenesch F, Wagner EG. The role of RNAs in the regulation of virulence-gene expression. Curr Opin Microbiol. 2006;9:229–236. doi: 10.1016/j.mib.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Samuels DS. Electrotransformation of the spirochete Borrelia burgdorferi. In: Nickoloff JA, editor. Electroporation Protocols for Microorganisms. Totowa, New Jersey: Humana Press; 1995. pp. 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS, Radolf JD. Who is the BosR around here anyway? Mol Microbiol. 2009;74:1295–1299. doi: 10.1111/j.1365-2958.2009.06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter C, Basquin J, Suck D. Sm-like proteins in Eubacteria: the crystal structure of the Hfq protein from Escherichia coli. Nucleic Acids Res. 2003;31:4091–4098. doi: 10.1093/nar/gkg480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Pearson RF, Møller T, Valentin-Hansen P, Brennan RG. Structures of the pleiotropic translational regulator Hfq and an Hfq-RNA complex: a bacterial Sm-like protein. EMBO J. 2002;21:3546–3556. doi: 10.1093/emboj/cdf322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serganov A, Patel DJ. Ribozymes, riboswitches and beyond: regulation of gene expression without proteins. Nat Rev Genet. 2007;8:776–790. doi: 10.1038/nrg2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Girschick HJ. Molecular survival strategies of the Lyme disease spirochete Borrelia burgdorferi. Lancet Infect Dis. 2004;4:575–583. doi: 10.1016/S1473-3099(04)01132-6. [DOI] [PubMed] [Google Scholar]
- Sittka A, Pfeiffer V, Tedin K, Vogel J. The RNA chaperone Hfq is essential for the virulence of Salmonella typhimurium. Mol Microbiol. 2007;63:193–217. doi: 10.1111/j.1365-2958.2006.05489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare JT, Carroll JA, Yang XF, Samuels DS, Akins DR. Gene regulation, transcriptomics and proteomics. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 67–101. [Google Scholar]
- Sledjeski DD, Whitman C, Zhang A. Hfq is necessary for regulation by the untranslated RNA DsrA. J Bacteriol. 2001;183:1997–2005. doi: 10.1128/JB.183.6.1997-2005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman A. The emergence of Lyme disease and human babesiosis in a changing environment. Ann N Y Acad Sci. 1994;740:146–156. doi: 10.1111/j.1749-6632.1994.tb19865.x. [DOI] [PubMed] [Google Scholar]
- Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Stevenson B, Schwan TG, Rosa PA. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G, Opdyke JA, Zhang A. Controlling mRNA stability and translation with small, noncoding RNAs. Curr Opin Microbiol. 2004;7:140–144. doi: 10.1016/j.mib.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhulin I, Wartell RM. Predicted structure and phyletic distribution of the RNA-binding protein Hfq. Nucleic Acids Res. 2002;30:3662–3671. doi: 10.1093/nar/gkf508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törö I, Basquin J, Teo-Dreher H, Suck D. Archaeal Sm proteins form heptameric and hexameric complexes: crystal structures of the Sm1 and Sm2 proteins from the hyperthermophile Archaeoglobus fulgidus. J Mol Biol. 2002;320:129–142. doi: 10.1016/S0022-2836(02)00406-0. [DOI] [PubMed] [Google Scholar]
- Törö I, Thore S, Mayer C, Basquin J, Séraphin B, Suck D. RNA binding in an Sm core domain: X-ray structure and functional analysis of an archaeal Sm protein complex. EMBO J. 2001;20:2293–2303. doi: 10.1093/emboj/20.9.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui HCT, Feng G, Winkler ME. Negative regulation of mutS and mutH repair gene expression by the Hfq and RpoS global regulators of Escherichia coli K-12. J Bacteriol. 1997;179:7476–7487. doi: 10.1128/jb.179.23.7476-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui HCT, Leung HC, Winkler ME. Characterization of broadly pleiotropic phenotypes caused by an hfq insertion mutation in Escherichia coli K-12. Mol Microbiol. 1994;13:35–49. doi: 10.1111/j.1365-2958.1994.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P, Eriksen M, Udesen C. The bacterial Sm-like protein Hfq: a key player in RNA transactions. Mol Microbiol. 2004;51:1525–1533. doi: 10.1111/j.1365-2958.2003.03935.x. [DOI] [PubMed] [Google Scholar]
- Večerek B, Moll I, Bläsi U. Translational autocontrol of the Escherichia coli hfq RNA chaperone gene. RNA. 2005;11:976–984. doi: 10.1261/rna.2360205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Večerek B, Moll I, Bläsi U. Control of Fur synthesis by the non-coding RNA RyhB and iron-responsive decoding. EMBO J. 2007;26:965–975. doi: 10.1038/sj.emboj.7601553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytvytska O, Moll I, Kaberdin VR, von Gabain A, Bläsi U. Hfq (HF1) stimulates ompA mRNA decay by interfering with ribosome binding. Genes Dev. 2000;14:1109–1118. [PMC free article] [PubMed] [Google Scholar]
- Wang G, Aguero-Rosenfeld ME, Wormser GP, Schwartz I. Detection of Borrelia burgdorferi. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 443–466. [Google Scholar]
- Waters LS, Storz G. Regulatory RNAs in bacteria. Cell. 2009;136:615–628. doi: 10.1016/j.cell.2009.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Goldberg MS, Popova TG, Schoeler GB, Wikel SK, Hagman KE, Norgard MV. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol Microbiol. 2000;37:1470–1479. doi: 10.1046/j.1365-2958.2000.02104.x. [DOI] [PubMed] [Google Scholar]
- Yang XF, Alani SM, Norgard MV. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc Natl Acad Sci USA. 2003;100:11001–11006. doi: 10.1073/pnas.1834315100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Lybecker MC, Pal U, Alani SM, Blevins J, Revel AT, et al. Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. J Bacteriol. 2005;187:4822–4829. doi: 10.1128/JB.187.14.4822-4829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med. 2004;199:641–648. doi: 10.1084/jem.20031960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Wassarman KM, Ortega J, Steven AC, Storz G. The Sm-like Hfq protein increases OxyS RNA interaction with target mRNAs. Mol Cell. 2002;9:11–22. doi: 10.1016/s1097-2765(01)00437-3. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bremer H. Control of the Escherichia coli rrnB P1 promoter strength by ppGpp. J Biol Chem. 1995;270:11181–11189. doi: 10.1074/jbc.270.19.11181. [DOI] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:1–10. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]