Abstract
Background and Purpose
HIV-associated dementia (HAD) has been extensively studied using magnetic resonance spectroscopy (MRS) at field strengths of 1.5 Tesla (T). Higher magnetic field strengths (such as 3T) allow for more reliable determination of certain compounds, such as glutamate (Glu) and glutamine (Gln). The current study was undertaken to investigate the utility of 3T MRS for evaluating HIV+ patients with different levels of cognitive impairment with emphasis on the measurement of Glu and Glx (the sum of Glu and Gln).
Methods
Eighty six HIV+ subjects were evaluated at 3T using quantitative short echo time single voxel MRS of frontal white matter (FWM) and basal ganglia (BG). Subjects were divided into 3 groups according to the Memorial Sloan Kettering (MSK) HIV dementia stage: 21 had normal cognition (NC) (MSK 0), 31 had mild cognitive impairment (MCI) without dementia, (clinical MSK stage = 0.5) and 34 had dementia (HAD) (MSK > 1). HIV+ subjects had also undergone standardized cognitive testing covering the domains of executive function, verbal memory, attention, information processing speed, and motor and psychomotor speed. Between group differences in metabolite levels in FWM and BG were evaluated using ANOVA. Pearson correlation coefficients were used to explore the associations between the Glu and Glx metabolites and neurocognitive (NP) results.
Results
FWM Glx (combined Glu and Gln) was lower in HAD (8.1±2.1 mM) compared to both the MCI (9.17±2.1 mM) and NC groups (10.0±1.6 mM), (P = 0.006). FWM myo-inositol (mI) was higher in HAD (4.15±0.75 mM) compared to both MCI (3.86±0.85 mM) and NC status (3.4±0.67 mM), (P = 0.006). FWM Glx/Creatine (Cr) was lower and FWM mI/Cr significantly higher in the HAD compared to MCI and NC groups (P = 0.01) and (P = 0.004) respectively. BG NAA was lower in the HAD group (6.79±1.53 mM), compared to the MCI (7.5±1.06 mM), and NC groups (7.6±1.01 mM), (P = 0.036). Significant negative correlations were observed between Glu, Glx, and NAA concentrations with Trail-making test B (P= 0.006, 0.0001, and 0.007 respectively) and significant positive correlation was found with Digit symbol test (P= 0.02, 0.002, and 0.008 respectively). FWM Glx and NAA concentrations showed negative correlation with Grooved pegboard non-dominant hand (P= 0.02 and 0.04 respectively).
Conclusion
Patients with HAD have lower levels of Glx concentrations and Glx/Cr ratio in FWM, which was associated with impaired performance in specific cognitive domains, including executive functioning, fine motor, attention and working memory performance. 3T MRS measurements of Glx may be a useful indicator of neuronal loss/dysfunction in patients with HIV infection.
Keywords: HIV Dementia, MR spectroscopy, Glutamate, Glx
Introduction
Human immunodeficiency virus (HIV) infection is commonly associated with neurological disease that occurs in the absence of extensive infection of neurons by HIV, suggesting that indirect mechanisms account for neuropathogenesis in the CNS. Viral replication occurs primarily in macrophages and microglia, resulting in macrophage/microglial activation, and perhaps changing the normal, neuroprotective functions of astrocytes (1). Histological and neuropathological studies have demonstrated reduced neuronal density in several cortical regions (2, 3), including neuronal loss and damaged neuronal structures in the frontal cortex (4, 5) in patients with HIV encephalitis. Despite frequent detection of the viral genome and proteins in the brains of HIV patients with and without HIV-associated dementia (HAD), only 5-10% of patients develop dementia (6).
Proton magnetic resonance spectroscopy is a non-invasive technique that indirectly gives information on brain pathophysiology through measurement of brain metabolite levels (7). The most common observations in patients with HIV are increased levels of choline (Cho) (8) and myo-inositol (mI) (9), thought to reflect glial cell proliferation, and decreased levels of N-acetyl aspartate (NAA), believed to be due to neuronal loss or metabolic dysfunction (8, 10-12). Metabolic abnormalities may be observed in regions of the brain with normal appearance on conventional MRI, even in subjects who are neurologically asymptomatic, and increase with increasing degrees of neurological involvement (13). In addition, metabolic abnormalities in mI and Cho have been reported to be reversible following antiretroviral therapy (9). For these reasons, MRS has been suggested to be a suitable tool of monitoring the degree of HIV involvement in the brain, and the effects of therapy (14, 15).
Most published MRS studies of HIV infection have used the conventional clinical MRI magnetic field strength of 1.5 Tesla (T). At higher magnetic field strengths (such as 3T, or above), and in combination with phased-array receiver coils (16, 17), increased sensitivity and chemical shift dispersion are expected to provide more reliable determination of metabolite concentrations, particularly for coupled spin systems (such as glutamate (Glu) and glutamine (Gln)) which are difficult to quantify at 1.5T (18). The current study was therefore undertaken to investigate the utility of 3T MRS in the evaluation of HIV patients with a range of neurological involvement, and to investigate the relationship of brain metabolite signals with neuropsychological (NP) test performance.
Material and Methods
Patients
Eighty six HIV+ subjects (59 male and 27 female; age: mean 46.9 ±5.8, range 31-60 years), who were undergoing highly active antiretroviral treatment (HAART) therapy, gave informed consent to participate in the study, which was conducted with the approval of the local institutional human research board (IRB). They underwent detailed neurological, neuropsychological, lab and functional assessments as described previously (19).
MR Spectroscopy
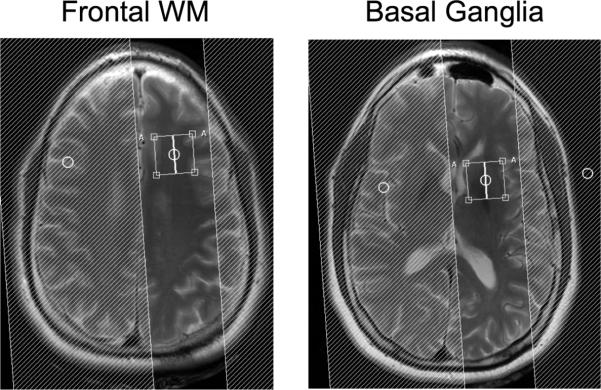
MR imaging and spectroscopy was performed on a 3T Philips Intera scanner with a 6-channel SENSE-head coil for signal reception. Spatial localization for MRS was achieved using the point-resolved spectroscopy sequence (PRESS), as well as 4 outer-volume suppression bands Single voxel spectra (TR/TE=2000/45 ms) were acquired from the left frontal white matter (FWM) and the left basal ganglia (BG) with and without water suppression. Voxel locations, indicated in Figure 1, were chosen on the basis of a prior MR spectroscopic imaging study which showed greatest involvement on the frontal lobe (12). Prior to data acquisition, field homogeneity was optimized using shim coils up to 2nd order. Water suppression was achieved using 3 frequency selective ‘CHESS’ pre saturation pulses of 120 Hz bandwidth. The voxel size was 2.2×2.2×2.2 cm3 and 128 averages were acquired with a total scan time of approximately 4.18 minutes. Representative spectra from two subjects are shown in Figure 2.
1.
PRESS voxel locations (2.2 cm in each dimension) used for MRS in the left frontal lobe white matter and basal ganglia, respectively. Shaded areas indicate locations of outer volume suppression pulses.
2.
Representative Spectra from the frontal lobe and basal ganglia in two HIV-positive subjects with MSK 0 (normal cognition) and MSK 1 (HIV dementia) respectively. NAA = N-acetyl aspartate, mI = myo-Inositol, Cho = choline, Cr = creatine, Glx = combined glutamate (glu) and glutamine (Gln). Spectra from the basal ganglia show increased line widths compared to frontal white matter, because of the high iron content of the basal ganglia.
Spectra were analyzed using the ‘LCModel’ algorithm (20) and quantified in millimole (mM) concentrations using the unsuppressed water signal as an internal intensity reference. In addition, metabolite ratios relative to creatine (Cr) were calculated. The following compounds were analyzed: NAA, Cho, Cr, mI, Glu, and Gln. In addition to reporting the individual values of Glu and Gln, the LCModel also reported ‘Glx’, the sum of Glu and Gln. Concentration and ratio values were only included if their Cramér-Rao lower bounds (CRB) were 20% or less (20). Group differences of the metabolite concentrations and ratios were evaluated using ANOVA. The level of statistical significance was set at P < 0.05. Pearson correlations were performed between the metabolites and NP tests.
Results
Based on clinical, functional, and neuropsychological assessment, the subjects were stratified into 3 groups: 21 were HIV+ with normal cognition (NC) (clinical Memorial Sloan Kettering (MSK) HIV dementia stage= 0), 31 with MCI without dementia [asymptomatic neurocognitive impairment (ANI) and mild neurocognitive disorder (MND)], (clinical MSK stage = 0.5) and 34 with HIV dementia (HAD) (clinical MSK stage >1). Patient demographics are given in Table 1; there were no significant differences in age, gender, level of education, race, CD4 cell count, or plasma or cerebrospinal fluid (CSF) HIV RNA between groups.
Table 1.
Patients’ demographic characteristics. Three HIV+ groups: Normal cognition (NC), mild cognitive impairment (MCI) and HIV associated dementia (HAD). N= total number of patients in each group. Values given are means ± standard deviation. n (%) is number and percentage in each group.
| HIV+ (NC) (N= 21) | MCI (N= 31) | HAD (N= 34) | P value (ANOVA) | |
|---|---|---|---|---|
| Age, years | 46.1 ± 5.7 | 47.4 ± 5.5 | 47.0 ± 6.3 | 0.75 |
| Male, n (%) | 14 (66 %) | 22 (71 %) | 23 (68 %) | 0.9 |
| Education, years | 11.9 ± 1.7 | 12.5 ± 1.9 | 12.6 ± 2.1 | 0.4 |
| Race (% African-American) | 18 (86 %) | 30 (97 %) | 32 (94 %) | 0.3 |
| CD4 cell count (cells/mm3) | 364 ± 188 | 369 ± 223 | 417 ± 239 | 0.6 |
| Plasma HIV RNA (Log10 copies/ml) | 3.0 ± 1.0 | 2.7 ± 1.4 | 3.4 ± 1.3 | 0.4 |
| CSF HIV RNA (Log10 copies/ml) | 0.68 ± 0.95 | 1.3 ± 1.4 | 1.3 ± 1.3 | 0.4 |
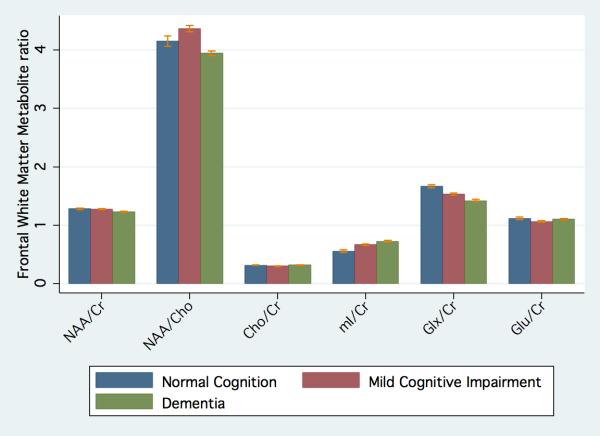
FWM metabolite concentrations and ratios are given in table 2 and figure 3, and those of the BG are in table 3. In the FWM, Glx was significantly lower in HAD (8.1±2.1 mM) as compared to both the MCI group (9.17±2.1 mM) and the NC group (10.0±1.6 mM) (P=0.006). There was a significant decrease in FWM Glx/Cr in the HAD group as compared to the MCI group and the NC group (P=0.01). FWM Glu and Gln concentrations separately were lowest in HAD; however, it did not reach statistical significance. Furthermore, there was a significant increase in FWM mI and mI/Cr ratio in the HAD group (P=0.006 and 0.004 respectively) as compared to the NC and MCI groups. FWM NAA tended to be lower in the HAD group (7.1 ± 0.93 mM) as compared to the MCI group (7.6 ± 1.04 mM) and the NC group (7.67± 0.93 mM); however, it did not reach statistical significance (P= 0.06).
Table 2.
Metabolites concentrations (millimole (mM)) and ratios mean ± standard deviation, in FWM.
| HIV+ (NC) (N= 21) | MCI (N= 31) | HAD (N=34) | P value (ANOVA) | Post-hoc NC versus MCI | Post-hoc NC versus HAD | Post-hoc MCI versus HAD | |
|---|---|---|---|---|---|---|---|
| mI (*) | 3.4 ± 0.67 | 3.86 ± 0.85 | 4.15±0.75 | 0.006 | ns | <0.05 | ns |
| Cho | 1.9 ± 0.39 | 1.78 ± 0.32 | 1.83 ± 0.32 | ns | ns | ns | ns |
| Cr | 6.04 ± 0.79 | 5.96 ± 0.8 | 5.79 ± 0.62 | ns | ns | ns | ns |
| Glu | 6.68 ± 1.2 | 6.3 ± 1.0 | 6.3 ± 1.1 | ns | ns | ns | ns |
| Gln | 4.22 ± 0.4 | 4.6 ± 1.0 | 3.82±0.21 | ns | sn | ns | ns |
| Glx (*) | 10.0 ± 1.62 | 9.17 ± 2.1 | 8.18 ± 2.1 | 0.006 | ns | <0.05 | ns |
| NAA(*) | 7.67 ± 0.93 | 7.6 ± 1.04 | 7.1 ± 0.93 | 0.06 | ns | ns | ns |
| mI/Cr (*) | 0.56 ± 0.19 | 0.67 ± 0.19 | 0.73 ± 0.15 | 0.004 | ns | <0.05 | ns |
| Cho/Cr | 0.32 ± 0.05 | 0.29 ± 0.04 | 0.32 ± 0.06 | ns | ns | ns | ns |
| Glu/Cr | 1.12 ± 0.2 | 1.06 ± 0.15 | 1.1 ± 0.2 | ns | ns | ns | ns |
| Glx/Cr (*) | 1.67 ± 0.24 | 1.53 ± 0.26 | 1.42 ± 0.34 | 0.01 | ns | <0.05 | ns |
| NAA/Cr | 1.28 ± 0.15 | 1.28 ± 0.09 | 1.23 ± 0.11 | ns | ns | ns | ns |
FWM = frontal white matter, Cho = choline, Cr = creatine, Glx = combined glutamate (Glu) and glutamine (Gln), NAA = sum of N-acetyl aspartate and N-acetyl aspartyl glutamate, mI = myo-Inositol. Normal cognition (NC), mild cognitive impairment (MCI) and HIV-associated dementia (HAD).
and values in bold = significant.
3.
Metabolite (a) Concentrations (b) Ratios in the FWM in HIV+ patients with normal cognition (NC), mild cognitive impairment (MCI) and with HIV associated dementia (HAD). NAA = N-acetyl aspartate, mI = myo-Inositol, Cho = choline, Cr = creatine, Glx = combined glutamate (glu) and glutamine (Gln).
Table 3.
Metabolites concentrations (millimole (mM)) and ratios mean ± standard deviation, in the BG.
| HIV+ (NC) (N= 21) | MCI (N= 31) | HAD (N= 34) | P value (ANOVA) | Post-hoc NC versus MCI | Post-hoc NC versus HAD | Post-hoc MCI versus HAD | |
|---|---|---|---|---|---|---|---|
| mI | 3.08 ± 0.87 | 3.06 ± 0.8 | 3.34 ± 0.69 | ns | ns | ns | ns |
| Cho | 2.11 ± 0.3 | 1.99 ± 0.3 | 1.98 ± 0.4 | ns | ns | ns | ns |
| Cr | 7.26 ± 1.1 | 7.19 ± 0.86 | 6.96 ± 0.97 | ns | ns | ns | ns |
| Glu | 7.49± 1.07 | 7.88 ± 1.8 | 7.87 ± 1.5 | ns | ns | ns | ns |
| Gln | 8.63 ± 0.08 | 7.04 ± 2.15 | 6.9±1.25 | ns | ns | ns | ns |
| Glx | 11.68 ± 2.57 | 12.6 ± 2.46 | 12.18 ± 2.1 | ns | ns | ns | ns |
| NAA (*) | 7.6 ±1.0 | 7.5 ± 1.06 | 6.79 ± 1.5 | 0.04 | ns | ns | ns |
| mI/Cr | 0.36 ± 0.19 | 0.35 ± 0.2 | 0.44 ± 0.16 | ns | ns | ns | ns |
| Cho/Cr | 0.29 ± 0.03 | 0.28 ± 0.04 | 0.28 ± 0.04 | ns | ns | ns | ns |
| Glu/Cr | 0.96± 0.28 | 1.09 ± 0.2 | 1.06 ± 0.38 | ns | ns | ns | ns |
| Glx/Cr | 1.61 ± 0.32 | 1.64 ± 0.56 | 1.74 ± 0.5 | ns | ns | ns | ns |
| NAA/Cr | 1.06 ± 0.17 | 1.05 ± 0.11 | 0.98 ± 0.19 | ns | ns | ns | ns |
BG = basal ganglia, Cho = choline, Cr = creatine, Glx = combined glutamate (Glu) and glutamine (Gln), NAA = sum of N-acetyl aspartate and N-acetyl aspartyl glutamate, mI = myo-Inositol. Normal cognition (NC), mild cognitive impairment (MCI) and HIV-associated dementia (HAD).
and values in bold = significant.
For the BG, there were fewer significant metabolic differences between groups. However, there was a significant decrease in BG NAA in the HAD group as compared to the NC and MCI groups, (P=0.04).
Table 4 shows a summary of NP tests performance in the 3 HIV+ groups. As expected, the HAD group had impaired performance in almost all of the NP tests compared to the NC and the MCI groups.
Table 4.
Summary of raw scores (mean ± standard deviation) of the NP test performance
| Test Executive function | NC | MCI | HAD | P value |
|---|---|---|---|---|
| Verbal fluency | 45.2±9.8 | 38.8± 12.0 | 32.3± 8.1 | 0.0004 |
| Trail Making B | 57.9 ±23.8 | 99.4 ±42.2 | 137.4 ±84.0 | 0.0002 |
| Odd Man Out total | 38.2 ±1.9 | 36.6 ±3.0 | 34.7 ±3.4 | 0.002 |
| Verbal memory | ||||
| RAVLT total | 52.6 ±7.8 | 50.7 ±7.3 | 39.6 ±8.0 | 0.0001 |
| RAVLT immediate recall | 10.8 ±3.2 | 10.86 ±2.39 | 7.3 ±2.7 | 0.0001 |
| RAVLT delayed recognition | 11.0±3.3 | 10.6± 2.4 | 7.1± 2.8 | 0.0001 |
| Visuo-construction and visual-memory | ||||
| Rey copy | 33.7 ±2.2 | 31.4± 4.3 | 27.8 ±7.3 | 0.0014 |
| Rey recall | 21.3± 5.3 | 16.7± 6.5 | 12.0 ±6.5 | 0.0001 |
| Attention and working memory | ||||
| Trail Making A | 24.3 ±7.7 | 30.0 ±9.2 | 37.0 ±11.4 | 0.0002 |
| Digit Symbol | 55.5 ±16.2 | 48.9 ±12.6 | 38.0 ±13.2 | 0.0002 |
| Letter Number sequencing | 17.3± 21.7 | 9.6± 2.3 | 8.2 ±2.4 | 0.02 |
| Information processing speed | ||||
| CALCAP_choice_mean | 429.2 ±50.2 | 446.25 ±42.3 | 482.2 ±81.4 | 0.016 |
| CALCAP_sequential_mean | 555.6±105.5 | 587.1±87.9 | 638.2 ±130.8 | 0.048 |
| CALCAP_choice_z score | -0.4 ±1.3 | -0.8 ±1.1 | -1.7 ±2.1 | 0.018 |
| CALCAP_sequential_z score | 0.03 ±1.09 | -0.3±0.9 | -0.9 ±1.3 | 0.05 |
| Motor and psychomotor speed | ||||
| Grooved Pegboard dominant hand | 69.5 ±9.2 | 77.8 ±15.9 | 88.2 ±19.9 | 0.0001 |
| Grooved Pegboard non dominant hand | 77.4 ±9.7 | 90.9± 22.1 | 104.0 ±28.9 | 0.001 |
| Timed Gait | 12.4 ±3.3 | 13.2 ±3.1 | 14.5± 3.3 | 0.11 |
| International HIV Dementia Scale | 11.4 ±1.8 | 10.6 ±1.4 | 10.3 ±1.7 | 0.15 |
Values in bold = significant.
NP= neuropsychological tests, RAVLT =Rey Auditory Verbal Learning Test, CALCAP=California Computerized Assessment Package. Normal cognition (NC), mild cognitive impairment (MCI) and HIV-associated dementia (HAD).
Scores for Trail Making Test A and B, Grooved Pegboard and Timed Gait are in seconds (higher scores indicating worse performance). Scores for the CALCAP Reaction Time subtests are in milliseconds (higher scores indicating slower performance). Digit Symbol lower scores indicate worse performance.
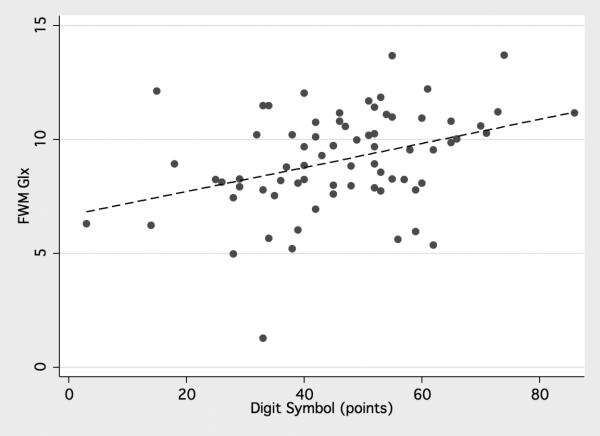
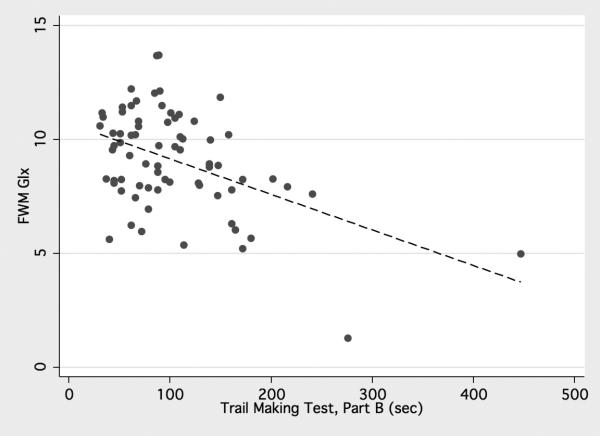
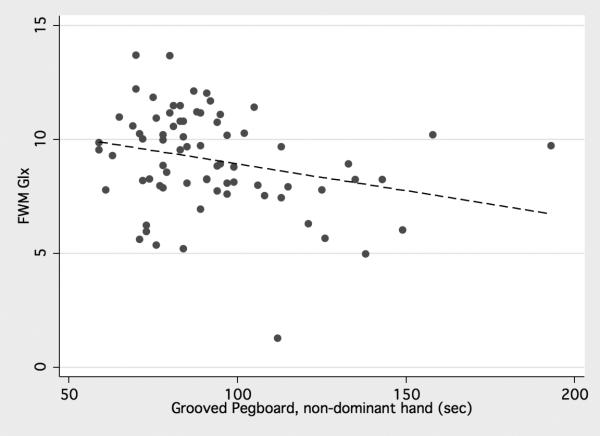
Significant negative correlations were observed for FWM Glu, Glx, and NAA and the Trail-Making test B (P= 0.006, 0.0001, and 0.007 respectively) and significant positive correlation was found with the Digit symbol test (P= 0.02, 0.002, and 0.008 respectively). FWM Glx and NAA concentrations showed negative correlation with Grooved pegboard non-dominant hand (P= 0.02 and 0.04 respectively) (table 5), (figure 4). Correlations of the glutamatergic metabolites and other NP tests were not significant. Regarding the basal ganglia, there was significant negative correlation of NAA concentration with Trail-Making test B (P= 0.004) and significant positive correlation with Digit symbol test (P= 0.002), however there was no significant correlation between basal ganglia Glu and Glx with the NP tests.
Table 5.
Pearson correlation coefficients: r and significance (P values) of the FWM and BG metabolite concentrations and NP tests.
| Trail-making test B | Grooved pegboard non-dominant hand | Digit symbol | |
|---|---|---|---|
| FWM NAA | -0.31 (0.007) | -0.24 (0.04) | 0.3 (0.008) |
| FWM Cho | ns | ns | ns |
| FWM mI | ns | ns | ns |
| FWM Glu | -0.32 (0.006) | ns | 0.28 (0.02) |
| FWM Glx | -0.47 (<0.0001) | -0.26 (0.02) | 0.36 (0.002) |
| BG NAA | -0.35 (0.004) | ns | 0.37 (0.002) |
| BG Cho | -0.26 (0.037) | ns | ns |
| BG mI | ns | 0.37 (0.006) | ns |
| BG Glu | ns | ns | ns |
| BG Glx | ns | ns | ns |
NP= neuropsychological tests, FWM = frontal white matter, BG = basal ganglia, NAA=N acetyl aspartate, Cho = Choline, mI=myoinositol, Glu= glutamate, Glx = combined Glu and glutamine (Gln).
Values in bold = significant, ns=non significant.
4.
(a) Positive correlation of frontal white matter (FWM) Glx with Digital symbol, (b) Negative correlations of FWM Glx with Trail Making Test, Part B, and (c) Negative correlations of FWM Glx with Grooved Pegboard non-dominant hand.
Discussion
The major finding of the current study is that brain MRS performed at 3T is able to detect decreased levels of Glx in the FWM of patients with HAD compared to those without dementia. Lower Glu and Glx concentrations were associated with worse performance on measures of executive function, measures of motor and psychomotor speed and attention and working memory (Trail Making Test B, Grooved Pegboard non-dominant hand, and Digit symbol). To our knowledge, this is the first study correlating Glx and Glu with performance on specific NP tests in HIV+ patients.
In spite of insignificant Cho differences between the groups, FWM mI and mI/Cr were found to be significantly higher in HAD as compared to NC and MCI groups which is in agreement with previous MRS studies (9, 21) and is suggestive of increased gliosis and/or altered astrocytic osmoregulation (22).
For the BG, there were fewer significant metabolic differences between groups, either due to smaller biological differences, and/or due to the lower spectral quality (increased line width) in the BG due to its high iron content, as opposed to the lack of a possible biological effect (Figure 2) (23).
Since Glu is the predominant component of the Glx signal under normal conditions, it appears that the reduction in Glx is most likely due to reduced Glu. Glu showed a trend to be reduced in patients with HAD, however because of its high co-variance in determination with Gln, this did not reach statistical significance.
Numerous factors may potentially influence brain Glx/Glu levels, including: 1) disruption of the Glu/Gln cycle, 2) decreased glutaminergic system function causing decreased Glu release to the synaptic site, 3) increased gliosis and thus increased uptake of excess Glu by glial cells and conversion to Gln, 4) oxidative stress, and 5) decreased oxygen and glucose metabolism, and therefore decreased synthesis of Glu.
Further studies will be required to determine the origin of the decreased Glx signal, although some explanations appear more likely than others – for instance, reduced Glu uptake has previously been demonstrated to occur in vitro in astrocytes exposed to HIV possibly through reduced expression of the glutamate transporter, excitatory amino acid transporter 2 (EAAT2) (24). However, it is not clear how this finding relates to the total pool of brain Glx as determined by in vivo MRS, which is the sum of all Glu pools (neuronal, astrocytic, as well as extracellular).
Because most prior MRS studies have mainly been performed at 1.5T where measurement of Glx is difficult, little literature exists about Glx in HIV. In agreement with the results of the current study, one recent study using 3T MRS also showed a decrease in Glu in the frontal lobe in HIV seropositive patients. The authors found that drug naïve and those under HAART were equally affected (25). In the current study, the majority of the patients were under HAART therapy. One other study measured Glx in HIV patients at 1.5T, and found a negative correlation between macrophage chemoattractant protein (MCP-1) in cerebrospinal fluid (CSF) and Glx in the basal ganglia in cases prior to highly-active anti-retroviral therapy (HAART) (26). They also found negative correlations between NAA in the frontal grey matter and MCP-1. A previous study of NAA and Glu in vitro in temporal lobe samples taken during epilepsy surgery found a strong correlation between NAA and Glu, also consistent with them both being ‘neuronal’ compounds (27).
Other in vitro studies also point to abnormal Glu metabolism in HIV. For instance, using brain tissue taken postmortem from the frontal cortex of AIDS patients and age-matched controls and with the use of saturable [3H] D-aspartate binding as a marker of the glutamate uptake site, the researchers found that N-methyl-D-Aspartate (NMDA) receptor density in the frontal cortex was reduced by 33% in the patients with AIDS, an effect that was more profound in patients with dementia. Ligand binding to the Glu uptake site was 50% reduced in patients with HAD compared to controls (28). These results are consistent with NMDA-receptor mediated neurotoxicity associated with the loss of neurons on which these receptors are found, as well as deficits in Glu uptake sites.
Brain Glx and Glu levels have not been measured in vivo in HIV patients by non MRS-methods. However, there have been studies of Glu in CSF in the years when HAART was first introduced; one study (29) found a 5-fold increase in CSF Glu compared to normal control subjects or patients with non-neurological disorders. It was also found that CSF Glu levels correlated with the degree of HIV dementia and brain atrophy, and were higher in patients who were not undergoing antiviral treatment. It has been proposed that increased extracellular Glu in the CNS could be linked to increased release from glutamatergic neurons or due to impaired reuptake (30). Both these mechanisms have been demonstrated to be present in astrocytic cultures treated with gp120 viral protein (31). However, in another study from the same time period (30), no differences were found between CSF Glu levels in patients with HIV dementia as compared to controls. The findings of normal or increased CSF Glu are opposite to the finding of decreased brain Glx and Glu observed here, although it should be recognized that the majority of patients in the current study were undergoing HAART therapy for many years.
In conclusion, in this large cohort of HAART experienced HIV+ individuals, progressively decreasing levels of FWM Glx were found in patients with normal cognition, MCI, and HAD. FWM Glx decreases were also associated with lower cognitive performance in HAD, specifically impaired executive, fine motor, attention and working memory performances. 3T MRS measurements of Glx may be a useful indicator of neuronal loss or dysfunction in patients with HIV infection. Future work is required to understand the underlying pathogenesis and clinical utility of this observation.
Acknowledgments
Supported by NIH P41RR015241 and R01MH071150.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Richard Skolasky, ScD, is affiliated with the Orthopedic Surgery Department of the Johns Hopkins University School of Medicine, conducted the statistical analysis.
“Disclosure: the authors report no conflict of interest.”
References
- 1.Fine SM, Angel RA, Perry SW, Epstein LG, Rothstein JD, Dewhurst S, Gelbard HA. Tumor necrosis factor alpha inhibits glutamate uptake by primary human astrocytes. Implications for pathogenesis of HIV-1 dementia. J Biol Chem. 1996;271:15303–15306. doi: 10.1074/jbc.271.26.15303. [DOI] [PubMed] [Google Scholar]
- 2.Everall IP, Luthert PJ, Lantos PL. Neuronal number and volume alterations in the neocortex of HIV infected individuals. J Neurol Neurosurg Psychiatry. 1993;56:481–486. doi: 10.1136/jnnp.56.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- 4.Masliah E, Ge N, Morey M, DeTeresa R, Terry RD, Wiley CA. Cortical dendritic pathology in human immunodeficiency virus encephalitis. Lab Invest. 1992;66:285–291. [PubMed] [Google Scholar]
- 5.Wiley CA, Masliah E, Morey M, Lemere C, DeTeresa R, Grafe M, Hansen L, Terry R. Neocortical damage during HIV infection. Ann Neurol. 1991;29:651–657. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]
- 6.Power C, McArthur JC, Nath A, Wehrly K, Mayne M, Nishio J, Langelier T, Johnson RT, Chesebro B. Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patients. J Virol. 1998;72:9045–9053. doi: 10.1128/jvi.72.11.9045-9053.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Zijl PC, Barker PB. Magnetic resonance spectroscopy and spectroscopic imaging for the study of brain metabolism. Ann N Y Acad Sci. 1997;820:75–96. doi: 10.1111/j.1749-6632.1997.tb46190.x. [DOI] [PubMed] [Google Scholar]
- 8.Chong WK, Sweeney B, Wilkinson ID, Paley M, Hall-Craggs MA, Kendall BE, Shepard JK, Beecham M, Miller RF, Weller IV. Proton spectroscopy of the brain in HIV infection: correlation with clinical, immunologic, and MR imaging findings. Radiology. 1993;188:119–124. doi: 10.1148/radiology.188.1.8099750. [DOI] [PubMed] [Google Scholar]
- 9.Chang L, Ernst T, Leonido-Yee M, Witt M, Speck O, Walot I, Miller EN. Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology. 1999;53:782–789. doi: 10.1212/wnl.53.4.782. [DOI] [PubMed] [Google Scholar]
- 10.Menon DK, Ainsworth JG, Cox IJ, Coker RC, Sargentoni J, Coutts GA, Baudouin CJ, Kocsis AE, Harris JR. Proton MR spectroscopy of the brain in AIDS dementia complex. J Comput Assist Tomogr. 1992;16:538–542. doi: 10.1097/00004728-199207000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Meyerhoff DJ, MacKay S, Poole N, Dillon WP, Weiner MW, Fein G. N-acetylaspartate reductions measured by 1H MRSI in cognitively impaired HIV-seropositive individuals. Magn Reson Imaging. 1994;12:653–659. doi: 10.1016/0730-725x(94)92460-0. [DOI] [PubMed] [Google Scholar]
- 12.Barker PB, Lee RR, McArthur JC. AIDS dementia complex: evaluation with proton MR spectroscopic imaging. Radiology. 1995;195:58–64. doi: 10.1148/radiology.195.1.7892496. [DOI] [PubMed] [Google Scholar]
- 13.Laubenberger J, Häussinger D, Bayer S, Thielemann S, Schneider B, Mundinger A, Hennig J, Langer M. HIV-related metabolic abnormalities in the brain: depiction with proton MR spectroscopy with short echo times. Radiology. 1996;199:805–810. doi: 10.1148/radiology.199.3.8638009. [DOI] [PubMed] [Google Scholar]
- 14.Navia BA, Rostasy K. The AIDS dementia complex: clinical and basic neuroscience with implications for novel molecular therapies. Neurotox Res. 2005;8:3–24. doi: 10.1007/BF03033817. [DOI] [PubMed] [Google Scholar]
- 15.Paul RH, Yiannoutsos CT, Miller EN, Chang L, Marra CM, Schifitto G, Ernst T, Singer E, Richards T, Jarvik GJ, Price R, Meyerhoff DJ, Kolson D, Ellis RJ, Gonzalez G, Lenkinski RE, Cohen RA, Navia BA. Proton MRS and neuropsychological correlates in AIDS dementia complex: evidence of subcortical specificity. J Neuropsychiatry Clin Neurosci. 2007;19:283–292. doi: 10.1176/jnp.2007.19.3.283. [DOI] [PubMed] [Google Scholar]
- 16.Jansen JF, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006 Aug;240(2):318–32. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- 17.Natt O, Bezkorovaynyy V, Michaelis T, Frahm J. Use of phased array coils for a determination of absolute metabolite concentrations. Magn Reson Med. 2005;53:3–8. doi: 10.1002/mrm.20337. [DOI] [PubMed] [Google Scholar]
- 18.Barker PB, Hearshen DO, Boska MD. Single-voxel proton MRS of the human brain at 1.5T and 3.0T. Magn Reson Med. 2001;45:765–769. doi: 10.1002/mrm.1104. [DOI] [PubMed] [Google Scholar]
- 19.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 20.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 21.Sacktor N, Skolasky RL, Ernst T, Mao X, Selnes O, Pomper MG, Chang L, Zhong K, Shungu DC, Marder K, Shibata D, Schifitto G, Bobo L, Barker PB. A multicenter study of two magnetic resonance spectroscopy techniques in individuals with HIV dementia. J Magn Reson Imaging. 2005 Apr;21(4):325–33. doi: 10.1002/jmri.20272. [DOI] [PubMed] [Google Scholar]
- 22.Strange K, Emma F, Parades A, Morrison R. Osmoregulation changes in myo-inositol content and Na+/Myo-inositol cotransport in rat cortical astrocytes. Glia. 1994;12(1):35–43. doi: 10.1002/glia.440120105. [DOI] [PubMed] [Google Scholar]
- 23.Barker PB, Szopinski K, Horska A. Metabolic heterogeneity at the level of the anterior and posterior commissures. Magn Reson Med. 2000;43:348–354. doi: 10.1002/(sici)1522-2594(200003)43:3<348::aid-mrm5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312:60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- 25.Sailasuta N, Kimberly Shriner K, Brian Ross B. Evidence of reduced glutamate inthe frontal lobe of HIV-seropositive patients. NMR in biomedicine. 2009;22:326–31. doi: 10.1002/nbm.1329. [DOI] [PubMed] [Google Scholar]
- 26.Chang L, Ernst T, St Hillaire C, Conant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir Ther. 2004 Jun;9(3):431–40. doi: 10.1177/135965350400900302. [DOI] [PubMed] [Google Scholar]
- 27.Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD. Neuronal and glial metabolite content of the epileptogenic human hippocampus. Ann Neurol. 2002;52:635–642. doi: 10.1002/ana.10360. [DOI] [PubMed] [Google Scholar]
- 28.Sardar AM, Hutson PH, Reynolds GP. Deficits of NMDA receptors and glutamate uptake sites in the frontal cortex in AIDS. NeuroReport. 1999;10:3513–3515. doi: 10.1097/00001756-199911260-00009. [DOI] [PubMed] [Google Scholar]
- 29.Ferrarese C, Aliprandi A, Tremolizzo L, Stanzani L, De Micheli A, Dolara A, Frattola L. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2001;57:671–675. doi: 10.1212/wnl.57.4.671. [DOI] [PubMed] [Google Scholar]
- 30.Espey MG, Ellis RJ, Heaton RK, Basile AS. Relevance of glutamate levels in the CSF of patients with HIV-1-associated dementia complex. Neurology. 1999;53:1144–1145. doi: 10.1212/wnl.53.5.1144. [DOI] [PubMed] [Google Scholar]
- 31.Vesce S, Bezzi P, Rossi D, Meldolesi J, Volterra A. HIV-1 gp120 glycoprotein affects the astrocyte control of extracellular glutamate by both inhibiting the uptake and stimulating the release of the amino acid. FEBS Lett. 1997;411:107–109. doi: 10.1016/s0014-5793(97)00674-1. [DOI] [PubMed] [Google Scholar]