Abstract
Background
Frequently, prescribers fail to account for changing kidney function when prescribing medications. We evaluated the use of a computerized provider order entry intervention to improve medication management during acute kidney injury (AKI).
Study Design
Quality improvement report with time series analyses.
Setting & Participants
1598 adult inpatients with a minimum 0.5 mg/dl increase in serum creatinine over 48 hours following an order for at least one of 122 nephrotoxic or renally cleared medications.
Quality Improvement Plan
Passive, non-interactive warnings about increasing serum creatinine appeared within the computerized provider order entry interface and on printed rounding reports. For contraindicated or high toxicity medications that should be avoided or adjusted, an interruptive alert within the system asked providers to modify or discontinue the targeted orders, mark the current dosing as correct and to remain unchanged, or defer the alert to reappear in the next session.
Outcomes & Measurements
Intervention effect on drug modification or discontinuation, time to modification or discontinuation, provider interactions with alerts.
Results
The modification or discontinuation rate per 100 events for medications included in the interruptive alert within 24 hours of increasing creatinine improved from 35.2 pre-intervention to 52.6 post-intervention (p<0.001); orders were modified or discontinued more quickly (p<0.001). During the post-intervention period, providers initially deferred 78.1% of interruptive alerts, although 54% of these were eventually modified or discontinued prior to patient death, discharge, or transfer. The response to passive alerts about medications requiring review did not significantly change when compared to baseline.
Limitations
Single tertiary care academic medical center; provider actions were not independently adjudicated for appropriateness.
Conclusions
A computerized provider order entry-based alerting system to support medication management following AKI significantly increased the rate and timeliness of modification or discontinuation of targeted medications.
Index Words: Clinical Decision Support Systems, Medical Order Entry Systems, Acute Kidney Failure, Computer-Assisted Drug Therapy
Acute kidney injury (AKI) is common in hospitalized patients and associated with increased morbidity and mortality1–8. Significant deterioration in kidney function with AKI may signal the onset of drug-induced nephrotoxicity or precipitate the accumulation of medications normally cleared by renal elimination9–11. Several retrospective studies of prescription error have shown that physicians do not reliably account for kidney function when prescribing new medications or monitoring existing prescriptions. Consequently, as many as 50% of patients with AKI receive inappropriate doses of nephrotoxic or renally cleared medications and are at risk for an adverse drug event3,4,12–20.
Clinical decision support capabilities embedded in computerized provider order entry and electronic medical record systems can successfully reduce medication errors, in part by ensuring the appropriate selection of drug dosing and monitoring for out-of-range laboratory values21–28. Guided dosing based on patient kidney function at the initial order time decreases the rate of inappropriate dosages and reduces the likelihood of patients receiving contraindicated medications14,17,20. Surveillance systems to monitor for changes in kidney function may also decrease the time to drug modification or discontinuation and the administration of excessive doses15,29.
Currently, no solution provides optimal support to providers when kidney function is changing. We developed a comprehensive medication safety system for patients with early-onset AKI. Our objective was to improve the timing and reliability of prescriber reaction to the AKI as compared to a historical control period. The system was integrated with computerized provider order entry to facilitate a rapid provider response. We report on the provider response to the intervention, including interactions with the clinical decision support system and the rates of discontinuation or dose modification of nephrotoxic and renally cleared medications.
Methods
Setting
Vanderbilt University Hospital is an academic, tertiary care facility with over 500 adult beds and 34,000 admissions annually. Over the past decade, Vanderbilt University Hospital care providers have used locally-developed and maintained inpatient computerized provider order entry and inpatient/outpatient electronic medical record systems with extensive integrated decision support30–32. Existing clinical decision support includes other alerts about drug safety and formulary substitutions and is described in greater detail by Miller, et al32. At baseline, the system assisted with the dosing of selected medications for patients with chronic kidney disease, delivering initial dosing advice and warnings for excessive doses.
Computerized Detection of Acute Kidney Injury
Two nephrologists (JPS, JBL) created the laboratory trigger for the intervention, defined as a 0.5 mg/dl or greater change in serum creatinine over any 48 hour period. The nephrologists considered AKIN and RIFLE consensus criteria33–35, measurement variability inherent in the local laboratory assay for serum creatinine, the need for early intervention to affect medication related outcomes, and the potential insignificance of transient changes in serum creatinine due to functional changes in glomerular filtration rate (GFR). At least one prior clinical decision support system has used a similar definition to activate quality or safety interventions29.
Intervention Description
The intervention alerts warned providers about existing inpatient medication orders that potentially required dose adjustment or discontinuation in the setting of a changing serum creatinine. The target list of drugs that triggered the intervention included all medications in the hospital formulary that are nephrotoxic or renally cleared, as determined by two nephrologists (JPS, JBL), pharmacists, and infectious disease specialists (Box 1). The expert panel divided target medications into three toxicity levels: drugs that were directly nephrotoxic or should be avoided with AKI, those requiring dose adjustments to avoid potentially toxic accumulation, and drugs with a low potential for toxicity but which should be reviewed for possible dose adjustments during prolonged episodes of AKI.
Box 1. Targeted Drugs for the AKI Intervention.
| Avoid with AKI | Adjust with AKI | Review with Prolonged AKI |
|---|---|---|
| Acarbose | Acyclovir | Amoxicillin |
| Acetazolamide | Adefovir | Ampicillin |
| Acetohexamide | Allopurinol | Azithromycin |
| Amikacin | Carboplatin | Bretylium |
| Amphotericin B | Cisplatin | Cefaclor |
| Capreomycin | Colchicine | Cefazolin |
| Celecoxib | Cycloserine | Cefepime |
| Chlorpropamide | Didanosine | Cefotaxime |
| Cidofovir | Digitoxin | Cefotetan |
| Diclofenac sodium | Digoxin | Cefoxitin |
| Diflunisal | Eptifibatide | Ceftazidime |
| Enoxaparin | Etoposide | Cefuroxime |
| Etodolac | Famciclovir | Cephalexin |
| Fenoprofen | Flucytosine | Chloroquine |
| Flurbiprofen | Foscarnet | Ciprofloxacin |
| Gallamine | Ganciclovir | Clarithromycin |
| Gentamicin | Imipenem-cilastatin | Clofibrate |
| Glyburide | Itraconazole | Daptomycin |
| Ibuprofen | Meropenem | Disopyramide |
| Immune globulin | Mitomycin | Doxacurium |
| Indomethacin | Penicillin-VK | Ertapenem |
| Ketoprofen | Pentostatin | Ethambutol |
| Ketorolac | Procainamide | Flecainide |
| Meloxicam | Pyridostigmine | Fluconazole |
| Meperidine | Stavudine | Gemfibrozil |
| Metformin | Temozolomide | Hydroxyurea |
| Methotrexate | Topotecan | Idarubicin |
| Nabumetone | Valacyclovir | Indinavir |
| Naproxen | Valganciclovir | Lamivudine |
| Nitrofurantoin | Vancomycin | Levofloxacin |
| Nitroprusside | Voriconazole | Melphalan |
| Pancuronium | Metocurine | |
| Piroxicam | Mivacurium | |
| Radiology exams with contrast dye | Morphine Neostigmine |
|
| Rofecoxib | Norfloxacin | |
| Sotalol | Ofloxacin | |
| Streptomycin | Penicillin-G | |
| Sulindac | Piperacillin | |
| Tenofovir | Pyrazinamide | |
| Tetracycline | Quinidine | |
| Tobramycin | Quinine | |
| Tolmetin | Rifampin | |
| Trimetrexate | Ticarcillin | |
| Tubocurarine | Tocainide | |
| Valdecoxib | Zidovudine |
AKI = Acute kidney injury
We developed two computerized provider order entry-based alerting mechanisms. The first, an initial passive alert, displayed when providers launched an order entry session on a patient who had a 0.5 mg/dl increase in serum creatinine and were prescribed a medication on the target list. The second alert was interruptive, appearing as providers attempted to exit from an ordering session when he or she had not adjusted medications as suggested by the passive alert. To reduce potential alert overload, which is common in computerized provider order entry systems36, the interruptive alert was limited to patients experiencing increasing creatinine levels, prescribed medications to be avoided or adjusted, and a baseline creatinine clearance greater than 30 ml/min, as estimated by the Cockcroft-Gault equation37. The baseline creatinine clearance requirement was instituted to avoid alerting on patients with pre-existing stage IV or V chronic kidney disease, who may have insignificant changes in GFR corresponding to a 0.5mg/dl change in serum creatinine, and who rarely need dose changes if the medication is appropriately dosed from the outset. Providers could receive one or both alert types when a change in a patient’s kidney function occurred in the presence of targeted drugs.
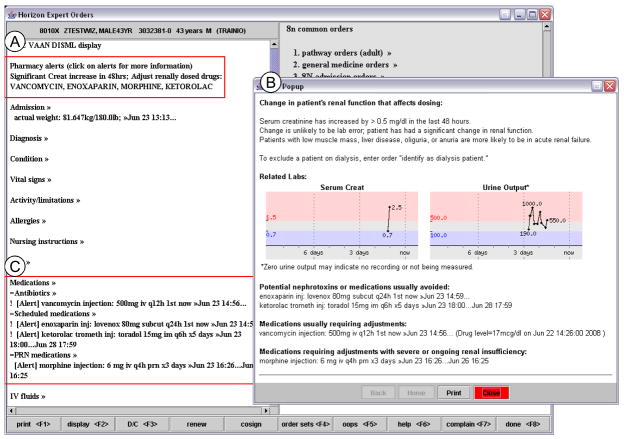
Figure 1 illustrates how the passive alerts appeared as descriptive text in a commonly viewed alert section of the interface (A). When a provider clicked the alert text, a popup window appeared with a graph of recent serum creatinine and recently recorded urine output, in addition to recommendations about which medications should be discontinued, dose-adjusted, or considered for changes (B). The passive alerts also appeared on computerized provider order entry-generated printed rounding reports. All forms of passive alerts persisted for 48 hours, or longer if the serum creatinine continued to change by 0.5 mg/dl within 48 hours.
Figure 1. Example of a Passive Acute Kidney Injury Intervention Alert.
The passive alert displayed as descriptive text in the “pharmacy alert” sections of the order entry environment (A) and printed rounding reports, and as a simple text notification marking each affected prescription in the “medication list” sections (C). When a provider clicked the “pharmacy alert” text, a popup window appeared (B) with detailed information about the order, displaying graphs of serum creatinine and recent recorded urine output, and recommendations about whether current prescriptions should be avoided, dose-adjusted, or reviewed.
The interruptive alert (Figure 2) required providers to modify the dose, discontinue the drug, confirm that the current medication dose was correct in order to suppress future alerts, or defer a definitive response to the alert until the next computerized provider order entry session. The provider could also identify a patient as receiving dialysis, suppressing all AKI alerts for the patient during future sessions. Deferred interruptive alerts persisted for 48 hours or until the patient’s kidney function stabilized, as defined above for passive alerts.
Figure 2. Example of an Interruptive Acute Kidney Injury Intervention Alert.
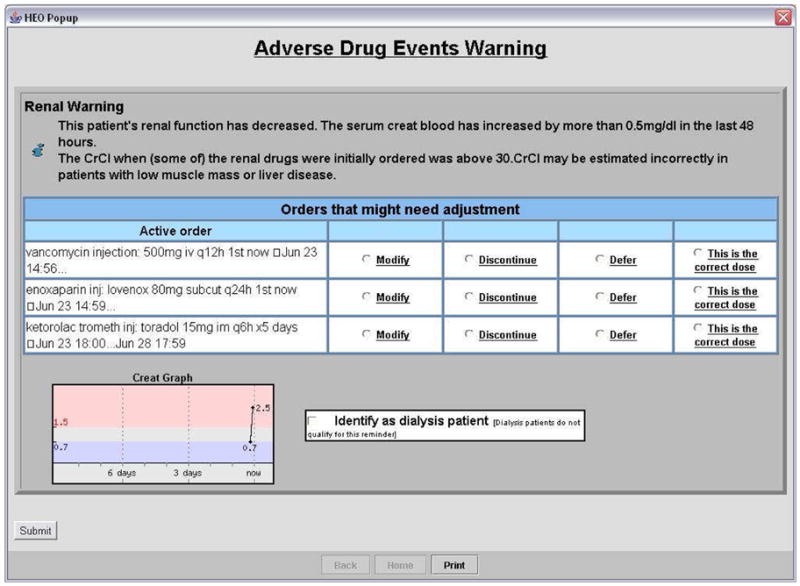
The interruptive alert appeared to providers for medications requiring discontinuation or adjustment when no immediate response was made to the passive alert. The alert interrupted the provider when he or she tried to exit the computerized provider order entry system, requiring the provider to modify or discontinue the drug, to suppress the alert by confirming that the dose was correct, or defer the alert until the next computerized provider order entry session.
Study Design
We conducted a before-after study of the effectiveness of the computerized provider order entry-based alerts at improving the management of medications in the setting of increasing serum creatinine. The analysis included all adult patients between October 14, 2006 and May 16, 2008 who experienced a 0.5 mg/dl increase in serum creatinine over 48 hours of hospitalization and had an active recurring order for one or more of 122 nephrotoxic or renally cleared medications (Box 1). Recurring orders were defined as having a frequency greater than one administration and were considered active until discontinued. We piloted the intervention from August 11, 2007 to October 13, 2007 and excluded data recorded during the pilot. Patients who had a baseline GFR less than 30 ml/min/1.73 m2, as estimated by the Modification of Diet in Renal Disease (MDRD) Study equation38, or were dialyzed prior to the first serum creatinine change event were excluded, as an increase of 0.5 mg/dl would not be considered clinically significant. Patients were also excluded if they died, transferred to an external facility, or were discharged immediately (within 24 hours) after the first change in creatinine. Such patients have all medications automatically discontinued within the computerized provider order entry system, and there is frequently no opportunity for the intervention to be delivered or for a response to be recorded. The Vanderbilt Institutional Review Board approved this study.
We used computerized provider order entry and electronic medical record historical log data to analyze changes in medical care in response to the AKI alert intervention. Data available from the log files included patient demographics, medication and radiology orders, laboratory results, and provider interactions with the order entry system. All data can be readily queried from a relational database.
The primary outcome for this phase of the study was discontinuation or modification of a target medication within 24 hours of a study event. Only the first episode of increasing serum creatinine during a patient’s admission was evaluated for each drug on the target list. Thus, a patient with two active orders for medications on the target list at the time of the first serum creatinine increase would contribute two events to the analysis. The secondary outcome was the time to drug discontinuation or modification. Because drug selection and dosing recommendations for patients with rapidly changing creatinine are not standardized, the intervention did not give specific dosing advice to providers. We considered both medication discontinuations and dose reductions as a potentially appropriate response to the AKI. However, the rate of drug discontinuation for medications to avoid and the rate of dose reductions for medications to adjust are also presented.
We analyzed providers’ specific responses to alerts presented during the post-intervention period as recorded in order entry log files. We determined the number of alerts displayed and categorized clinicians’ responses to each alert type. We tracked all providers’ responses to subsequent alerts if the initial alert was deferred. We also examined the number of times providers clicked on the passive alert to view the more informative popup window.
Statistical Analysis
Univariate comparisons between the pre- and post-intervention time periods were made with the Pearson chi-square test for categorical variables and the log-rank test for the time from event to provider response variables. To formally evaluate the effect of the intervention on provider response rates, we applied logistic regression. The dependent variable was binary, set to one if a provider responded to an event within 24 hours of its occurrence and zero otherwise. Independent variables included: a post-intervention indicator, which was one during the post-intervention period and zero during the pre-intervention period, day of the study period, which ranged from 1 to 300 during the pre-intervention period and 1 to 215 during the post-intervention period, and the interaction between intervention and time. Inclusion of the intervention by day interaction variable permitted examination of time trends in addition to simple intervention effects. Due to the potential for correlation within individual providers and over time, we used robust or “sandwich” standard error estimates39. Analyses were conducted with Intercooled Stata 9.2 (Stata, www.stata.com) and with the R programming language (www.r-project.org)40,41.
Results
The pre-intervention and post-intervention periods were not statistically different for patient age, sex, admitting service, need for ICU care, and median number of target drug orders, though patient ethnicities differed slightly (Table 1). Data analyzed in the pre-intervention period included 914 patients with 1920 orders for a target drug in the setting of an initial creatinine increase. Excluded were 39 patients identified as receiving dialysis and 158 patients who died, transferred, or were discharged within 24 hours of the initial change in kidney function. Data analyzed in the post-intervention period included 745 patients with 1598 orders for a target drug in the setting of an initial creatinine increase. Excluded were 54 patients identified as receiving dialysis and 171 patients who died, transferred, or were discharged within 24 hours of the initial change in creatinine.
Table 1.
Study Population
| Pre-Intervention (10/15/06 – 8/10/07) | Post-Intervention (10/14/07 – 5/16/08) | p | |
|---|---|---|---|
| Age (y) | 57.9 +/− 18 | 57.9 +/− 17.1 | 0.9 |
| Sex (%) | |||
| Female, % | 41.2 | 41.7 | 0.7 |
| Male, % | 56.6 | 55.7 | 0.6 |
| Unknown, % | 2.2 | 2.6 | 0.5 |
| Race, % | |||
| White | 58.3 | 62.5 | 0.009 |
| Black | 23.0 | 22.2 | 0.6 |
| Hispanic | 0.8 | 1.4 | 0.06 |
| Other | 1.5 | 0.6 | 0.009 |
| Unknown | 16.5 | 13.3 | 0.006 |
| Admitting service, % | |||
| Cardiology | 14.2 | 14.0 | 0.9 |
| Critical Care | 12.6 | 13.6 | 0.4 |
| Geriatrics | 1.7 | 2.4 | 0.1 |
| Hematology/Oncology | 8.0 | 7.3 | 0.4 |
| Hepatology | 1.4 | 1.5 | 0.8 |
| Infectious Disease | 2.8 | 3.9 | 0.07 |
| Medicine | 10.6 | 10.1 | 0.6 |
| Other | 9.2 | 8.8 | 0.7 |
| Renal | 7.6 | 6.8 | 0.4 |
| Surgery | 23.9 | 23.7 | 0.9 |
| Trauma | 7.9 | 7.8 | 0.9 |
| Unknown | 0.1 | 0.1 | 0.9 |
| Intensive Care Unit, % | 46.2 | 46.2 | 0.9 |
Note: values shown are mean +/− standard deviation or percent.
Effect of Intervention on Drug Modification and Discontinuation during Acute Kidney Injury
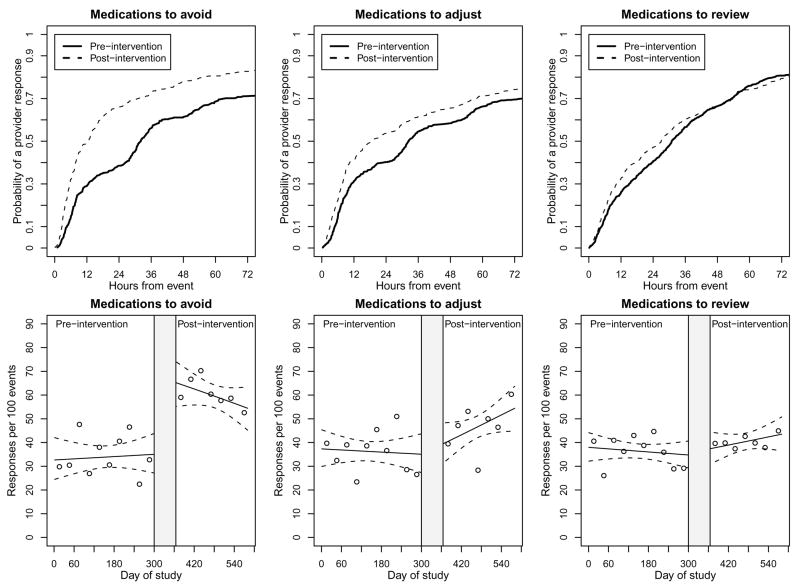
Table 2 summarizes univariate analyses of pre- and post- intervention outcomes. The impact was greatest with medications to avoid, as the rate of response within 24 hours for the pre-and post intervention time periods was 33.9 and 59.5 responses per 100 events respectively (p<0.001), and with the log-rank test indicating significant reductions in response times (p<0.001). Of these responses, 95.6% of pre-intervention and 77.6% of post-intervention responses reflected discontinued medications. Smaller yet significant effects of the intervention were observed for medications requiring adjustment, with response rates within 24 hours increasing from 36.2 to 46.4 per 100 events (p=0.001), and with the log-rank test indicating significant reductions in response times (p=0.01). Of these responses, 40.3% of pre-intervention and 56.4% of post-intervention responses reflected reduced dosing. While there was some evidence to suggest a higher response rate within 24 hours of an event (36.3 to 40.4 responses per 100 events, p=0.08), the time to response did not change over the course of hospitalization (p=0.6 for the log-rank test). Kaplan-Meier curves for the univariate, time to response analyses are shown in the first row of Figure 3. In support of the log-rank test results, we observed a large intervention effect on medications to be avoided, a smaller effect on medications requiring adjustment, and little to no effect on medications to be reviewed.
Table 2.
Univariate Analyses of Intervention Effect on Drug Modification and Discontinuation
| Pre- Intervention | Post- Intervention | Δ | p | |
|---|---|---|---|---|
| Patient Admissions | 914 | 745 | ||
| Modifications/Discontinuations per 100 Events (Study Events*) | ||||
| Medications to Avoid | 33.9 (410) | 59.5 (398) | 25.6 | < 0.001 |
| Medications to Adjust | 36.2 (527) | 46.4 (450) | 10.2 | 0.001 |
| Medications to Review | 36.3 (983) | 40.4 (750) | 4.1 | 0.08 |
Medication orders active during the first changing serum creatinine event.
Figure 3. Kaplan-Meier Curves for Time to Provider Response and Multivariate Time Series Analyses of Intervention Effect on Rate of Provider Response.
The first row shows Kaplan-Meieir curves for the univariate time to response analysis. A response is defined as modification of discontinuation of a medication order prior to patient death, discharge, or transfer. In the second row, results are based on logistic regression analyses that included as predictors: a post-intervention indicator, day of the study period, and the interaction between time by intervention interaction. Solid and dotted lines describe the model derived number of response per 100 events and pointwise confidence intervals, respectively. The points on the plots depict observed rates of responses per 100 event averaged over 30 day time periods. The shaded area between study periods represents implementation and piloting of software where no outcome data was analyzed. The response rate is defined as the proportion of eligible medication orders modified or discontinued within 24 hours of a study event in response to an intervention alert.
The second row of panels in Figure 3 displays the results from logistic regression analyses. For medications to avoid, the impact of the intervention was immediate and stable, with a highly significant effect for the intervention (p<0.001) and non-significant effects for both time and time by intervention interaction variables (p=0.8 and 0.2 respectively). With medications to adjust, the effects of the intervention and time variables were non-significant (p=0.7 and 0.7 respectively), but the time by intervention interaction variable was marginally significant (p=0.09). This led to the modest but increasing trend in response rates during the post-intervention period observed in Figure 3. The intervention did not influence provider response rate with medications requiring review (p=0.9 for intervention, 0.5 for time, and 0.2 for time by intervention).
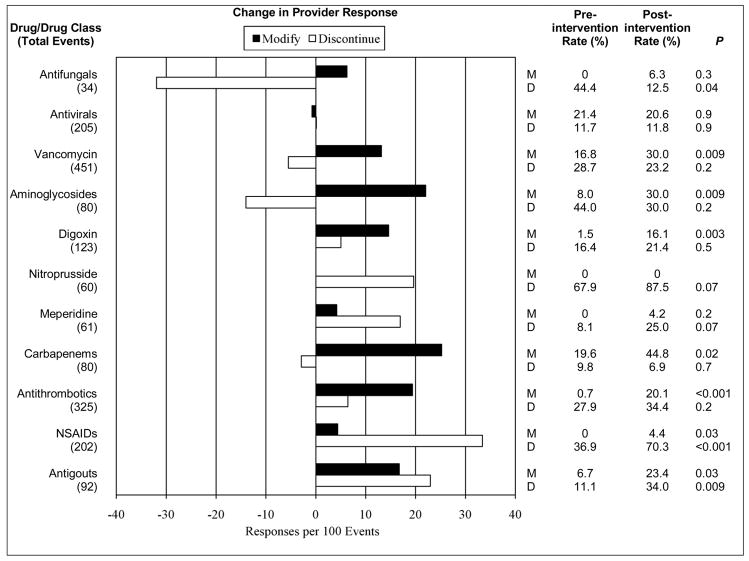
Figure 4 shows the change in rate of provider response for medications to be avoided or adjusted. Medications for gout and non-steroidal anti-inflammatory drugs were most frequently altered due to the intervention. While the net change for antimicrobials was low, the rate of dose modifications increased for many groups.
Figure 4. Change in Rate of Drug Modification or Discontinuation by Individual Drug or by Drug Class.
Response rates represent the proportion of eligible medication orders modified (M) or discontinued (D) within 24 hours of an increase in serum creatinine following an order for a target nephrotoxic or renally cleared drug. Drugs or drug classes with fewer than 10 increasing serum creatinine events were excluded.
Provider Responses to Alerts in Post-Intervention Period
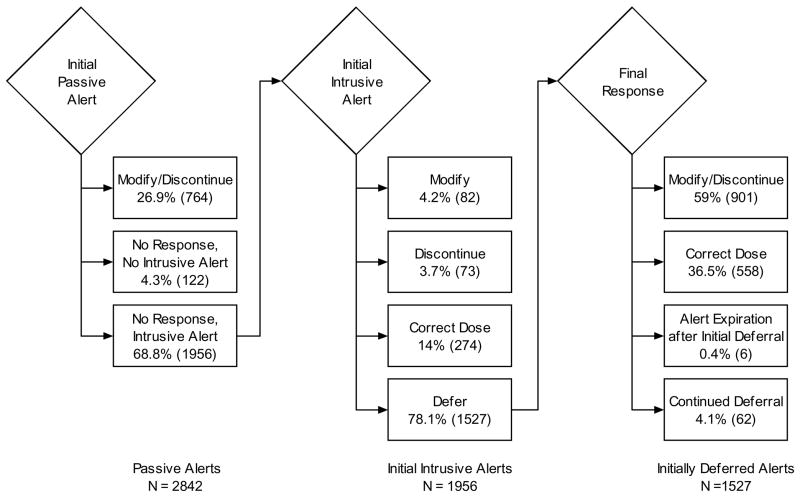
We evaluated the provider response to alerts generated by eligible medication orders in the 31 week post-intervention period. Study events triggered 1956 passive alert/interruptive alert pairs and an additional 886 passive alerts without an accompanying interruptive alert. Figure 5 illustrates the multiple pathways through which providers responded to alerts. After viewing only a passive alert, providers modified or discontinued 26.9% of alerted medication orders. Providers clicked the passive alert to view the detailed information screen for less than 1% of passive alerts.
Figure 5. Provider Responses to Alerts in Post-Intervention Period.
Displayed are provider responses to passive and interruptive alerts generated from drug orders with medications requiring discontinuation or adjustment during acute kidney injury. The provider’s interaction are categorized as response (modifying or discontinuing the alerted order) after viewing only a passive alert, immediate response after an interruptive alert, or delayed response Also represented is the number of alerts which expired after 48 hours, or when the patient died or was discharged from the hospital. N represents the number of alerts displayed, or the opportunities to respond.
For those orders not immediately modified or discontinued following the passive alerts, providers most frequently (78.1%) chose to defer response within the interruptive alert. Providers selected the “modify” or “discontinue” options during 4.2% and 3.7% of initial interruptive alerts respectively, and selected the “correct dose” option for 14% of initial interruptive alerts. Following an initial deferral, providers subsequently modified or discontinued 59% of medication orders and marked 36.5% as correct; 4.1% of orders were not modified or discontinued prior to patient death or discharge.
Alerts were often viewed and deferred by multiple team members over the course of hours to days. For each event-drug pair, the passive alert displayed to one or more providers a median of 24 times. For those orders eligible for an interruptive alert, the median number of deferred alerts was 4 (25th–75th percentiles, 2–10; range, 1–56) prior to a more definitive response.
Discussion
We developed an alerting system integrated with computerized provider order entry to continuously monitor and inform health care providers about medication safety in the setting of acute kidney injury. The intervention significantly improved provider response to rising creatinine levels, increasing the frequency at which nephrotoxic and renally cleared medications were modified or discontinued. The greatest effect occurred for medications with the highest potential for toxicity. Response rates exhibited a modest increase during the post-intervention period, possibly demonstrating a learning effect. The findings of our evaluation are important in defining how clinical decision support can reduce drug selection and dosing errors that are a significant proportion of overall ordering and monitoring errors27,28. Improved management of medications in patients with AKI may reduce drug toxicity and represents improved compliance with the standard of care for hospitalized patients.
The alert system featured two levels of workflow intrusiveness to maximize provider attention to important prescribing issues while avoiding an over-abundance of interruptive alerting for medication dosing problems of lower urgency or clinical significance. The passive alerts for medications requiring review, which persisted on the main screen until the alert expired or an expected drug action was taken, did not change the prescribing behavior of providers. Similar to previous studies using clinical decision support, the interruptive alerts were more effective at producing change in prescribing behavior than passive alerts42,43. We could not determine whether the low response rates to passive alerts were due to provider unawareness of the alert or deliberate judgment that no action was required.
The system allowed deferral of the alert so that users not primarily responsible for medication decisions could pass responsibility for responding to the alert to the next user. Providers frequently used this option, sometimes to apparent excess, which may signal that the medication decision needs to be escalated to a more knowledgeable member of the care team, such as the attending physician or rounding pharmacist. Targeting the alert only to the responsible team member (i.e. all members of the primary team and covering teams) would be a desirable enhancement to the current approach, where all users receive the alert.
The high rate of deferred and overridden alerts is similar to other evaluations of clinical decision support. Chertow, et al.14 and Galanter, et al.17 also found high rates of alert overrides (42% and 48% respectively) in systems which targeted medication safety in chronic or acute kidney disease. In our study, some of the overrides appeared to have been appropriate due to low initial dosing such as a prophylactic dose of acyclovir in transplant patients. Patients may have been experiencing transient changes in serum creatinine, and providers may have anticipated that the changes would be reversed with fluid administration. When deferring or overriding the alerts, providers may also be demonstrating alert fatigue or uncertainty about what therapeutic changes to make in the face of AKI. Our analysis of provider interactions showed that although initial deferral rates were high, more than half of alerted drugs are eventually modified or discontinued.
The decision support capabilities developed for this intervention have not previously been combined in a single system. A number of systems successfully provided guidance for renally dosed drugs when providers initially prescribed medications14,17,20,44. Other systems have implemented surveillance to alert providers about kidney function changes but have distinct differences to our system15,29,45. One developed by Rind, et al.29 generated alerts for 55 medications that appeared to providers as e-mail messages outside of the workflow. The study found a 21.6 hour reduction in physician response time, compared to our finding of 19.6 hours for a comparable set of high toxicity drugs. Systems developed by Evans, et al.15 and Kilbridge, et al.45 also include a surveillance approach, though the systems assessed for changes only once daily, and the alerts appeared to pharmacists rather than ordering providers. Alerts displayed to pharmacists may complement those displayed to physicians or other prescribers, as pharmacists may filter alerts for priority and clinical significance. However, they may not always have updated knowledge of the patient condition, or do not have sufficient staffing to monitor all patients at all times. By continuously monitoring for updated lab results and alerting ordering providers directly in the computerized provider order entry system, we gave prescribers the opportunity to make dosing changes more quickly.
Our study has a number of limitations. The measured outcomes may have been affected by the changing case mix of patients admitted during the two sequential study periods or parallel efforts within the institution to improve prescribing quality. However, the regression analysis suggests that the change was abrupt and associated with activating the intervention. The attempt to exclude dialysis patients from the intervention using a user-defined flag and a filter for low baseline calculated GFR was imperfect and may have led to both false positive and false negative alerting. A more reliable coded method to identify dialysis patients is needed.
Because recommendations for patients with rapidly changing creatinine are difficult to provide without significant error, the intervention did not give specific dosing advice, and we considered both medication discontinuations and dose reductions as responses. We are taking steps to further evaluate the intervention by retrospectively reviewing cases and adjudicating the appropriateness of alert responses. Approaches to providing recommendations in the future may include providing approximate dosing recommendations for mild, moderate, or severe AKI, or facilitating the expert dosing advice of a pharmacist or nephrologist.
Finally, it is not known whether the intervention prevented adverse drug event rates. The interactions between kidney function and medications are numerous and difficult to measure in a comprehensive fashion for 122 medications. Additionally, there is no validated method to distinguish nephrotoxic drug effects from native kidney disease in patients selected for a changing creatinine.
In conclusion, we developed, implemented, and evaluated an intervention to continuously monitor for and alert providers within the computerized provider order entry system about AKI in the presence of target nephrotoxic and renally cleared drugs. The intervention significantly increased the rate and timeliness of provider modification or discontinuation of target drugs.
Acknowledgments
The authors thank Mark Sullivan, Cori Nelsen, and Titus Daniels for their clinical expertise and Randolph Miller for his review and insight. Preliminary results were presented as a poster at the 2008 AMIA Annual Symposium.
Support: The authors were funded in part by National Library of Medicine grants T15 LM007450-08 (ABM, CSG), R01 LM009965-02 (ABM, JFP, LRW, ID), and R03 LM009238-02 (JFP).
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clermont G, Acker CG, Angus DC, et al. Renal failure in the ICU: comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int. 2002;62(3):986–996. doi: 10.1046/j.1523-1755.2002.00509.x. [DOI] [PubMed] [Google Scholar]
- 2.Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74(2):243–248. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- 3.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 4.Cantú TG, Ellerbeck EF, Yun SW, Castine SD, Kornhauser DM. Drug prescribing for patients with changing renal function. Am J Hosp Pharm. 1992;49(12):2944–2948. [PubMed] [Google Scholar]
- 5.Menashe PI, Ross SA, Gottlieb JE. Acquired renal insufficiency in critically ill patients. Crit Care Med. 1988;16(11):1106–1109. doi: 10.1097/00003246-198811000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 7.Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17(4):1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 8.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3(3):844–861. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 9.Aronson JK. Drugs and Renal Insufficiency. Medicine. 2003;31(7):103–109. [Google Scholar]
- 10.Kappel J, Calissi P. Nephrology: 3. Safe drug prescribing for patients with renal insufficiency. CMAJ. 2002;166(4):473–477. [PMC free article] [PubMed] [Google Scholar]
- 11.Le Sher DA. Considerations in the use of drugs in patients with renal failure. J Clin Pharmacol. 1976;16(10 Pt 2):570–576. doi: 10.1177/009127007601601019. [DOI] [PubMed] [Google Scholar]
- 12.Davidman M, Olson P, Kohen J, Leither T, Kjellstrand C. Iatrogenic renal disease. Arch Intern Med. 1991;151(9):1809–1812. [PubMed] [Google Scholar]
- 13.Shusterman N, Strom BL, Murray TG, et al. Risk factors and outcome of hospital-acquired acute renal failure. Clinical epidemiologic study. Am J Med. 1987;83(1):65–71. doi: 10.1016/0002-9343(87)90498-0. [DOI] [PubMed] [Google Scholar]
- 14.Chertow GM, Lee J, Kuperman GJ, et al. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286(22):2839–2844. doi: 10.1001/jama.286.22.2839. [DOI] [PubMed] [Google Scholar]
- 15.Evans RS, Pestotnik SL, Classen DC, Burke JP. Evaluation of a computer-assisted antibiotic-dose monitor. Ann Pharmacother. 1999;33(10):1026–1031. doi: 10.1345/aph.18391. [DOI] [PubMed] [Google Scholar]
- 16.Falconnier AD, Haefeli WE, Schoenenberger RA, Surber C, Martin-Facklam M. Drug dosage in patients with renal failure optimized by immediate concurrent feedback. J Gen Intern Med. 2001;16(6):369–375. doi: 10.1046/j.1525-1497.2001.016006369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galanter WL, Didomenico RJ, Polikaitis A. A trial of automated decision support alerts for contraindicated medications using computerized physician order entry. J Am Med Inform Assoc. 2005;12(3):269–274. doi: 10.1197/jamia.M1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu KT, Matayoshi A, Stevenson FT. Calculation of the estimated creatinine clearance in avoiding drug dosing errors in the older patient. Am J Med Sci. 2001;322(3):133–136. doi: 10.1097/00000441-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Salomon L, Deray G, Jaudon MC, et al. Medication misuse in hospitalized patients with renal impairment. Int J Qual Health Care. 2003;15(4):331–335. doi: 10.1093/intqhc/mzg046. [DOI] [PubMed] [Google Scholar]
- 20.Oppenheim MI, Vidal C, Velasco FT, et al. Impact of a computerized alert during physician order entry on medication dosing in patients with renal impairment. Proc AMIA Symp. 2002:577–581. [PMC free article] [PubMed] [Google Scholar]
- 21.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280(15):1311–1316. doi: 10.1001/jama.280.15.1311. [DOI] [PubMed] [Google Scholar]
- 22.Bates DW, Teich JM, Lee J, et al. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc. 1999;6(4):313–321. doi: 10.1136/jamia.1999.00660313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teich JM, Merchia PR, Schmiz JL, et al. Effects of computerized physician order entry on prescribing practices. Arch Intern Med. 2000;160(18):2741–2747. doi: 10.1001/archinte.160.18.2741. [DOI] [PubMed] [Google Scholar]
- 24.Evans RS, Pestotnik SL, Classen DC, et al. Preventing adverse drug events in hospitalized patients. Ann Pharmacother. 1994;28(4):523–527. doi: 10.1177/106002809402800417. [DOI] [PubMed] [Google Scholar]
- 25.Bates DW, O'Neil AC, Boyle D, et al. Potential identifiability and preventability of adverse events using information systems. J Am Med Inform Assoc. 1994;1(5):404–411. doi: 10.1136/jamia.1994.95153428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bobb A, Gleason K, Husch M, et al. The epidemiology of prescribing errors: the potential impact of computerized prescriber order entry. Arch Intern Med. 2004;164(7):785–792. doi: 10.1001/archinte.164.7.785. [DOI] [PubMed] [Google Scholar]
- 27.Leape LL, Bates DW, Cullen DJ, et al. Systems analysis of adverse drug events. ADE Prevention Study Group. JAMA. 1995;274(1):35–43. [PubMed] [Google Scholar]
- 28.Lesar TS, Briceland L, Stein DS. Factors related to errors in medication prescribing. JAMA. 1997;277(4):312–317. [PubMed] [Google Scholar]
- 29.Rind DM, Safran C, Phillips RS, et al. Effect of computer-based alerts on the treatment and outcomes of hospitalized patients. Arch Intern Med. 1994;154(13):1511–1517. [PubMed] [Google Scholar]
- 30.Geissbühler A, Miller RA. A new approach to the implementation of direct care-provider order entry. Proc AMIA Annu Fall Symp. 1996:689–693. [PMC free article] [PubMed] [Google Scholar]
- 31.Giuse DA. Provider order entry with integrated decision support: from academia to industry. Methods Inf Med. 2003;42(1):45–50. [PubMed] [Google Scholar]
- 32.Miller RA, Waitman LR, Chen S, Rosenbloom ST. The anatomy of decision support during inpatient care provider order entry (CPOE): empirical observations from a decade of CPOE experience at Vanderbilt. J Biomed Inform. 2005;38(6):469–485. doi: 10.1016/j.jbi.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellomo R, Kellum J, Ronco C. Acute renal failure: time for consensus. Intensive Care Med. 2001;27(11):1685–1688. doi: 10.1007/s00134-001-1120-6. [DOI] [PubMed] [Google Scholar]
- 34.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of Drug Safety Alerts in Computerized Physician Order Entry. J Am Med Inform Assoc. 2006;13(2):138–147. doi: 10.1197/jamia.M1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 38.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 39.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 40.R Development Core Team. R: A language and environment for statistical computing. Foundation for Statistical Computing; Vienna, Austria: 2005. [Google Scholar]
- 41.Zeileis A. Object-oriented computation of sandwich estimators. Journal of Statistical Software. 2006;16(9):1–16. [Google Scholar]
- 42.Rosenbloom ST, Geissbuhler AJ, Dupont WD, et al. Effect of CPOE user interface design on user-initiated access to educational and patient information during clinical care. J Am Med Inform Assoc. 2005;12(4):458–473. doi: 10.1197/jamia.M1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamblyn R, Huang A, Taylor L, et al. A randomized trial of the effectiveness of on-demand versus computer–triggered drug decision support in primary care. J Am Med Inform Assoc. 2008;15(4):430–438. doi: 10.1197/jamia.M2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sellier E, Colombet I, Sabatier B, et al. Effect of alerts for drug dosage adjustment in inpatients with renal insufficiency. J Am Med Inform Assoc. 2009;16(2):203–210. doi: 10.1197/jamia.M2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kilbridge PM, Alexander L, Ahmad A. Implementation of a system for computerized adverse drug event surveillance and intervention at an academic medical center. J Clin Outcomes Manage. 2006;13(2):94. [Google Scholar]