Abstract
Background
Warfarin, a drug primarily metabolized by the cytochrome P450 system, is initiated at similar doses and managed similarly in patients with kidney impairment as in the general medical population. Unfortunately, few data exist to guide dose adjustment in patients with reduced kidney function. Herein we determine the degree of warfarin dose reduction associated with kidney impairment and make recommendations for warfarin dosing.
Study Design
Cross-sectional analysis.
Setting & Participants
Chronic warfarin users followed at anticoagulation clinics (n=980); 708 participants from the University of Alabama (UAB) and 272 participants from the University of Chicago (UIC).
Predictor
No/mild (eGFR≥60ml/min/1.73 m2), moderate (eGFR=30–59ml/min/1.73 m2) and severe (eGFR<30ml/min/1.73 m2) kidney impairment, CYP2C9 and VKORC1 genotype, age, race, gender, body mass, socio-demographic factors, smoking status, alcohol, vitamin K intake, comorbid conditions (e.g. CHF, etc.) and drug interactions (e.g. amiodarone, statins, etc.).
Outcome & Measurement
Warfarin dose (mg/day) was evaluated using linear regression after adjustment for clinical demographic and genetic factors.
Results
The prevalence of moderate kidney impairment (31.8% and 27.6%) and severe kidney impairment (8.9% and 6.6%) was similar in the UAB and UIC cohorts. Warfarin dose requirements were significantly lower in patients with moderate and severe kidney impairment compared to those with none/mild kidney impairment in the UAB (p<0.001) and UIC (p<0.001) cohorts. Compared to patients with no/mild kidney impairment, patients with moderate kidney impairment required 9.5% lower doses (p<0.001) and patients with severe kidney impairment required 19% lower doses (p<0.001).
Limitations
No measurement of warfarin, serum albumin, vitamin K and clotting factor levels, no evaluation of other markers (e.g. cystatin).
Conclusion
Moderate and severe kidney impairment were associated with a reduction in warfarin dose requirements.
Keywords: Warfarin, Dose adjustment, Pharmacogenetics, CYP2C9, VKORC1, Kidney impairment
Chronic kidney disease (CKD) has emerged as a major public health concern, with about 26 million adults affected in the US.1, 2 These patients are at a substantially increased risk of cardiovascular disease, anemia, and bone disease, and require multiple drugs to treat these complications.3–7 Not surprisingly, drugs with primarily renal excretion require substantial dose reductions in patients with kidney impairment. However, even among drugs eliminated primarily by metabolism or non-renal transport, 25% have a ~two-fold increase in area-under-the-curve in patients with severe kidney impairment, requiring significant dose reductions.8 Although the mechanisms of altered pharmacokinetics in kidney impairment are not well understood, animal studies suggest down-regulation of various cytochrome (CYP) enzymes and transporters, thereby influencing the response to drugs with primarily non-renal clearance. 9–11
Warfarin, the most commonly prescribed oral-anticoagulant, exhibits large inter-patient variability in dose requirements.12, 13 Initiation and maintenance of therapy is challenging due to the multitude of factors (diet, medications, genetics, etc.) that influence warfarin pharmacokinetics and pharmacodynamics.12 Although clinicians recognize that anticoagulation management is even more challenging among patients with kidney impairment, warfarin therapy is initiated at similar doses and managed similarly in patients with kidney impairment as in the general medical population.14–16 Unfortunately, few published data exist to guide dose adjustments in patients with reduced kidney function. We recently reported that patients with reduced kidney function require lower warfarin doses, even after adjustment for clinical and genetic factors known to affect warfarin metabolism. These observations suggest that warfarin may need to be instituted at a lower dose in patients with moderate or severe kidney impairment, as compared to those with mild/no kidney impairment.17
This secondary cross-sectional analysis assesses the degree of warfarin dose reduction associated with moderate or severe kidney impairment in two independent cohorts and derives recommendations for warfarin dosing in patients with kidney impairment.
Methods
Cohorts
University of Alabama at Birmingham (UAB) cohort
The Pharmacogenetic Optimization of Anticoagulation Therapy (POAT) and the Genetic and Environmental Determinants of Warfarin (GEDWR) are ongoing prospective cohort studies aimed at defining the influence of polymorphisms in CYP2C9 and other genes on warfarin response. Patients ≥20 years of age were considered eligible if the intended duration of anticoagulation therapy was ≥ 2 years, therapy was managed at the anticoagulation clinic and the INR was 2–3. The study was conducted under the approval of the Institutional Review Boards of the University of Alabama at Birmingham and Jefferson County Health System.
University of Illinois in Chicago (UIC) cohort
The UIC cohort comprised of participants ≥18 years of age who achieved stable warfarin dosing, defined as the dose that produced stable anticoagulation (INR within 0.2 units of the therapeutic range) for at least 3 consecutive clinic visits. The patients were recruited at the University of Illinois at Chicago (UIC) under the approval of the Institutional Review Board. Patients with a documented history of liver dysfunction or amiotransferase levels at least twice the upper limit of normal were excluded.
Data Collection
A detailed history documented clinical information including self-reported race, age, height and weight, serum urea nitrogen (SUN), serum creatinine (SCr), warfarin dose, INR, indication for therapy, co-morbid conditions, medications, smoking, alcohol use as detailed in recent publications.18, 19 Concurrent therapy with medications such as non-steroidal anti-inflammatory drugs (NSAIDs) or antiplatelet agents or drugs that alter warfarin pharmacokinetics including CYP2C9 inhibitors (e.g. amiodarone), CYP2C9 inducers (e.g. rifampin), or CYP2C9 substrates (e.g. losartan) 20, 21 were documented. Both cohorts documented information on clinical, demographic and genetic factors in a similar fashion.
Kidney function
The glomerular filtration rate (eGFR) was estimated by using the 4-variable MDRD Study equation.22 Patients were categorized into 3 groups based on eGFR as recommended by the National Kidney Foundation. Patients with eGFR ≥60 ml/min/1.73 m2 were categorized as having no/mild kidney impairment, those with eGFR =30–59 ml/min/1.73 m2 were categorized as having moderate kidney impairment and those with eGFR <30 ml/min/1.73 m2 were categorized as having severe kidney impairment. Patients receiving maintenance dialysis were categorized as having severe kidney impairment.23, 24
CYP2C9 and VKORC1 Genotypes
Genotypes were determined using PCR-RFLP (polymerase chain reaction– restriction fragment length polymorphism) analysis, pyrosequencing, and iPLEX technology (a single-base extension multiplex PCR assay with mass spectrometric readout from Sequenom Inc [www.sequenom.com], and performed at the Broad Institute [Cambridge, MA]) from DNA extracted from whole blood or buccal cells as detailed in recent manuscripts.19, 25, 26 Specifically, in the CYP2CP gene, we assessed the single-nucleotide polymorphisms (SNPs) with reference SNP (rs) identification numbers rs1799853, rs1057910, rs28371686, rs9332131, and rs28371685. These correspond to CYP2C9 alleles *2, *3, *5, *6, and *11, respectively, which are polymorphisms 430C/T, 1075A/C, 1080C/G, 818delA, and 1003C/T in the CYP2C9 cDNA. In the VKORC1 gene (which encodes vitamin K epoxide reductase complex, subunit 1), we assessed SNPs −1639G>A (rs9923231) and 1173C/T(rs9934438).
Outcome Definitions and Statistical Methods
Analysis of variance was used to assess group differences for continuous variables and χ2 test of independence for categorical variables. The assumption of Hardy-Weinberg equilibrium was tested using the χ2 test of independence.
Warfarin dosewas defined as the average mainte nance dose required to maintain therapeutic anticoagulation for the duration of therapy (UAB cohort) or the dose that produced stable anticoagulation for at least 3 consecutive clinic visits (UIC cohort). To improve model fit and limit heteroscedascity, we used a logarithmic transformation of warfarin dose. Evaluation of the effects of individual predictor variables on warfarin dose employed both univariate and multivariable linear regression.
Linear-regression analysis was conducted to assess the influence of CKD, CYP2C9 and VKORC1 genotype, age, race, gender, body mass, socio-demographic factors, smoking status, alcohol, vitamin K intake, comorbid conditions (e.g. CHF, etc.) and drug interactions (amiodarone and statins). Backward elimination technique was used to select influential predictors (p<0.2). CYP2C9 and VKORC1 genotypes were assessed in both additive and dominant models. To assess model fit, we examined residuals, and median prediction error (mg/day). The influence of predictor variables was determined in the UAB cohort and UIC cohorts separately and then combined the two data sets to perform analysis (as described above) to provide robust estimates of the influence of genetic and clinical predictors. All analyses were performed using SAS version 9.1 (SAS Institute, www.sas.com) at a non-directional alpha level of 0.05.
Results
Of the 797 eligible participants at the University of Alabama at Birmingham (UAB cohort), 76 participants (9.5%) declined participation in the study, and thirteen were excluded due to missing serum creatinine values. The remaining 708 participants (mean age 61±15 years, 50.0% men) comprised the UAB cohort (327 African Americans, 377 European Americans, 3 Hispanic and 1 Asian). Of the 303 eligible participants at UIC, 31 (10.2%) declined participation. The UIC cohort (n=272) was comprised of 207 African Americans, 23 European Americans, 42 Hispanic participants (mean age 56±16 years, 25.7% men).
Clinical, demographic and genetic characteristics for participants are presented in Table 1. Genotype distributions for CYP2C9 and VKORC1 were in within each race group (all p-values >0.2). As reported previously, European Americans had a higher frequency of variant CYP2C9 (35.4%) and VKORC1 1173 (59.7%)genotypes as compared to African Americans (11.2% and 18.4%, respectively <0.001). Genotype frequencies did not differ across GFR categories (Table 1).
Table 1.
Baseline cohort characteristics by level of kidney function
| eGFR ≥ 60 (n=599) | eGFR 30–≤59 (n=300) | eGFR <30 (n=81) | P | |
|---|---|---|---|---|
| Age (years) | 56.8 ±15.7 | 66.3 ± 13.4 | 56.9 ± 15.3 | <0.001 |
| BMI (kg/m2) | 31.2 ± 8.5 | 30.1 ± 7.7 | 30.2 ± 6.3 | 0.3 |
| Height (in inches) | 67.2 ± 4.1 | 66.2 ± 4.1 | 66.9 ± 3.9 | 0.07 |
| Weight (in lbs) | 199.9 ± 55.1 | 190 ± 80.9 | 193.1 ± 47.1 | 0.1 |
| Ideal Body Weight (kg) | 61.2 ± 11.3 | 62.5 ± 11.0 | 63.3 ± 10.4 | 0.09 |
| Warfarin dose (mg/day) | 6.1 ± 2.6 | 5.0 ± 2.2 | 4.6 ± 1.9 | <0.001 |
| Serum urea nitrogen (mg/dl) | 13.2 ± 5.0 | 21.9 ± 11.8 | 38.2 ± 18.4 | <0.001 |
| Serum creatinine (mg/dl) | 0.93 ± 0.2 | 1.37 ± 0.3 | 5.9 ± 4.1 | <0.001 |
| Race | <0.001 | |||
| European American | 219 (36.6%) | 156 (52.0%) | 22 (27.1%) | |
| African American | 353 (58.9%) | 126 (42.0%) | 55 (67.9%) | |
| Hispanic or Asian | 27 (4.5%) | 18 (6.0%) | 4 (0.05%) | |
| Gender | 0.4 | |||
| Male | 269 (44.9%) | 120 (40.0%) | 35 (43.2%) | |
| Female | 330 (55.1%) | 180 (60.0%) | 46 (56.8%) | |
| Indication/comorbidities | ||||
| Venous thromboembolism | 283 (47.1%) | 102 (34.0%) | 38 (46.9%) | <0.001 |
| Stroke/TIA | 108 (18.0%) | 51 (16.9%) | 7 (8.7%) | 0.1 |
| Atrial Fibrillation | 185 (30.8%) | 147 (46.7%) | 32 (40.0%) | <0.001 |
| Congestive heart failure | 88 (14.7%) | 78 (26.0%) | 20 (24.7%) | <0.001 |
| Coronary artery disease | 141 (23.5%) | 112 (37.3%) | 29 (35.8%) | <0.001 |
| Diabetes mellitus | 162 (27.0%) | 112 (37.3%) | 36 (44.4%) | <0.001 |
| Hypertension | 329 (54.9%) | 202 (67.3%) | 76 (93.8%) | <0.001 |
| Cancer | 71 (11.8%) | 41 (13.7%) | 10 (12.3%) | 0.7 |
| Current smokers | 84 (14.0%) | 30 (10.0%) | 15 (18.5%) | 0.08 |
| Current alcohol use | 175 (29.3%) | 70 (23.3%) | 10 (12.3%) | 0.002 |
| Concurrent amiodarone | 33 (5.6%) | 33 (11.0%) | 8 (9.9%) | 0.01 |
| Concurrent statin | 241 (40.5%) | 135 (45.0%) | 40 (49.4%) | 0.2 |
| VKORC1 varianta | 170 (32.4%) | 101 (39.8%) | 26 (37.1%) | 0.1 |
| CYP2C9 variantb | 108 (20.1%) | 57 (22.1%) | 16 (21.9%) | 0.8 |
| Site | 0.1 | |||
| UAB | 420 (70.1%) | 225 (75.0%) | 63 (77.8%) | |
| UIC | 179 (29.9%) | 75 (25.0%) | 18 (22.2%) |
Note: Values shown are mean ± standard deviation or number (percentage). P-values for continuous variables are based on t-test/Kruskal-Wallis test; P values for categorical variables based on chi-square test. eGFR (estimated glomerular filtration rate) is based on National Kidney Foundation staging using the Modification of Diet in Renal Disease Study equation, and is reported in mL/min/1.73 m2 (factor for conversin to mL/s/1.73 m2, ×0.01667). Patients eGFR ≥60 were categorized as having no/mild kidney impairment, those with eGFR =30–59 as having moderate kidney impairment and those with eGFR <30 as having severe kidney impairment. Patients receiving maintenance dialysis were categorized as having severe kidney impairment. For UAB participants, information missing for SUN (n=3), statin (n=3), amiodarone (n=3), alcohol (n=2). For UIC participants, information missing for SUN (n=13), statin (n=1), amiodarone (n=1).For UAB participants VKORC1 (n=131) CYP2C9 (n=113) remain to be determined.
VKORC1 variant refers to −1173C>T allele, and includes genotypes TT or CT.
CYP2C9 variant genotype includes *2, *3 alleles among European Americans and *2, *3, *5, *6 and *11 alleles among African Americans.
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; UAB, University of Alabama at Birmingham; UIC, University of Illinois at Chicago; TIA, transient ischemic attack.
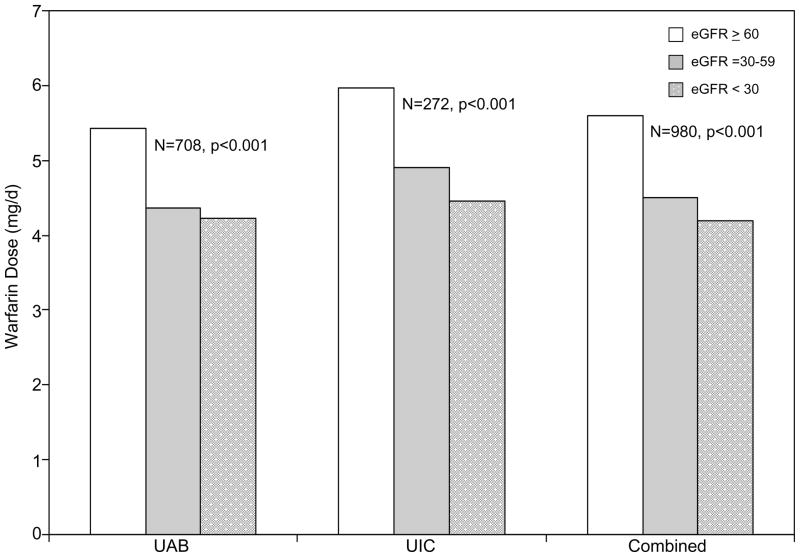
Estimation of kidney function based on eGFR categorized the majority of UAB and UIC participants (59.3% and 65.8%) as no/mild kidney impairment, 31.8% and 27.6% as moderate kidney impairment, and 8.9% and 6.6% as severe kidney impairment, respectively. The distribution of kidney impairment categories did not differ across the UAB and UIC cohorts (Table 1, p=0.2). Decreased kidney function was associated with lower dose requirements among participants of the UAB and UIC cohorts (Figure 1). Warfarin dose requirements were significantly lower in patients with moderate (p<0.001) and severe (p<0.001) kidney impairment compared to those with none/mild kidney impairment.
Figure 1. Influence of kidney function on warfarin dose requirements.
Average warfarin dose by stratified by cohort. UAB denotes cohort of participants from the University of Alabama at Birmingham. UIC denotes participants from the University of Illinois in Chicago. The combined cohort includes participants from UAB and UIC. Patients eGFR ≥60 were categorized as having no/mild kidney impairment, those with eGFR =30–59 were categorized as having moderate kidney impairment and those with eGFR <30 ml/min/1.73 m2 were categorized as having severe kidney impairment. Patients receiving maintenance dialysis were categorized as having severe kidney impairment.
This association remained significant after adjustment for clinical and genetic factors. Among participants of the UAB cohort, moderate kidney impairment was associated with a 10.9% (95% CI: 4.2%–17.1%) and severe kidney impairment was associated with a 21.3% (95% CI, 11.9%–29.6%) decrease in warfarin dose requirements. This kidney impairment-warfarin dose association was consistent in the UIC cohort. Among participants of the UIC cohort, moderate kidney impairment was associated with a 7.2% (95% CI, 2.6%–13.7%) and severe kidney impairment was associated with a 13.9% (95% CI, 5.1%–25.5%) decrease in warfarin dose requirements (p=0.04).
In the combined UAB-UIC cohort, alcohol (p=0.1), concomitant use of statins (p=0.9), current smoking (p=0.6), gender (p=0.4), race (p=0.2) and site (UAB vs. UIC, p=0.5) did not have a significant influence on warfarin dose. Table 2 displays dose requirements and percent dose-changes accounted for by significant clinical and genetic predictors. As compared to patients with no/mild kidney impairment, patients with moderate kidney impairment required 9.5% lower doses (p<0.001) and patients with severe kidney impairment required 19.1% lower doses (p<0.001). Reduced kidney function, was associated with lower warfarin dose requirements independently of CYP2C9 and VKORC1 genotype and clinical factors. In the combined cohort (Table 3), incorporation of kidney function improved prediction of the variance in warfarin dose requirements (F2,966=16.7; p<0.001) over that explained by other clinical variables only. Consistent with previous reports, incorporation of CYP2C9 and VKORC1 genotypes significantly improved prediction and decreased mean prediction error (F2,834=83.3; p<0.001).
Table 2.
Influence of kidney function, clinical factors, andVKORC1 and CYP2C9 genotype on warfarin dose requirements in the combined cohort
| Warfarin Dosed (mg/d) | % Dose Change | p value | |
|---|---|---|---|
| Referent patienta | 7.0 (6.5–7.7) | ||
| Variable | |||
| Age (per decade increase over 40) | 6.5 (6.0–6.9) | −7.2 (−5.6 to −8.7) | <0.001 |
| Weight (per 10 lb increase over 180) | 7.0 (6.5–7.7) | 1.7 (1.5 to 2.3) | <0.001 |
| Height (per inch increase over 68 inches) | 7.0 (6.5–7.7) | 1.4 (0.7 to 2.0) | <0.001 |
| Concurrent amiodarone | 5.8 (5.0–6.6) | −17.2 (−9.1 to −24.5) | <0.001 |
| VKORC1 variantb | 5.2 (4.7–5.7) | −25.6 (−21.6 to −29.3) | <0.001 |
| CYP2C9 variantc | 5.5 (4.7–6.4) | −19.9 (−15.0 to −24.6) | <0.001 |
| Moderate kidney impairment (eGFR 30–59) | 6.4 (5.5–7.3) | −9.5 (−4.4 to −13.6) | <0.001 |
| Severe kidney impairment (eGFR<30) | 5.7 (4.8–6.8) | −19.1 (−11.4 to −26.1) | <0.001 |
Note: Combined cohort includes University of Alabama at Birmingham and University of Illinois at Chicago participants. Values in parentheses are 95% confidence intervals. eGFR (estimated glomerular filtration rate) is based on National Kidney Foundation staging using the Modification of Diet in Renal Disease Study equation, and is reported in mL/min/1.73 m2 (factor for conversin to mL/s/1.73 m2, ×0.01667). Patients eGFR >60 were categorized as having no/mild kidney impairment, those with eGFR =30–59 as having moderate kidney impairment and those with eGFR <30 as having severe kidney impairment. Patients receiving maintenance dialysis were categorized as having severe kidney impairment.
The referent patient is a 40 year old man weighing 180 pounds, 68” tall, with wild-type CYP2C9 and VKORC1 genotype, GFR ≥60, not on current amiodarone therapy.
VKORC1 variant refers to −1173C>T allele, and includes genotypes TT or CT.
CYP2C9 variant genotype includes *2, *3 alleles among European Americans and *2, *3, *5, *6 and *11 alleles among African Americans.
Abbreviations: eGFR, estimated glomerular filtration rate.
Dose is equal to the exponent of 1.94–0.074 (for each decade of age over 40) + 0.0174 (for each 10 pound increment over 180lbs) + 0.0137 (for each inch of height over 68”) – 0.188 (if concurrent amiodarone therapy) – 0.222 (if CYP2C9 variant) – 0.295 (if VKORC1 variant) – 0.094 (if eGFR=30–59) – 0.212 (if eGFR <30).
Table 3.
Dosing accuracy in the combined cohort
| Clinical variables1 | Clinical + eGFR 2 | Clinical + eGFR + Genes3 | |
|---|---|---|---|
| UAB cohort R2 | 22.6% | 25.2% | 37.8% |
| UIC cohort R2 | 28.0% | 29.0% | 42.6% |
| Combined cohort R2 | 22.8% | 25.3% | 38.0% |
Note: Combined cohort includes University of Alabama at Birmingham and University of Illinois at Chicago participants. eGFR (estimated glomerular filtration rate) is based on National Kidney Foundation staging using the Modification of Diet in Renal Disease Study equation. Patients eGFR >60 in mL/min/1.73 m2 were categorized as having no/mild kidney impairment, those with eGFR =30–59 in mL/min/1.73 m2 as having moderate kidney impairment and those with eGFR <30 in mL/min/1.73 m2 ml/min/1.73 m2 as having severe kidney impairment. Patients receiving maintenance dialysis were categorized as having severe kidney impairment.
Clinical variables include age, gender, race, weight and concurrent amiodarone use
Clinical + eGFR includes clinical variables and eGFR category.
Clinical + eGFR + genes includes clinical variables, eGFR category and CYP2C9 and VKORC1 genotype (CYP2C9 variant genotype includes *2, *3 alleles among European Americans and *2, *3, *5, *6 and *11 alleles among African Americans; VKORC1 variant refers to −1173C>T allele, and includes genotypes TT or CT).
Discussion
The influence of kidney function on disposition of drugs excreted by the kidney is widely recognized, and used to derive dosing reductions in patients with kidney impairment. However, there is now an increasing appreciation that kidney impairment can also reduce non-renal clearance and alter the bioavailability of and response to drugs predominantly metabolized by the liver.8, 24 The current study demonstrates that dose requirement for warfarin, a drug primarily metabolized by the hepatic cytochrome P450 system, is influenced by kidney function. Patients with moderate and severe kidney impairment require lower (~10% and 20%, respectively) warfarin doses compared to those with none/mild kidney impairment. To our knowledge, this is the first report that provides guidance on warfarin dose adjustments in patients with impaired kidney function.
Animal studies in end-stage-renal-disease (ESRD) have shown a significant down-regulation (40–85%) of hepatic cytochrome P450 metabolism.27, 28 Invitro-invivo correlations in human subjects have also demonstrated a substantial decrease in non-renal clearance and increase in the area under the curve 8, 29, 30 of hepatically cleared drugs. These findings are corroborated by clinical data demonstrating significantly higher systemic exposure of hepatically cleared drugs at equivalent doses among patients with kidney impairment and may at least partially account for the high rates of drug toxicity in this population.8, 30 Experience with rosuvastatin and telithromycin highlight that dosing adjustments are warranted in spite of a drug’s non-renal route of elimination.24 Most of these data are derived from observational studies and post-marketing analysis as patients with severe kidney impairment are routinely excluded from (or underrepresented in) randomized clinical trials.31–34 Thus, observational studies are valuable in optimizing drug therapy management in the patients with kidney impairment.
In plasma, the ratio of the warfarin enantiomers (S)-warfarin and (R)-warfarin (ie, the warfarin S/R ratio) offers a convenient in vivo probe for monitoring relative changes in CYP2C9 activity, because (S)-warfarin is metabolized almost exclusively by CYP2C9 whereas (R)-warfarin is metabolized by multiple CYP and non-CYP pathways.35, 36 Utilizing this approach, Dreisbach et al14 demonstrated a 50% increase in the plasma warfarin S/R ratio among ESRD patients compared to those without ESRD after accounting for CYP2C9 genotype, providing supportive evidence of decrease in hepatic CYP2C9 activity in kidney failure. This may explain why patients with reduced kidney function require lower warfarin doses. We previously showed the 2.5-fold higher risk of hemorrhage among warfarin users with severe kidney impairment after accounting for genetic and clinical factors.17 The current study demonstrates that both moderate and severe kidney impairment is associated with significantly lower warfarin dose requirements in order to maintain therapeutic anticoagulation.
The current FDA recommendations suggest kidney function staging can be based on eGFR22 or CCr,37 but, ideally, adjustments should be provided for both methods of staging.24 Historically creatinine clearance (CCr) estimated by using the Cockcroft–Gault equation was widely used as a measurement of kidney function to provide guidance on dosing in patients with impaired renal function.37 However, in the past few years the estimated glomerular filtration rate (eGFR) based on the Modification of Diet in Renal Disease Study (MDRD) equation 22 has supplanted the CCr as the best overall measure of kidney function 38, 39 Therefore we provide warfarin dose adjustments staging kidney function based on eGFR. The high prevalence of kidney impairment (34%–40%) in our cohorts of chronic warfarin users highlights the size of the population that stands to benefit from incorporating kidney function in dosing decisions. The value of eGFR is further enhanced as it can be easily calculated using the standardized serum creatinine reported as part of the fluid balance profile which is available in most patients. Moreover as eGFR reporting is a key component of a public-health strategy for CKD,40–42 more than 75% of laboratories now reporting eGFR (along with SCr) in the US.43 This enhances the ease of application of the eGFR in clinical practice, patient care, and public health.
This study has limitations worth noting. First, the UAB and UIC cohorts did not routinely collect blood samples for warfarin (enantiomers and metabolites) or albumin concentration determinations. Thus we could not conduct analysis to understand alterations in albumin binding and resultant changes in warfarin pharmacokinetics.44 Second, as vitamin K levels or levels of coagulation factors were not measured we could not evaluate their contribution on differences in warfarin dosing noted herein. Third, we did not assess other biomarkers, such as cystatin level45–49 or eGFR calculated using the CKD Epidemiology Collaboration (CKD-Epi) equation,50 which have demonstrated more accurate prediction of kidney function. However, the disparity between temporal trends when kidney function is assessed with different measurements suggests that estimating trends in disease burden remains an open question.47 Moreover, eGFR is a clinically feasible method of estimating kidney function, and thus, our data are readily applicable to warfarin dosing decisions.
The significance of these findings is underscored by the increasing (10 to 13% from 1988 to 2004) prevalence of reduced kidney fucntion.1 The prevalence of CKD in persons aged 64 years or older varies from 23.4% to 35.8%.51 The higher kidney impairment prevalence in our cohort is perhaps explained by the higher cardiovascular comorbidity associated with warfarin candidacy.4, 5, 52–56 Our racially diverse cohort is representative of the aging population of warfarin users. Moderate and severe kidney impairment were associated with a reduction in warfarin dose requirements. The high prevalence of kidney impairment in our cohort highlights that diminished kidney function may have implications for a larger proportion of warfarin users than previously estimated. Moreover, as the prevalence of disease (e.g. atrial fibrillation) and risk factors (e.g. obesity, diabetes) associated with thrombosis increases,57 the use of warfarin in individuals with reduced kidney function will likely increase as well.
In conclusion, moderate and severe kidney impairment was associated with a reduction in warfarin dose required to maintain target international normalized ratio (INR). The increasing prevalence of CKD in the general population and the high prevalence in patients with cardiovascular morbidity suggests that diminished kidney function may have implications for a larger proportion of warfarin users than previously estimated.
Acknowledgments
The authors thank Dr. Joyce Goldstein and Joyce Blaisdell for their work with CYP2C9 genotyping. We are grateful to all the patients that participated in the study. We thank Janice Ware, Joseph Huffstutler, Roberta Hill and Alison Del Carmen, Edith Nutescu, and Nancy Shapiro for their untiring efforts with patient recruitment and the staff of the Anticoagulation Clinic at The Kirklin Clinic, the Cooper Green Hospital and Jefferson Clinic P.C for their help with identification of potential participants. We also thank the physicians, especially Drs. Mark Wilson, and Melissa Baird; at the University of Alabama at Birmingham and the Health Service Foundation for their support of this research. We appreciate the work of Steve Duncan and Darlene Green and the Office of Data Resources related to the database and quality assurance.
This study has contributed samples to the National Institute of Neurological Disorders and Stroke (NINDS) Human Genetics Resource Center DNA and Cell Line Repository (http://ccr.coriell.org/ninds); NINDS repository sample numbers corresponding to the samples used are ND04466, ND04556, ND04604, ND04605, ND04626, ND04869, ND04907, ND04934, ND04951, ND05036, ND05108, ND05175, ND05176, ND05239, ND05605, ND05606, ND05701, ND05702, ND05735, ND06147, ND06207, ND06385, ND06424, ND06480, ND06706, ND06814, ND06871, ND06983, ND07057, ND07234, ND07304, ND07494, ND07602, ND07711, ND07712, ND08065, ND08596, ND08864, ND08932, ND09079, ND09172, ND09760, ND09761, ND09809.
Support: This work was supported in part by grants from the National Heart Lung and Blood Institute (R01 HL092173), NINDS (K23NS45598), and the NIH National Center for Research Resources (5UL1RR025777-02). Additional support was provided by the University of Illinois, Chicago; American Association of Colleges of Pharmacy New Investigator Award; and a University of Illinois at Chicago Hans Vahlteich Research Award. Work performed at the Broad Institute Center for Genotyping and Analysis is supported by grant U54 RR020278-01 from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007 Nov 7;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Bash LD, Coresh J, Kottgen A, et al. Defining incident chronic kidney disease in the research setting: The ARIC Study. American Journal of Epidemiology. 2009 Aug 15;170(4):414–424. doi: 10.1093/aje/kwp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner DE. Public health consequences of chronic kidney disease. Clinical Pharmacology & Therapeutics. 2009 Nov;86(5):566–569. doi: 10.1038/clpt.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiner DE, Rifkin DE. Kidney function and the risk of cardiovascular disease. BMJ. 2009;338:b1307. doi: 10.1136/bmj.b1307. [DOI] [PubMed] [Google Scholar]
- 5.Reinecke H, Brand E, Mesters R, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol. 2009 Apr;20(4):705–711. doi: 10.1681/ASN.2007111207. [DOI] [PubMed] [Google Scholar]
- 6.Knauf F, Aronson PS. ESRD as a window into America’s cost crisis in health care. J Am Soc Nephrol. 2009 Oct;20(10):2093–2097. doi: 10.1681/ASN.2009070715. [DOI] [PubMed] [Google Scholar]
- 7.Anderson S, Halter JB, Hazzard WR, et al. Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol. 2009 Jun;20(6):1199–1209. doi: 10.1681/ASN.2008080860. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Zhang L, Abraham S, et al. Assessment of the impact of renal impairment on systemic exposure of new molecular entities: evaluation of recent new drug applications. Clinical Pharmacology & Therapeutics. 2009 Mar;85(3):305–311. doi: 10.1038/clpt.2008.208. [DOI] [PubMed] [Google Scholar]
- 9.Nolin TD. Altered nonrenal drug clearance in ESRD. Curr Opin Nephrol Hypertens. 2008 Nov;17(6):555–559. doi: 10.1097/MNH.0b013e3283136732. [DOI] [PubMed] [Google Scholar]
- 10.Michaud J, Naud J, Chouinard J, et al. Role of parathyroid hormone in the downregulation of liver cytochrome P450 in chronic renal failure. J Am Soc Nephrol. 2006 Nov;17(11):3041–3048. doi: 10.1681/ASN.2006010035. [DOI] [PubMed] [Google Scholar]
- 11.Dreisbach AW, Lertora JJ. The effect of chronic renal failure on drug metabolism and transport. Expert Opin Drug Metab Toxicol. 2008 Aug;4(8):1065–1074. doi: 10.1517/17425255.4.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008 Jun;133(6 Suppl):160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 13.Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007 Dec 4;147(11):755–765. doi: 10.7326/0003-4819-147-11-200712040-00006. [DOI] [PubMed] [Google Scholar]
- 14.Dreisbach AW, Japa S, Gebrekal AB, et al. Cytochrome P4502C9 activity in end-stage renal disease. Clinical Pharmacology & Therapeutics. 2003 May;73(5):475–477. doi: 10.1016/s0009-9236(03)00015-8. [DOI] [PubMed] [Google Scholar]
- 15.Elliott MJ, Zimmerman D, Holden RM. Warfarin anticoagulation in hemodialysis patients: a systematic review of bleeding rates. Am J Kidney Dis. 2007 Sep;50(3):433–440. doi: 10.1053/j.ajkd.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Genovesi S, Vincenti A, Rossi E, et al. Atrial fibrillation and morbidity and mortality in a cohort of long-term hemodialysis patients. Am J Kidney Dis. 2008 Feb;51(2):255–262. doi: 10.1053/j.ajkd.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 17.Limdi NA, Beasley TM, Baird MF, et al. Kidney Function Influences Warfarin Responsiveness and Hemorrhagic Complications. J Am Soc Nephrol. 2009 Feb 18;20:912–921. doi: 10.1681/ASN.2008070802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limdi NA, Beasley TM, Crowley MR, et al. VKORC1 polymorphisms, haplotypes and haplotype groups on warfarin dose among African-Americans and European-Americans. Pharmacogenomics. 2008 Oct;9(10):1445–1458. doi: 10.2217/14622416.9.10.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Momary KM, Shapiro NL, Viana MA, Nutescu EA, Helgason CM, Cavallari LH. Factors influencing warfarin dose requirements in African-Americans. Pharmacogenomics. 2007 Nov;8(11):1535–1544. doi: 10.2217/14622416.8.11.1535. [DOI] [PubMed] [Google Scholar]
- 20.Miners JO, Birkett DJ. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol. 1998;45(6):525–538. doi: 10.1046/j.1365-2125.1998.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rettie AE, Jones JP. Clinical and toxicological relevance of CYP2C9: drug-drug interactions and pharmacogenetics. Annual Review of Pharmacology & Toxicology. 2005;45:477–494. doi: 10.1146/annurev.pharmtox.45.120403.095821. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006 Aug 15;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003 Jul 15;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 24.Huang SM, Temple R, Xiao S, Zhang L, Lesko LJ. When to conduct a renal impairment study during drug development: US Food and Drug Administration perspective. Clinical Pharmacology & Therapeutics. 2009 Nov;86(5):475–479. doi: 10.1038/clpt.2009.190. [DOI] [PubMed] [Google Scholar]
- 25.Limdi N, Goldstein J, Blaisdell J, Beasley T, Rivers C, Acton R. Influence of CYP2C9 Genotype on warfarin dose among African American and European Americans. Per Med. 2007 May 1;4(2):157–169. doi: 10.2217/17410541.4.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Limdi NA, McGwin G, Goldstein JA, et al. Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Clinical Pharmacology & Therapeutics. 2008 Feb;83(2):312–321. doi: 10.1038/sj.clpt.6100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dreisbach AW, Lertora JJ. The effect of chronic renal failure on hepatic drug metabolism and drug disposition. Semin Dial. 2003 Jan-Feb;16(1):45–50. doi: 10.1046/j.1525-139x.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- 28.Leblond F, Guevin C, Demers C, Pellerin I, Gascon-Barre M, Pichette V. Downregulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol. 2001 Feb;12(2):326–332. doi: 10.1681/ASN.V122326. [DOI] [PubMed] [Google Scholar]
- 29.Elston AC, Bayliss MK, Park GR. Effect of renal failure on drug metabolism by the liver. British Journal of Anaesthesia. 1993 Aug;71(2):282–290. doi: 10.1093/bja/71.2.282. [DOI] [PubMed] [Google Scholar]
- 30.Nolin TD, Frye RF, Le P, et al. ESRD impairs nonrenal clearance of fexofenadine but not midazolam. J Am Soc Nephrol. 2009 Oct;20(10):2269–2276. doi: 10.1681/ASN.2009010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ezekowitz MD, Connolly S, Parekh A, et al. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009 May;157(5):805–810. e801–802. doi: 10.1016/j.ahj.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Young AM, Billingham LJ, Begum G, et al. Warfarin thromboprophylaxis in cancer patients with central venous catheters (WARP): an open-label randomised trial. Lancet. 2009 Feb 14;373(9663):567–574. doi: 10.1016/S0140-6736(09)60205-1. [DOI] [PubMed] [Google Scholar]
- 33.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. New England Journal of Medicine. 2005 Jul 21;353(3):238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 34.Strippoli GF, Navaneethan SD, Johnson DW, et al. Effects of statins in patients with chronic kidney disease: meta-analysis and meta-regression of randomised controlled trials. BMJ. 2008 Mar 22;336(7645):645–651. doi: 10.1136/bmj.39472.580984.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaminsky LS, Zhang ZY. Human P450 metabolism of warfarin. Pharmacology & Therapeutics. 1997;73(1):67–74. doi: 10.1016/s0163-7258(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 36.Rettie AE, Korzekwa KR, Kunze KL, et al. Hydroxylation of warfarin by human cDNA-expressed cytochrome P-450: a role for P-4502C9 in the etiology of (S)-warfarin-drug interactions. Chemical Research in Toxicology. 1992 Jan-Feb;5(1):54–59. doi: 10.1021/tx00025a009. [DOI] [PubMed] [Google Scholar]
- 37.Spruill WJ, Wade WE, Cobb HH., 3rd Continuing the use of the Cockcroft-Gault equation for drug dosing in patients with impaired renal function. Clinical Pharmacology & Therapeutics. 2009 Nov;86(5):468–470. doi: 10.1038/clpt.2009.187. [DOI] [PubMed] [Google Scholar]
- 38.Stevens LA, Levey AS. Use of the MDRD study equation to estimate kidney function for drug dosing. Clinical Pharmacology & Therapeutics. 2009 Nov;86(5):465–467. doi: 10.1038/clpt.2009.124. [DOI] [PubMed] [Google Scholar]
- 39.Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 2009 Nov;20(11):2305–2313. doi: 10.1681/ASN.2009020171. [DOI] [PubMed] [Google Scholar]
- 40.Jain AK, McLeod I, Huo C, et al. When laboratories report estimated glomerular filtration rates in addition to serum creatinines, nephrology consults increase. Kidney International. 2009 Aug;76(3):318–323. doi: 10.1038/ki.2009.158. [DOI] [PubMed] [Google Scholar]
- 41.Levey AS, Schoolwerth AC, Burrows NR, Williams DE, Stith KR, McClellan W. Comprehensive public health strategies for preventing the development, progression, and complications of CKD: report of an expert panel convened by the Centers for Disease Control and Prevention. American Journal of Kidney Diseases. 2009 Mar;53(3):522–535. doi: 10.1053/j.ajkd.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 42.Stevens LA, Levey AS. Impact of reporting estimated glomerular filtration rate: it’s not just about us. Kidney International. 2009 Aug;76(3):245–247. doi: 10.1038/ki.2009.143. [DOI] [PubMed] [Google Scholar]
- 43.Miller WG. Reporting estimated GFR: a laboratory perspective. American Journal of Kidney Diseases. 2008 Oct;52(4):645–648. doi: 10.1053/j.ajkd.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 44.Meijers BK, Bammens B, Verbeke K, Evenepoel P. A review of albumin binding in CKD. American Journal of Kidney Diseases. 2008 May;51(5):839–850. doi: 10.1053/j.ajkd.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 45.Selvin E, Kottgen A, Coresh J. Kidney function estimated from serum creatinine and cystatin C and peripheral arterial disease in NHANES 1999–2002. European Heart Journal. 2009 Aug;30(15):1918–1925. doi: 10.1093/eurheartj/ehp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taglieri N, Koenig W, Kaski JC. Cystatin C and cardiovascular risk. Clinical Chemistry. 2009 Nov;55(11):1932–1943. doi: 10.1373/clinchem.2009.128397. [DOI] [PubMed] [Google Scholar]
- 47.Foley RN, Wang C, Snyder JJ, Collins AJ. Cystatin C levels in U.S. adults, 1988–1994 versus 1999–2002: NHANES. Clin J Am Soc Nephrol. 2009 May;4(5):965–972. doi: 10.2215/CJN.05281008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. American Journal of Kidney Diseases. 2008 Mar;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tidman M, Sjostrom P, Jones I. A Comparison of GFR estimating formulae based upon s-cystatin C and s-creatinine and a combination of the two. Nephrol Dial Transplant. 2008 Jan;23(1):154–160. doi: 10.1093/ndt/gfm661. [DOI] [PubMed] [Google Scholar]
- 50.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wattanakit K, Cushman M, Stehman-Breen C, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008 Jan;19(1):135–140. doi: 10.1681/ASN.2007030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wattanakit K, Folsom AR, Selvin E, Coresh J, Hirsch AT, Weatherley BD. Kidney function and risk of peripheral arterial disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol. 2007 Feb;18(2):629–636. doi: 10.1681/ASN.2005111204. [DOI] [PubMed] [Google Scholar]
- 54.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004 May;15(5):1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe H, Watanabe T, Sasaki S, Nagai K, Roden DM, Aizawa Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J. 2009 Oct;158(4):629–636. doi: 10.1016/j.ahj.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 56.Mahajan A, Qiu J, Stark PC, et al. Prevalence of ICD-9-CM codes for chronic kidney disease in individuals with cardiovascular disease risk factors. J Nephrol. 2009 Jul-Aug;22(4):523–533. [PubMed] [Google Scholar]
- 57.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009 Jan 27;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]