Abstract
Background
Chronic kidney disease (CKD) and albuminuria are associated with increased risk of all-cause mortality.
Study Design
Prospective observational cohort study
Setting and Participants
17,393 participants (mean age, 64.3 ± 9.6 years) in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study.
Predictor
Estimated glomerular filtration rate (eGFR), urinary albumin-creatinine ratio (ACR).
Outcome
All-cause mortality (710 deaths); median duration of follow-up: 3.6 years.
Measurements and Analysis
Categories of eGFR (90– <120, 60–<90, 45–<60, 30–<45, and 15–<30 mL/min/1.73 m2) and urinary ACR (<10 mg/g or normal, 10–<30 mg/g or high normal, 30–300 mg/g or high, and >300 mg/g or very high). Cox’s proportional hazards models were adjusted for demographic factors, cardiovascular covariates, and hemoglobin.
Results
The background all-cause mortality rate for participants with normal ACR, eGFR of 90–<120 mL/min/1.73 m2 and no CHD was 4.3 deaths/1,000 person-years. Higher ACR was associated with an increased multivariable adjusted hazard ratio for all-cause mortality within each eGFR category. Reduced eGFR was associated with higher adjusted hazard ratio for all-cause mortality for participants with high normal (P value = 0.01) and high (P value <0.001) ACR values, but not for those with normal or very high ACR values.
Limitations
Only one laboratory assessment for serum creatinine and ACR was available
Conclusions
Increased albuminuria was an independent risk factor for all-cause mortality. Reduced eGFR was associated with increased mortality risk among those with high normal and high ACR. The mortality rate was low in the normal ACR group and increased in the very high ACR group but did not vary with eGFR in these groups.
Chronic kidney disease (CKD) is defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, or urine albumin-creatinine ratio >30 mg/g.1 Even though the definition of CKD is debated,2 reduced eGFR is associated with an increased risk for death, cardiovascular events, and hospitalization rates.3,4 Patients with coronary heart disease (CHD) have been shown to have worse outcomes if they also have CKD.5–7 Thus, the “clinical intersection” between CKD and CHD represents a high-risk state for death and organ failure.8
Albuminuria has been associated with CKD progression in adults,9,10 and is also associated with risk for all-cause mortality,11 especially in the elderly12 and patients with heart failure.13 The independent contributions of reduced eGFR and albuminuria, while only recently addressed in large scale epidemiologic studies of progression to end-stage renal disease10,14 and mortality,14,15 represent another “clinical intersection” that identifies patients with high risk for adverse outcomes. We hypothesized that reduced eGFR and albuminuria are independently associated with all-cause mortality, and that this mortality risk would be increased among individuals with a history of CHD. To test this hypothesis, we analyzed data from the population-based REGARDS (Reasons for the Geographic and Racial Differences in Stroke) study.
METHODS
Study Design and Population
Renal REGARDS is a subcohort of the ongoing REGARDS cohort study.16,17 The purpose of REGARDS is to identify factors that contribute to the excess stroke mortality among black individuals compared to their white counterparts, and in the southeastern United States, compared to the rest of the country.
Participants
The REGARDS study includes 30,239 black and white individuals over age 45 years.16 Participants were recruited using a combination of mail and telephone contact. Medical and risk factor history was obtained by computer-assisted telephone interview. Physical measures were subsequently collected at a single in-home visit and examination, and included blood pressure, blood and urine samples, and electrocardiogram. Study methods were reviewed and approved by the UAB Institutional Review Board, and all participants gave written informed consent for participation in this study.
Participants were recruited between January 2003 and October 2007. After the first 8,608 participants were recruited, additional measures were obtained, including complete blood count, serum albumin, blood urea nitrogen, and 12-lead electrocardiograms in 21,658 participants (Renal REGARDS cohort).16 Values for urine albumin, urine creatinine, or serum creatinine were not available for 1,724 participants; we also excluded 495 participants with eGFR ≥120 or <15 ml/min/1.73 m2; 1,498 with missing covariates (age, gender, race, educational status, current smoking status, body mass index, hypertension, diabetes, dyslipidemia, baseline CHD status or hemoglobin); 515 participants who did not have any follow-up contact following their initial home visit; and 33 other participants on dialysis by self-report. Remaining were 17,393 participants with 710 confirmed deaths as of January 1, 2010.
Data and Data Collection
Data were obtained during the telephone interview included age; race; gender; self-report of previous stroke or transient ischemic attack, myocardial infarction, and hyperlipidemia; smoking status; health insurance status; and assessment of socioeconomic status (primarily education and income). During the subsequent in-home examination, data and samples were obtained that included blood pressure, height and weight, blood and urine samples, and electrocardiogram. Subsequent follow-up is made by telephone contact every 6 months.
Laboratory Measures
Serum creatinine assays were performed at the University of Vermont, and calibrated with an isotope dilution mass spectroscopic standard.18 The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to obtain estimated GFR (eGFR).19 Hemoglobin was measured as part of a complete blood count, as described elsewhere.20 Urinary albumin was measured at the Department of Laboratory Medicine and Pathology at the University of Minnesota, using the BN ProSpec Nephelometer from Dade Behring (www.dadebehring.com). The observed assay range was 2.4 to 76.9 mg/L on initial sampling. The inter-assay coefficients of variations were 2.2% at 109.9 mg/L and 4.3% at 12.7 mg/L. Urinary creatinine was measured with a rate-blanked Jaffé procedure, using the Modular-P analyzer (Roche/Hitachi;labsystems.roche.com). The observed assay range was 1 to 650 mg/dL on initial sampling. The inter-assay coefficients of variations were 2.4% at 66.6 mg/dL and 7.8% at 15.6 mg/dL. The results were expressed for each participant as the urinary albumin-creatinine ratio (ACR).
Definition of eGFR, and Urinary ACR Categories
The eGFR categories (mL/min/1.73 m2) were defined as: 90–<120; 60– <90; 45–<60; 30– <45; and 15– <30. Urinary ACR categories (mg/g) were defined as: normal (<10); high normal (10 to <30); high (30 to 300) and very high (>300).12,21
Definition of CHD and Other Covariates
Cardiovascular covariates were defined by the parent REGARDS study.22 History of CHD included self-reported myocardial infarction, or by electrocardiographic criteria, or previous coronary artery by-pass, angioplasty or stent procedure. The electrocardiographic criteria for previous myocardial infarction was based on the Minnesota classification.23 Hypertension was defined as elevated blood pressure (systolic ≥140 or diastolic ≥90 mm Hg) or self-reported medications for blood pressure; diabetes was defined as fasting glucose ≥126 mg/dL, non-fasting glucose ≥200 mg/dL, or self-reported diabetes or use of medications or insulin for diabetes; dyslipidemia was defined as serum total cholesterol ≥240 mg/dL, or low-density lipoprotein cholesterol ≥160 mg/dL, or high density lipoprotein cholesterol <40 mg/dL, or self-reported medications for dyslipidemia.
Study Outcome
The primary outcome was all-cause mortality. Death status was queried through active follow-up with participants or with contacts provided at recruitment and online searches of the Social Security Administration’s Death Master File (ssdi.rootsweb.ancestry.com). Of the 710 deaths included in the current analysis, 483 (68%) were identified through proxy contact and verified through online searches, 104 (15%) were identified through online searches, and 123 (18%) were identified via proxy contact alone.
Statistical analysis
Baseline characteristics of study participants are reported as mean ± standard deviation (SD), or as counts with percentages. Mortality-free survival curves using the Kaplan Meier method were used to assess time to death stratified by eGFR and urinary ACR categories. Using a Cox’s proportional hazards regression, restricted quadratic splines with 4 knots were used to model the age-adjusted hazard ratio (HR) for all-cause mortality associated with urinary ACR or eGFR as separate variables. Cox’s proportional hazards models were also used to calculate the multivariable-adjusted HRs for all-cause mortality associated with the eGFR and urinary ACR categories. Multivariable adjustment included age, race, gender, educational status, current smoking status, body mass index, hypertension, diabetes, dyslipidemia and hemoglobin. As it may be an intermediate factor in the pathway between CKD and mortality, analyses were repeated without adjustment for hemoglobin. For analysis of mortality risk stratified by prevalent CHD, eGFR was adjusted for urinary ACR, and urinary ACR was adjusted for eGFR. Cox’s proportional hazards regression analysis, performed as described by Li and Chambless, was used to calculate the HRs for all-cause mortality associated with the cross-classification of eGFR and ACR categories.24 The standard deviations, and 95% confidence intervals (CI) of the joint probabilities of all-cause mortality were calculated as described previously.24,25 Linear trends in the HRs for mortality associated with eGFR and ACR, modeled as categorical variables, used the median values of the categories as the independent variable. Differences in the association between lower eGFR categories and higher mortality by ACR categories were assessed by comparing the log likelihood of models with and without interaction terms. A similar approach was used to assess differences in the association between higher ACR categories and higher mortality by eGFR categories. We evaluated the proportional hazards assumption of the Cox’s models by analyzing and inspecting the generalized linear regressions of the scaled Schoenfeld residuals as a function of time. Hypertension was the only covariable that appeared to violate the proportional hazard assumption. Therefore, in addition to hypertension, a hypertension × log(follow-up time) interaction term was included in the multivariable adjusted models. SAS, version 9.2 (SAS Institute, www.sas.com) was used for primary analyses, and Stata (version 11, www.stata.com) and R (Version 2.8, www.r-project.org) for graphical support. Statistical significance was set at 5%.
RESULTS
Of 17,393 REGARDS participants in the present analysis, 6,656 (38.3%) were male, and 10,856 (62.4%) were white and 3,842 (22.1%) had a history of CHD at baseline (Table 1). The mean age was 64.3 ± 9.6 years, the mean eGFR was 85.5 ± 18.7 mL/min/1.73 m2, and the median (25th, 75th percentile) urinary ACR was 7.1 mg/g (4.6, 14.6 mg/g). The prevalence of traditional cardiovascular risk factors are presented in Table 1 for all participants, as well as stratified by a history of CHD. The overall mortality rate was 11.4 deaths per 1,000 person-years. All-cause mortality was higher for those with versus without a history of CHD (20.5 and 8.9 deaths per 1,000 person-years). The background mortality rate was 4.26 deaths/1,000 person-years among 4,628 participants with eGFR of 90–<120 mL/min/1.73 m2, normal ACR and no history of CHD during 3.5 ± 1.2 years of follow-up (69 deaths). The number of participants, deaths, follow-up duration, and death rates per 1,000 person-years are presented for each eGFR and urinary ACR category, stratified by CHD status in Table S1 (provided as online supplementary material available with this article at www.ajkd.org).
Table 1.
Baseline Characteristics of Study Participants, by CHD History
| All Participants (N=17,393) | No History of CHD (n=13,551) | History of CHD (n=3,842) | |
|---|---|---|---|
| Demographic factors | |||
| Age (yr) | 64.3 ± 9.6 | 63.4 ± 9.4 | 67.3 ± 9.3 |
| Male (%) | 6,656 (38.3) | 4,744 (35.0) | 1,912 (49.8) |
| White race (%) | 10,856 (62.4) | 8,394 (61.9) | 2,462 (64.1) |
| Education ≥ High School (%) | 15,501 (89.1) | 12,206 (90.1) | 3,295 (85.8) |
| Kidney-specific predictors | |||
| eGFR (mL/min/1.73 m2) | 85.5 ± 18.7 | 87.0 ± 18.0 | 80.5 ± 20.1 |
| ACR | 7.1 (4.6, 14.6) | 6.9 (4.5, 13.5) | 8.7 (5.2, 20.0) |
| Other Covariates | |||
| Current Smoker | 2,392 (13.8) | 1,791 (14.1) | 601 (15.6) |
| Body Mass Index (kg/m2) | 29.3 ± 6.2 | 29.3 ± 6.2 | 29.3 ± 6.1 |
| Hemoglobin (g/dL) | 13.7 ± 1.4 | 13.7 ± 1.4 | 13.7 ± 1.5 |
| Diabetes (%) | 3,336 (19.2) | 2,297 (17.0) | 1,039 (27.0) |
| Hypertension (%) | 10,022 (57.6) | 7,381 (54.5) | 2,641 (68.7) |
| Dyslipidemia (%) | 9,943 (57.2) | 7,238 (53.4) | 2,704 (70.4) |
| All-Cause Mortality | |||
| deaths (%) | 710 (4.1) | 428 (3.2) | 282 (7.3) |
| Follow-up duration (year) | 3.6 ± 1.2 | 3.6 ± 1.2 | 3.6 ± 1.3 |
| Deaths/1,000 person-year | 11.4 | 8.9 | 20.5 |
Note: Values shown as means ± SD, number (column percentage), or median (25th, 75th percentile). Conversion factor for eGFR in mL/min/1.73 m2 to mL/s/1.73 m2, x0.01667.
Abbreviations: ACR, albumin-creatinine ratio; CHD, coronary heart disease (self-reported myocardial infarction, coronary artery procedure or electrocardiographic evidence of myocardial infarction); eGFR, estimated glomerular filtration rate by the CDK-EPI equation 18.
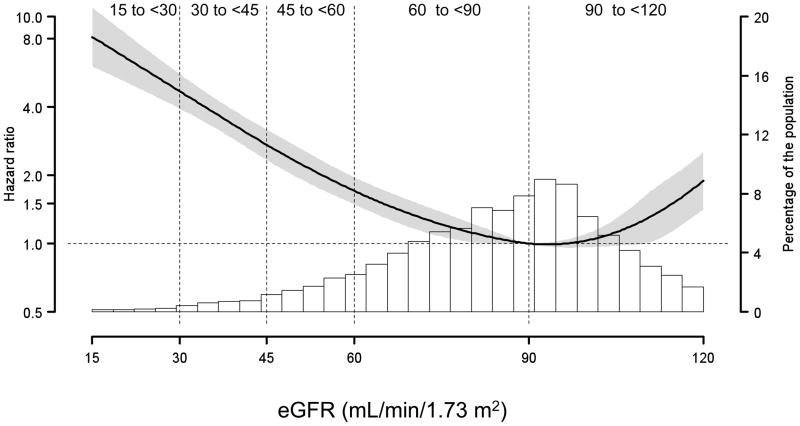
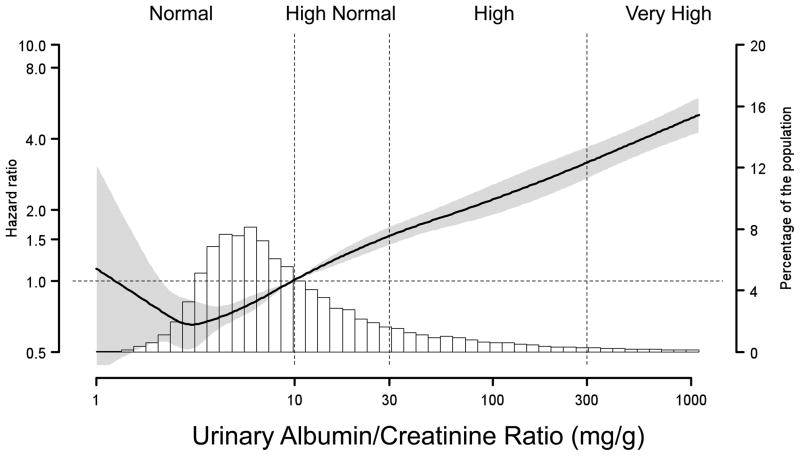
Figure 1 shows the age-adjusted HR for all-cause mortality, plotted as a function of eGFR (first panel) and urinary ACR (second panel; log scale) as continuous variables. With an eGFR of 90 mL/min/1.73 m2 set as the reference, a progressive increased HR for all-cause mortality was detected at lower eGFR levels. There was also an increased risk for all-cause mortality among participants with eGFR >110 mL/min/1.73 m2. The urinary ACR reference value was set at 10 mg/g in the second panel of Figure 1, corresponding to the cut-point between the normal and high normal ACR categories. There was a continuous direct relation of higher mortality risk at higher ACR.
Figure 1. Hazard Ratios for All-Cause Mortality Among 17,393REGARDS Participants.
Estimated glomerular filtration rate (eGFR; first panel), and urinary albumin-creatinine ratio (ACR; second panel), were treated as continuous variables and fitted in a Cox’s proportional Hazards Model using restricted quadratic spline regression, adjusted for age. Knots for the spline were placed at the 10th, 35th, 65th and 90th percentiles. The dashed horizontal lines correspond to the reference values. The reference points were set at 90 mL/min/1.73 m2, and 10 mg/g. Shaded area represents 95% CI for the hazard ratios. Histograms present the distributions of eGFR and ACR among the REGARDS participants. The dotted vertical lines separate the eGFR and ACR categories used for subsequent analysis.
Figure 2 shows the survival curves for urinary ACR categories, across eGFR categories. There was significantly lower survival for individuals with high-normal, high and very high ACR, compared to normal ACR, within each eGFR strata (all log rank P values <0.001). The corresponding numbers at risk are shown in Table 2.
Figure 2. Survival Plots for 17,393REGARDS Participants: All-Cause Mortality Stratified by Urinary Albumin/Creatinine Ratio (ACR), within estimated Glomerular Filtration Rate (eGFR) Categories.
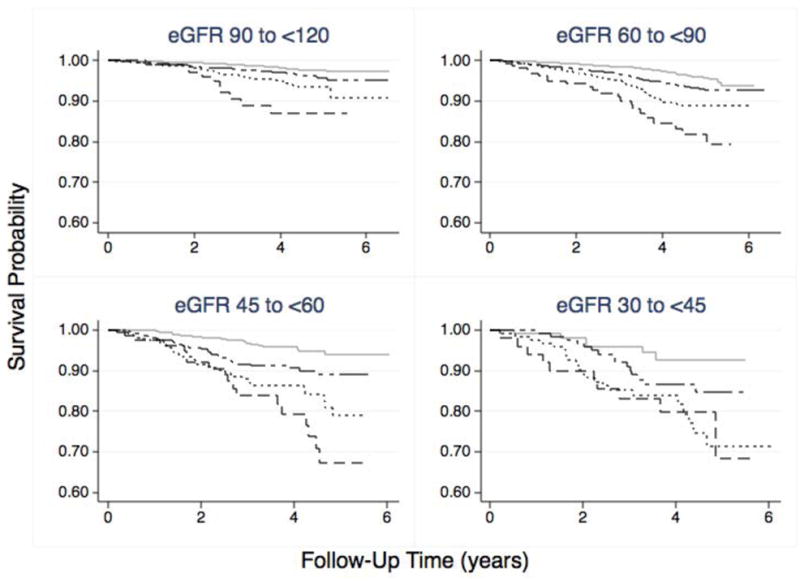
The survival probability curves were obtained by Kaplan-Meier analysis. The solid gray lines represent the survival probabilities for participants with Normal ACR (<10 mg/g). The long-short dashed lines represent participants with High Normal ACR (10 to <30 mg/g), the dotted lines represent participants with High ACR (30 to 300 mg/g) and the dashed lines represent survival probability for participants with Very High ACR (>300 mg/g). The eGFR categories were defined at the baseline evaluation, and the follow-up time was the difference between initial enrollment date and date of confirmed death. There were 710 deaths over an average follow up of 3.6 years. The log rank tests were significant (<0.001) for the entire cohort, as well as each of the ACR categories in each eGFR strata. The numbers at risk for each category are shown below the figure.
Table 2.
Kaplan Meier Survival Analysis, stratified by UACR and eGFR Categories.
| Numbers at Risk | ||||
|---|---|---|---|---|
| 0 y | 2 y | 4 y | 6 y | |
| Normal UACR (<10 mg/g) | ||||
| eGFR categories | ||||
| 90–<120 | 4,292 | 3,963 | 2,043 | 19 |
| 60–<90 | 3,951 | 3,716 | 1,922 | 11 |
| 45–<60 | 454 | 427 | 228 | 2 |
| 30–<45 | 109 | 97 | 51 | 0 |
| 15–<30 * | 14 | 14 | 6 | 0 |
| High Normal UACR (10–<30 mg/g) | ||||
| eGFR categories | ||||
| 90–<120 | 2,817 | 2,574 | 1,349 | 12 |
| 60–<90 | 2,529 | 2,339 | 1,231 | 7 |
| 45–<60 | 390 | 347 | 160 | 0 |
| 30–<45 | 124 | 115 | 55 | 0 |
| 15–<30 * | 21 | 16 | 7 | 1 |
| High UACR (30–300 mg/g) | ||||
| eGFR categories | ||||
| 90–<120 | 831 | 751 | 386 | 5 |
| 60–<90 | 955 | 852 | 433 | 1 |
| 45–<60 | 278 | 241 | 120 | 0 |
| 30–<45 | 126 | 105 | 43 | 1 |
| 15–<30 * | 30 | 23 | 10 | 0 |
| Very High UACR (>300 mg/g) | ||||
| eGFR categories | ||||
| 90–<120 | 113 | 95 | 42 | 0 |
| 60–<90 | 163 | 138 | 70 | 0 |
| 45–<60 | 80 | 66 | 35 | 0 |
| 30–<45 | 50 | 43 | 24 | 0 |
| 15–<30 * | 65 | 55 | 26 | 0 |
Note: eGFR is given in mL/min/1.73 m2; factor for conversion to ml/s/1.73m2, x0.01667.
Abbreviations: eGFR, estimated glomerular filtration rate; UACR, Urinary Albumin-Creatinine Ratio
This category is not shown in Figure 2.
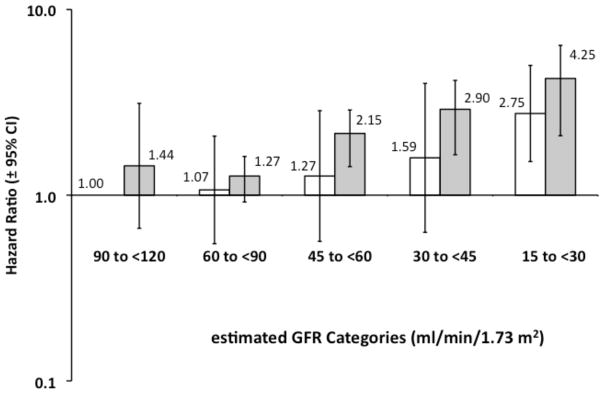
The multivariable adjusted HR for mortality was higher at higher urinary ACR levels for participants with and without a history of CHD (Figure 3, first panel). For example, the HR for mortality associated with high normal ACR compared to normal ACR was 1.63 (95% CI, 1.29–2.05) and 2.00 (95% CI, 1.45–2.55) for those without and with a history of CHD, respectively. The P values for the linear trends across ACR categories for those without and with CHD were 0.01 and <0.001, respectively. Compared to those with an eGFR of 90–<120 ml/min/1.73m2, the multivariable adjusted HR for all-cause mortality was increased for eGFR levels <60 mL/min/1.73 m2 (Figure 3, second panel) among individuals with prevalent CHD and <30 ml/min.17.3 m2 among individuals without prevalent CHD. The P values for the linear trends across eGFR categories for those without and with CHD were <0.001.
Figure 3. Hazard Ratios for All-Cause Mortality Among 17,393 REGARDS Participants: Stratified by history of Coronary Heart Disease (CHD) within Urinary Albumin-Creatinine Ratio (ACR) Categories and estimated Glomerular Filtration Rate (eGFR) categories.
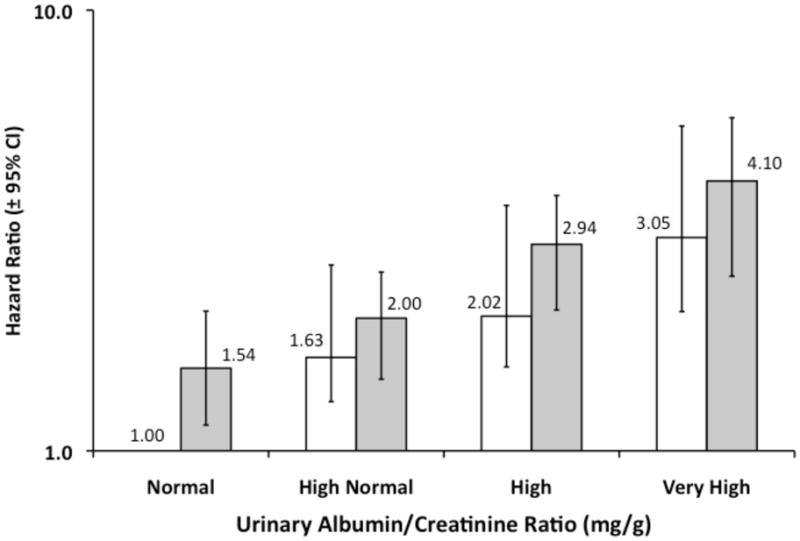
The hazard ratios were obtained with Cox’s proportional hazards regression for categorical analysis,24 and were adjusted for age, race, gender, educational status, current smoking status, body mass index, hypertension, diabetes, dyslipidemia and hemoglobin, and eGFR (upper panel) or ACR (lower panel). Open bars represent the participants without a history of CHD, and gray bars represent participants with prevalent CHD. The Hazard Ratio is presented above each bar with 95% Confidence Intervals indicated by the error bars. Linear trend analysis was significant for all 4 categorical groups. Identical results were observed when hemoglobin was omitted as a covariate.
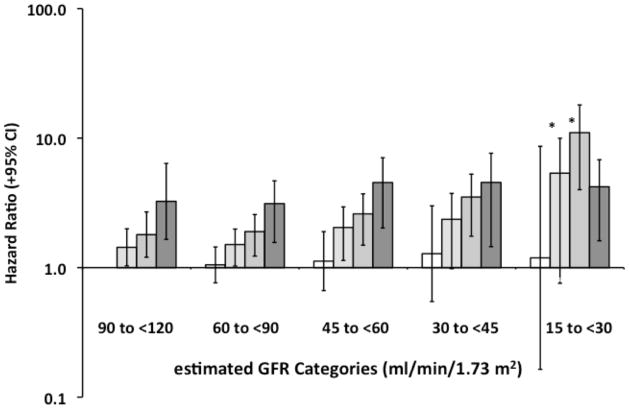
The HR for all-cause mortality associated with eGFR and ACR categories are presented in Figures 4 and 5. The association between lower eGFR and higher mortality differed significantly across ACR categories (P value = 0.02). The HR for all-cause mortality increased progressively with lower eGFR categories for participants with high normal (P value for linear trend = 0.01) and high ACR categories (P value for linear trend <0.001). The HR was modest and did not vary substantially between eGFR categories for participants with normal ACR (P value for trend = 0.4). The HR for mortality associated with very high ACR was increased, but also did not vary by eGFR category (P value for trend = 0.5). Similar results were obtained if participants above and below the median age were compared (data not shown). The association between higher ACR categories and higher mortality was similar across eGFR categories (P value = 0.4). Results were markedly consistent when hemoglobin was omitted from the multivariable adjusted model with the exception of a significant association with increased mortality being present at lower eGFR for participants with very high ACR values.
Figure 4. Hazard Ratios for All-Cause Mortality Among 17,393REGARDS Participants: estimated Glomerular Filtration Rate (eGFR) and Urinary Albumin-Creatinine Ratio (ACR) Categories.
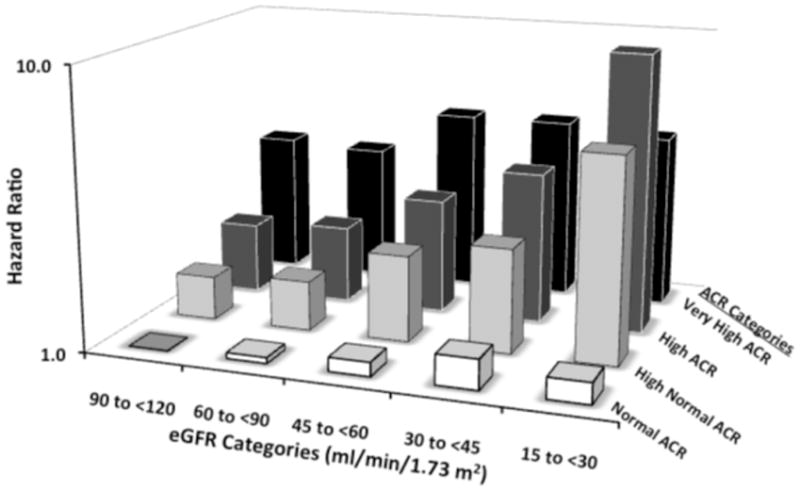
The hazard ratios were obtained with Cox’s proportional hazards regression for categorical analysis,24 and were adjusted for age, race, gender, educational status, current smoking status, body mass index, hypertension, diabetes, dyslipidemia and hemoglobin, as well as interactions between each eGFR and ACR category. Urinary ACR categories (mg/g): Normal, <10; High Normal, 10 to <30; High, 30 to 300; Very High, >300. The P values for albuminuria trends without and with prevalent CHD were significant (P=0.006, and 0.005, respectively).
Figure 5. Hazard Ratios for All-Cause Mortality Among 17,393REGARDS Participants: Stratified by Urinary Albumin/Creatinine Ratio (ACR) Categories within each estimated Glomerular Filtration Rate (eGFR) category.
The hazard ratios were obtained with Cox’s proportional hazards regression for categorical analysis,23 and were adjusted for age, race, gender, educational status, current smoking status, body mass index, hypertension, time-dependent effect of hypertension, diabetes, dyslipidemia and hemoglobin, as well as interactions between each eGFR and ACR category. Open bars represent the normal ACR category (ACR <10 mg/g), and the darker bars represent high normal, high and very high ACR categories in each eGFR strata. The high normal and high ACR series had significant P values (<0.05) for linear trends when regressed on the median eGFR for each category (*). The linear trend analyses for the normal and very high ACR categories were not significant (P value >0.4).
Tables S2–S4 (online supplementary material) show the maximum likelihood estimates assessing the relationship between each of the covariates included in the multivariable model and all-cause mortality, as well as the cross-product terms between the eGFR and urinary ACR categories.
DISCUSSION
Go et al.3 described a significant association between eGFR and the risk of death, cardiovascular events and hospitalization events. There was a risk gradient for eGFR with a step-up in risk for eGFR <45 ml/min/1.73 m2, supporting the division of the eGFR category between 60 and 30 into Stage 3a and Stage 3b CKD. Subsequently, Weiner et al.26 examined the associations between reduced eGFR (<60 mL/min/1.73 m2) and cardiovascular disease (including CHD, stroke, transient ischemic attacks and peripheral vascular disease) in 4 large community-based epidemiologic studies, with a composite outcome of cardiac events, stroke and death. They found that reduced eGFR and prevalent cardiovascular disease were independently associated with the primary outcome, but did not evaluate the risks associated with albuminuria.26 In the current study, we observed a risk gradient for mortality such that the mortality risk was higher with higher albuminuria strata within each eGFR category. This association was observed among participants with and without a history of CHD, and when the joint effects of ACR and eGFR were considered. These findings support the assessment of urinary ACR as well as reduced eGFR for mortality risk in patients with a history of CHD. The association between eGFR and mortality differed by level of ACR. Specifically, lower levels of eGFR were not associated with a significant increase in mortality among individuals with normal and very high ACR. These findings should be confirmed in future studies, and with meta-analyses of combined cohorts.
Post-hoc analyses of previous results26 suggested that an “additive interaction” accounted for excess risk of mortality in patients with cardiovascular disease.27 We observed a similar interaction in preliminary analyses with eGFR stratified at 60 mL/min/1.73 m2 (data not shown), but this effect was not seen when eGFR was stratified into the 5 categories used in the present analysis. We suggest that this apparent interaction27 may have resulted from dichotomizing eGFR into a limited set of categorical variables.28
We, and others15,29–32 have observed a “U” shaped association between eGFR and mortality risk with a minimally adjusted risk model (Figure 2). However, the apparent excess mortality risk associated with eGFR >110 ml/min/1.73 m2 was not present after multivariable adjustment. The adjusted HR was the same for the highest eGFR category (120 to ≥90 mL/min/1.73 m2) when compared to the eGFR category of 60– <90 mL/min/1.73 m2 for all 4 urinary ACR categories.
Increased mortality risk has been reported for high normal ACR, compared to urinary ACR <10 mg/day.12,33–35 Our results confirm these findings. Reduced eGFR has also been associated with increased mortality. In the current analysis, this association was present among participants with high normal and high ACR levels, but the risk for all-cause mortality did not increase at lower eGFR among participants in the normal and very high urinary ACR categories; among those with normal ACR, the mortality risk was low, and for very high ACR, the mortality risk was substantially increased but did not vary with eGFR category.
Hemmelgarn et al. have reported that reduced eGFR and increased albuminuria independently contributed to the cumulative probability of all-cause mortality.14 In agreement with these findings, we observed >2-fold mortality risk among participants in the REGARDS study with eGFR <60 mL/min/1.73 m2 or urinary ACR >30 mg/g in a model adjusted only for age, with the highest mortality risks observed among participants in the lower eGFR and higher urinary ACR categories. The associations of higher mortality risk with lower eGFR and higher urinary ACR was observed for participants with compared to those without a history of CHD, and persisted after full multivariable adjustment for demographic factors, traditional cardiovascular risk factors and hemoglobin.
In addition to confirming the findings of Hemmelgarn et al.14 our analysis extends the characterization of the independent effects on death associated with albuminuria and reduced eGFR. All-cause mortality was the primary outcome for both studies, and was similar (6.3 and 7.84 deaths/1000 person-years) for participants with eGFR ≥60 mL/min/1.73 m2 and urinary ACR ≤30 mg/g. An analytic difference needs to be addressed: Hemmelgarn et al.14 did not stratify urinary ACR ≤30 mg/g, and reported increased risk of all-cause mortality associated with this urinary ACR category. With stratification of urinary ACR into normal (≤10 mg/g) and high normal (10–≤30 mg/g) categories, we can assign the associated risk of mortality to the high normal ACR category, in agreement with previous studies12,33–35 and an extension beyond what Hemmelgarn et al. described14 for the 102,701 subjects with measurements of urinary ACR in the Alberta cohort. There is also a difference in the recruitment strategy for the REGARDS study cohort compared to participants in the Alberta Health System. Urinary ACR was measured for all of the REGARDS participants at the same time as serum creatinine, while “dip-stick” proteinuria was measured in the larger Alberta cohort. Approximately 10% of that cohort also had urinary ACR measurements, apparently as part of a best-practices approach for diabetic patients: the prevalence of diabetes was 19.2% among REGARDS participants, and 54.1% among the Alberta cohort with urinary ACR measurements.14 This difference may account for the risk gradient for all-cause mortality with very high ACR described by Hemmelgarn et al.,14 while among the REGARDS study cohort, the risk gradient associated with very high ACR was flat.
Further examination of this issue is described in a recently published meta-analysis of several large community-based cohorts, including the REGARDS and Alberta cohorts,14 in an effort that is described as the Chronic Kidney Disease Prognosis Consortium.15 While the overall results reported herein are similar to the meta-analysis, there were significant trends for all-cause mortality with ACR across all eGFR categories in the larger scale analysis. This difference may simply reflect the larger number of participants with more advanced CKD in the meta-analysis, although that analysis used the MDRD equation36 and we used the CKD-EPI estimating equation.19 The pooled results were adjusted for the same covariates as the present analysis, with the exception that adjustment for hemoglobin was not carried out in the meta-analysis. Whether or not adjustment for hemoglobin will affect the significance of the ACR trends in the meta-analysis could be evaluated in future investigations. Finally, we stratified the present results for CHD status, while this was adjusted for as a covariate in the larger meta-analysis.15
As proposed in the Steno Hypothesis, albuminuria may be a marker for microangiopathy, glomerular vascular damage and generalized vascular dysfunction in type 1 diabetes.37 Perkovic et al.38 confirmed the association between proteinuria and subsequent risk of coronary heart disease. Jackson et al.13 have associated albuminuria with all-cause mortality as well as poor cardiac outcomes in patients with congestive heart failure. Albuminuria and GFR are strongly associated with progression of CKD 10,39,40 cardiovascular and cerebrovascular events,40,41 and all-cause mortality.14 de Jong and Gansevoort have emphasized that albuminuria is not a “risk factor”, but rather provides evidence of early target organ damage; as such, the kidney serves as a mirror of the systemic vasculature.42
We used the recently developed CKD-EPI estimating equation19 to avoid uncertainties associated with the Modification of Diet in Renal Disease Study equation,19,36 and thereby could address albuminuria as an independent risk for all-cause mortality. Hence, both urinary ACR and eGFR could be used to assess mortality risk rather than introducing potential confounding attributable to including urinary ACR ≥30 mg/g in the classification of CKD Stage 1 and 2.
Our study has several limitations that need to be acknowledged. The laboratory assessments included single determinations at baseline. Due to random measurement error associated with the use of a single baseline measurement, misclassification could bias our results toward the null. Therefore, our reported estimates of the associations between eGFR, albuminuria, CHD and all-cause mortality reported are likely to be conservative. Also, we assumed that the baseline covariates persisted throughout the follow-up period. Additional assessments would be desirable, especially to evaluate risk that vary over time While the Renal REGARDS cohort is relatively large, there are not enough participants and events in the lower eGFR categories to examine the joint effects between eGFR and urinary ACR when the cohort is stratified by co-morbidities in the present report. Increased number of outcome events, including cause-specific mortality and incident end-state renal disease, as well as further meta-analysis of several community-based cohorts will buttress the conclusions presented herein.
Patients with CKD, especially if they are elderly, are more likely to die from CHD than progress to end-stage renal disease.3,4,43 Epidemiological studies can identify the risk factors for all-cause mortality in CKD, with the implication that targeted interventions could improve outcomes. It is notable that the trends for all-cause mortality associated with lower eGFR were relatively flat among participants with normal and very high ACR, indicating that participants with normal ACR have a low mortality risk throughout the entire range of eGFR. Conversely, a graded association of higher mortality with higher ACR levels was present at each eGFR level, suggesting that mortality risk might be lessened if urinary ACR could be reduced in a systematic fashion.
Supplementary Material
Table S1: Number of participants, deaths, follow-up duration, and death rates per 1,000 person-years for each eGFR and urinary ACR category, stratified by CHD status (January 2010 Data Freeze)
Table S2: Analysis of Maximum Likelihood Estimates: 5 × 4 Table; eGFR and ACR Categorical Analysis (January 2010 Data Freeze)
Table S3: Analysis of Maximum Likelihood Estimates: 2×4 Table; CHD and ACR Categorical Analysis (January 2010 Data Freeze)
Table S4: Analysis of Maximum Likelihood Estimates: 2×4 Table; CHD and eGFR Categorical Analysis (January 2010 Data Freeze)
Acknowledgments
A list of the REGARDS Investigators and staff, by institution, follows. The University of Alabama at Birmingham (Study PI, Statistical and Data Coordinating Center, Survey Research Unit): V. Howard PhD, L. Wagner MA, V. Wadley PhD, R. Go PhD, M. Safford MD, E. Temple PhD, M. Stewart MSPH, J. D. Rhodes BSN; Wake Forest University (ECG Reading Center): R. Prineas MD, PhD; University of Vermont (Central Laboratory): Rebekah Boyle; Alabama Neurological Institute (Stroke Validation Center, Medical Monitoring): C. Gomez MD, S. Bowling MD; University of Arkansas for Medical Sciences (Survey Methodology): L. Pulley PhD; University of Cincinnati (Clinical Neuroepidemiology): B. Kissela MD, D. Kleindorfer MD; Examination Management Services, Inc (In-Person Visits): A. Graham; Medical University of South Carolina (Migration Analysis Center): D. Lackland DrPH; Indiana University School of Medicine (Neuropsychology Center): F. Unverzagt PhD; National Institute of Neurological Disorders and Stroke (NINDS; primary funding agency): C. Moy Ph.D.
Support: This research project is supported by a cooperative agreement U01 NS041588 from NINDS (National Institutes of Health [NIH]), with Dr Howard as Principal Investigator. The content is solely the responsibility of the authors and does not necessarily represent the official views of NINDS or NIH. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Additional funding was provided by an investigator-initiated grant-in-aid from Amgen, Inc to Dr Warnock. The manuscript was sent to Amgen for internal review prior to submission for publication, but the company did not have any role in the design and conduct of the study, the collection, management, data analysis, or interpretation of the data.
Footnotes
N section: A list of the REGARDS Investigators appears in the Acknowledgements.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org.
Descriptive Text for Online Delivery
Hyperlink: Supplementary Table S1 (PDF)
About: Number of participants, deaths, follow-up duration, and death rates per 1,000 person-years for each eGFR and urinary ACR category, stratified by CHD status (January 2010 Data Freeze)
Hyperlink: Supplementary Table S2 (PDF)
About: Analysis of Maximum Likelihood Estimates: 5 × 4 Table; eGFR and ACR Categorical Analysis (January 2010 Data Freeze)
Hyperlink: Supplementary Table S3 (PDF)
About: Analysis of Maximum Likelihood Estimates: 2×4 Table; CHD and ACR Categorical Analysis (January 2010 Data Freeze)
Hyperlink: Supplementary Table S4 (PDF)
About: Analysis of Maximum Likelihood Estimates: 2×4 Table; CHD and eGFR Categorical Analysis (January 2010 Data Freeze)
Financial Disclosure: Dr Cushman reports receipt of research support from AMGEN; Dr Kewalramani is a full-time AMGEN employee; Dr McClellan is consultant for and receives research support from AMGEN; Dr Newsome receives research support from AMGEN, Genzyme, Genetech, and Shire; Dr Warnock is a consultant for AMGEN, Genzyme, AMAG, Gilead Sciences and Parion Pharmaceuticals, and receives research support from Genzyme and AMGEN. The remaining authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 2.Winearls CG, Glassock RJ. Dissecting and refining the staging of chronic kidney disease. Kidney Int. 2009;75:1009–1014. doi: 10.1038/ki.2009.49. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 5.Beattie JN, Soman SS, Sandberg KR, et al. Determinants of mortality after myocardial infarction in patients with advanced renal dysfunction. Am J Kidney Dis. 2001;37:1191–1200. doi: 10.1053/ajkd.2001.24522. [DOI] [PubMed] [Google Scholar]
- 6.Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med. 2002;137:555–562. doi: 10.7326/0003-4819-137-7-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 8.McCullough PA. Cardiovascular disease in chronic kidney disease from a cardiologist’s perspective. Curr Opin Nephrol Hypertens. 2004;13:591–600. doi: 10.1097/00041552-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 9.National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49:S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gansevoort RT, de Jong PE. The Case for Using Albuminuria in Staging Chronic Kidney Disease. J Am Soc Nephrol. 2009;20:465–468. doi: 10.1681/ASN.2008111212. [DOI] [PubMed] [Google Scholar]
- 12.Hallan S, Astor B, Romundstad S, Aasarod K, Kvenild K, Coresh J. Association of Kidney Function and Albuminuria With Cardiovascular Mortality in Older vs Younger Individuals: The HUNT II Study. Arch Intern Med. 2007;167:2490–2496. doi: 10.1001/archinte.167.22.2490. [DOI] [PubMed] [Google Scholar]
- 13.Jackson CE, Solomon SD, Gerstein HC, et al. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. 2009;374:543–550. doi: 10.1016/S0140-6736(09)61378-7. [DOI] [PubMed] [Google Scholar]
- 14.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation Between Kidney Function, Proteinuria, and Adverse Outcomes. JAMA. 2010;303:423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 15.Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality: a collaborative meta-analysis of general population cohorts. Lancet. 2010 doi: 10.1016/S0140-6736(10)60674-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard VJ, Cushman M, Pulley L, et al. The Reasons for Geographic and Racial Differences in Stroke Study: Objectives and Design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 17.Warnock DG, McClellan W, McClure LA, et al. Prevalence of chronic kidney disease and anemia among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study: baseline results. Kidney Int. 2005;68:1427–1431. doi: 10.1111/j.1523-1755.2005.00553.x. [DOI] [PubMed] [Google Scholar]
- 18.Kurella Tamura M, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008;52:227–234. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zakai NA, McClure LA, Prineas R, et al. Correlates of anemia in American blacks and whites: the REGARDS Renal Ancillary Study. Am J Epidemiol. 2009;169:355–364. doi: 10.1093/aje/kwn355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54:205–226. doi: 10.1053/j.ajkd.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Howard G, Cushman M, Prineas RJ, et al. Advancing the hypothesis that geographic variations in risk factors contribute relatively little to observed geographic variations in heart disease and stroke mortality. Prev Med. 2009;49:129–132. doi: 10.1016/j.ypmed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prineas R, Crow R, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. Littleton, MA: John Wright-PSG, Inc; 1982. [Google Scholar]
- 24.Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17:227–236. doi: 10.1016/j.annepidem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Zou GY. On the estimation of additive interaction by use of the four-by-two table and beyond. Am J Epidemiol. 2008;168:212–224. doi: 10.1093/aje/kwn104. [DOI] [PubMed] [Google Scholar]
- 26.Weiner DE, Tabatabai S, Tighiouart H, et al. Cardiovascular outcomes and all-cause mortality: exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis. 2006;48:392–401. doi: 10.1053/j.ajkd.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 27.de Mutsert R, Jager KJ, Zoccali C, Dekker FW. The effect of joint exposures: examining the presence of interaction. Kidney Int. 2009;75:677–681. doi: 10.1038/ki.2008.645. [DOI] [PubMed] [Google Scholar]
- 28.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 30.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 31.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 32.Cox HJ, Bhandari S, Rigby AS, Kilpatrick ES. Mortality at Low and High Estimated Glomerular Filtration Rate Values: A “U” Shaped Curve. Nephron Clinical Practice. 2008;110:c67–c72. doi: 10.1159/000151720. [DOI] [PubMed] [Google Scholar]
- 33.Janssen WM, Hillege H, Pinto-Sietsma SJ, Bak AA, De Zeeuw D, de Jong PE. Low levels of urinary albumin excretion are associated with cardiovascular risk factors in the general population. Clin Chem Lab Med. 2000;38:1107–1110. doi: 10.1515/CCLM.2000.165. [DOI] [PubMed] [Google Scholar]
- 34.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 35.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 36.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 37.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32:219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 38.Perkovic V, Verdon C, Ninomiya T, et al. The Relationship between Proteinuria and Coronary Risk: A Systematic Review and Meta-Analysis. PLoS Medicine. 2008;5:e207. doi: 10.1371/journal.pmed.0050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halbesma N, Kuiken DS, Brantsma AH, et al. Macroalbuminuria is a better risk marker than low estimated GFR to identify individuals at risk for accelerated GFR loss in population screening. J Am Soc Nephrol. 2006;17:2582–2590. doi: 10.1681/ASN.2005121352. [DOI] [PubMed] [Google Scholar]
- 40.van der Velde M, Halbesma N, de Charro FT, et al. Screening for albuminuria identifies individuals at increased renal risk. J Am Soc Nephrol. 2009;20:852–862. doi: 10.1681/ASN.2008060655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brantsma AH, Bakker SJ, Hillege HL, de Zeeuw D, de Jong PE, Gansevoort RT. Cardiovascular and renal outcome in subjects with K/DOQI stage 1–3 chronic kidney disease: the importance of urinary albumin excretion. Nephrol Dial Transplant. 2008 doi: 10.1093/ndt/gfn356. [DOI] [PubMed] [Google Scholar]
- 42.de Jong PE, Gansevoort RT. Albuminuria in non-primary renal disease: risk marker rather than risk factor. Nephrol Dial Transplant. 2010;25:656–658. doi: 10.1093/ndt/gfp691. [DOI] [PubMed] [Google Scholar]
- 43.O’Hare AM, Choi AI, Bertenthal D, et al. Age Affects Outcomes in Chronic Kidney Disease. J Am Soc Nephrol. 2007;18:2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Number of participants, deaths, follow-up duration, and death rates per 1,000 person-years for each eGFR and urinary ACR category, stratified by CHD status (January 2010 Data Freeze)
Table S2: Analysis of Maximum Likelihood Estimates: 5 × 4 Table; eGFR and ACR Categorical Analysis (January 2010 Data Freeze)
Table S3: Analysis of Maximum Likelihood Estimates: 2×4 Table; CHD and ACR Categorical Analysis (January 2010 Data Freeze)
Table S4: Analysis of Maximum Likelihood Estimates: 2×4 Table; CHD and eGFR Categorical Analysis (January 2010 Data Freeze)