Abstract
Background
The role of donor specific antibodies (DSA) to mismatched human leukocyte antigen (HLA) and antibodies (Abs) to cardiac self-antigens myosin (MYO) and vimentin (VIM) in the pathogenesis of acute antibody mediated rejection (AMR) in the early posttranplant period (EP,<12months) and cardiac allograft vasculopathy (CAV) in the late posttransplant period (LP,>12months) following heart transplantation (HTx) was studied.
Methods
148 HTx recipients (65 EP, 83 LP) were enrolled in the study. Development of DSA was determined by LUMINEX. Circulating Abs against MYO and VIM in sera were measured using ELISA. Frequency of CD4+ T helper cells (CD4+TH) secreting IFN-γ, IL-17, IL-10 or IL-5 specific to either MYO or VIM were analyzed in vitro using ELISPOT assays.
Results
AMR patients were more likely DSA positive (AMR−:15%, AMR+:70%, p=0.03) and demonstrated increased Abs to MYO (AMR−:144±115mcg/ml, AMR+:285±70 mcg/ml, p=0.033) and VIM (AMR−:37±19mcg/ml, AMR+:103±43mcg/ml, p=0.014). AMR patients demonstrated increased IL-5 CD4+TH specific to MYO (5.2±0.9fold, p=0.003) and VIM (7.3±2.9fold, p=0.004) and decreased IL-10 CD4+TH specific to MYO (2.2±0.4 fold, p=0.009) and VIM (1.7±0.2 fold, p=0.03). CAV patients were more likely DSA positive (CAV−: 25%, CAV+:79%, p=0.03) and demonstrated increased Abs to MYO (CAV−:191±120ug/ml, CAV+:550±98ug/ml, p=0.025) and VIM (CAV−: 55±25ug/ml, CAV+: 255±49ug/ml, p=0.001). CAV patients demonstrated increased IL-17 CD4+TH specific to MYO (10.5±7.3fold, p=0.002) and VIM (7.0±3.9 fold, p=0.003).
Conclusions
The presence of DSA in AMR and CAV is significantly associated with development of Abs to MYO and VIM in post-HTx patients. The induction of high CD4+TH specific to cardiac self-antigens that predominantly secrete IL-5 and IL-17 play a significant role in the development of Abs to self-antigens leading to AMR and CAV respectively.
Introduction
Acute antibody mediated rejection (AMR) is recognized as a major cause of allograft dysfunction following heart transplantation (HTx) and its overall prevalence during the initial postoperative phase has been reported to exceed 40% (1–7). The clinical diagnosis of AMR is heralded by the onset of hemodynamic instability in the absence of cellular rejection or graft atherosclerosis following HTx (3, 8). Recent International Society of Heart and Lung Transplantation (ISHLT) guidelines have proposed AMR as a distinct clinico-pathological entity characterized by presence of allograft dysfunction in concert with histological findings for capillary injury, positive immunofluorescence for C4d in endomyocardial biopsies and detection of circulating donor specific antibodies (DSA) (8, 9). There is now accumulating evidence which suggests that early posttransplant events promote development of a chronic inflammatory process which subsequently leads to transplant rejection (2, 10). Previous studies from our laboratory have demonstrated that lung transplant recipients with primary graft dysfunction have elevated proinflammatory mediators including IP-10, MCP-1, interleukin (IL) IL-1, IL-2, IL-12, IL-15, IL-17 and interferon-gamma (IFN-γ) during the early posttransplant period (10, 11). The increase in proinflammatory mediators is associated with the development of donor-specific human leukocyte antigen (HLA) alloimmunity (12). While there is increasing evidence for the role of circulating Abs in mediating allograft rejection post-HTx, the exact mechanisms by which the alloimmune response leads to rejection still remain nebulous (13, 14).
A major limitation to the long term prognosis of cardiac transplantation is the development of chronic cardiac allograft vasculopathy (CAV) (12, 15, 16). While recent advances in early postoperative management has significantly improved the 1 year post-HTx survival rate, long term survival remains low at 43% at 7 years (17). Cardiac allograft vasculopathy is characterized by obliterative arteriosclerosis with chronic inflammation, medial thickening and concentric fibrous intimal hyperplasia (12, 16). It likely results from an initial injury to the allograft endothelium which sets the stage for a chronic inflammatory state (18). The key determinants contributing to the endothelial cell inflammation include both immunologic factors and non-immunologic factors. It has been suggested that an alloimmune response to mismatched donor major histocompatibility complex (MHC) antigens can break the tolerance to self-antigens (19). Recent studies, both from our laboratory and others, have demonstrated that Abs to self-antigens develop following solid-organ transplantation (20–22). Post-HTx patients have been shown to develop anti-phospholipid antibodies (Abs), anti-ribosomal Abs, anti-muscle protein Abs and anti-intracellular adhesion molecule-1 Abs (23–26). However, the clinical relevance of Abs to donor specific mismatched HLA and cardiac self antigens in the development of AMR and CAV in HTx recipients have yet to be established.
In the present study, we evaluated the role of DSA to mismatched HLA and serum levels of Abs against two important cardiac self antigens, myosin (MYO) and vimentin (VIM) in post-HTx patients who were diagnosed with AMR and CAV in the early and late postoperative period respectively. In addition, we identified immune mechanisms which contribute to the induction of Abs to self antigens in post-HTx patients. Our study results highlight a significant association for Abs to both MYO and VIM in patients with AMR and CAV compared to pts without AMR and CAV respectively. Importantly, serial monitoring of postoperative sera indicated that detection of DSA precedes the detection of Abs to MYO and VIM by 1.7±0.3 and 3.0±0.4 months respectively, suggesting a temporal relationship between DSA and development of Abs to self antigens in post-HTX patients. Furthermore, the development of Abs to self antigens was associated with induction of predominantly IL-5 secreting CD4+ T cells and IL-17 secreting CD4+ T cells against corresponding self antigens in patients with AMR and CAV respectively. This was in parallel to a concomitant loss of IL-10 secreting CD4+T cells in patients with AMR and CAV indicating a breakdown of peripheral tolerance to self antigens.
Material and Methods
Study Population
One hundred and forty eight HTx patients at Barnes-Jewish Hospital/Washington University were prospectively enrolled in the study in accordance with a protocol approved by the Institutional Review Board. Serum and peripheral blood lymphocytes were isolated from whole blood as described previously (27, 28) and stored at −135°C. Concurrent with blood samples collection, endomyocardial biopsies were performed in 65 patients in the early (<12 months) post-HTx period and coronary angiograms were performed in 83 patients in the late (>12 months) post-HTx period. A diagnosis of AMR was made based on ISHLT recommended guidelines on clinical, histological and serological criteria. The features which were evaluated to reach a clinical diagnosis included patient symptoms (fatigue, palpitations), echocardiographic findings of cardiac dysfunction (decreased ejection fraction, restrictive physiology), need for inotropic support, histological evidence (acute capillary injury manifest by capillary endothelial swelling, macrophages or neutrophils in capillaries, absence of features consistent with cellular rejection), immunopathologic evidence (C4d capillary staining, CD68 positivity for capillary macrophages) and serological evidence of donor specific HLA Ab. Treatment was initiated within 24 hours of reaching a diagnosis of AMR by the attending transplant cardiologist. Our institutional preference for initial treatment for AMR includes plasmapheresis, intravenous immunoglobulins (IVIG), and intravenous methylprednisone. Secondary therapy includes the use of plasmapheresis, rituximab, IVIG and methylprednisone. Cardiac allograft vasculopathy was diagnosed based on angiographic criteria. Angiographic evidence of coronary artery stenosis (>50% luminal diameter) of 1 vessel or less was termed none/minimal while coronary artery stenosis (>50% luminal diameter) of 2 vessels or more was termed moderate/severe.
Detection of Abs to HLA
The presence of DSA in sera was identified using a solid-phase assay by Luminex technology (Biosource International Inc, CA). In brief, primary Ab coated beads and incubation buffer were placed into 96-well filter plates. Both samples and standards were incubated with the primary Ab beads at room temperature on an orbital shaker. The wells were then washed and biotinylated secondary Abs were added for a further incubation period of 30 minutes. The wells were washed again and strepatividin-R-phyocoerythrin solution was added and incubated for 15 minutes. Following this, the wells were washed and data read utilizing a dual-laser flow analyzer, the Luminex-100 system version 1.7. Data analysis was performed using the MasterPlex QT 1.0 system (MiraiBio) and a five-parameter regression formula was utilized to allow detection compared to standard curves.
Detection of Abs to self antigens, MYO and VIM
The sera were tested for the development of Abs to MYO and VIM by enzyme linked immunosorbent assay (ELISA) developed in our laboratory. In brief, 96-well plates (Nunc, NY) were coated with 1 ug/mL of commercially available porcine MYO or recombinant purified VIM in phosphate-buffer solution (PBS) overnight at 4°C. The antigen coated wells were blocked for non-specific binding with 1% bovine serum albumin for 2 hours. Sera from post-HTx patients and normal volunteers were tested at dilutions (1:750 and 1:1500) for the presence of Abs against MYO and VIM. Commercially available anti-MYO and anti-VIM were used as positive controls. Specific binding were detected with anti-human IgG, IgM bound to horseradish peroxidase (Jackson ImmunoResearch Laboratory In, PA) and developed with tetramethylbenzidine substrate (Millipore, CA). Immunosorbance was detected at 460nm. Concentration of Abs was calculated based on a standard curve using the binding of known concentration of commercial anti-MYO and anti-VIM Abs (Santacruz Biotechnology, Inc. CA). A two standard deviation from the mean concentration of Abs to MYO and VIM in health control subjects was used as cutoff to determine positive titers of auto-Abs in experimental samples.
Antigen Stimulation
Freshly isolated peripheral blood mononuclear cells (PBMC) were stimulated with individual antigens (20μg/ml) in 24-well culture plates (Fischer Scientific, PA) resuspended in Complete-RPMI 1640 (Gibco, NY) supplemented with 10% heat inactivated AB negative human sera, 2Mm L-Glutamine, 20Mm HEPES, 100 U/mL Penicillin, and 100μg/mL Streptomycin for 3–5 days in a humidified 5% Co2 incubator at 37C. CD4+ T cells were then purified from stimulated PBMCs using immunomagnetic separation cocktails by negative selection (Stem Cell, Canada). The purity of CD4+ T cells obtained by this method were >95% (data not shown).
ELISPOT
CD4+ T cells purified from PBMC cultures stimulated with individual antigens were utilized for performing IFN-γ, IL-5, IL-10 and IL-17 ELISPOT assays as described in our earlier publications (10, 11, 22). In brief, a multi-screen 96-well filtration plate (Millipore) was used to coat with monoclonal Ab to IFN-γ, IL-5, IL-10 and IL-17 (5μg/ml, BD biosciences). The plates were blocked with 5% BSA for one hour and washed three times with phosphate-buffered saline (PBS). CD4+ T cells (3 X 105) were cultured in triplicate in the presence of either MYO (20μg/ml) or VIM (20μg/ml) and autologous irradiated CD4 depleted PBMCs as APCs (1:1) in complete RPMI-1640 medium in a humidified 5% CO2 incubator at 37°C. After 72 hours, the plates were washed three times with PBS and subsequently with 0.05% PBS/Tween 20 three times. Following this, 3ug/mL of biotinylated monoclonal antibodies specific for IFN-γ, IL-5, IL-10 and IL-17 (BD biosciences) resuspended in PBS/BSA/Tween-20 were added. After 12 h at 4°C, the plates were washed three times and horseradish peroxidase labeled streptavidin (1:2000, Pharmigen) was added for 2 hours at room temperature. The spots were developed using Amino-9-ethylcarbazole solution (Pharmigen). Spots were analyzed in an ImmunoSpot Series 1 analyzer (Cellular Technology) and the results were reported as spots per million cells (spm). CD4+ T cells cultured alone in medium with APCS were used as a negative control. CD4+ T cells cultured in the presence of PHA (5μg/ml) were used as positive control. The number of spots observed in the negative control was subtracted from the number of spots observed in experimental wells.
Statistical Correlation
Graphpad Prism version 4.03 software was used to analyze data. The Mann-Whitney test was used to determine the differences in CD4+ T cell responses specific to individual antigens between the two groups. The correlation analysis was done using Spearman rank test. The two sided p-values less than 0.05 were considered to be statistically significant.
Results
Patient Demographics
The study cohort consists of 148 patients who underwent HTx and their demographics are given in Table 1. Mean age at transplant was 51.5±3.5. 119 patients (81%) were male. 122 patients (82%) were Caucasian, 21 (14%) were African-American and 5 (4%) were classified as Other. The indication for HTx included ischemic cardiomyopathy and dilated cardiomyopathy. Mean (± standard deviation) follow-up after HTx was 3.2 ± 2.7 years and the median follow-up was 3.5 years. Of 148 patients, 65 patients were followed for development of acute AMR in the early post-HTx period (<12 months) while 83 patients were followed for development of CAV in the late post-HTx period (>12 months). 10 patients had diagnostic criteria consistent with acute AMR (AMR+) and 14 patients had angiographic evidence of moderate or severe CAV (CAV+). While patients with active infection at time of study enrollment met exclusion criteria, 8 patients developed systemic infections (4 with bacterial and 2 with viral) following study enrollment.
Table 1.
Patient Demographics
| Variable n (%) | Early HTx (n=65) | Late HTx (n=83) | ||||
|---|---|---|---|---|---|---|
| Total patients n= 148 | AMR (n=10) | No AMR (n=55) | p value | CAV (n=14) | No CAV (n=69) | p value |
| Age at HTx (yrs) | 49.9 ± 13.4 | 52.3 ± 11.8 | 0.25 | 57.6 ± 10.3 | 55.2 ± 9.5 | 0.32 |
| Male Gender(M:F) | 7:3 | 44:11 | 0.18 | 11:3 | 57: (83%) | 0.29 |
| Ethnicity | ||||||
| Caucasian | 8 (80%) | 47 (85%) | 0.12 | 11 (79%) | 56 (81%) | 0.67 |
| African American | 2 (20%) | 6 (11%) | 0.17 | 2 (14%) | 11 (16%) | 0.35 |
| Other | 0 (0%) | 2 (4%) | 1.0 | 1 (7%) | 2 (3%) | 0.43 |
| LVAD prior to HTx | 2 (20%) | 10 (18%) | 0.71 | 3 (21%) | 17 (25%) | 0.12 |
| Mean f/u time (yrs) | 1.7 ± 0.9 | 1.6 ± 0.7 | 0.55 | 8.9 ± 4.9 | 10.1 ± 6.4 | 0.31 |
Values presented as mean ± stdev or (%)
Abs to self-antigens, MYO and VIM are significantly increased in post-HTx recipients with AMR compared to stable cardiac transplant patients
Using ELISA, we determined the development of Abs in the sera to cardiac self antigens, MYO and VIM in post-HTx patients during the early postoperative period (≤12months). As shown in Figure 1A, patients with AMR develop Abs to MYO (Control:87±66mcg/ml, AMR−:144±115mcg/ml, AMR+: 285±70mcg/ml) at significantly higher titers compared to stable cardiac transplant patients without AMR (p=0.033). Antibodies to MYO were present 3.5±0.0 months prior to the diagnosis of acute AMR. Furthermore, detection of DSA in patients with AMR preceded the detection of anti-MYO Abs by 1.7± 0.3 months (Figure 5). Examination of patient sera for the presence of Abs to VIM as seen in Figure 1B (Control:26±16mcg/ml, AMR−:37±19mcg/ml, AMR+:103±43mcg/ml) also revealed a statistically significant increase in Abs to VIM in patients with AMR (p=0.014). Antibodies to VIM were present 2.1±0.2 months prior to the diagnosis of acute AMR. Furthermore, detection of DSA in patients with AMR preceded the detection of anti-VIM Abs by 3.0±0.4 months (Figure 5). In summary, DSA followed by Abs to self-antigens, MYO and VIM precedes the diagnosis of AMR in post-HTx patients.
Figure 1.
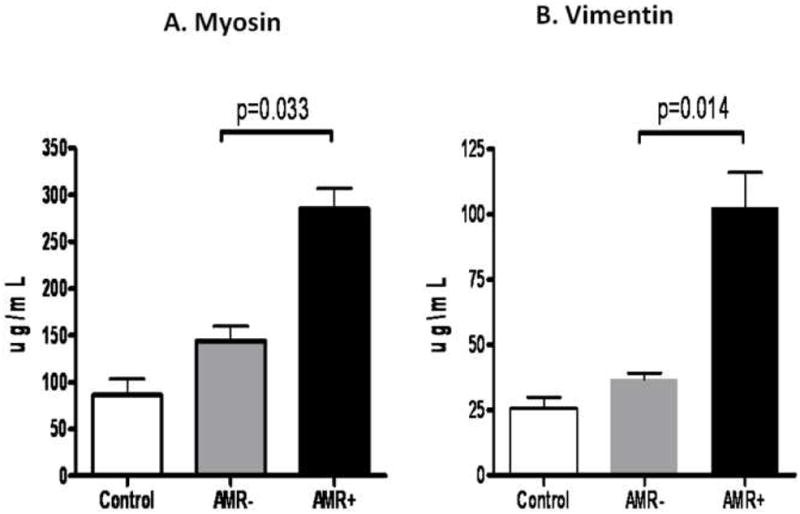
A) Patients with AMR develop Abs to MYO at significantly higher titers compared to stable cardiac transplant patients without AMR. B) Patients with AMR develop Abs to VIM at significantly higher titers compared to stable cardiac transplant patients without AMR.
Figure 5.
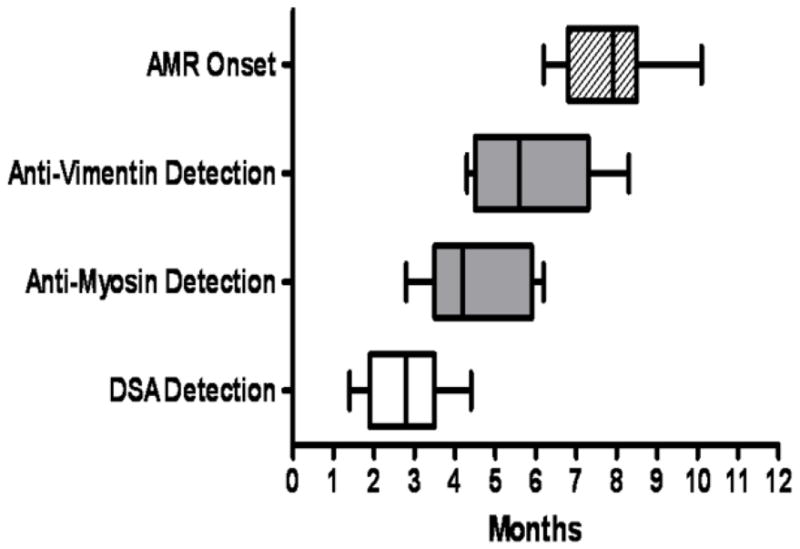
Serial monitoring of DSA, Abs to MYO and VIM in the early post-HTx period in 7 DSA+ patients indicated that DSA was detectable at 2.8±1.0 months, followed by MYO at 4.5±1.3 months and VIM at 5.8±1.5 months in patients diagnosed with AMR at 8.0±1.3 months.
Increased frequencies of IL-5, IFN-γ and decreased frequencies of IL-10 secreting CD4+ T cells specific to MYO and VIM in post HTx recipients with AMR
In order to identify the immune responses that contribute to the development of Abs to self antigens, MYO and VIM in post-HTx patients, the frequency of CD4+ T cells secreting IFN-γ, IL-5, IL-17 and IL-10 specific to either MYO and VIM were determined using ELISPOT. As presented in Figure 2A and 2B, patients with AMR demonstrated increased frequencies of IL-5 and IFN-γ against both MYO (IFN-γ: AMR−:17±6spm, AMR+:46±24spm, p=0.008; IL-5: AMR−:25±12spm, AMR+:120±41spm, p=0.003) and VIM (IFN-γ: AMR−:12±8spm, AMR+:40±25spm, p=0.03; IL-5: AMR−:15±10spm, AMR+:80±29spm, p=0.004). Concurrently, patients with AMR demonstrated decreased frequencies of IL-10 secreting CD4+ T cells specific to MYO (AMR−:440±105spm, AMR+:220±89spm, p = 0.009) and VIM (AMR−:320±124spm, AMR+:200±100spm, p = 0.03) compared to patients without AMR. However, no significant difference was noted in the frequencies of CD4+ T cells secreting IL-17 specific to MYO (AMR−:35±27spm, AMR+:40±35spm, p=0.35) and VIM (AMR−:21±15spm, AMR+:25±20spm, p=0.29) between AMR− and AMR+ post-HTx patients. These results indicate that patients with AMR demonstrate high frequencies of CD4+ T helper cells specific to self antigens which predominantly secrete the Th2 cytokine, IL-5. Therefore, the induction of IL-5 by self reactive CD4+T cells in patients with AMR may play an important role in the activation and differentiation of B cells involved in autoantibody production.
Figure 2.
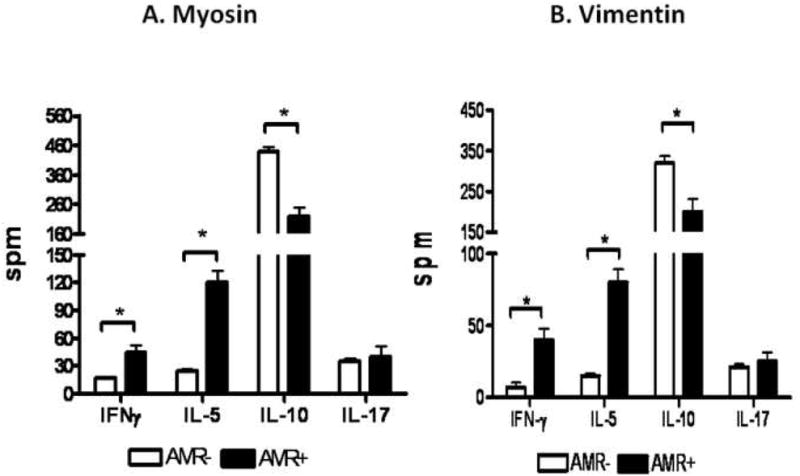
A) Patients with AMR demonstrated increased frequencies of IL-5 and IFN-γ and decreased frequency of IL-10 secreting CD4+ T cells specific to MYO compared to patients without AMR. There is no significant difference in the frequency of IL-17 secreting CD4+ T cells specific to MYO when comparing the two cohorts. B) Patients with AMR demonstrated increased frequencies of IL-5 and IFN-γ and decreased frequency of IL-10 secreting CD4+ T cells specific to VIM compared to patients without AMR. There is no significant difference in the frequency of IL-17 secreting CD4+ T cells specific to VIM when comparing the two cohorts.
Increased levels of Abs to MYO and VIM are significantly associated with presence of DSA in post-HTx patients with AMR compared to post-HTx patients without AMR
Table 2 depicts the features of AMR and self-antigen profile in all 10 recipients who were AMR positive in our study. As indicated in Table 3, patients with AMR who tested positive for DSA (DSA+AMR+) had increased levels of Abs to MYO (DSA−AMR+:174±65mcg/ml, DSA+AMR+:332±34mcg/ml, p=0.03) and VIM (DSA−AMR+:48±10mcg/ml, DSA+AMR+:126±21mcg/ml, p=0.003) compared to patients diagnosed with AMR without DSA (DSA−AMR+). No significant difference in the levels of Abs to MYO (DSA−AMR−:142±121mcg/ml, DSA+AMR−:155±88mcg/ml, p=0.92) and VIM (DSA−AMR−:35±18mcg/ml, DSA+AMR−:45±23mcg/ml, p=0.89) were identified between patients without AMR who tested either positive (DSA+AMR−) or negative (DSA−AMR−) for DSA. These results strongly suggest that DSA to mismatched HLA are significantly associated with and can play an important role in the development of Abs to self antigens in patients with AMR following HTx.
Table 2.
Characteristics of AMR+ Patients
| Patient | Symptoms | Abnormal Echo | Inotropes | Histology | Immunopathology | DSA | Anti-MYO | Anti-VIM | |
|---|---|---|---|---|---|---|---|---|---|
| CD4 | CD68 | ||||||||
| 1 | + | + | + | + | + | − | + | + | + |
| 2 | + | + | − | + | + | + | + | + | + |
| 3 | − | + | + | + | + | + | − | + | − |
| 4 | + | + | + | + | − | + | − | + | + |
| 5 | + | + | + | − | + | + | + | − | − |
| 6 | − | + | + | − | − | + | + | + | + |
| 7 | − | + | + | + | + | + | + | + | + |
| 9 | + | + | + | + | + | − | + | + | + |
| 9 | + | − | + | + | + | + | − | + | + |
| 10 | − | + | + | + | − | + | + | + | + |
Table 3.
Increased levels of Abs to Myosin and Vimentin are significantly associated with the development of DSA in AMR following HTx
| DSA+ | DSA− | P value | ||
|---|---|---|---|---|
| Myosin | AMR+ | 332 ± 34 (n=7) | 174 ± 65 (n=3) | 0.03 |
| AMR− | 155 ± 88 (n=10) | 142 ± 121 (n=45) | 0.92 | |
| Vimentin | AMR+ | 126 ± 21 (n=7) | 48 ± 10 (n=3) | 0.003 |
| AMR− | 45 ± 23(n=10) | 35 ± 18 (n=45) | 0.89 |
Abs to self-antigens, MYO and VIM are significantly increased in post-HTx recipients with CAV compared to stable cardiac transplant patients
Using ELISA, we determined the development of Abs in the sera to cardiac self antigens, MYO and VIM in post-HTx patients. As shown in Figure 3A and 3B, patients with CAV develop Abs to MYO (Control:87±66mcg/ml, CAV−:191±120mcg/ml, CAV+:550±98mcg/ml, p=0.025) and VIM (Control:26±16mcg/ml, CAV−:55±25mcg/ml, CAV+:255±49, p=0.001) significantly higher in comparison to stable cardiac transplant patients without CAV. These results indicate that patients with CAV develop significant increase in the levels of Abs to MYO and VIM compared to stable post-HTx recipients without CAV.
Figure 3.
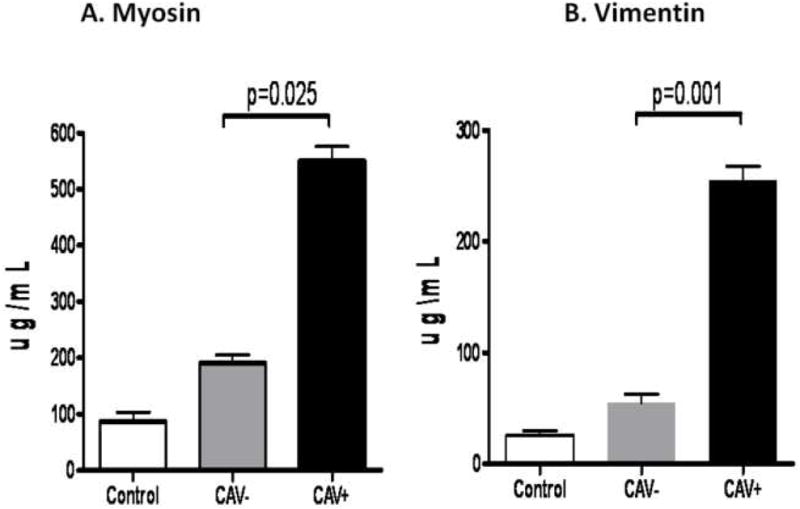
A) Patients with CAV develop Abs to MYO at significantly higher titers compared to stable cardiac transplant patients without CAV. B) Patients with CAV develop Abs to VIM at significantly higher titers compared to stable cardiac transplant patients without CAV.
Increased frequencies of IL-17 and decreased frequencies of IL-10 secreting CD4+ T cells specific to MYO and VIM in post HTx recipients with CAV
In order to identify the immune responses that contribute to the development of Abs to self antigens, MYO and VIM in post-HTx patients with CAV, the frequency of CD4+ T cells secreting IFN-γ, IL-5, IL-17 and IL-10 specific to either MYO and VIM were determined using ELISPOT. As presented in Figure 4A and 4B, patients with CAV demonstrated increased frequencies of IL-17 secreting CD4+ T cells against both MYO (CAV−:25±20spm, CAV+:115±26spm, p=0.002) and VIM (CAV−: 20±13spm, CAV+:70±34spm, p=0.003). Concurrently, patients with CAV demonstrated decreased frequencies of IL-10 secreting CD4+ T cells specific to MYO (CAV−:420±120spm, CAV+:220±89spm, p=0.008) and VIM (CAV−:500±220spm, CAV+:260±130spm, p=0.005) compared to patients without CAV. However, no significant difference was noted in the frequencies of CD4+ T cells secreting IFN-γ and IL-5 specific to MYO (IFN-γ: CAV−:25±15spm, CAV+:45±24spm, p=0.55; IL-5: CAV−:24±19spm, CAV+:34±25spm, p=0.54) and VIM (IFN-γ: CAV−: 17±12spm, CAV+:38±17spm, p=0.64; IL-5: CAV−:25±20spm, CAV+:30±18spm, p=0.24) between CAV− and CAV+ post-HTx patients. These results indicate that patients with CAV demonstrate high frequencies of CD4+ T helper cells specific to self antigens which predominantly secrete the Th17 cytokine, IL-17. Therefore, the induction of IL-17 by self reactive CD4+T cells in patients with CAV may play an important role in the facilitation of autoantibody development by driving neo-germinal center formation.
Figure 4.
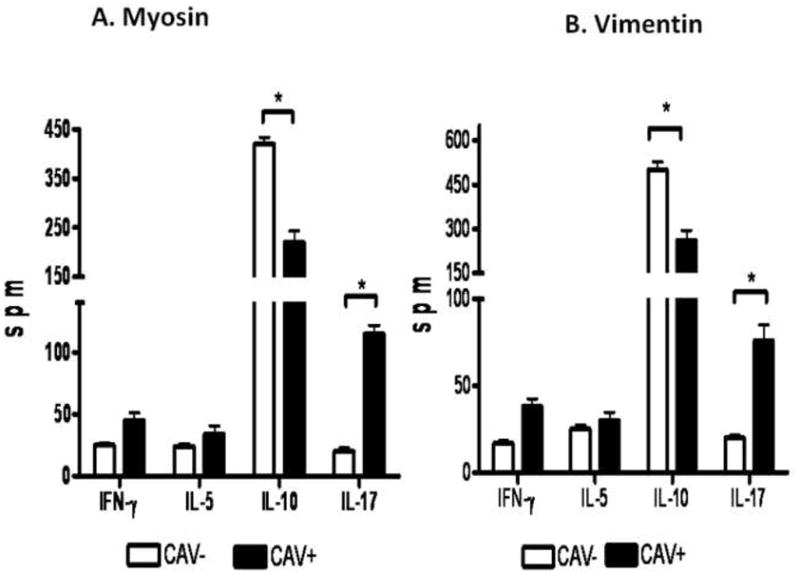
A) Patients with CAV demonstrated increased frequency of IL-17 and decreased frequency of IL-10 secreting CD4+ T cells specific to MYO compared to patients without CAV. There is no significant difference in the frequencies of IFN-γ and IL-5 secreting CD4+ T cells specific to MYO when comparing the two cohorts. B) Patients with CAV demonstrated increased frequency of IL-17 and decreased frequency of IL-10 secreting CD4+ T cells specific to VIM compared to patients without CAV. There is no significant difference in the frequencies of IFN-γ and IL-5 secreting CD4+ T cells specific to VIM when comparing the two cohorts.
Increased levels of Abs to MYO and VIM are significantly associated with presence of DSA in post-HTx patients with CAV compared to post-HTX patients without CAV
As indicated in Table 4, patients with CAV who tested positive for DSA (DSA+CAV+) had increased levels of Abs to MYO (DSA−CAV+:357±38mcg/ml, DSA+CAV+:603±84mcg/ml, p=0.001) and VIM (DSA−CAV+:173±29mcg/ml, DSA+CAV+:277±48mcg/ml, p=0.02) compared to patients diagnosed with CAV without DSA (DSA−CAV+). No significant difference in the levels of Abs to MYO (DSA−CAV−:180±86mcg/ml, DSA+CAV−:224±190mcg/ml, p=0.63) and VIM (DSA−CAV−:51±49mcg/ml, DSA+CAV−:67±56mcg/ml, p=0.78) were identified between patients without CAV who tested either positive (DSA+CAV−) or negative (DSA−CAV−) for DSA. These results indicate that DSA to mismatched HLA are significantly associated with and can play an important role in the development of Abs to self antigens in patients with CAV following HTx.
Table 4.
Increased levels of Abs to Myosin and Vimentin are significantly associated with the development of DSA in patients with CAV following HTx
| DSA+ | DSA− | P value | ||
|---|---|---|---|---|
| Myosin | CAV + | 603 ± 84 (n=11) | 357 ± 38(n=3) | 0.001 |
| CAV− | 224 ± 190 (n=17) | 180 ± 86(n=52) | 0.63 | |
| Vimentin | CAV + | 277 ± 48 (n=11) | 173 ± 29(n=3) | 0.02 |
| CAV− | 67 ± 56 (n=17) | 51 ± 49 (n=52) | 0.78 |
Discussion
Cardiac allograft dysfunction contributes to nearly 25% of the mortality within 10 years following transplantation (16, 29). During the early transplant period (<12 months), acute rejection is a major contributor of allograft dysfunction in HTx recipients and the frequency of acute rejection episodes generally tend to decline after the first HTx year (9, 17). Conversely, the incidence of cardiac allograft atherosclerosis in the form of vasculopathy is significantly increased after the first year and accounts for allograft dysfunction during the late HTx period (>12 months) (16, 29). It has been estimated that 30–50% of cardiac transplant recipients will demonstrate histological evidence of cardiac allograft vasculopathy at the end of 5 years post-HTx (29). The risk factors which promote allograft dysfunction leading to AMR and CAV in HTx recipients include circulating DSA to HLA and non-HLA antigens such as ABO and cardiac self antigens (7, 9, 12). Studies have demonstrated that recipient T cells recognize cardiac myosin presented in the context of recipient MHC Class II molecules and can mediate rejection of cardiac allografts, in the absence of an alloimmune response (30). In this study, we sought to address the role of DSA to mismatched HLA and their association with Abs to self antigens, MYO and VIM in the pathogenesis of acute AMR in the early post-HTX period and CAV in late post-HTx period. To determine the mechanism contributing to the development of immune response to self antigens, we also assessed the frequency of T cell mediated responses to self antigens, MYO and VIM as well as their cytokine profiles.
We enrolled 148 patients who underwent HTx and 65 patients were followed in the early (<12 months) period for development of acute AMR and 83 patients were followed in the late (>12 months) period for development of CAV. Of these, 10 patients fulfilled the ISHLT criteria for diagnosis of acute AMR. The mean time to diagnosis of acute AMR in these patients was 7.5±0.9 months. There was no significant difference in the clinical and demographic characteristics of patients with AMR and without AMR, including age, gender, ethnicity, mean follow up time, the number of acute rejection episodes, dosage of pulse steroids used for treating acute rejection, and maintenance immunosuppressive regimen used. Of the 10 patients who were diagnosed with AMR, 7 (70%) tested positive for DSA to mismatched HLA.
It has been suggested that Abs to donor specific HLA antigens mediate allograft injury during episodes of acute rejection. Specifically, the binding of anti-HLA Abs to the graft results in activation of complement cascade and endothelial cell activation accompanied by neutrophil and CD68 macrophage infiltration (31–33). Such an inflammatory milieu is conducive for autoimmunity to self-antigens. Furthermore, anti-MHC Class I Abs have been demonstrated to directly induce the development of autoimmunity to self-antigens such as Col- V and K-alpha-1-Tubulin following human lung transplantation (21, 22, 34, 35). In this context, we monitored the levels of Abs to cardiac antigens, MYO and VIM in post-HTx patients with and without evidence of AMR. In contrast to control subjects and post-HTx patients without AMR, there was a significant increase in the serum levels of Abs to MYO and VIM (Figure 1A, 1B). Interestingly, patients with AMR who tested positive for DSA (DSA+ AMR+) had increased levels of Abs to MYO and VIM compared to patients diagnosed with AMR without DSA (DSA− AMR+) (Table 3). This indicates that DSA is significantly associated with the development of Abs to self antigens. Serial monitoring of DSA, Abs to MYO and VIM in the early post-HTx period in patients who were DSA+ indicated that DSA was detectable by 2.8±1.0 months, followed by MYO by 4.5±1.3 months, VIM by 5.8±1.5 months in patients diagnosed with AMR at 8.0±1.3 months (Figure 5).
In order to determine the immune mechanisms which lead to the development of Abs to cardiac self antigens, we determined the frequencies of CD4+ T helper cells specific to MYO and VIM secreting IFN-γ, IL-5, IL-17 and IL-10. An increased frequency of CD4+ T cells that predominately secrete IL-5 followed by IFN-γ specifically against MYO and VIM were noted in patients with AMR. A significant decline in the frequency of IL-10 secreting CD4+ T cells was also noted in patients with AMR. This strongly suggests that the loss of IL-10 secreting CD4+ T cells specific to cardiac self antigens may result in the breakdown of peripheral tolerance accompanied by the development of both IL-5 and IFN-γ dependent CD4+ T cell dependent immune responses specific to the individual antigens in post-HTX patients with AMR. These results are in agreement with other reports that indicate that IL-5 is a critical cytokine involved in the induction and augmentation of B cell dependent autoantibody responses (36–38). Further, IFN-γ dependent immune responses to self antigens play an important role in the pathogenesis of de novo autoimmunity following heart transplantation (38).
We also analyzed patients for the development of CAV based on the angiographic evidence of patients in the follow-up period. We determined the levels of Abs to cardiac antigens, MYO and VIM, in post-HTx patients with and without evidence of CAV. In contrast to control subjects and post-HTx patients without CAV, there was a significant increase in the serum levels of Abs to MYO and VIM in patients with CAV (Fig 3A, 3B). We also determined CD4+T cell responses to MYO and VIM by analyzing the frequencies of CD4+ T cells that secrete IFN-γ, IL-5, IL-17 or IL-10 in patients with CAV. An increased frequency of CD4+ T cells that predominantly secrete IL-17 specifically against MYO and VIM were noted in patients with CAV (Figure 4A, 4B). A significant decline in the frequency of IL-10 secreting CD4+ T cells indicating the breakdown of peripheral tolerance was also noted in patients with CAV (Figure 4A, 4B). The development of predominantly IL-17 dependent CD4+ T cell responses to cardiac self antigens in post-HTX patients with CAV are in agreement with studies indicating an important role for IL-17 in the development of cardiac allograft vasculopathy and other autoimmune diseases in humans (21, 39, 40). These findings are supported by recent observations that in the absence of Th-1 mediated responses, CD4 Th17 responses mediate an accelerated proinflammatory responses leading to allograft rejection and CAV (40).
Investigators have demonstrated that in the posttransplant period, higher anti-myosin IgG levels were detected in sera collected during acute rejection than in sera collected during the rejection-free period and that in absence of an alloimmune response, sensitization of recipient mice with MYO is sufficient to induce rejection of cardiac allografts (30, 41, 42). The role of antibodies to vimentin leading to accelerated cardiac rejection has also been demonstrated in a recent study using a mouse model (43). Furthermore, imaging studies have suggested that anti-MYO Abs could be used as a predictor of transplant survival (44). While the role of anti-MYO and anti-VIM Abs in the development of rejection (mostly chronic) following HTx have been demonstrated in studies with small patient cohorts, we have demonstrated these findings in a large group of human heart transplant patients who developed acute AMR and CAV, with a concomitant immune response to self antigens, MYO and VIM in the early and late post-HTX period respectively (42, 45). The evidence in the literature in conjunction with our study findings lead to a strong suggestion that autoimmune mediated responses play a critical role in the development of chronic rejection post-HTx. Recently IL-5 and IL-17 have been proposed to mediate key proinflammatory response in the pathogenesis of autoimmune responses (46, 47). We have shown an increased frequency of IL-5 and IL-17 producing T-lymphocytes in the peripheral blood lymphocytes of posttransplant patients with acute AMR and CAV. Interleukin -17 in particular, induces the production of numerous cytokines (such as IL-6, G-CSF, GM-CSF, IL-1β, TGF-β, TNF-α), chemokines (including IL-8, GRO-α and MCP-1) and prostaglandins (e.g. PGE2) from many cell types (fibroblasts, endothelial cells, epithelial cells, keratinocytes and macrophages) (48).
In our study, the presence of increased levels of IL-5 and IL-17 demonstrate the role of autoimmunity in the pathogenesis of acute AMR and CAV respectively. We provide evidence which strongly suggests that both alloimmune responses as well as autoimmune responses play an important role in the processes of AMR and CAV in post-HTx patients. Our results also suggest cross talk between DSA and autoimmunity mediated by IL-17 in the pathogenesis of CAV. Therefore, IL-17 pathway mediated autoimmunity to self antigens can represent an important therapeutic option in the management of CAV. Future studies examining the mechanistic basis and signaling pathways involved in this pathogenesis are warranted.
Acknowledgments
There is no financial or professional conflict of interest by any of the authors of this manuscript. DSN was supported by NIH Training Grant T32 HL07776. We would like to thank Deepti Saini, PhD for her assistance in providing technical support for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kfoury AG, Hammond ME. Controversies in defining cardiac antibody-mediated rejection: Need for updated criteria. J Heart Lung Transplant. doi: 10.1016/j.healun.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Michaels PJ, Fishbein MC, Colvin RB. Humoral rejection of human organ transplants. Springer Semin Immunopathol. 2003;25:119–40. doi: 10.1007/s00281-003-0139-x. [DOI] [PubMed] [Google Scholar]
- 3.Kfoury AG, Hammond ME, Snow GL, et al. Early screening for antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 2007;26:1264–9. doi: 10.1016/j.healun.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Almuti K, Haythe J, Dwyer E, et al. The changing pattern of humoral rejection in cardiac transplant recipients. Transplantation. 2007;84:498–503. doi: 10.1097/01.tp.0000278094.41131.9f. [DOI] [PubMed] [Google Scholar]
- 5.Fishbein MC, Kobashigawa J. Biopsy-negative cardiac transplant rejection: etiology, diagnosis, and therapy. Curr Opin Cardiol. 2004;19:166–9. doi: 10.1097/00001573-200403000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Wu GW, Kobashigawa JA, Fishbein MC, et al. Asymptomatic antibody-mediated rejection after heart transplantation predicts poor outcomes. J Heart Lung Transplant. 2009;28:417–22. doi: 10.1016/j.healun.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reed EF, Demetris AJ, Hammond E, et al. Acute antibody-mediated rejection of cardiac transplants. J Heart Lung Transplant. 2006;25:153–9. doi: 10.1016/j.healun.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Uber WE, Self SE, Van Bakel AB, Pereira NL. Acute antibody-mediated rejection following heart transplantation. Am J Transplant. 2007;7:2064–74. doi: 10.1111/j.1600-6143.2007.01900.x. [DOI] [PubMed] [Google Scholar]
- 9.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–20. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Bharat A, Kuo E, Steward N, et al. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86:189–95. doi: 10.1016/j.athoracsur.2008.03.073. discussion 96–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bharat A, Narayanan K, Street T, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83:150–8. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 12.Kobashigawa JA. Cardiac allograft vasculopathy in heart transplant patients: pathologic and clinical aspects for angioplasty/stenting. J Am Coll Cardiol. 2006;48:462–3. doi: 10.1016/j.jacc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Hornick PI, Mason PD, Yacoub MH, Rose ML, Batchelor R, Lechler RI. Assessment of the contribution that direct allorecognition makes to the progression of chronic cardiac transplant rejection in humans. Circulation. 1998;97:1257–63. doi: 10.1161/01.cir.97.13.1257. [DOI] [PubMed] [Google Scholar]
- 14.Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Cook HT, Lechler RI. Loss of direct and maintenance of indirect alloresponses in renal allograft recipients: implications for the pathogenesis of chronic allograft nephropathy. J Immunol. 2001;167:7199–206. doi: 10.4049/jimmunol.167.12.7199. [DOI] [PubMed] [Google Scholar]
- 15.Uretsky BF, Murali S, Reddy PS, et al. Development of coronary artery disease in cardiac transplant patients receiving immunosuppressive therapy with cyclosporine and prednisone. Circulation. 1987;76:827–34. doi: 10.1161/01.cir.76.4.827. [DOI] [PubMed] [Google Scholar]
- 16.Ramzy D, Rao V, Brahm J, Miriuka S, Delgado D, Ross HJ. Cardiac allograft vasculopathy: a review. Can J Surg. 2005;48:319–27. [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor DO, Edwards LB, Boucek MM, Trulock EP, Keck BM, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twenty-first official adult heart transplant report--2004. J Heart Lung Transplant. 2004;23:796–803. doi: 10.1016/j.healun.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Davis SF, Yeung AC, Meredith IT, et al. Early endothelial dysfunction predicts the development of transplant coronary artery disease at 1 year posttransplant. Circulation. 1996;93:457–62. doi: 10.1161/01.cir.93.3.457. [DOI] [PubMed] [Google Scholar]
- 19.Fedoseyeva EV, Tam RC, Popov IA, Orr PL, Garovoy MR, Benichou G. Induction of T cell responses to a self-antigen following allotransplantation. Transplantation. 1996;61:679–83. doi: 10.1097/00007890-199603150-00001. [DOI] [PubMed] [Google Scholar]
- 20.Haque MA, Mizobuchi T, Yasufuku K, et al. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169:1542–9. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- 21.Fukami N, Ramachandran S, Saini D, et al. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182:309–18. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180:4487–94. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn MJ, Rose ML, Latif N, et al. Demonstration by western blotting of antiheart antibodies before and after cardiac transplantation. Transplantation. 1991;51:806–12. doi: 10.1097/00007890-199104000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Linke AT, Marchant B, Marsh P, Frampton G, Murphy J, Rose ML. Screening of a HUVEC cDNA library with transplant-associated coronary artery disease sera identifies RPL7 as a candidate autoantigen associated with this disease. Clin Exp Immunol. 2001;126:173–9. doi: 10.1046/j.1365-2249.2001.01654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laguens RP, Argel MI, Chambo JG, et al. Anti-skeletal muscle glycolipid antibodies in human heart transplantation as markers of acute rejection. Correlation with endomyocardial biopsy. Transplantation. 1996;62:211–6. doi: 10.1097/00007890-199607270-00011. [DOI] [PubMed] [Google Scholar]
- 26.Lawson C, Holder AL, Stanford RE, Smith J, Rose ML. Anti-intercellular adhesion molecule-1 antibodies in sera of heart transplant recipients: a role in endothelial cell activation. Transplantation. 2005;80:264–71. doi: 10.1097/01.tp.0000165433.88295.4c. [DOI] [PubMed] [Google Scholar]
- 27.Golocheikine A, Nath DS, Basha HI, et al. Increased erythrocyte C4D is associated with known alloantibody and autoantibody markers of antibody-mediated rejection in human lung transplant recipients. J Heart Lung Transplant. 2009 doi: 10.1016/j.healun.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fields RC, Bharat A, Steward N, et al. Elevated soluble CD30 correlates with development of bronchiolitis obliterans syndrome following lung transplantation. Transplantation. 2006;82:1596–601. doi: 10.1097/01.tp.0000241076.46033.4c. [DOI] [PubMed] [Google Scholar]
- 29.Dhaliwal A, Thohan V. Cardiac allograft vasculopathy: the Achilles’ heel of long-term survival after cardiac transplantation. Curr Atheroscler Rep. 2006;8:119–30. doi: 10.1007/s11883-006-0049-1. [DOI] [PubMed] [Google Scholar]
- 30.Fedoseyeva EV, Kishimoto K, Rolls HK, et al. Modulation of tissue-specific immune response to cardiac myosin can prolong survival of allogeneic heart transplants. J Immunol. 2002;169:1168–74. doi: 10.4049/jimmunol.169.3.1168. [DOI] [PubMed] [Google Scholar]
- 31.Jindra PT, Hsueh A, Hong L, et al. Anti-MHC class I antibody activation of proliferation and survival signaling in murine cardiac allografts. J Immunol. 2008;180:2214–24. doi: 10.4049/jimmunol.180.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horne PH, Zimmerer JM, Fisher MG, et al. Critical role of effector macrophages in mediating CD4-dependent alloimmune injury of transplanted liver parenchymal cells. J Immunol. 2008;181:1224–31. doi: 10.4049/jimmunol.181.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wasowska BA, Lee CY, Halushka MK, Baldwin WM., 3rd New concepts of complement in allorecognition and graft rejection. Cell Immunol. 2007;248:18–30. doi: 10.1016/j.cellimm.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braun RK, Molitor-Dart M, Wigfield C, et al. Transfer of tolerance to collagen type V suppresses T-helper-cell-17 lymphocyte-mediated acute lung transplant rejection. Transplantation. 2009;88:1341–8. doi: 10.1097/TP.0b013e3181bcde7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada Y, Sekine Y, Yoshida S, et al. Type V collagen-induced oral tolerance plus low-dose cyclosporine prevents rejection of MHC class I and II incompatible lung allografts. J Immunol. 2009;183:237–45. doi: 10.4049/jimmunol.0804028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herron LR, Coffman RL, Bond MW, Kotzin BL. Increased autoantibody production by NZB/NZW B cells in response to IL-5. J Immunol. 1988;141:842–8. [PubMed] [Google Scholar]
- 37.Hsu LW, Goto S, Lin YC, et al. Prolongation of heart allograft survival of rats treated by a Th2 inhibitor. Transpl Immunol. 2003;11:385–8. doi: 10.1016/S0966-3274(02)00152-1. [DOI] [PubMed] [Google Scholar]
- 38.Braun MY, Desalle F, Le Moine A, et al. IL-5 and eosinophils mediate the rejection of fully histoincompatible vascularized cardiac allografts: regulatory role of alloreactive CD8(+) T lymphocytes and IFN-gamma. Eur J Immunol. 2000;30:1290–6. doi: 10.1002/(SICI)1521-4141(200005)30:5<1290::AID-IMMU1290>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 39.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205:3133–44. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fedoseyeva EV, Zhang F, Orr PL, Levin D, Buncke HJ, Benichou G. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol. 1999;162:6836–42. [PubMed] [Google Scholar]
- 42.Morgun A, Shulzhenko N, Unterkircher CS, et al. Pre- and post-transplant anti-myosin and anti-heat shock protein antibodies and cardiac transplant outcome. J Heart Lung Transplant. 2004;23:204–9. doi: 10.1016/S1053-2498(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 43.Mahesh B, Leong HS, McCormack A, Sarathchandra P, Holder A, Rose ML. Autoantibodies to vimentin cause accelerated rejection of cardiac allografts. Am J Pathol. 2007;170:1415–27. doi: 10.2353/ajpath.2007.060728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isobe M, Haber E, Khaw BA. Early detection of rejection and assessment of cyclosporine therapy by 111In antimyosin imaging in mouse heart allografts. Circulation. 1991;84:1246–55. doi: 10.1161/01.cir.84.3.1246. [DOI] [PubMed] [Google Scholar]
- 45.Jurcevic S, Ainsworth ME, Pomerance A, et al. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71:886–92. doi: 10.1097/00007890-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 46.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 47.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]


