Summary
Background
Formation of epithelial sheets requires that cell division occurs in the plane of the sheet. During mitosis, spindle poles align so the astral microtubules contact the lateral cortex. Confinement of the mammalian Pins protein to the lateral cortex is essential for this process. Defects in signaling through Cdc42 and atypical protein kinase C (aPKC) also cause spindle misorientation. When epithelial cysts are grown in 3D cultures, mis-orientation creates multiple lumens.
Results
We now show that silencing of the polarity protein Par3 causes spindle mis-orientation in MDCK cell cysts. Silencing of Par3 also disrupts aPKC association with the apical cortex, but expression of an apically-tethered aPKC rescues normal lumen formation. During mitosis, Pins is mislocalized to the apical surface in the absence of Par3, or by inhibition of aPKC. Active aPKC increases Pins phosphorylation on Ser401, which recruits 14-3-3 protein. 14-3-3 binding inhibits association of Pins with Gαi, through which Pins attaches to the cortex. A Pins S401A mutant mislocalizes over the cell cortex and causes spindle orientation and lumen defects.
Conclusions
The Par3/aPKC polarity proteins ensure correct spindle pole orientation during epithelial cell division by excluding Pins from the apical cortex. Apical aPKC phosphorylates Pins, which results in the recruitment of 14-3-3 and inhibition of binding to Gαi, so the Pins falls off the cortex. In the absence of a functional exclusion mechanism, astral microtubules can associate with Pins over the entire epithelial cortex, resulting in randomized spindle pole orientation.
Introduction
Orientation of the mitotic spindle is essential for asymmetric stem cell divisions and for tissue morphogenesis [1]. In the Drosophila neuroblast the polarity proteins Par3, Par6, and aPKC, form a complex that is organized into a crescent at the apical cortex [2, 3]. Par3 binds to an adapter protein called Inscuteable, which in turn recruits Partner of Inscuteable (Pins) to the apical crescent. A second pathway involving the heterotrimeric G-protein GαI, Discs large (Dlg), and microtubules, also helps ensure localized enrichment of Pins [4]. Pins is believed to attach astral microtubules to the cortex, ensuring correct spindle orientation so that the apical daughter retains the Par and Pins proteins while cell fate determinants are segregated into the basal daughter. A related process controls spindle orientation in the C. elegans zygote [5, 6], but the mechanisms in other cell types are less well understood.
Epithelial monolayers are a basic unit of organization in many tissues, and emerge through a combination of intercellular adhesion and oriented cell division [7, 8]. Epithelial cells possess an apical-basal polarity, and intercellular adhesion occurs through the lateral membranes. Extension of epithelial sheets requires that cell division occurs in the plane of the sheet. Several polarity proteins have been implicated recently in spindle pole orientation during epithelial cell division, including Cdc42 [9], the Cdc42-specific exchange factors Tuba [10] and Intersectin-2 [11], aPKC [10] and the mammalian Pins protein, also called LGN [12]. Cdc42-GTP can bind to the Par6/aPKC complex, and activate aPKC [13]. Downstream of aPKC, Pins/LGN has to be excluded from the apical cortex so as to ensure the correct orientation of the mitotic spindle. Either the inhibition of aPKC or the forced tethering of Pins to the apical surface will severely disrupt spindle orientation. However, the underlying mechanism that controls Pins exclusion from the apical cortex remains unclear.
When MDCK or Caco-2 epithelial cells are grown in Matrigel 3D cultures, they form highly polarized cysts in which the apical surface faces a single central lumen [8, 14]. This system has proven to be a valuable in vitro model of epithelial morphogenesis, and recapitulates many of the processes that occur during the formation of ducts. Mitosis occurs in the plane of the cyst surface, such that the cysts maintain a single layer of cells as they enlarge. Inhibition of aPKC or the loss of Cdc42 disrupts spindle pole orientation, which causes the formation of multiple lumens [9–11].
Using this system, we show that silencing of Par3 expression in MDCK cells also disrupts spindle pole orientation, through the mislocalization of aPKC away from the apical surface. Atypical PKC can phosphorylate Pins on Ser401, which enhances binding of 14-3-3. In most cells, Pins is recruited to the cell cortex through association not with Inscuteable but with the heterotrimeric G-protein Gαi, to which it binds via GoLoco domains in its C-terminal region [15–17]. We find that 14-3-3 binding to Pins inhibits this association, which will result in the release of Pins from the cortex. Thus, aPKC-mediated exclusion of Pins from the apical cortex ensures that astral microtubules will not attach to the apical surface and that mitosis occurs only in the plane of the epithelial sheet.
Results
Silencing of Par3 causes a spindle orientation defect
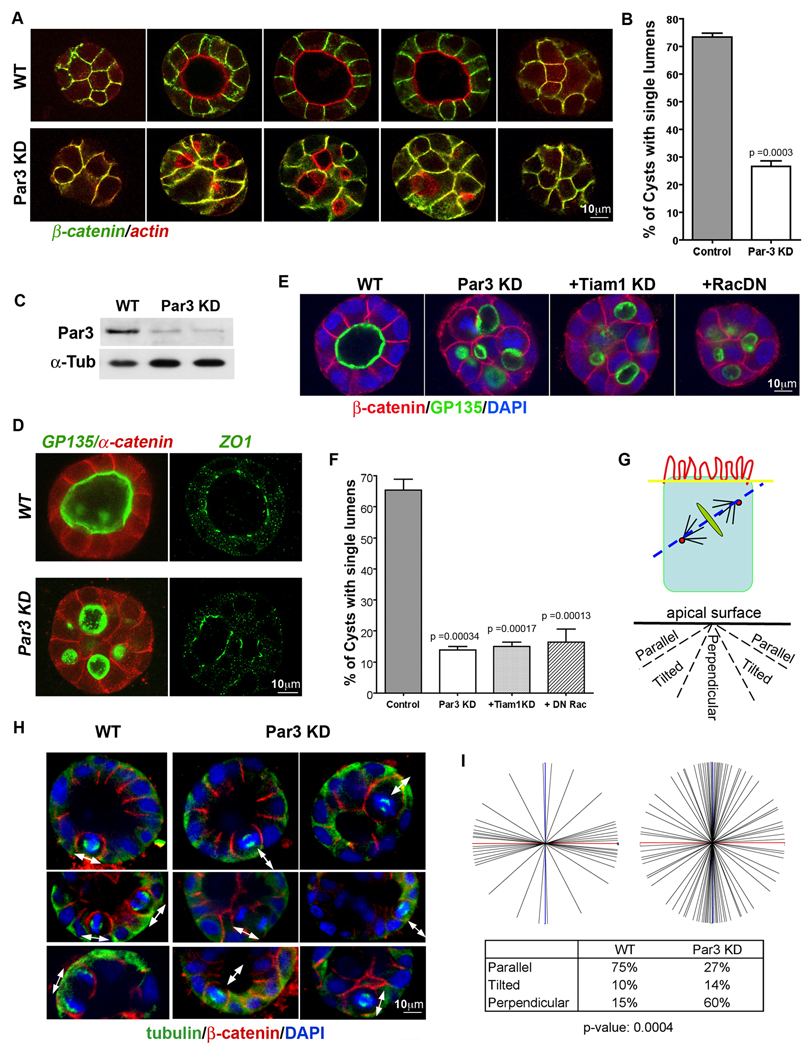
The polarity protein Par3 was silenced in MDCK cells by expression of an shRNA from a plasmid or lentivirus. As reported previously [18], depletion of Par3 resulted in a robust defect in lumen formation when the cells were grown as cysts in Matrigel cultures, (Fig. 1A, B). Two different shRNAs gave similar phenotypes and efficiently silenced Par3 expression (Fig. 1C). Importantly, however, the cells still retained normal apical-basal polarity, as assessed by the apical markers podocalyxin/gp135 and actin, tight junction marker ZO-1, and the lateral markers E-cadherin, α-catenin, and β-catenin (Fig. 1A, D).
Figure 1. Loss of Par3 causes defects in lumen formation and spindle orientation through a Rac/TIAM1-independent mechanism.
(A and B) Silencing of Par3 expression produces cysts that retain apical/basal polarity but form multiple lumens. Images in (A) show z-sections through cysts stained for β-catenin (green) and actin (red). Cysts were scored for lumen formation and compared using an unpaired t-test (n>100 cysts per condition).
(C) Knockdown efficiency of the Par3 shRNAs. Two plasmid shRNAs against different canine Par3 DNA sequences were used [20] and gave similar levels of knockdown. The phenotypes were indistinguishable.
(D) Apical and tight junction markers are not disrupted by silencing of Par3. Cysts expressing conrol or Par3 shRNAs were stained for α-catenin (red) as a lateral marker and gp135/podocalyxin (green) as an apical marker, or ZO-1 (green) to mark the tight junctions.
(E and F) The lumen defect caused by silencing of Par3 is not reversed by co-silencing of Tiam1 or co-expression of a dominant negative mutant of Rac [20]. Representative images and quantification of lumen formation are shown (n > 50 cysts per condition; error bars are +/− 1 SD).
(G – I) Loss of Par3 disrupts mitotic orientation. Angles of mitosis were measured relative to the plane of the apical surface (G). Representative images are shown (blue = DAPI; green = tubulin; red = β-catenin) in (H) and the quantification in (I). Data were analyzed by the Mann-Whitney test. Means were significantly different, P = 0.0004 (n = 66).
In 2D monolayer cultures, loss of Par3 causes a delay in tight junction assembly, through the inappropriate activation of the Rac GTPase and LIMK2 [19, 20]. The C-terminal region of Par3 can bind directly to the Rac exchange factor Tiam1, thereby restricting its distribution in the cell and its activation of Rac. This restriction is lost upon knockdown of Par3. To test whether a similar signaling pathway controls lumen formation in MDCK cell cysts, we co-expressed the Par3 shRNA with a dominant-negative Rac(N17) mutant, and shRNA against Tiam1, both of which we had shown previously to rescue tight junction assembly in the absence of Par3 [20]. However, neither approach restored single lumen formation (Fig. 1E, F; and Supplementary Fig. S1A, B). We conclude, therefore, that Par3 does not act through the Tiam1/Rac pathway in the organization of lumens.
The multi-lumen phenotype can occur if epithelial cells undergo mitosis in the wrong plane [9]. Therefore, we next tested whether spindle orientation might be defective when cells are depleted of Par3. It is difficult to assess spindle angles when cysts have multiple lumens. However, by immediately plating the cells into Matrigel after transduction with a Par3-shRNA lentivirus, cysts grow and begin to form lumens before depletion of the Par3 protein has occurred, and at 3d post-transduction most of these cysts still retain a single lumen, so spindle orientation of mitotic cells can be easily assessed by reference to the apical surface of the cyst (Fig. 1G). Using this method, we found that silencing of Par3 expression causes a strong defect in spindle orientation, with a large fraction of the cells dividing in a direction perpendicular to the plane of the cyst surface (Fig. 1H, I). Based on these data, we suggest that multiple lumens arise because of the spindle orientation defect caused by loss of Par3.
Spindle misorientation is caused by loss of apical aPKC
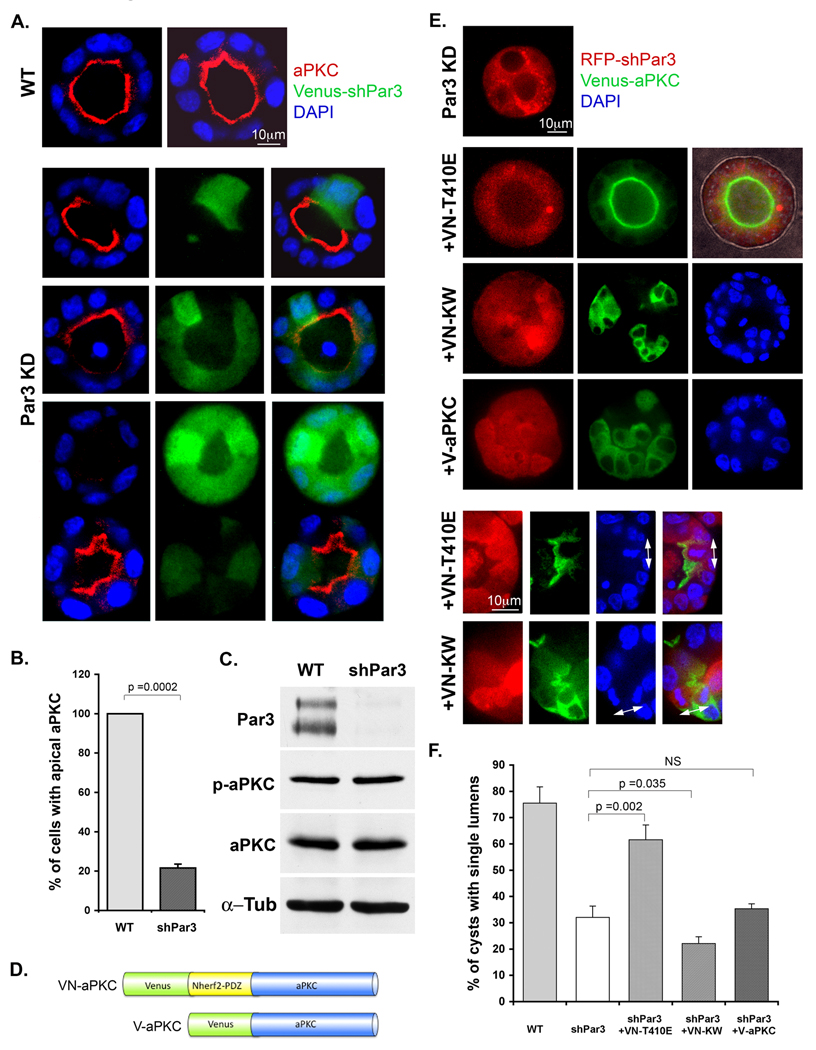
In mammary gland luminal epithelial cells the depletion of Par3 causes a loss of aPKC from the apical surface [21]. A similar phenotype was observed in MDCK cysts, in which lentivirally-driven knockdown of Par3 eliminated aPKC from the apical surface, but with no change in total level of the kinase (Fig. 2A, B, C). We next asked if this mislocalization is causally related to a spindle orientation defect. For this purpose, we constructed a fusion of full-length, constitutively active aPKC to the PDZ domains of NHERF2 (Fig 2D, Supplementary Fig. S1C). NHERF1/2 are regulators of the Na/H exchanger and localize to the apical surface through a PDZ domain-mediated interaction with the C-terminus of podocalyxin/gp135 [22, 23]. Because apical-basal polarity of the MDCK cells is not lost when Par3 is depleted (Fig. 1), we reasoned that expression of this fusion protein would tether aPKC to the apical cortex in a Par3-independent manner. When the construct was co-expressed with the Par3 shRNA, it was enriched at the apical cortex (Fig. 2E) and the majority of cysts developed single lumens (Fig. 2E, F). In addition, spindle pole orientation was normalized (Fig. 2E). Expression of a kinase-dead aPKC increased the number of defective cysts, while an untethered active aPKC had no effect, confirming that apical localization is important to maintain normal spindle orientation. We conclude that active aPKC at the apical cortex is required to prevent mitosis from occurring in the wrong plane.
Figure 2. Lumen organization requires apical aPKC, which is recruited by Par3.
(A, B and C) Loss of Par3 results in the disappearance of aPKC from the apical cortex, but with no change in the overall level of aPKC expression. Cysts were scored for cells with apical aPKC localization and compared using an unpaired t-test (n=60; error bars +/− 1 SD).
(D, E and F) An apically-tethered aPKC rescues normal cyst morphogenesis in the absence of Par3. A schematic of the aPKC fusion proteins is shown. The NHERF2-PDZ domains bind pococalyxin, thereby recruiting the fusion protein to the apical cortex. An activated aPKC fusion (VN-T410E) rescues normal lumen formation while the kinase-dead mutant (VN-KW) and wild type untethered aPKC (V-aPKC) do not. Error bars +/− 1 SD.
Pins is excluded from the apical cortex by aPKC-dependent phosphorylation
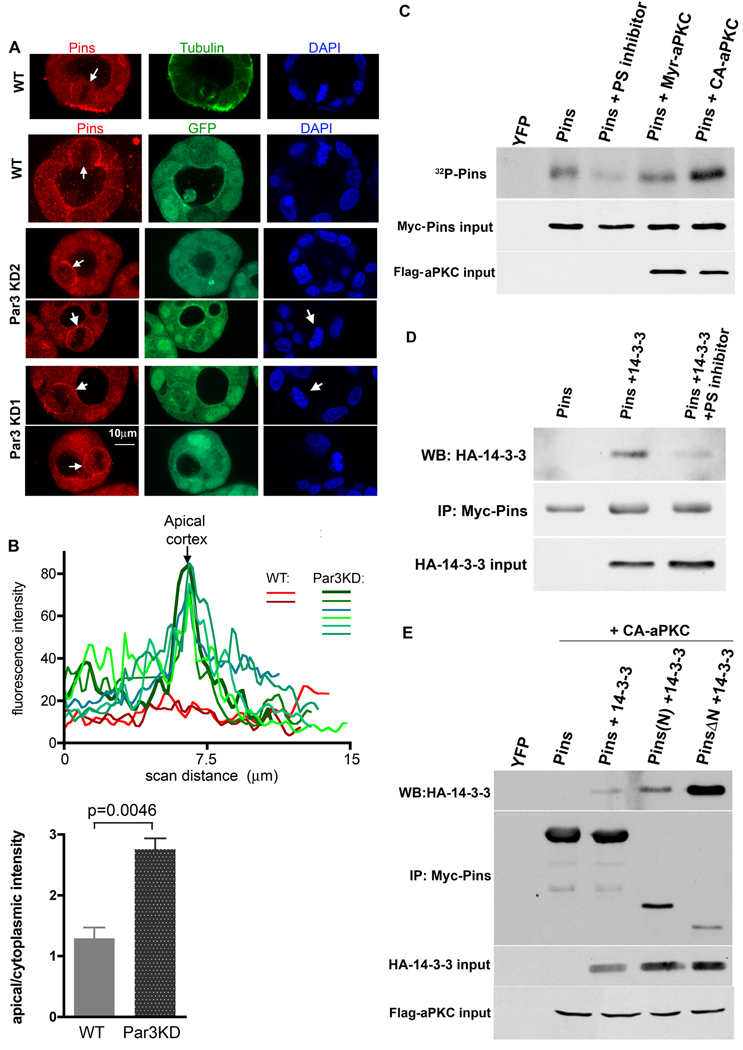
The mammalian Pins protein (Pins or LGN) plays essential roles in spindle orientation during mitosis of both stem cells and epithelial cells [1, 12]. Pins/LGN is confined to the lateral cortex in dividing MDCK cells, and the expression of an apically-tethered Pins causes a profound orientation defect [12]. Moreover, the apical exclusion of endogenous Pins is lost when aPKC is inhibited. Together, these data suggested that Pins might be phosphorylated by aPKC. Consistent with this idea, depletion of Par3 results in the mislocalization of Pins in mitotic MDCK cells, such that it is no longer excluded from the apical surface (Fig 3A, B). To test whether Pins can be phosphorylated by aPKC, we expressed Myc-tagged Pins in 293T cells together with a membrane-tethered aPKC (myr-aPKC) or a T410E constitutively-active mutant (CA-aPKC), or with added myristoylated pseudosubstrate to inhibit endogenous aPKC. The cells were incubated in medium containing 32P-phosphate, and the Pins was purified from lysates over Myc-antibody-conjugated agarose beads. The incorporation of 32P into Pins was increased in the presence of activated aPKC as compared to wild type aPKC, and was reduced by the pseudosubstrate inhibitor (Fig. 3C).
Figure 3. Loss of Par3 causes mislocalization of Pins, which is phosphorylated by aPKC and binds 14-3-3.
A Endogenous Pins localization in mitotic MDCK cells. Cysts were fixed and stained for Pins, tubulin, and DNA. White arrows mark the enrichment of Pins on the cortex of mitotic cells.
B Line scans across the apical cortex of mitotic cells stained for endogenous Pins. Histogram shows mean ratios of apical/cytoplasmic intensity of Pins staining. P value calculated using an unpaired, 2-tailed t-test; error bars +/− 1 SD.
C Pins is phosphorylated by aPKC. Myc-tagged Pins was immunoprecipitated from 293T cells incubated with 32P-phosphate. Co-expression of activated aPKC increased Pins phosphorylation, and a pseudosubstrate (PS) aPKC inhibitor peptide reduced Pins phosphorylation.
D Active aPKC promotes binding of 14-3-3 to Pins. Myc-Pins was immunoprecipitated from cells expressing HA-14-3-3 +/− activated aPKC or (delete?) pseudosubstrate inhibitor.
E A fragment of Pins lacking the N-terminal TPR repeats binds strongly to 14-3-3. Full-length Pins or the isolated N-terminal region (Pins(N)), or the linker plus GoLoCo motifs (Pins ΔN) were expressed with HA-14-3-3 plus activated aPKC, immunoprecipitated, and blotted for the HA tag. (The Pins(N) might associate with more 14-3-3 than the full length protein by associating with the C-terminus of endogenous Pins and forcing it into the open conformation).
Ser401-phosphorylated Pins binds 14-3-3
A subset of sites phosphorylated by aPKC is recognized by the 14-3-3 family of phospho-Ser binding proteins [24, 25]. We found that HA-tagged 14-3-3 co-precipitated with myc-Pins but this interaction was blocked by the aPKC pseudosubstrate inhibitor (Fig. 3D). Robust binding was also observed when a constitutively active aPKC was expressed with the Pins and 14-3-3; and importantly, aPKC itself did not bind to Pins (Supplementary Figure S3A).
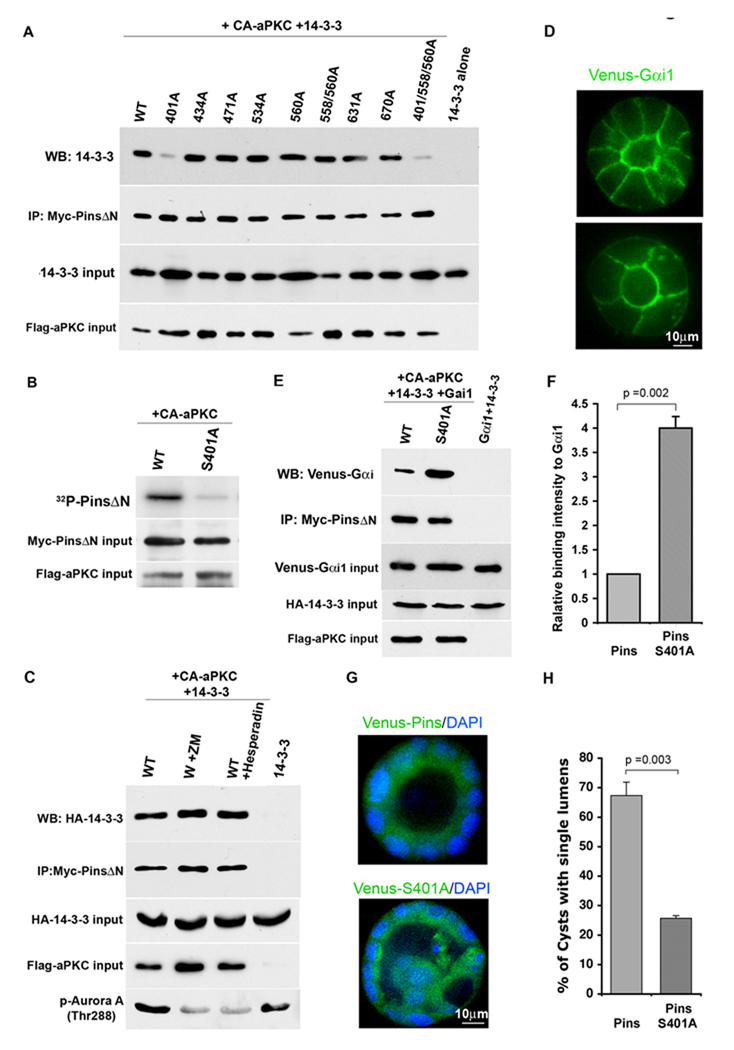
The N-terminal TPR repeat domain of Pins associates in an intramolecular interaction with the C-terminal domain, which contains GoLoco motifs [26], and can switch between an open and closed conformational states [16,17]. NuMA interacts with the TPR repeats while Gαi-GDP binds the GoLoco motifs. The closed state binds only inefficiently to GαI subunits, while NuMa stabilizes Pins in its open conformation and enhances Gαi binding. Deletion of the N-terminal region also enhances Gαi binding [17]. To identify the region of Pins that interacts with 14-3-3 we expressed the isolated N-terminus, the Pins ΔN fragment, or full-length Pins together with HA-tagged 14-3-3 and active aPKC. As shown in Fig. 3E, although Pins ΔN was expressed less efficiently than the other 2 constructs, it bound 14-3-3 much more robustly than either of them (Fig. 3D). (The weak but detectable binding of the N-terminal fragment might arise from its association with endogenous Pins, which would be forced into the open conformation). These data show that 14-3-3 recognizes phosphorylated Pins, and binds to the linker and/or C-terminal domain. Moreover, this interaction seems to be suppressed by the N-terminal domain. LS-MS/MS analysis revealed multiple phosphorylations of Ser and Thr residues in S-tagged full-length Pins co-expressed with activated aPKC (not shown). All phosphorylated residues were situated in the linker region or C-terminal domain. Further analysis using the PinsΔN fragment identified the same sites (supplementary fig. S1D,S2 and Table 1).Notably S401 was stoichiometrically phosphorylated (~98%; Supplementary Fig. S2). Seven of the mapped phosphorylation sites (S401, S434, S471, S534, S560, S631 and S670) were mutated to Ala residues, and tested for their effects on 14-3-3 binding. None of the mutations had any effect except for S401A, which almost completely abolished 14-3-3 binding (Fig. 4A). This mutant also showed no increase in 32P incorporation when co-expressed with activated aPKC (Fig. 4B). As expected, the phosphomimetic mutant Pins(401E) also did not bind to 14-3-3 (Fig. S3C). We conclude, therefore, that aPKC phosphorylates (either directly or indirectly) S401 in the linker region of Pins, which permits the association of Pins with 14-3-3.
Figure 4. Pins is phosphorylated on Ser401 by aPKC, which regulates Gαi binding and lumen formation.
A Binding of 14-3-3 to Pins requires phosphorylation of S401 but not of other phospho-sites.
B Mutation of S401 to A reduces aPKC-dependent incorporation of 32P into Pins.
C Aurora kinase inhibitors do not block 14-3-3 binding to Pins.
D Venus-Gαi is enriched on the apical and lateral cortex of MDCK cells in cysts.
(E and F) The S401A mutant of Pins binds to Venus-Gαi more robustly than the wild type Pins. Myc-PinsΔN was co-expressed with HA-14-3-3 and activated aPKC. Cell lysates were mixed with lysates from cells expressing Venus-Gαi. Myc immunoprecipitates were blotted for YFP. Venus-Gαi binding intensities were quantified and values were normalized to the intensity of wild type. Error bars +/− 1 SD.
(G and H) Expression of the Pins S401A mutant causes lumen defects in MDCK cell cysts. Error bars +/− 1 SD.
This Ser is conserved between mammals and insects, and in mitotic Drosophila S2 cells is phosphorylated by Aurora-A [27]. We tested whether aPKC might indirectly phosphorylate S401 through the activation of Aurora-A, but pre-treatment with the Aurora inhibitors ZM447439 or Hesparadin had no effect on aPKC-dependent 14-3-3 binding to Pins (Fig. 4C). These inhibitors were functional, because they reduced the auto-phosphorylation of T288 in Aurora A (FIg. 4C). Most likely, therefore, the phosphorylation is direct.
The non-phosphorylatable Pins S401A mutant causes lumen defects and binds constitutively to Gαi
Pins is recruited to the cell cortex by association of the C-terminal GoLoco motifs with Gαi subunits in the GDP-bound state [26]. Gαi isoforms are myristoylated and are constitutively localized to the plasma membrane, but it is unknown whether they are polarized in epithelial cells. We examined, therefore, the expression of a Gαi1-YFP fusion protein in MDCK cysts, and found that the protein appears enriched on the apical cortex, but is also expressed on the baso-lateral membranes (Fig. 4D). Thus Gαi does not provide information on polarity, and Pins could in principle be recruited by the G-protein to the apical cortex (Fig. 4C). Exclusion of Pins might occur if its phosphorylation by apical aPKC and recruitment of 14-3-3 reduces the association with Gαi. To test this hypothesis, we expressed wild type or S401A myc-PinsΔN with activated aPKC and 14-3-3, then added cell lysate containing YFP-Gαi.
Immunoprecipitates of the myc-Pins were blotted for YFP. As shown in Fig. 4E, F, the S401A mutant, which cannot interact with 14-3-3, bound GαI more robustly than the wild type protein, consistent with the idea that 14-3-3 reduces the affinity of Pins for Gαi. In addition, we asked if the co-expression of 14-3-3 would reduce the amount of Gαi immunoprecipitated with Pins from transfected cells. Endogenous 14-3-3 will interfere with this experiment, but nonetheless a significant decrease in Gαi binding was detected (Supplementary Fig. S3C).
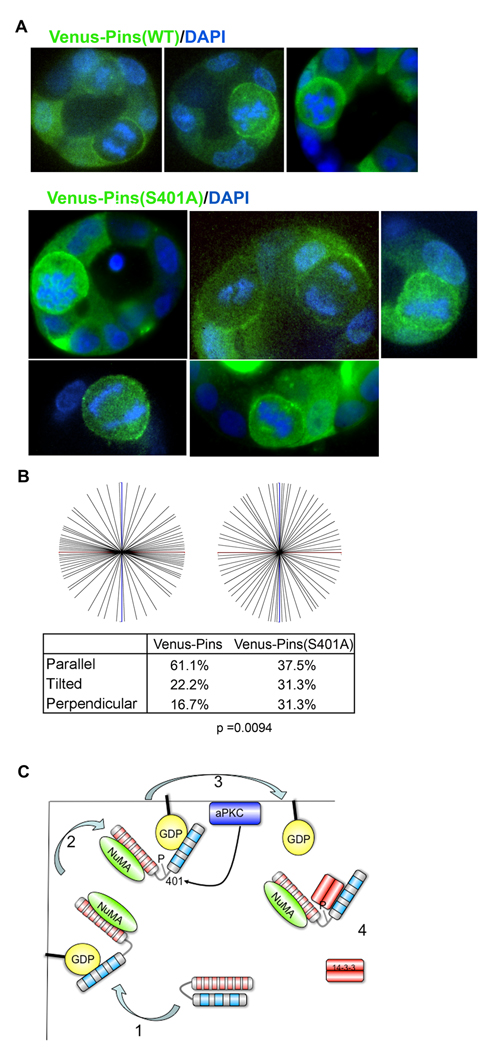
Taken together, these results predict that the S401A point mutant of Pins should mislocalize over the apical surface during mitosis, and thereby cause spindle pole orientation defects and multiple lumen formation. To test this prediction, we expressed either wild-type or S401A YFP-Pins from lentiviruses and examined the cysts after 3 – 4 days. Strikingly, the S401A mutant caused a strong lumen defect (Fig. 4G, H; note that Pins is diffusely cytoplasmic in interphase cells). Moreover, in mitotic cells, although wild type YFP-Pins was excluded from the apical cortex the S401A mutant was not (Fig. 5A). In addition, the point mutant caused a significant defect in spindle orientation (Fig. 5B), strongly supporting the idea that phosphorylation of S401 by aPKC is crucial for epithelial cell division in the correct plane.
Figure 5. The Pins S401A mutant is mislocalized and causes spindle pole misorientation in MDCK cell cysts.
(A and B) Cells were infected with lentivirus expressing wild type or mutant Venus-Pins and grown as cysts in Matrigel. Mitotic cells were imaged to localize the fusion proteins. DAPI staining was used to score the spindle pole orienation, and angles were analyzed by the Mann-Whitney test (n = 68).
C Schematic showing the mechanism by which Pins is excluded from the apical surface. Pins in the closed state is cytoplasmic. During mitosis, it binds NuMA, which switches Pins to an open conformation that can associate with Gαi at the cell cortex. Any Pins bound to apical Gαi can be phosphorylated on Ser401 by aPKC (which is bound to Par6, and activated by Cdc42-GTP). 14-3-3 is recruited to Pins by phospho-Ser401, which displaces Pins from Gαi into the cytoplasm, thereby excluding it from the apical surface.
Discussion
A central question in epithelial biology is how the organization of epithelial sheets is maintained during growth. Cadherin-based intercellular adhesions normally prevent cells from migrating out of the layer, but cells could escape during mitosis unless the spindles are oriented in the same plane as the sheet, so that the daughter cells are retained in the monolayer. Spindle orientation in Drosophila stem cells is specified by the Par polarity proteins, and by Pins, which by some unknown mechanism attaches astral microtubules to the cell cortex [2, 3, 5]. Recent data have implicated mammalian Pins/LGN in the orientation of mitosis in epithelial cell division [12], but the mechanism that excludes Pins from the apical surface has not been understood.
We have now found that Par3 is an essential upstream component of the epithelial spindle orientation mechanism, and acts through recruitment of aPKC to the apical cortex. Importantly, Par3 does not colocalize with apical aPKC in epithelial cells from either mammals or Drosophila [21, 27], and the recruitment mechanism has not been fully resolved. However, it is clear that phosphorylation of Par3 by aPKC is necessary for kinase disassociation from Par3; and the Crumbs polarity protein and Par6 also play key roles, at least in Drosophila [27]. Atypical PKC is activated at the apical cortex by Par6/Cdc42-GTP, where we propose that it can phosphorylate Pins on S401 (Fig. 5C). Interestingly, this site is also a target in Drosophila for phosphorylation by Aurora-A [28], and the phosphorylation is required for recruitment of Dlg and dynein-dependent spindle orientation. However, PhosphoNet (http://www.phosphonet.ca/) predicts that the site might be the target of numerous kinases, including the classical and atypical PKCs.
In interphase cells Pins is diffusely cytoplasmic and is in its closed conformation, but after nuclear envelope breakdown NuMA is released and associates with Pins, triggering its switch to the open conformation such that a fraction of the protein can bind to Gαi-GDP at the cell cortex [17]. Any Pins binding to the apical cortex will be phosphorylated, however, by aPKC. We propose that the phosphorylated Pins recruits 14-3-3, which reduces the affinity of Pins for GαI, so the apical Pins is released into the cytoplasm (Fig. 5C). A similar mechanism has been identified previously for the exclusion of the Par1 polarity protein from the apical surface. [29,30]. Pins on the lateral membrane will not be released and can function to tether astral microtubules, ensuring that mitosis will occur only in the plane of the epithelial sheet. A striking confirmation of this model is the lumen and spindle orientation defects caused by expression of the Pins S401A point mutant in MDCK cell cysts, which acts dominantly over the endogenous protein, as would be predicted if it cannot be excluded from the apical cortex. It will be of interest to know if a similar mechanism maintains spindle orientation in the epithelia of Drosophila and C. elegans, given the high degree of conservation among the polarity proteins involved in this process.
One puzzling feature of our data is that silencing of Par3 results in a shift to a predominantly perpendicular spindle orientation in the cysts, rather than to a random orientation as might be expected if Pins localization is randomized. This might result from the enrichment of Gαi on the apical surface, which in the absence of aPKC activity would recruit more Pins than the lateral membranes. In addition, however, silencing of Par3 expression causes a more pronounced lumen defect than does the silencing of Pins itself [12]. We suspect, therefore, that Par3 and/oraPKC play additional roles in spindle orientation, possibly through effects on Pins-associated proteins.
We have recently discovered that Par3 is essential for normal mammary gland morphogenesis, and that silencing of Par3 in mammary glands results in a loss of aPKC from the apical surface of the luminal epithelium and the accumulation of multiple layers of cells in the ducts [21]. In addition, loss of Par3 increases the proportion of dual-positive progenitor cells in the ducts. We propose that these effects may arise because of defects in the orientation of mitosis by epithelial progenitors, which disrupts the organization of the single luminal epithelial layer and may prevent asymmetric cell divisions of progenitors. Such defects could be important contributors to the invasive behavior of epithelial breast cancers.
Experimental Procedures
Plasmids and antibodies
Modified versions of lentivector pLVTHM (provided by D. Trono from Ecole Polytechnique Federale de Lausanne, Lausanne, Switzerland) were used for stable expression of shRNAs and cDNAs. To generate Venus-NHERF(PB1,2)-aPKC constructs, the PB1 and PB2 domains of NHERF2 was amplified by PCR to introduce a 5’ BamHI site and 3’ EcoRI site and cloned into pLVTHM-Venus. Then the constitutively-active aPKC and kinase-dead aPKC were amplified to introduce a 5’EcoRI and 3’SpeI and cloned downstream of NHERF2(PB1,2). To make Venus-aPKC, wild type aPKC was amplified to introduce EcoRI and SpeI and cloned into pLVTHM. Venus-Pins and Venus-Pins401A were cloned into pLVTHM through NotI sites. The Pins deletion constructs have been described previously [17]. For mass spectroscopy, S-tag-Pins and -PinsΔN were cloned into a pK mammalian expression vector containing an S-peptide tag and multiple cloning site. For mutant screening, Myc-tagged mutants of Pins were prepared using QuikChange XL Site-Directed Mutagenesis Kit (Stratagene).
Anti-β-catenin antibody was from BD Biosciences. Rabbit anti-PKCζ antibody was from Santa Cruz Biotechnology. Mouse anti-α-Tubulin was from Sigma-Aldrich. TXR-Phalloidin was from Molecular Probes. Rabbit anti-GFP antibody was from Invitrogene. Rabbit anti-phospho-PKCλ/ζ were from Cell-Signaling Technology. Mouse anti-ZO1 was generated by Daniel Goodenough (Harvard Medical School) from the Hybridoma Center, and rabbit anti-Par3 was from Millipore (Fig. 1) or was a custom antibody generated by Cocalico, Inc., against a peptide (GenScript) coupled to keyhole limpet hemocyanin (Fig 2). Mouse anti-podocalyxin/gp135 was a gift from G. Ojakian (State University of New York Downstate Medical Center, Brooklyn, NY) Myrstoylated PKC-ζ pseudosubstrate inhibitor was from Invitrogen. Hairpin RNA sequences used for silencing Par3 and other genes, and transfection methods, have been described previously [20].
Cysts culture in Matrigel and lentivirus infection
MDCK cells were trypsinized to produce a single cell suspension and plated in MEM+5%FBS+2% Matrigel solution in Matrigel-coated 8-well chambers. Lentivirus was produced by transfection of pLVTHM lentivector and packaging system (psPAX2 and pMD2G) into 293LT cells, as described previously [21]. To infect cells, lentivirus was added to cells in suspension, and the cells were grown for 1 – 2 d before plating cells on Matrigel. Cysts were cultured at 370°C and 5% CO2.
Immunofluoresence staining and microscopy
Cysts were washed twice with PBS, and fixed in 4% paraformaldehyde, then permeabilized with 0.25% Triton X-100 for 10 min [31]. After blocking with 0.7% gelatin+0.1% saponin, cysts were incubated with primary antibody in blocking buffer overnight at 4°C. After 3 washes with blocking buffer, cysts were incubated with Alexa Fluor-488, 546 and 633 for 1–2 hrs at room temperature. Cysts were then stained with DAPI for nuclei, and mounted in Prolong Gold Antifade Reagent (Invitrogen). Cysts were imaged using a Yokogawa Spinning Disc Confocal System (Solamere Technologies, Inc.) using a 60× NA 1.40 oil-immersion objective lens, a 1K intensified CCD camera (Stanford Photonics) and InVivo acquisition software (Media Cybernetics). Images were processed using Volocity (PerkinElmer) and assembled using Photoshop 7.0 (Adobe).
LC-MS/MS Analysis of S-tagged Pins
S-tagged Pins proteins were expressed in 293LT cells and purified using S-protein Agarose beads (Novagen). Mass spectrometric analysis was performed as described previously by Udeshi et al [32]. Briefly, S-tagged Pins was reduced and carbamidomethylated using dithiothreitol and iodoacetamide respectively, prior to proteolytic digestion. Peptides amenable to mass analysis were generated using endoproteinase LysC. To achieve optimal sequence coverage a portion of the LysC digest was subdigested with endoproteinase AspN. The Pins peptides were subsequently loaded on to a C18 column, separated by reverse-phase HPLC and analyzed on a Front End ETD (FETD) enabled high resolution LTQ-FT mass spectrometer. The raw data was searched against a human Pins sequence (Accession: AAN01266) containing database using the Open Mass Spectrometry Search Algorithm Version 2.1.1 (OMSSA). The CAD (b- and y-type ions) and ETD (c- and z-type ions) data sets were searched using the following parameters: ±0.01 Da precursor mass tolerance, ±0.35 Da fragment ion mass tolerance, protease specificity of LysC, AspN or no specificity with 3 possible missed cleavages where applicable. Variable modifications included were carbamidomethylation of Cys, oxidation of Met, phosphorylation of Ser, Thr and Tyr in addition to GlcNAcylation of Ser and Thr residues. OMSSA performed the removal of reduced charge species from ETD data sets prior to searching. All site assignments were manually validated.
Measurement of mitotic spindle pole angles
To measure spindle orientations, lentiviruses were added to cells immediately before plating cells on Matrigel and fixed after 3–4 d. Using this approach, lumens begin to form before silencing has occurred, and most cysts retain single lumens for at least 3 d, which facilitates spindle analysis. Confocal sections of cysts containing mitotic spindles at different phases of mitosis were collected. Spindle angles between spindle axis and the monolayer surface were measured using Image J, for sections of mitotic cells in which both spindles were visible, or both sets of daughter chromosomes. Data were analyzed by the nonparametric Mann-Whitney test, using Prism software. An unpaired, two-tailed t-test gave similar p values.
In-Cell 32P-phosphate labeling and immunoprecipitation
MDCK cells or 293LT cells were transfected with Myc-tagged Pins in the presence of different forms of aPKC or aPKC pseudosubstrate inhibitor, or transfected with Myc-tagged Pins and Pins(S401A). After 36 hrs growth, cells were washed with phosphate-free medium containing DMEM+10%FBS. Then 32P-phosphate (American Radiolabled Chemicals, Inc) was added to a final concentration of 0.2 mCi/ml in phosphate-free medium. After 2 hrs, cells were treated with 20nM CalyculinA for 10min. Then cells were washed with cold PBS and lysed in RIPA buffer (1% NP-40, 1% Sodium Deoxycholate, 0.1% SDS, 150mM NaCl, 10mM Sodium phosphate, pH7.2, 2mM EDTA, 50mM sodium fluoride, 0.2mM Sodium vanadate, 1uM Microcystin LR. 0.5mM β-Glycerophosphate, protease inhibitor aprotinin, pepstatin, and leupeptin, and 1mM PMSF). Immunoprecipitation was performed using Protein G Sepharose 4 Fast Flow beads (GE Healthcare) conjugated with monoclonal 9E10 antibody (for Myc tag). 32P-phosphate labeling of bound proteins was detected by SDS-PAGE gel and exposure to X-ray film.
Highlights
Par3 is required for spindle pole orientation in mitotic epithelial cells
Par3 functions to recruit aPKC to the apical surface, which in turn excludes Pins
aPKC mediates the phosphorylation of Pins on S401, which recruits 14-3-3
14-3-3 binding reduces Pins association with Gαi at the cell cortex.
Supplementary Material
Acknowledgements
We thank the Trono lab and G. Ojakian for reagents, and the Macara lab for advice. This work was supported by grants GM070902 (to IGM), GM 037537 (to DFH), and GM079506 (to QD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siller KH, Doe CQ. Spindle orientation during asymmetric cell division. Nat Cell Biol. 2009;11:365–374. doi: 10.1038/ncb0409-365. [DOI] [PubMed] [Google Scholar]
- 2.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Yu F, Kuo CT, Jan YN. Drosophila neuroblast asymmetric cell division: recent advances and implications for stem cell biology. Neuron. 2006;51:13–20. doi: 10.1016/j.neuron.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Siegrist SE, Doe CQ. Microtubule-induced Pins/Galphai cortical polarity in Drosophila neuroblasts. Cell. 2005;123:1323–1335. doi: 10.1016/j.cell.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Gonczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol. 2008;9:355–366. doi: 10.1038/nrm2388. [DOI] [PubMed] [Google Scholar]
- 6.Cowan CR, Hyman AA. Asymmetric cell division in C. elegans: cortical polarity and spindle positioning. Annu Rev Cell Dev Biol. 2004;20:427–453. doi: 10.1146/annurev.cellbio.19.111301.113823. [DOI] [PubMed] [Google Scholar]
- 7.Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol. 2008;183:625–633. doi: 10.1083/jcb.200807121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin Y, Meisen WH, Hao Y, Macara IG. Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J Cell Biol. 2010;189:661–669. doi: 10.1083/jcb.201002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Fraticelli AE, Vergarajauregui S, Eastburn DJ, Datta A, Alonso MA, Mostov K, Martin-Belmonte F. The Cdc42 GEF Intersectin 2 controls mitotic spindle orientation to form the lumen during epithelial morphogenesis. J Cell Biol. 189:725–738. doi: 10.1083/jcb.201002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Z, Zhu H, Wan Q, Liu J, Xiao Z, Siderovski DP, Du Q. LGN regulates mitotic spindle orientation during epithelial morphogenesis. J Cell Biol. 2010;189:275–288. doi: 10.1083/jcb.200910021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montesano R, Schaller G, Orci L. Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell. 1991;66:697–711. doi: 10.1016/0092-8674(91)90115-f. [DOI] [PubMed] [Google Scholar]
- 15.Schaefer M, Petronczki M, Dorner D, Forte M, Knoblich JA. Heterotrimeric G proteins direct two modes of asymmetric cell division in the Drosophila nervous system. Cell. 2001;107:183–194. doi: 10.1016/s0092-8674(01)00521-9. [DOI] [PubMed] [Google Scholar]
- 16.Nipper RW, Siller KH, Smith NR, Doe CQ, Prehoda KE. Galphai generates multiple Pins activation states to link cortical polarity and spindle orientation in Drosophila neuroblasts. Proc Natl Acad Sci U S A. 2007;104:14306–14311. doi: 10.1073/pnas.0701812104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du Q, Macara IG. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell. 2004;119:503–516. doi: 10.1016/j.cell.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Horikoshi Y, Suzuki A, Yamanaka T, Sasaki K, Mizuno K, Sawada H, Yonemura S, Ohno S. Interaction between PAR-3 and the aPKC-PAR-6 complex is indispensable for apical domain development of epithelial cells. J Cell Sci. 2009;122:1595–1606. doi: 10.1242/jcs.043174. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Macara IG. Par-3 mediates the inhibition of LIM kinase 2 to regulate cofilin phosphorylation and tight junction assembly. J Cell Biol. 2006;172:671–678. doi: 10.1083/jcb.200510061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 2005 doi: 10.1038/ncb1226. [DOI] [PubMed] [Google Scholar]
- 21.McCaffrey LM, Macara IG. The Par3/aPKC interaction is essential for end bud remodeling and progenitor differentiation during mammary gland morphogenesis. Genes Dev. 2009;23:1450–1460. doi: 10.1101/gad.1795909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeda T. Podocyte cytoskeleton is connected to the integral membrane protein podocalyxin through Na+/H+-exchanger regulatory factor 2 and ezrin. Clin Exp Nephrol. 2003;7:260–269. doi: 10.1007/s10157-003-0257-8. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Li J, Straight SW, Kershaw DB. PDZ domain-mediated interaction of rabbit podocalyxin and Na(+)/H(+) exchange regulatory factor-2. Am J Physiol Renal Physiol. 2002;282:F1129–F1139. doi: 10.1152/ajprenal.00131.2001. [DOI] [PubMed] [Google Scholar]
- 24.Kusakabe M, Nishida E. The polarity-inducing kinase Par-1 controls Xenopus gastrulation in cooperation with 14-3-3 and aPKC. EMBO J. 2004;23:4190–4201. doi: 10.1038/sj.emboj.7600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, Kishikawa M, Hirose H, Amano Y, Izumi N, et al. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr Biol. 2004;14:1425–1435. doi: 10.1016/j.cub.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Willard FS, Kimple RJ, Siderovski DP. RETURN OF THE GDI: The GoLoco Motif in Cell Division. Annu Rev Biochem. 2004;73:925–951. doi: 10.1146/annurev.biochem.73.011303.073756. [DOI] [PubMed] [Google Scholar]
- 27.Morais-de-Sa E, Mirouse V, St. Johnston D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell. 2010;141:509–523. doi: 10.1016/j.cell.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston CA, Hirono K, Prehoda KE, Doe CQ. Identification of an Aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell. 2009;138:1150–1163. doi: 10.1016/j.cell.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kusakabe M, Nisihida E. The polarity-inducing kinase Par-1 controls Xenopus gastrulation in cooperation with 14-3-3 and aPKC. EMBO J. 2004;23:4190–4201. doi: 10.1038/sj.emboj.7600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, Kishikaw aM, Hirose H, Amano Y, Izumi N, Miwa Y, Ohno S. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr Biol. 2004;14:736–741. doi: 10.1016/j.cub.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov K. Nature Cell Biol. 2001;11:4259–4275. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- 32.Udeshi ND, Compton PD, Shabanowitz J, Hunt DF, Rose KL. Methods for analyzing peptides and proteins on a chromatographic timescale by electron-transfer dissociation mass spectrometry. Nat. Protoc. 2008;3:1709–1717. doi: 10.1038/nprot.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.