Abstract
Purpose
To describe the five-year outcomes of patients with cytomegalovirus (CMV) retinitis and AIDS in the era of highly active antiretroviral therapy (HAART).
Design
Prospective, multicenter, observational study
Participants
503 patients with AIDS and CMV retinitis
Methods
Follow-up every 3 months with medical history, ophthalmologic examination, laboratory testing, and retinal photographs. Participants were classified as having previously-diagnosed CMV retinitis and immune recovery (CD4+ T cells >100 cells/µL), previously-diagnosed retinitis and immune compromise, and newly-diagnosed CMV retinitis (diagnosis < 45 days prior to enrollment).
Main outcome measures
Mortality, retinitis progression (movement of the border of a CMV lesion ≥ ½ disc diameter or occurrence of a new lesion), retinal detachment, immune recovery uveitis (IRU), and visual loss (to worse than 20/40 and to 20/200 or worse),
Results
Overall mortality was 9.8 deaths/100 person-years (PY). Rates varied by group at enrollment from 3.0/100 PY for those with previously-diagnosed retinitis and immune recovery to 26.1/100 PY for those with newly-diagnosed retinitis. The rate of retinitis progression was 7.0/100 PY and varied from 1.4/100 PY for those with previously-diagnosed retinitis and immune recovery to 28.0/100 PY for those with newly-diagnosed retinitis. The rate of retinal detachment was 2.3/100 eye-years (EY) and varied from 1.2/100 EY for those with previously-diagnosed retinitis and immune recovery to 4.9/100 EY for those with newly-diagnosed retinitis. The rate of IRU was 1.7/100 PY and varied from 1.3/100 PY for those with previously-diagnosed retinitis and immune recovery at enrollment to 3.6/100 PY for those with newly-diagnosed retinitis who subsequently experienced immune recovery. The rates of visual loss to worse than 20/40 and to 20/200 or worse were 7.9/100 EY and 3.4/100 EY, respectively; they varied from 6.1/100 EY and 2.7/100 EY for those with previously-diagnosed retinitis and immune recovery to 11.8/100 EY and 5.1/100 EY for those with newly-diagnosed retinitis. Although the event rates tended to decline with time, in general, at no time did they reach zero.
Conclusions
Despite the availability of HAART, patients with AIDS and CMV retinitis remain at increased risk for mortality, retinitis progression, complications of the retinitis, and visual loss over a 5-year period.
Cytomegalovirus (CMV) retinitis is the most frequent ocular opportunistic infection among patients with the acquired immune deficiency syndrome (AIDS) and is a major cause of visual impairment and blindness.1–7 Although highly active antiretroviral therapy (HAART) has reduced the incidence of CMV retinitis by 80 to 90%,8–12 CMV retinitis remains a substantial cause of visual impairment among patients with AIDS.6,7 In addition, CMV infection, as evidenced by CMV retinitis, is a risk factor for mortality among patients without immune recovery.13 Although patients with immune recovery due to HAART (evidenced by a sustained rise in CD4+ T cells to >100 cells/µL) typically can have anti-CMV therapy discontinued without relapse of the retinitis,14–19 immune recovery will not control the retinitis in all patients,20–24 and the progression rate among patients with CD4+ T cells >100 cells/µL has been reported as 3/100 person-years (PY), necessitating ongoing regular ophthalmologic follow-up.23 These data come from analyses of the Longitudinal Study of the Ocular Complications of AIDS (LSOCA) cohort with about two years of follow-up.6,7,23,24 Human Immunodeficiency Virus (HIV)-infected patients with immune recovery now have survivals predicted to be longer than 10 years;25 therefore, longer follow-up of the outcomes of patients with CMV retinitis is needed to guide clinical management. In this manuscript we analyze the five-year mortality and ocular outcomes of patients with AIDS and CMV retinitis, particularly in relationship to time since CMV retinitis diagnosis and immunologic status.
Patients and Methods
LSOCA is a prospective observational study of patients with AIDS that begun in September 1998 and is designed to document the incidence of ocular complications of AIDS in the era of HAART and on their impact on vision.11,26 Eligible patients have AIDS diagnosed according to the 1993 Centers for Disease Control and Prevention case surveillance definition of AIDS.27 Recruitment was performed at 19 clinical centers across the United States, typically located in urban areas with large HIV-infected populations.11 Participants were enrolled with a “rolling cohort” approach (ongoing enrollment) so that there are participants in the cohort with features of the evolving AIDS epidemic. For this study, the data base was frozen as of 31 December 2008. Patients were enrolled with and without ocular opportunistic infections and across a spectrum of immunologic function (as determined by CD4+ T cell count).11,26 Because the study enrolled at AIDS ophthalmology clinics, the study was enriched with patients with cytomegalovirus retinitis. The study protocol was reviewed and approved at the individual clinical centers and at the resource centers by the institutional review boards, and the study was conducted in accordance with the principles of the Declaration of Helsinki. All participants gave written informed consent.
Participants with CMV retinitis were seen every three months for follow-up. At enrollment and follow-up visits, all participants gave a detailed medical history, including information on AIDS-related illnesses, nadir CD4+ T cell count (lowest count prior to enrollment), and antiretroviral therapy. A complete ophthalmologic examination was performed, including best corrected visual acuity with logarithmic visual acuity charts,28,29 slit lamp examination, and dilated indirect ophthalmoscopy. Standardized fundus photographs were obtained and forwarded to a centralized reading center for grading, as previously described.23,28 Laboratory testing included hematology, chemistry, CD4+ T cells, and assessment of the amount of HIV RNA in plasma (HIV load).
Cytomegalovirus retinitis was diagnosed by study-certified ophthalmologists with expertise in AIDS.11,23 Newly-diagnosed retinitis was defined as either the CMV retinitis diagnosed ≤45 days prior to study enrollment or diagnosed during follow-up (incident cases). Previously-diagnosed retinitis was defined as CMV retinitis diagnosed >45 days prior to enrollment. Retinitis progression was graded at the Reading Center, as previously described, and defined as the movement of a border of a CMV lesion at least ½ standard disc diameter along a front ½ disc diameter in size or the occurrence of a new lesion ¼ disc area in size.23,28 Retinal detachments and immune recovery uveitis (IRU) were diagnosed clinically. Immune recovery uveitis was defined as either the occurrence of new intraocular inflammation or an increase in intraocular inflammation (cells in the anterior chamber or vitreous) in an eye with CMV retinitis in the setting of immune recovery.30–33 Immune recovery was defined as an increase in CD4+ T cells from <100 cells/µL to a level ≥100 cells/µL, as this level is the one used to recommend discontinuation of anti-CMV therapy.19,23,33 The incidence of IRU was calculated from the date that the CD4+ T cells rose to >100 cells/µL. For those participants with immune recovery at enrollment, the date the CD4+ T cells became >100 cells/µL was estimated, assuming a linear increase from the nadir CD4+ T cells to the enrollment CD4+ T cells with the increase beginning at the time HAART was started.
Enrollment characteristics were compared among the subsets of patients with CMV retinitis with the chi-square test for categorical variables or the Kruskal-Wallis test for continuous variables. The P-values reported for comparisons across subgroups and for pairwise comparisons between subgroups are nominal and were not adjusted for multiple comparisons or multiple outcomes. Analyses were censored as of the last completed follow-up visit. Thirty-three of the 503 patients (6.6%) were considered lost to follow-up, as their vital status was missing subsequent to the last visit. Event rates are expressed either as the rate per 100 person-years (PY) for patient-related events (e.g., mortality) or the rate per 100 eye-years (EY) for eye-related events (e.g., visual loss in an eye with CMV retinitis).34 The outcomes of retinitis progression and of immune recovery uveitis were determined by the occurrence in either eye and, therefore, are patient-related events and expressed as rate per 100 PY, in order to be consistent with previous reporting methods. Because event rates did not differ significantly between participants with newly-diagnosed retinitis at enrollment and those with incident retinitis during follow-up, these two groups were combined for analyses of follow-up event rates. Kaplan-Meier curves,35 using staggered entry where appropriate, were plotted to portray cumulative probability of an event over time.36 Staggered-entry Kaplan-Meier curves were anchored to the date of CMV retinitis diagnosis, except for the analysis of IRU, which was anchored to the date of immune recovery. The relative risk of selected outcomes comparing groups of CMV patients was estimated with the test for trend in Cox proportional hazard regression models, accounting for the correlation between eyes when necessary.37 For comparisons between groups of eye-related events, P-values were adjusted for the correlation between eyes.38 Statistical analyses were performed using SAS (SAS/STAT User’s Guide, Version 9.1, Cary, NC:SAS Institute) and Stata (StataCorp. 2007, Stata Statistical Software: Release 10, College Station, TX: StataCorp LP) statistical packages.
Results
As of 31 December 2008, 2718 participants with AIDS were enrolled in LSOCA, of whom 503 had CMV retinitis at enrollment (474 participants) or developed CMV retinitis during follow-up (29 participants), and these 503 patients constitute the basis for this report.
Characteristics of the Study Population
The characteristics of the 503 participants with CMV retinitis are listed as Table 1. Patients were classified as: 1) previously diagnosed CMV retinitis at enrollment and immune recovered (n=217); 2) previously diagnosed and immune compromised, defined as CD4+ T cells <100 cells/µL (n=126); 3) newly-diagnosed at enrollment (n=131); and 4) newly-diagnosed during follow-up, i.e., incident (n=29). Participants with previously-diagnosed CMV retinitis generally had long-standing disease with a median time from diagnosis of CMV retinitis to enrollment of 1108 days (interquartile range 1496 to 638 days) for those with immune recovery and 664 days for those with persistent immune compromise (interquartile range 1242 to 181 days). Demographic differences among the four groups were consistent with the evolution of the AIDS epidemic and previous studies of demographics of patients with CMV retinitis.1,3,4,11,39–41 CMV retinitis tended to occur more often among men (80.7%), particularly men who have sex with men (54.5%), and affected all races and ethnicities. Characteristics of the CMV lesions among the four CMV retinitis groups were not statistically significantly different with one exception: lesions were smaller in the incident, newly-diagnosed group (P=0.0002). As might be expected, median CD4+ T cells were low in the three groups with immune compromise and significantly higher among those with immune recovery. Moreover, over 70% of participants with immune recovery had CD4+ T cells above 200 cells/µL, indicating substantive immune recovery. Although the median CD4+ T cell count among those with immune recovery was 289 cells/µL, the median nadir count among this group was 12 cells/µL, indicating a history of substantial immune compromise. About 17% of participants with newly-diagnosed CMV retinitis in both subsets (i.e., at enrollment and incident) had CD4+ T cells >100 cells/µL at enrollment, and among the incident group, nearly 7% of participants had CD4+ T cells >100 cells/µL at the time of diagnosis of CMV retinitis. The incidence of CMV retinitis among the 2215 participants without CMV retinitis at enrollment was 0.24/100 PY. The incidence by enrollment CD4+ T cells was: for participants with <50 cells/µL at enrollment, 0.95/100 PY; 50–99 cells/µL, 0.14/100 PY; 100–199 cells/µL, 0.12/100 PY; and ≥200 cells/µL, 0.04/100 PY.
Table 1.
Enrollment Characteristics of the Study Population
| Previously-diagnosed | Newly-diagnosed | |||||
|---|---|---|---|---|---|---|
| Characteristic | Total | Immune-recovered | Immune- compromised |
At enrollment | During follow- up |
P-value |
| Number patients | 503 | 217 | 126 | 131 | 29 | |
| Age (years) | 0.006 | |||||
| Median | 41 | 42 | 41 | 38 | 40 | |
| Interquartile range | 35–46 | 37–47 | 35–45 | 32–45 | 37–45 | |
| Range | 17–68 | 18–66 | 25–61 | 17, 68 | 26, 52 | |
| Gender (%) | 0.004 | |||||
| Men | 80.7 | 83.9 | 84.1 | 70.2 | 89.7 | |
| Women | 19.3 | 16.1 | 15.9 | 29.8 | 10.3 | |
| Race & ethnicity (%) | 0.08 | |||||
| White, non-Hispanic | 46.9 | 52.5 | 45.2 | 36.6 | 58.6 | |
| Black, non-Hispanic | 31.8 | 24.0 | 36.5 | 40.5 | 31.0 | |
| Hispanic | 17.3 | 19.8 | 14.3 | 17.6 | 10.3 | |
| Asian/Pacific Islander | 2.0 | 2.3 | 0.8 | 3.0 | 0.0 | |
| American Indian/Alaskan Native | 0.8 | 0.9 | 1.6 | 0.0 | 0.0 | |
| Other | 1.2 | 0.5 | 1.6 | 2.3 | 0 | |
| HIV* exposure (%) | 0.07 | |||||
| Men having sex with men | 54.5 | 59.9 | 57.9 | 38.9 | 69.0 | |
| Injection drug use | 1.8 | 2.3 | 1.6 | 1.5 | 0.0 | |
| MSM & injection drug use† | 2.6 | 2.3 | 2.4 | 3.1 | 3.5 | |
| Heterosexual contact | 23.7 | 21.2 | 21.4 | 31.3 | 17.2 | |
| Other/missing | 17.3 | 14.3 | 16.7 | 24.4 | 10.3 | |
| Cytomegalovirus retinitis (%) | ||||||
| Bilateral retinitis | 32.4 | 33.2 | 34.9 | 31.3 | 20.7 | 0.5 |
| Area CMV‡ retinitis > 25% | 31.8 | 32.7 | 43.7 | 24.4 | 6.9 | 0.0002 |
| retinal area in either eye | ||||||
| Zone 1 CMV in either eye§ | 44.5 | 46.1 | 43.7 | 44.3 | 37.9 | 0.9 |
| HAART** | ||||||
| Receiving at enrollment (%) | 78.3 | 88.9 | 72.2 | 68.7 | 69.0 | <0.0001 |
| Median years since initiation | 3.1 | 3.0 | 2.9 | 4.2 | 3.7 | 0.002 |
| Range in years since initiation | 0–15.8 | 0–12.5 | 0.1–10.2 | 0–15.8 | 0.1–6.8 | |
| CD4+ T cells | <0.0001 | |||||
| Median (cells/µL) | 94 | 289 | 22 | 28 | 16 | |
| Count (%) | ||||||
| < 50 cells/µL | 39.2 | 0.0 | 71.4 | 64.9 | 75.9 | |
| 50–99 cells/µL | 12.3 | 0.0 | 28.6 | 18.3 | 6.9 | |
| 100–199 cells/µL | 15.5 | 29.5 | 0.0 | 8.4 | 10.3 | |
| ≥ 200 cells/µL | 33.0 | 70.5 | 0.0 | 8.4 | 6.9 | |
| HIV load | <0.0001 | |||||
| Median (log10[copies/mL]) | 3.65 | 2.60 | 4.86 | 4.88 | 5.39 | |
| Log10(copies/mL) (%)†† | ||||||
| < 2.60 | 34.6 | 57.6 | 22.2 | 14.5 | 6.9 | |
| 2.60–4.00 | 15.9 | 20.7 | 10.3 | 16.0 | 3.4 | |
| 4.00–5.00 | 17.1 | 12.0 | 18.3 | 24.4 | 17.2 | |
| >5.00 | 27.0 | 6.0 | 44.4 | 37.4 | 62.1 | |
HIV = human immunodeficiency virus.
MSM = men having sex with men.
CMV = cytomegalovirus.
Zone 1 = an area within one disc-diameter of the edge of the optic nerve or two disc-diameters of the center of the fovea.
HAART = highly active antiretroviral therapy.
Columns do not add up to 100% as 5.4% of HIV load measurements are missing.
Mortality
The overall mortality rate was 9.8/100 PY, and the rate varied by patient group (Table 2) and immunologic status (Table 3), from a low of 3.0/100 PY for those with CMV retinitis and immune recovery to a high of 26.1/100 PY for those with newly-diagnosed retinitis (Figure 1, logrank P<0.0001). Compared to those with previously-diagnosed CMV retinitis and immune recovery, the hazard ratio for death was 4.5 for those with previously-diagnosed retinitis and immune compromise (95% confidence interval [CI] 3.0, 6.7; P<0.01) and 7.6 for those with newly-diagnosed CMV retinitis (95% CI 5.2, 11.2; P<0.01). By five years after diagnosis of CMV retinitis, nearly 75% of patients with CMV retinitis without immune recovery had died. However, even among those with immune recovery, the Kaplan-Meier curve for mortality continued to increase out to five years (Figure 1).
Table 2.
Rates of select outcomes among patients with cytomegalovirus retinitis.
| Previously diagnosed | ||||
|---|---|---|---|---|
| Overall | Immune recovered* |
Immune compromised* |
Newly diagnosed† |
|
| Mortality | ||||
| Patients at risk | 470 | 203 | 119 | 148 |
| Events | 213 | 39 | 68 | 106 |
| Person-years | 2178 | 1306 | 466 | 406 |
| Rate (/100 PY‡) | 9.8 | 3.0 | 14.6 | 26.1 |
| Hazard ratio¶ | 1.0 | 4.5 | 7.6 | |
| 95% confidence interval¶ | 3.0, 6.7 | 5.2, 11.2 | ||
| P value¶ | <0.01 | <0.01 | ||
| Retinitis progression | ||||
| Patients at risk | 449 | 202 | 110 | 137 |
| Events | 115 | 15 | 34 | 66 |
| Person-years | 1651 | 1047 | 367 | 236 |
| Rate (/100 PY) | 7.0 | 1.4 | 9.3 | 28.0 |
| Hazard ratio | 1.0 | 5.5 | 10.6 | |
| 95% confidence interval | 3.0, 10.2 | 6.0, 18.6 | ||
| P value | <0.01 | <0.01 | ||
| Retinal detachment | ||||
| Eyes at risk | 614 | 261 | 156 | 197 |
| Events | 64 | 18 | 18 | 28 |
| Eye-years | 2808 | 1534 | 709 | 566 |
| Rate (/100 EY§) | 2.3 | 1.2 | 2.5 | 4.9 |
| Hazard ratio | 1.0 | 2.0 | 2.9 | |
| 95% confidence interval | 1.0, 3.8 | 1.6, 5.0 | ||
| P value | 0.04 | <0.01 | ||
| 2nd eye involvement | ||||
| Eyes at risk | 290 | 113 | 64 | 113 |
| Events | 40 | 6 | 11 | 23 |
| Eye-years | 1322 | 717 | 289 | 316 |
| Rate (/100 EY) | 3.0 | 0.8 | 3.8 | 7.3 |
| Hazard ratio | 1.0 | 3.8 | 5.9 | |
| 95% confidence interval | 1.4, 10.3 | 2.4, 14.7 | ||
| P value | 0.01 | <0.01 | ||
| Immune recovery uveitis | ||||
| Patients at risk | 229 | 134 | 51 | 44 |
| Events | 26 | 14 | 7 | 5 |
| Person-years | 1494 | 1098 | 258 | 138 |
| Rate (/100 PY) | 1.7 | 1.3 | 2.7 | 3.6 |
| Hazard ratio | 1.0 | 1.9 | 2.0 | |
| 95% confidence interval | 0.7, 4.9 | 0.7, 5.7 | ||
| P value | 0.18 | 0.21 | ||
Immune recovered = CD4+ T cells > 100 cells/µL; immune compromised = CD4+ T cells < 100 cells/µL.
Includes cytomegalovirus retinitis patients newly diagnosed at enrollment as well as those diagnosed under follow-up.
PY = person-years.
Hazard ratios, 95% confidence intervals, and P values obtained through Cox proportional hazards regression using previously diagnosed immune-recovered patients as reference group.
EY = eye-years.
Table 3.
Outcomes of Patients with Cytomegalovirus Retinitis by CD4+ T cells
| Event rate Outcome |
Overall (/100 PY) |
Enrollment CD4+ T cells (cells/µL) | ||||
|---|---|---|---|---|---|---|
| < 50 | 50–99 | 100–199 | ≥ 200 | P-value | ||
| Mortality | 9.8 | 27.0 | 6.8 | 5.3 | 2.7 | <0.01 |
| Retinitis progression | 7.0 | 23.3 | 7.2 | 3.3 | 1.2 | <0.01 |
| Retinal detachment in involved eyes* | 2.3 | 4.2 | 1.9 | 1.7 | 1.1 | <0.01 |
| 2nd eye involvement | 3.0 | 7.1 | 3.2 | 0.8 | 0.8 | <0.01 |
| VA worse than 20/40, involved eyes* | 7.9 | 10.9 | 6.4 | 6.4 | 6.0 | 0.04 |
| VA 20/200 or worse, involved eyes* | 3.4 | 6.0 | 3.9 | 4.8 | 3.0 | 0.03 |
VA = visual acuity; for these analyses, events expressed as rate/100 eye-years.
Figure 1.
Mortality among patients with cytomegalovirus retinitis. CMV=cytomegalovirus.
Retinitis Progression
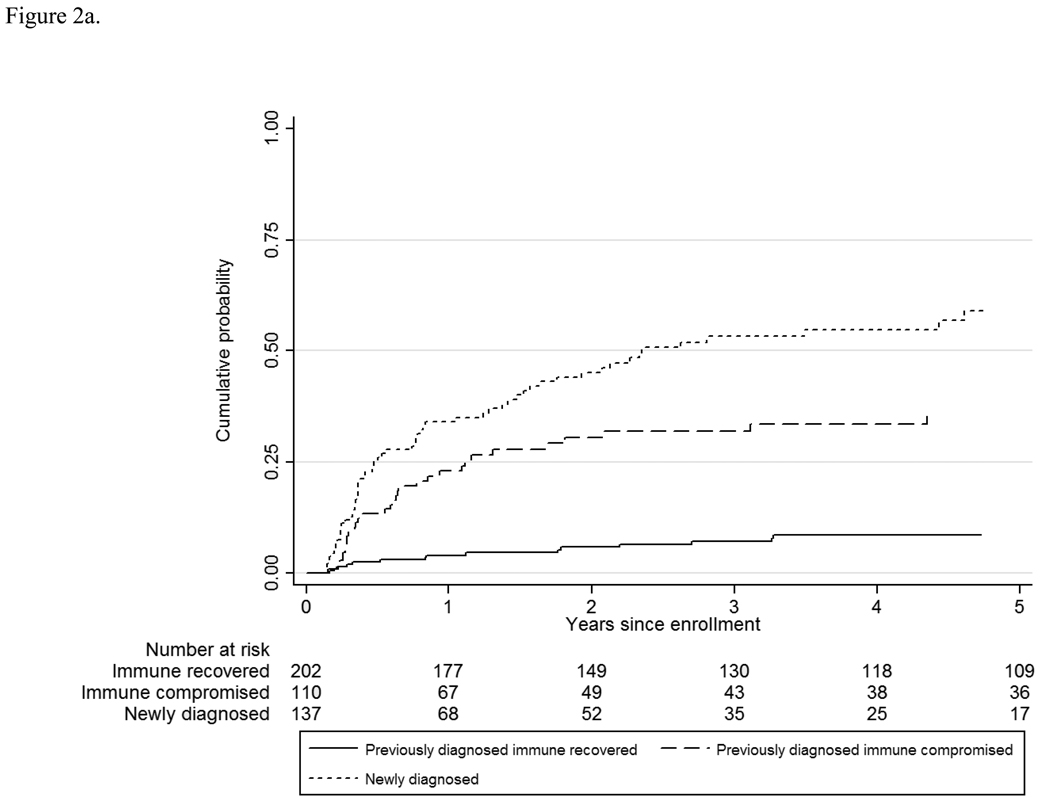
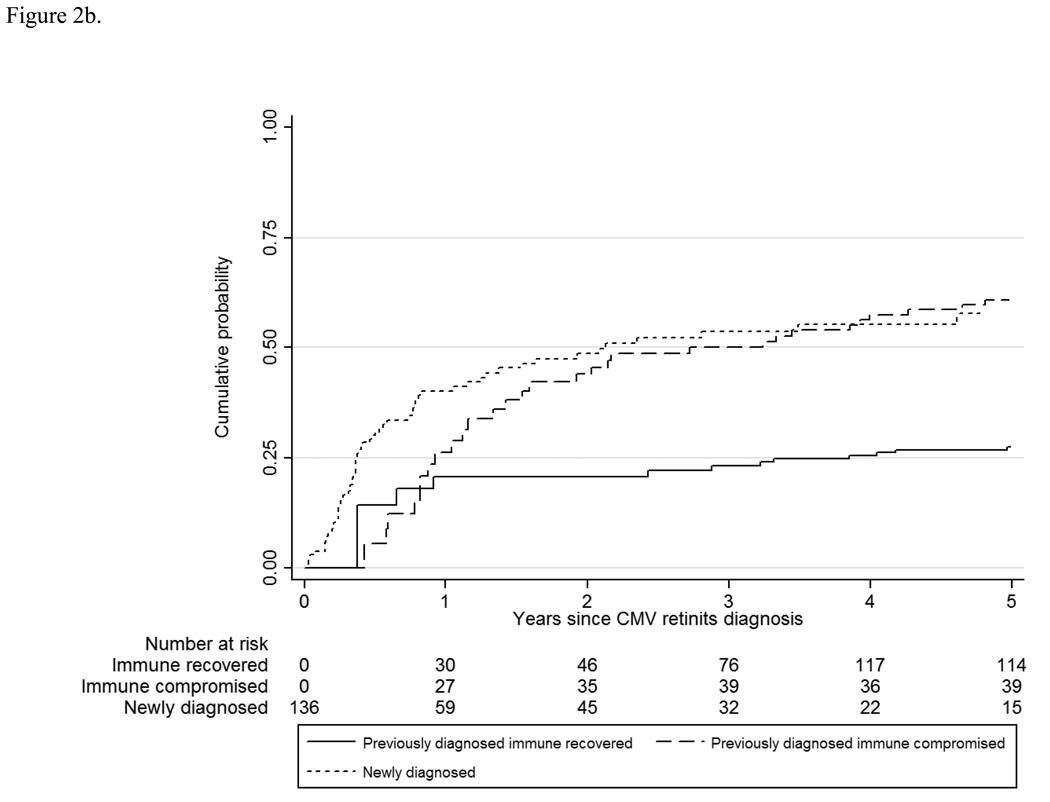
The overall rate of retinitis progression was 7.0/100 PY, and the rate varied by CMV retinitis group and by immunologic status (Table 2 and Table 3) from a low of 1.4/100 PY among those with immune recovery to 28.0/100 PY among those with newly-diagnosed retinitis. Compared to those with immune recovery, the hazard ratio for retinitis progression was 5.5 for those with previously-diagnosed retinitis and immune compromise (95% CI 3.0, 10.2; P<0.01) and 10.6 for those with newly-diagnosed retinitis (95% CI 6.0, 18.6; P<0.01). Although most of the progressions appeared to occur in the first three years after enrollment (Figure 2a), the Kaplan-Meier curve for retinitis progression continued to increase out to five years for all three groups. Because retinitis progression can be a recurrent event, and because data on progression prior to enrollment were not available for those patients with previously-diagnosed retinitis, the staggered entry approach cannot capture retinitis progression prior to immune recovery among patients enrolled with immune recovery; nevertheless, it can be used to compare the long-term cumulative progression rate between the two groups with immune compromise (Figure 2b). These data show a similar long-term cumulative risk of progression (~60% at 5 years after the diagnosis of CMV retinitis) between the two groups.
Figure 2.
a. Retinitis progression among patients with cytomegalovirus retinitis.
b. Retinitis progression among patients with cytomegalovirus retinitis. CMV= cytomegalovirus.
Retinal Detachment
The overall rate of retinal detachment was 2.3/100 EY, and the rate varied by CMV retinitis group and by immunologic status (Table 2 and Table 3) from a low of 1.2/100 EY for those with immune recovery to 4.9/100 EY for those with newly-diagnosed retinitis (Figure 3). Compared to those with immune recovery, the hazard ratio for retinal detachment was 2.0 for those with previously-diagnosed retinitis and immune compromise (95% CI 1.0, 3.8; P=0.04) and 2.9 for those with newly-diagnosed retinitis (95% CI 1.6, 5.0; P<0.01).
Figure 3.
Retinal detachment among patients with cytomegalovirus retinitis.
Immune Recovery and Immune Recovery Uveitis
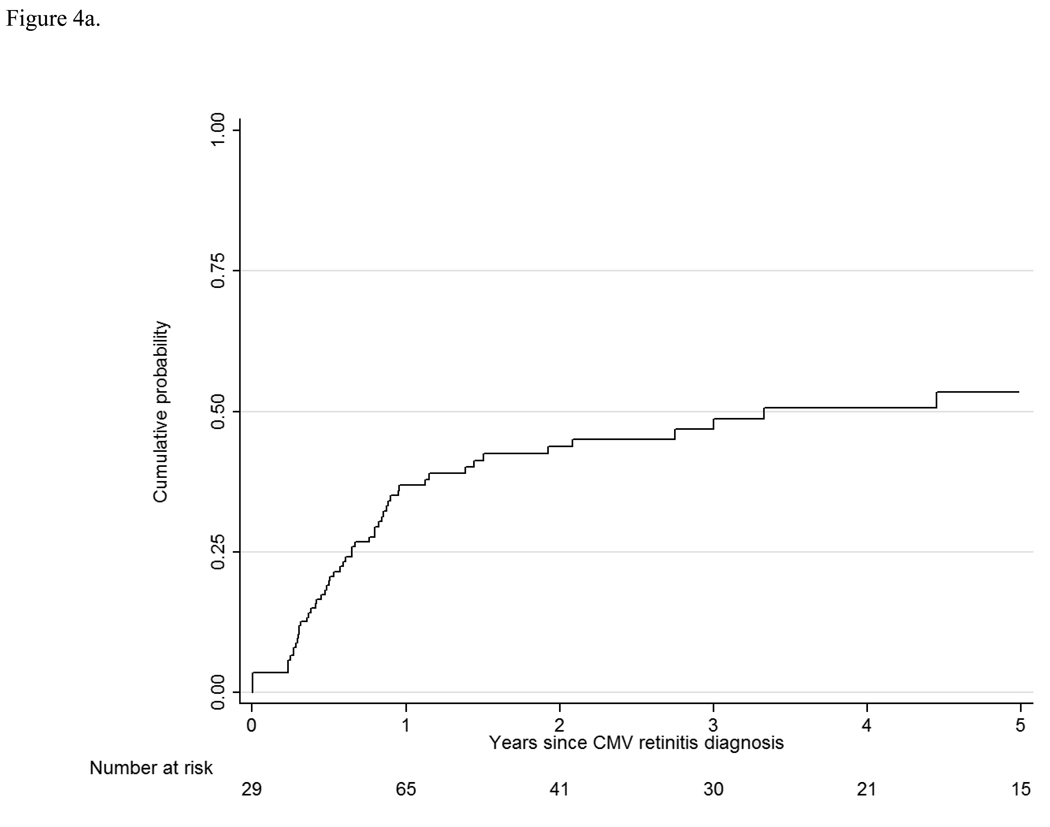
Forty percent of the patients with previously-diagnosed retinitis and immune compromise at enrollment and 39% of the patients with newly-diagnosed retinitis experienced immune recovery to a CD4+ T cell level ≥ 100 cells/µL. For those patients with newly-diagnosed retinitis the rate of immune recovery was 21.2/100 PY, and the cumulative probability of immune recovery was ~50% at five years after the diagnosis of CMV retinitis (Figure 4a). Among patients with newly-diagnosed CMV retinitis who experienced immune recovery, the estimated one- and five-year survivals from the diagnosis of CMV retinitis were 97.9% and 63.7%, respectively, whereas among those who did not experience immune recovery, the one- and five-year survivals were 43.6% and 1.4%, respectively.
Figure 4.
a. Immune recovery among patients with newly-diagnosed cytomegalovirus retinitis. CMV= cytomegalovirus.
b. Immune recovery uveitis among patients with newly-diagnosed cytomegalovirus retinitis.
The overall rate or IRU was 1.7/100 PY and varied from an estimated 1.3/100PY among those with immune recovery at enrollment to 3.6/100 PY among those with newly-diagnosed retinitis and subsequent immune recovery (Table 2 and Figure 4b). The rates among the three groups did not differ significantly; compared to those with immune recovery, the hazard ratio for IRU was 1.9 for those with previously-diagnosed retinitis and immune compromise (95% CI 0.7, 4.9; P=0.18) and 2.0 for those with newly-diagnosed retinitis (95% CI 0.7, 5.7; P=0.21). A time-updated analysis of CD4+ T cells and IRU did not demonstrate any significant difference in the rates of IRU relative to the amount of immune recovery; compared to CD4+ T cells >500 cells/µL, the hazard ratios for those with CD4+ T cells 100–199 cells/µL were 1.4 (P=0.54) and 0.6 for those with CD4+ T cells 200–499 cells/µL (P=0.44).
Visual Loss
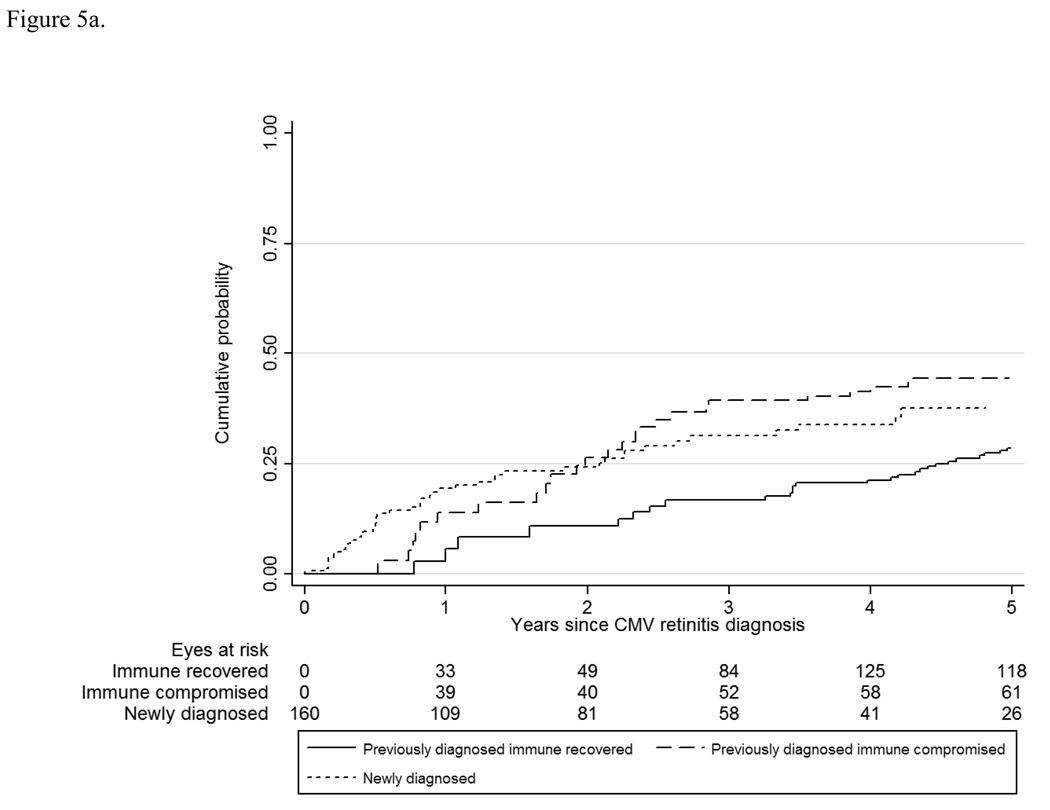
The overall rates of visual impairment (worse than 20/40) and legal blindness (20/200 or worse) were 7.9/100 EY and 3.4/100 EY, respectively. Rates of visual impairment and blindness varied by retinitis group and immunologic status from 6.1/100 EY to 11.8/100 EY, and 2.7/100 EY to 5.1/100 EY for those with immune recovery to those with newly-diagnosed retinitis (Table 3 and Table 4, Figure 5). Fortunately, rates of bilateral visual impairment and bilateral blindness (the better eye analyses) were about one-half of those for an eye with CMV retinitis (overall 3.7/100 PY and 1.4/100 PY). These rates did not differ significantly by CMV retinitis group at enrollment (Table 4).
Table 4.
Rates of visual outcomes among patients with cytomegalovirus retinitis.
| Previously diagnosed | ||||
|---|---|---|---|---|
| Overall | Immune recovered* |
Immune compromised* |
Newly diagnosed† |
|
| VA** worse than 20/40 | ||||
| Better eye | ||||
| Patients at risk | 465 | 199 | 111 | 155 |
| Events | 76 | 31 | 22 | 23 |
| Person-years | 2046 | 1113 | 475 | 458 |
| Rate (/100 PY‡) | 3.7 | 2.8 | 4.6 | 5.0 |
| Hazard ratio¶ | 1.0 | 1.5 | 1.4 | |
| 95% confidence interval¶ | 0.9, 2.7 | 0.8, 2.4 | ||
| P value¶ | 0.12 | 0.23 | ||
| Involved eyes | ||||
| Eyes at risk | 486 | 198 | 125 | 163 |
| Events | 146 | 60 | 37 | 49 |
| Eye-years | 1857 | 980 | 462 | 415 |
| Rate (/100 EY§) | 7.9 | 6.1 | 8.0 | 11.8 |
| Hazard ratio | 1.0 | 1.2 | 1.5 | |
| 95% confidence interval | 0.8, 1.8 | 1.0, 2.1 | ||
| P value | 0.44 | 0.05 | ||
| VA 20/200 or worse | ||||
| Better eye | ||||
| Patients at risk | 497 | 215 | 122 | 160 |
| Events | 32 | 12 | 10 | 10 |
| Person-years | 2403 | 1309 | 577 | 517 |
| Rate (/100 PY) | 1.3 | 0.9 | 1.7 | 1.9 |
| Hazard ratio | 1.0 | 1.7 | 1.6 | |
| 95% confidence interval | 0.8, 4.0 | 0.7, 3.7 | ||
| P value | 0.20 | 0.30 | ||
| Involved eyes | ||||
| Eyes at risk | 607 | 264 | 154 | 189 |
| Events | 94 | 40 | 25 | 29 |
| Eye-years | 2752 | 1493 | 688 | 570 |
| Rate (/100 EY) | 3.4 | 2.7 | 3.6 | 5.1 |
| Hazard ratio | 1.0 | 1.3 | 1.5 | |
| 95% confidence interval | 0.8, 2.1 | 1.0, 2.5 | ||
| P value | 0.31 | 0.08 | ||
Immune recovered = CD4+ T cells ≥ 100 cells/µL; immune compromised = CD4+ T cells µ 100 cells/µL.
Includes cytomegalovirus retinitis patients newly diagnosed at enrollment as well as those diagnosed under follow-up.
PY = person-years.
Hazard ratios, 95% confidence intervals, and P values obtained through Cox proportional hazards regression using previously diagnosed immune-recovered patients as reference group.
EY = eye-years.
VA = Visual acuity.
Figure 5.
a. Visual loss to worse than 20/40 among patients with cytomegalovirus retinitis. CMV= cytomegalovirus.
b. Visual loss to 20/200 or worse among patients with cytomegalovirus retinitis. CMV= cytomegalovirus.
Discussion
Although HAART has lowered dramatically mortality and the incidence of opportunistic infections among patients with HIV infection,8–12 over one-third of patients (38.3%) with newly-diagnosed HIV infection in the United States still will progress to AIDS within one year of diagnosis of HIV infection and 45% within three years, presumably due to late testing for HIV infection.42 Therefore, a substantial portion of patients with HIV infection are at risk for opportunistic infections, including CMV retinitis. Among those with CMV retinitis, treatment with HAART can lead to immune recovery and the possibility of prolonged survival. Thus, data on long-term outcomes among these patients will provide useful prognostic information. These data extend those reported in our previous publications on CMV retinitis progression, mortality, and visual loss, which had mean follow-ups of two to three years.
These updated data show lower rates of mortality, retinitis progression, and visual loss than previous SOCA publications in the HAART era (Table 5), consistent with the “flattening” of the Kaplan-Meier curves in Figure 1, Figure 2, and Figure 5. Nevertheless, the cumulative incidences of these events continue to rise, consistent with ongoing risk out to five years. In general, the rates do not appear to decrease to zero, even with long-term follow-up among those with immune recovery, suggesting that ongoing monitoring will be needed, at least for five years. Rates for progression and complications of CMV retinitis were lower among those with previously-diagnosed retinitis and immune compromise than among those with newly-diagnosed CMV retinitis. Reasons for this difference were not evident among enrollment characteristics but likely reflect survivor bias. Because the rate of retinitis progression declines over time, and because those with previously-diagnosed CMV retinitis had the retinitis for a median of 1.8 years, the observed rate of retinitis progression after enrollment in this group might be expected to be lower than that in the newly-diagnosed group (Figure 2a). This explanation is supported by the staggered-entry analysis of progression, which showed similar cumulative incidences by five years from diagnosis of CMV retinitis (Figure 2b). The five-year cumulative mortality from diagnosis of CMV retinitis was substantial and similar between those with previously-diagnosed CMV retinitis and immune compromise and those with newly-diagnosed retinitis; by five years nearly 75% of each group had died (Figure 1).
Table 5.
Comparison of Selected Two- to Three-Year v Five-Year Outcomes among Patients with Cytomegalovirus Retinitis
| Outcome | 2–3-year rate | 5-year rate |
|---|---|---|
| Mortality | 12/100 PY* | 9.8/100 PY |
| Retinitis progression | ||
| Overall | 10/100 PY | 7.0 /100 PY |
| CD4+ T cells < 50 cells/µL† | 27/100 PY | 23.3/100 PY |
| CD4+ T cells > 100 cells/µL | 3/100 PY | 1.8/100 PY |
| Visual acuity loss to | ||
| worse than 20/40 | ||
| Overall | 10/100 EY‡ | 7.9/100 EY |
| CD4+ T cells < 50 cells/µL† | 19/100 EY | 8.8/100 EY |
| CD4+ T cells > 100 cells/µL | 10/100 EY | 5.4/100 EY |
| 20/200 or worse | ||
| Overall | 6/100 EY | 3.4/100 EY |
| CD4+ T cells < 50 cells/µL† | 7/100 EY | 5.1/100 EY |
| CD4+ T cells > 100 cells/µL | 4/100 EY | 2.5/100 EY |
As previously reported,6,21–24 no level of CD4+ T cells is fully protective against progression or the complications of CMV retinitis. Progression, complications, and visual loss all occurred even among patients with CD4+ T cells >200 cells/µL. The rate of a retinal detachment in either eye in patients with immune recovery was 1.6/100 PY, which is over 100-fold greater than the population estimate of ~10/100,000 PY.43 Furthermore, immune recovery was not fully protective against CMV retinitis. About 1/6 of patients with newly-diagnosed retinitis at enrollment had CD4+ T cells >100 cells/µL, and about 1/6 of patients who developed CMV retinitis during follow-up had CD4+ T cells >100 cells/µL at enrollment. Although the incidence of CMV retinitis declined with increasing CD4+ T cells, the rate among those with CD4+ T cells ≥200 cells/µL was 0.04/100 PY.
Soon after the introduction of HAART, the phenomenon of IRU was described.30–33 Early studies estimated the incidence of IRU among those with CMV retinitis who experienced immune recovery at 10.9/100 PY and the prevalence at 17.6/100 patients with CMV retinitis and immune recovery.30,33 Substantially higher rates were reported by the group at the University of California, San Diego,32 but this group pioneered the use of intravitreous cidofovir therapy, and previous therapy with intravitreous cidofovir is a substantial risk factor for IRU (odds ratio=10.6).33 Our data suggest an ongoing but lower risk of IRU, which was not significantly different among the three groups of patients. Although the rates of IRU were not statistically different among the three groups, the number of IRU events was limited, limiting the power to detect smaller hazard ratios. Furthermore, the estimated incidence of IRU among patients presenting with immune recovery required assumptions that could limit its precision. Therefore, the observed rates of IRU of 2.7–3.6/100 PY may represent a better estimate of the current rate in the HAART era. Nevertheless, these rates are lower than that previously reported.30 Although it had been speculated that the phenomenon of IRU largely resulted from the introduction of HAART into a population of patients with long-standing CMV retinitis,44 IRU occurred among those with CMV retinitis newly-diagnosed in the HAART era, albeit at a lower rate than reported previously .
The LSOCA cohort includes over 500 participants with CMV retinitis. Despite the size of this cohort and the length of follow-up, some event rates, such as those for IRU, were estimated from a small number of events, limiting the precision of the estimates and the ability to compare among groups. The cohort is reasonably representative of the AIDS epidemic in the HAART era, but it is somewhat under representative of patients with HIV infection due to injection drug use.11 Nevertheless, the data should be reasonably generalizable, as the major determinants of outcomes among patients with AIDS are immunologic and virologic; given its size, duration of follow-up, and breadth of immunologic function, the LSOCA cohort appears to provide the best opportunity to describe long-term ocular outcomes among patients with AIDS and CMV retinitis.11
In conclusion, patients with CMV retinitis remain at increased risk for mortality, retinitis progression, complications of CMV retinitis, and visual loss for at least five years, even among those with immune recovery from HAART.
Acknowledgments
Financial support: Supported by cooperative agreements from the National Eye Institute, the National Institutes of Health, Bethesda, MD to the Mount Sinai School of Medicine, New York, NY (U10 EY08052); the Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD (U10 EY08057); and the University of Wisconsin, Madison, Madison, WI (U10 EY08067).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Authors with conflicting interests are listed after references.
Research Group membership available at http://aaojournal.org.
This article contains online-only material. The following should appear online-only: Research Group
REFERENCES
- 1.Jabs DA. Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc. 1995;93:623–683. [PMC free article] [PubMed] [Google Scholar]
- 2.Gallant JE, Moore RD, Richman DD, et al. Zidovudine Epidemiology Study Group. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated with zidovudine. J Infect Dis. 1992;166:1223–1227. doi: 10.1093/infdis/166.6.1223. [DOI] [PubMed] [Google Scholar]
- 3.Pertel P, Hirschtick R, Phair J, et al. Risk of developing cytomegalovirus retinitis in persons infected with the human immunodeficiency virus. J Acquir Immune Defic Syndr. 1992;5:1069–1074. [PubMed] [Google Scholar]
- 4.Hoover DR, Peng Y, Saah A, et al. Occurrence of cytomegalovirus retinitis after human immunodeficiency virus immunosuppression. Arch Ophthalmol. 1996;114:821–827. doi: 10.1001/archopht.1996.01100140035004. [DOI] [PubMed] [Google Scholar]
- 5.Studies of Ocular Complications of AIDS Research Group, AIDS Clinical Trials Group. Foscarnet-Ganciclovir Cytomegalovirus Retinitis Trial 4: visual outcomes. Ophthalmology. 1994;101:1250–1261. [PubMed] [Google Scholar]
- 6.Thorne JE, Jabs DA, Kempen JH, et al. Studies of Ocular Complications of AIDS Research Group. Incidence of and risk factors for visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology. 2006;113:1432–1440. doi: 10.1016/j.ophtha.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Thorne JE, Jabs DA, Kempen JH, et al. Studies of Ocular Complications of AIDS Research Group. Causes of visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology. 2006;113:1441–1445. doi: 10.1016/j.ophtha.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Palella FJ, Jr, Delaney KM, Moorman AC, et al. HIV Outpatient Study Investigators. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 9.Holtzer CD, Jacobson MA, Hadley WK, et al. Decline in the rate of specific opportunistic infections at San Francisco General Hospital, 1994–1997. AIDS. 1998;12:1931–1933. [PubMed] [Google Scholar]
- 10.Jacobson MA, Stanley H, Holtzer C, et al. Natural history and outcome of new AIDS-related cytomegalovirus retinitis diagnosed in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30:231–233. doi: 10.1086/313612. [DOI] [PubMed] [Google Scholar]
- 11.Jabs DA, Van Natta ML, Holbrook JT, et al. Studies of the Ocular Complications of AIDS Research Group. Longitudinal Study of the Ocular Complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology. 2007;114:780–786. doi: 10.1016/j.ophtha.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Jabs DA. AIDS and ophthalmology, 2008. Arch Ophthalmol. 2008;126:1143–1146. doi: 10.1001/archopht.126.8.1143. [DOI] [PubMed] [Google Scholar]
- 13.Jabs DA, Holbrook JT, Van Natta ML, et al. Studies of Ocular Complications of AIDS Research Group. Risk factors for mortality in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology. 2005;112:771–779. doi: 10.1016/j.ophtha.2004.10.049. [DOI] [PubMed] [Google Scholar]
- 14.Jabs DA, Bolton G, Dunn JP, Palestine AG. Discontinuing anticytomegalovirus therapy in patients with immune reconstitution after combination antiretroviral therapy. Am J Ophthalmol. 1998;126:817–822. doi: 10.1016/s0002-9394(98)00285-2. [DOI] [PubMed] [Google Scholar]
- 15.Tural C, Romeu J, Sirera G, et al. Long-lasting remission of cytomegalovirus retinitis without maintenance therapy in human immunodeficiency virus-infected patients. J Infect Dis. 1998;177:1080–1083. doi: 10.1086/517399. [DOI] [PubMed] [Google Scholar]
- 16.Macdonald JC, Torriani FJ, Morse LS, et al. Lack of reactivation of cytomegalovirus (CMV) retinitis after stopping CMV maintenance therapy in AIDS patients with sustained elevations in CD4 T cells in response to highly active antiretroviral therapy. J Infect Dis. 1998;177:1182–1187. doi: 10.1086/515281. [DOI] [PubMed] [Google Scholar]
- 17.Whitcup SM, Fortin E, Lindblad AS, et al. Discontinuation of anticytomegalovirus therapy in patients with HIV infection and cytomegalovirus retinitis. JAMA. 1999;282:1633–1637. doi: 10.1001/jama.282.17.1633. [DOI] [PubMed] [Google Scholar]
- 18.Kirk O, Reiss P, Uberti-Foppa C, et al. Safe interruption of maintenance therapy against previous infection with four common HIV-associated opportunistic pathogens during potent antiretroviral therapy. Ann Intern Med. 2002;137:239–250. doi: 10.7326/0003-4819-137-4-200208200-00008. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan JE, Benson C, Holmes KK, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–198. [PubMed] [Google Scholar]
- 20.Torriani FJ, Freeman WR, Macdonald JC, et al. CMV retinitis recurs after stopping treatment in virological and immunological failure of potent antiretroviral therapy. AIDS. 2000;14:173–180. doi: 10.1097/00002030-200001280-00013. [DOI] [PubMed] [Google Scholar]
- 21.Johnson SC, Benson CA, Johnson DW, Weinberg A. Recurrences of cytomegalovirus retinitis in a human immunodeficiency virus-infected patient despite potent antiretroviral therapy and apparent immune reconstitution. Clin Infect Dis. 2001;32:815–819. doi: 10.1086/319219. [DOI] [PubMed] [Google Scholar]
- 22.Komanduri KV, Feinberg J, Hutchins RK, et al. Loss of cytomegalovirus-specific CD4+ T cell responses in human immunodeficiency virus type 1-infected patients with high CD4+ T cell counts and recurrent retinitis. J Infect Dis. 2001;183:1285–1289. doi: 10.1086/319683. [DOI] [PubMed] [Google Scholar]
- 23.Jabs DA, Van Natta ML, Thorne JE, et al. Studies of Ocular Complications of AIDS Research Group. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: 1. Retinitis progression. Ophthalmology. 2004;111:2224–2231. doi: 10.1016/j.ophtha.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 24.Jabs DA, Van Natta ML, Thorne JE, et al. Studies of Ocular Complications of AIDS Research Group. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: 2. Second eye involvement and retinal detachment. Ophthalmology. 2004;111:2232–2239. doi: 10.1016/j.ophtha.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 25.Murphy EL, Collier AC, Kalish LA, et al. Viral Activation Transfusion Study Investigators. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 26.Jabs DA, Van Natta ML, Holbrook JT, et al. Studies of the Ocular Complications of AIDS Research Group. Longitudinal Study of the Ocular Complications of AIDS: 2. Ocular examination results at enrollment. Ophthalmology. 2007;114:787–793. doi: 10.1016/j.ophtha.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 27.Castro KG, Ward JW, Slutsker L, et al. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 28.Studies of Ocular Complications of AIDS (SOCA) Research Group, AIDS Clinical Trials Group (ACTG) Studies of Ocular Complications of AIDS Foscarnet-Ganciclovir Cytomegalovirus Retinitis Trial: 1. Rationale, design and methods. Control Clin Trials. 1992;13:22–39. doi: 10.1016/0197-2456(92)90027-w. [DOI] [PubMed] [Google Scholar]
- 29.Ferris FL, III, Kassof A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–96. [PubMed] [Google Scholar]
- 30.Nguyen QD, Kempen JH, Bolton SG, et al. Immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis after highly active antiretroviral therapy. Am J Ophthalmol. 2000;129:634–639. doi: 10.1016/s0002-9394(00)00356-1. [DOI] [PubMed] [Google Scholar]
- 31.Robinson MR, Reed G, Csaky KG, et al. Immune-recovery uveitis in patients with cytomegalovirus retinitis taking highly active antiretroviral therapy. Am J Ophthalmol. 2000;130:49–56. doi: 10.1016/s0002-9394(00)00530-4. [DOI] [PubMed] [Google Scholar]
- 32.Karavellas MP, Plummer DJ, MacDonald JC, et al. Incidence of immune recovery vitritis in cytomegalovirus retinitis patients following institution of successful highly active antiretroviral therapy. J Infect Dis. 1999;179:697–700. doi: 10.1086/314639. [DOI] [PubMed] [Google Scholar]
- 33.Kempen JH, Min YI, Freeman WR, et al. Studies of Ocular Complications of AIDS Research Group. Risk of immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis. Ophthalmology. 2006;113:684–694. doi: 10.1016/j.ophtha.2005.10.067. [DOI] [PubMed] [Google Scholar]
- 34.Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall; 1991. pp. 213–215.pp. 241–256. [Google Scholar]
- 35.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 36.Tarwater PM, Mellors J, Gore ME, et al. Methods to assess population effectiveness of therapies in human immunodeficiency virus incident and prevalent cohorts. Am J Epidemiol. 2001;154:675–681. doi: 10.1093/aje/154.7.675. [DOI] [PubMed] [Google Scholar]
- 37.Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med. 1994;13:2233–2247. doi: 10.1002/sim.4780132105. [DOI] [PubMed] [Google Scholar]
- 38.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 39.Jabs DA, Van Natta ML, Kempen JH, et al. Characteristics of patients with cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2002;133:48–61. doi: 10.1016/s0002-9394(01)01322-8. [DOI] [PubMed] [Google Scholar]
- 40.Holland GN, Vaudaux JD, Jeng SM, et al. CMV Retinitis Study Group. Characteristics of untreated AIDS-related cytomegalovirus retinitis. I. Findings before the era of highly active antiretroviral therapy (1988 to 1994) Am J Ophthalmol. 2008;145:5–11. doi: 10.1016/j.ajo.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 41.Holland GN, Vaudaux JD, Shiramizu KM, et al. Southern California HIV/Eye Consortium. Characteristics of untreated AIDS-related cytomegalovirus retinitis. II. Findings in era of highly active antiretroviral therapy (1997 to 2000) Am J Ophthalmol. 2008;145:12–22. doi: 10.1016/j.ajo.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention (CDC) Late HIV testing – 34 states, 1996–2005. MMWR Morb Mortal Wkly Rep. 2009;58:661–665. [PubMed] [Google Scholar]
- 43.Mitry D, Charteris DG, Fleck BW, et al. The epidemiology of rhegmatogenous retinal detachment – geographic variation and clinical associations. Br J Ophthalmol. doi: 10.1136/bjo.2009.157727. In press. [DOI] [PubMed] [Google Scholar]
- 44.Nussenblatt RB, Lane HC. Human immunodeficiency virus disease: changing patterns of intraocular inflammation. Am J Ophthalmol. 1998;125:374–382. doi: 10.1016/s0002-9394(99)80149-4. [DOI] [PubMed] [Google Scholar]