Abstract
Objective
Increased interleukin-6 plasma levels have been reported in metabolic syndrome (MS); nevertheless, it is unclear whether interleukin-6 activity is exerted through direct signalling only or also through the “trans-signalling”. This issue is important to clarify since signalling and “trans-signalling” affect different tissues. We investigated the relationship between MS and the interleukin-6 system in an older population.
Methods
Data from 997 older community dwelling individuals (age ≥ 65 years; females: 56.2%) enrolled the InChianti study were analyzed. Interleukin-6, soluble interleukin-6 receptor (sIL-6r), and soluble glycoprotein 130 (sgp130) were measured on plasma by ELISA. MS was defined by the NCEP-ATPIII criteria; 309 individuals (31%) resulted affected by MS.
Results
Subjects with MS had higher interleukin-6 and sgp130 levels compared to controls; a trend toward higher levels of sIL-6R was also observed. The risk of having MS was increased in individuals with high sIL-6r or/and sgp130 levels, independent of age, gender, and interleukin-6 levels. Elevated sgp130 levels were associated with higher plasma glucose, HOMA, triglycerides, and with diabetes both in subjects with and without MS. Although the risk of high sgp130 levels was generally associated with MS (O.R.:1.77, 95%C.I.: 1.39-2.25), this excess of risk was not present in MS phenotypes excluding the criteria “elevated glucose” or “elevated triglycerides”. Furthermore, the association between sgp130 and MS disappeared after adjustment for HOMA.
Conclusions
We found that older individuals with MS have increased sgp130 plasma levels compared with controls; nevertheless, our data suggest that this association might be mediated by insulin resistance.
Keywords: metabolic syndrome, interleukin-6, sgp130, pathway, trans-signalling
INTRODUCTION
Metabolic syndrome (MS) is a common clinical condition characterized by the clustering of cardiovascular risk factors related to insulin-resistance, including impaired glucose tolerance, central obesity, hypertension, and dyslipidemia (1,2).
MS and its components have been consistently associated with “low grade” systemic inflammation (LGSI), manifested as increased levels of C reactive protein (CRP), interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α) (3-6). Recently, we confirmed the association between MS and LGSI in older individuals (7); furthermore, we demonstrated that this association was mainly driven by a strong correlation between waist circumference and LGSI (7). This observation supports the notion that also in MS there is increased release of cytokines such as IL-6 by the visceral adipose tissue (8).
IL-6 is a pleiotropic pro-inflammatory cytokine acting on a large variety of cells. Its biological activity is mediated by a membrane-bound receptor which consists of two subunits: a cognate receptor subunit (IL-6r) which specifically recognize IL-6, and a signal-transducing element, the glycoprotein 130 (gp130) (9). While IL-6r is functionally expressed by hepatocytes and leukocytes, gp130 represents the ubiquitous signaling receptor for the IL-6 family, and is expressed in almost all organs (9). In addition to the membrane-bound receptors, two soluble receptors are present in serum, the soluble IL-6r (sIL-6r), and the soluble gp130 (sgp130). sIL-6r binds to IL-6 prolonging its half-life; furthermore, the complex sIL-6r/IL-6 is capable of activating gp130 (10). In this way, sIL-6r has the ability to widen the repertoire of cells responsive to IL-6. The activation of the IL-6 pathway by the sIL-6r/IL-6 complex is called “trans-signalling”, and it accounts for most of the biological activity of IL-6. The trans-signalling mechanism is tightly regulated by the sgp130 which can bind and inactivate the sIL-6r/IL-6 complex (10). While IL-6 plasma levels change remarkably over time, sIL-6r and sgp130 levels fluctuate much less, and give more precise information about the activation of IL-6 pathway. Interestingly, it has been suggested that during an inflammatory process, the trans-signalling mechanism is critical in the switch from the initial acute inflammation (recruitment of neutrophils) to the chronic phase (recruitment of lymphocytes), typical of several conditions associated with aging (9,11).
The previous findings of a chronic LGSI associated with visceral fat deposition in older individuals with MS (7,8) suggest the possible involvement of the IL-6 “trans-signalling” mechanism in these individuals. To test this hypothesis, we investigated the relationship between the IL-6 system, assessed by plasma levels of IL-6, sIL-6r, and sgp130, and metabolic syndrome, in a large sample of older individuals.
MATERIALS AND METHODS
This study is part of the “Invecchiare in Chianti” (Aging in the Chianti area, InCHIANTI) study, a prospective population-based study of older persons, designed by the Laboratory of Clinical Epidemiology of the Italian National Research Council of Aging (INRCA, Florence, Italy). The study included participants randomly selected from the residents in two towns of the Chianti area (Greve in Chianti and Bagno a Ripoli, Tuscany, Italy)(12). A detailed description of the sampling procedure and data collection method has been previously published (12). Overall, 1453 subjects were recruited. Of these, 634 men (≥65yrs: 492) and 802 women (≥65yrs: 648) with an age range of 21–103 years, underwent a complete dietary interview and were included in the final analytical sample. Clinical visits and assessments were performed in the study clinic and were preceded by an interview conducted at the participants’ homes. Trained interviewers administered two structured questionnaires. One collected data on dietary intakes and the other included questions on household composition, social networks, economical status, education, and general information on health and functional status. The INRCA Ethical Committee ratified the entire study protocol.
The analyses presented in this report are based on data from 997 individuals over 65 years in which all metabolic parameters and inflammatory mediators had been measured at baseline. Metabolic syndrome was defined by the presence of at least three out of five criteria established by the NCEP-ATP III - AHA/NHLBI statement (13).
Clinical chemistry parameters
All parameters were measured on fresh serum after 12 hours overnight fasting; the patient had been sedentary in sitting or supine position for 15 minutes. Commercial enzymatic colorimetric tests using the peroxidase-chromogen reaction and the Roche Hitachi 917 Analyzer (Roche Diagnostics, GmbH, Mannheim, Germany) were used for determining total cholesterol (TC), triglycerides (TG), and fasting glucose.
HDL Cholesterol (HDL-C) levels were measured by enzymatic colorimetric method (Liquid Homogeneous HDL-c reagent, ALI FAX, Padova, Italy).
Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald’s formula: LDL-C: TC – (TG/5) – HDL-C.
Fasting insulin was determined on plasma sample stored −80°C by using a commercial double-antibody, solid-phase radioimmunoassay (Sorin Biomedica, Milan, Italy).
Insulin resistance was calculated by the Homeostasis Model Assessment (HOMA) as follows: fasting insulin (U/L) x fasting glucose (mmol/L) / 22.5.
Inflammatory markers
Sampled blood was transferred into the tube avoiding hemolysis. After having been aliquoted, plasma was frozen and stored at −80°C until the tests were performed.
Interleukin-6 (IL-6) levels (pg/ml)
ultrasensitive ELISA (CytoScreen Human IL-6, Biosource International Inc., Camarillo, CA, USA). Minimum detectable threshold: 0.10 pg/mL
Soluble IL-6 receptor (sIL-6r) (ng/ml)
ELISA (cytoscreen human sIL-6r kit, BioSource International Inc., Camarillo, CA). The intrassay and interassay coefficients of variation were <6% and 7%, respectively.
Soluble glycoprotein 130 (sGP130) (ng/ml)
ELISA on plasma (Quantikine Human soluble gp130 Immunoassay, R&D Systems, Inc, Minneapolis, MN, USA). Lower detection level: approximately 25 ng/mL (at a 1:100 dilution). Inter-assay CV was approximately 10%
Interleukin-1β (IL-1β) (pg/ml)
ELISA using an ultrasensitive commercial kit (Human Ultrasensitive; Biosource International Inc., Camarillo, CA, USA). Minimum detectable threshold: 0.01 pg/mL. The intra assay CV was < 7%; the inter-assay CV was < 9%.
Interleukin-1 Receptor Antagonist (IL-1RA) (pg/ml)
ELISA kit (EASIA™ ELISA Human IL-1ra; Biosource International Inc., Camarillo, CA, USA).
The intra-assay CV was < 5%; the inter-assay CV was < 7%.
Interleukin 18 (IL-18) (pg/ml)
sandwich ELISA (Human IL-18 ELISA Kit; Medical and Biological Laboratories Co., Ltd., Nagoya, Japan; distributed by R&D Systems, Minneapolis, MN, USA). Minimum detectable concentration: 100 pg/mL (at a 1:5 dilution). Inter-assay CVs: < 10%.
Tumor Necrosis Factor-alpha (TNF-α) (pg/ml)
UltraSensitive ELISA (R&D systems, Minneapolis, USA, Quantikine HS HSTA00C) and LINCOplex kit, Luminex (HADK2-61K-B). The intrassay and interassay coefficients of variation were 7% and <21%, respectively.
C Reactive Protein (CRP)
high sensitivity ELISA using purified protein and polyclonal anti-CRP antibodies. The minimum detectable concentration was 0.03 mg/L. The average of two measures performed on each sample was used in the analysis. The intrassay and interassay coefficients of variation were 5%.
Dietary variables
Data on alcohol intake were collected by the food-frequency questionnaire created for the European Prospective Investigation into Cancer and nutrition (EPIC) study.
Statistical analysis
Continuous variables were expressed as mean (SD) or median (interquartile range). Means were compared by ANOVA, while medians were compared by non-parametric tests (Mann-Whitney). Correlations between continuous variables were tested by multivariate linear regression analysis. Prevalences were compared by the χ2 test.
Due to the skewed distribution of values, sGP130, hs.CRP, cytokines, insulin, TG, and HOMA were log-transformed to approximate normal distribution before entering regression analysis.
Subjects with MS were stratified by the presence of low (< median value: 307.2 ng/ml) or high (≥ median value) levels of plasma sgp130.
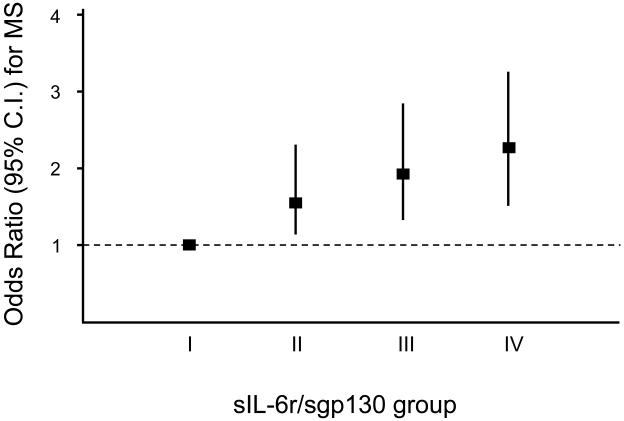
The risk (Odds Ratio - O.R.; 95% Confidence Interval (C.I.); age and gender adjusted) of having the diagnosis of MS, based on different combinations of sIL-6r and sGP130 plasma levels, was calculated by multivariate logistic regression analysis (Figure 1). Levels of sIL-6r were defined as “high” when over the median value (≥93.8 ng/ml). The groups included into the analysis were:
Group I: sIL-6r: low / sgp130: low (reference)
Group II: sIL-6r: high / sgp130: low
Group III: sIL-6r low / sgp130: high
Group IV: sIL-6r; high / sgp130: high
FIGURE 1.
Age and gender adjusted Odds Ratio (95% C.I.) for metabolic syndrome (by the NCEP ATP III criteria) in 997 older community dwelling individuals from the InChianti study, divided by plasma levels of sIL-6r and sgp130 (Group I: sIL-6r: low / sgp130: low; Group II: sIL-6r: high / sgp130: low; Group III: sIL-6r low / sgp130: high; Group IV: sIL-6r; high / sgp130: high)
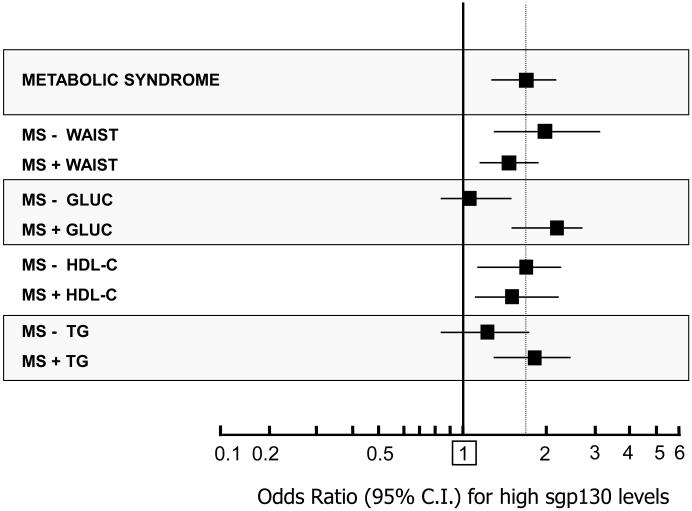
The probability (O.R.; age and gender adjusted) of having elevated sGP130 levels in different MS phenotypes was evaluated by multivariate logistic regression analysis (Figure 2). For each MS criterion, a new variable was created by including the diagnosis of MS plus the absence (−) or the presence (+) of the single criterion.
FIGURE 2.
Age and gender adjusted Odds Ratio (95% C.I.) for the presence of high sgp130 plasma levels 309 in community dwelling older subjects with MS (by the NCEP ATP III criteria) from the InChianti study, according to the absence (−) of presence (+) of the single MS components.
Systat for Windows, version 5.0, and SPSS for Windows, version 7.0 (SPSS, Inc, Chicago, IL) statistical packages were used.
RESULTS
The principal characteristics of the sample according to presence or absence of MS are described in TABLE 1. Subjects with MS were younger and more likely to be female; their alcohol intake was lower, while the prevalence of diabetes and coronary hearth disease (CHD) was higher compared to subjects without MS. The levels of IL-6, sIL-6r, sgp130, and other inflammatory mediators according to MS status are reported in TABLE 2. Compared to normal, subjects with MS had higher IL-6, sgp130, hs.CRP, IL-18, IL-1β, and IL-1ra. The levels of sIL-6r were slightly higher in subjects with MS compared with controls (not significant). In subjects with MS, median values of IL-6 progressively increased from 1.46 pg/ml to 1.68 pg/ml to 2.73 pg/ml in individuals bearing 3, 4, or 5 MS criteria, respectively (p<0.003). On the contrary, median values of sIL-6r and sgp130 did not change according to the number of MS criteria.
TABLE 1.
Principal characteristics of 997 community dwelling older individuals from the InChianti study, according to the absence (n° 688) or presence (n° 309) of metabolic syndrome (MS) by the NCEP ATP III criteria
| VARIABLE | CONTROLS (n. 688) |
MS + (n. 309) |
P |
|---|---|---|---|
| AGE (yrs) | 76.2± 7.9 | 75.3± 6.8 | 0.009 |
| GENDER (F%) | 53.8 | 64.3 | 0.001 |
| ATP III MS CRITERIA (%) | |||
| - INCREASED WAIST | 26.2 | 79.0 | 0.001 |
| - HIGH TRIGLYCERIDES | 9.3 | 60.5 | 0.001 |
| - LOW HDL-C | 9.6 | 54.1 | 0.001 |
| - HYPERTENSION | 90.9 | 98.0 | 0.001 |
| - HYPERGLYCEMIA | 12.1 | 57.8 | 0.001 |
| GLUCOSE (mg/dL) | 88.2±18 | 108.1±35 | 0.001 |
| INSULIN (U/L) | 9.30 (6.65-13.2) | 11.20 (8.5-16.6) | 0.001 |
| HOMA | 2.03 (1.41-2.94) | 3.03 (2.03-4.55) | 0.001 |
| TOT. CHOL. (mg/dL) | 212±39 | 221±40 | 0.04 |
| TRIGL. (mg/dL) | 110±42 | 175±85 | 0.001 |
| LDL-C (mg/dL) | 133.8±34.0 | 139.5±36.1 | 0.08 |
| HDL-C (mg/dL) | 58±14 | 47±10 | 0.001 |
| WBC (x 1000/mmc) | 6.20±1.51 | 6.42±1.80 | 0.001 |
| AST (U/L) | 20 (17-24) | 19 (17-22) | 0.07 |
| ALT (U/L) | 16 (13-21) | 18 (14-23) | 0.001 |
| ALP (U/L) | 200 (167-244) | 207 (170-254) | 0.13 |
| γ GT (U/L) | 18 (14-27) | 21 (16-33) | 0.001 |
| WAIST (cm) | 89±9.0 | 99±8.5 | 0.001 |
| BMI (kg/m2) | 26.4±3.48 | 29.8±4.37 | 0.001 |
| SBP (mm/Hg) | 148.5±18.5 | 154±17 | 0.001 |
| DBP mm/Hg) | 83.8±8.3 | 85±8..8 | 0.01 |
| ALCOHOL (g/day) | 7.61 (0-26.2) | 5.71 (0-12.7) | 0.02 |
| DIABETES (%) | 8.2 | 21.6 | 0.001 |
| CHD (%) | 6.6 | 10.3 | 0.04 |
| STROKE (%) | 6.7 | 6.9 | 0.48 |
| SMOKING (%) | 0.07 | ||
| Never | 58.0 | 63.5 | |
| Former | 30.4 | 27.5 | |
| Current | 11.6 | 9.0 | |
WBC: white blood cells count, BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; CHD: coronary heart disease.
For plasma cytokines, insulin, HOMA, AST, ALT, ALP, γ-GT, and alcohol intake: median (interquartile range)
TABLE 2.
Plasma levels (median - interquartile range) of IL-6, sIL-6r, sgp130, and other inflammatory mediators in 997 older community dwelling individuals from the InChianti study, according to the absence (MS− / controls) or presence (MS+) of metabolic syndrome by the NCEP ATP III criteria
| CONTROLS (n° 688) |
MS + (n° 309) |
p | |
|---|---|---|---|
| IL-6 (pg/ml) | 1.39 (0.82-2.07) | 1.68 (0.96-2.75) | 0.001 |
| sIL-6r (ng/ml) | 92.5 (67.6-125.8) | 97.5 (72.0-125.2) | 0.15 |
| sgp130 (ng/ml) | 303.8 (270.5-344.0) | 313.5 (277.4-352.2) | 0.01 |
| hs.CRP (mg/L) | 2.40 (1.20-5.45) | 3.38 (1.81-6.46) | 0.001 |
| IL-1β (pg/ml) | 0.12 (0.08-0.18) | 0.13 (0.08-0.19) | 0.03 |
| IL-1 ra (pg/ml) | 122.8 (91.4-169.0) | 164.0 (120.2-230.0) | 0.001 |
| TNF-α (pg/ml) | 5.00 (2.79-7.48) | 5.25 (3.23-7.96) | 0.19 |
| IL-18 (ug/ml) | 378.1 (287.7-474.6) | 404.2 (312.8-517.1) | 0.002 |
| TGF-β (pg/ml) | 12246 (5401-17380) | 11837 (4673-17387) | 0.31 |
By multivariate logistic regression analysis we were able to demonstrate that plasma levels of IL-6 (III vs I tertile O.R. 1.50; 95%C.I. 1.01-2.26) and sgp130 (III vs I tertile O.R. 1.49; 95%C.I. 1.05-2.12), but not sIL-6r (III vs I tertile O.R. 1.12; 95%C.I. 0.80-1.59) were significantly associated with MS independent of age, gender, hs.CRP, and IL-1β, IL-18, and IL-1ra (data not shown).
In order to evaluate the contribution of the trans-signalling mechanism to the risk of having MS, the Odds Ratio (age and gender adjusted) for MS was calculated based on different combinations of sIL-6r and sgp130 plasma levels (FIGURE 1). Compared to subjects with low levels of both sIL-6r and sgp130 (group I) the risk for MS progressively increased from group II (O.R. 1.62; 95%C.I. 1.12-2.33), to group III (O.R. 1.95; 95%C.I. 1.34-2.82), to group IV (O.R. 2.25; 95%C.I. 1.57-3.22). Interestingly, after adjustment for IL-6 levels, the risk for MS was slightly reduced but still significant in subjects with high sgp130 levels (group II: O.R. 1.38; 95%C.I. 0.95-2.01; group III: O.R. 1.72; 95%C.I. 1.18-2.52, and group IV: O.R. 1.98; 95%C.I. 1.38-2.85).
Since the association of sgp130 with MS was stronger compared to sIL-6r, and was independent from other mediators of inflammation including IL-6, we successively focused our analysis on sgp130.
A positive a significant correlation between sgp130 plasma levels and fasting glucose, HOMA score, fasting insulin, and triglycerides levels was found, both in subjects with and in subjects without MS (data not shown).
In TABLE 3 are reported the characteristics of subjects with MS stratified by presence of low or high levels of plasma sgp130. Compared to individuals with MS and low sgp130 levels, subjects with MS and elevated sgp130 levels had higher levels of TNF-α, IL-18, blood fasting glucose, HOMA score, total cholesterol, triglycerides, γ-GT, and ALP, and higher prevalence of diabetes.
TABLE 3.
Principal characteristics of 309 community dwelling older individuals from the InChianti study affected by metabolic syndrome (by the NCEP ATP III criteria), and stratified by low (< 307.2 ng/ml) or high (≥ 307.2 ng/ml) levels of plasma sgp130
| MS + | |||
|---|---|---|---|
| VARIABLE | Low sgp130 (n. 151) |
High sgp130 (n. 158) |
P |
| AGE (yrs) | 74.6±7.0 | 75.3±6.5 | 0.36 |
| GENDER (F%) | 66.4 | 64.3 | 0.38 |
| IL-6 (pg/ml) | 1.66 (0.97-2.88) | 1.69 (0.93-2.57) | 0.81 |
| sIL-6r (ng/ml) | 92 (73-119) | 103 (69-107) | 0.53 |
| sgp130 (ng/ml) | 273 (252-291) | 346 (324-375) | 0.001 |
| hs.CRP (mg/L) | 3.71 (1.66-7.0) | 3.33 (1.84-6.36) | 0.87 |
| IL-1β (pg/ml) | 0.13 (0.08-0.19) | 0.13 (0.08-0.20) | 0.93 |
| IL-1 ra (pg/ml) | 163 (119-233) | 165 (121-227) | 0.91 |
| TNF-α (pg/ml) | 4.66 (2.44-7.64) | 5.62 (3.56-8.14) | 0.04 |
| IL-18 (ug/ml) | 376 (304-468) | 418 (339-530) | 0.01 |
| TGF-β (pg/ml) | 12508 (4681-17198) | 10958 (4536-17687) | 0.77 |
| GLUCOSE (mg/dL) | 104±28 | 114±39 | 0.006 |
| INSULIN (U/L) | 11.8 (8.1-16.3) | 11.4 (8.6-16.7) | 0.44 |
| HOMA | 2.81 (1.87-4.33) | 3.21 (2.21-4.60) | 0.007 |
| TOT. CHOL. (mg/dL) | 214±40 | 226±40 | 0.01 |
| TRIGL. (mg/dL) | 161±71 | 189±98 | 0.004 |
| LDL-C (mg/dL) | 145.5±36 | 141±35 | 0.17 |
| HDL-C (mg/dL) | 46.8±11 | 47±12 | 0.70 |
| WBC (x 1000/mmc) | 6.6±1.7 | 6.3±1.6 | 0.17 |
| AST (U/L) | 19 (17-22) | 20 (17-24) | 0.65 |
| ALT (U/L) | 18 (13-21) | 18 (14-23) | 0.97 |
| ALP (U/L) | 193 (161-231) | 216 (174-260) | 0.005 |
| γ GT (U/L) | 18 (14-27) | 20 (14-30) | 0.007 |
| WAIST (cm) | 99.4±9.5 | 98.9±9 | 0.66 |
| BMI (kg/m2) | 29.8±4.1 | 29.9±4.1 | 0.87 |
| SBP (mm/Hg) | 154±19 | 156±18 | 0.24 |
| DBP (mm/Hg) | 85±8.4 | 85.5±8.4 | 0.66 |
| ALCOHOL (g/day) | 5.73 (0-13.6) | 6.04 (0-19.3) | 0.60 |
| DIABETES | 17.2 | 25.4 | 0.04 |
| CHD (%) | 9.3 | 10.7 | 0.41 |
| STROKE (%) | 6.0 | 7.7 | 0.35 |
| SMOKING (%) | 0.71 | ||
| Never | 64.5 | 62.6 | |
| Former | 27 | 28.1 | |
| Current | 8.5 | 9.4 | |
WBC: white blood cells count, BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; CHD: coronary heart disease.
For plasma cytokines, insulin, HOMA, AST, ALT, ALP, γ-GT, and alcohol intake: median (interquartile range)
In FIGURE 2 is reported the probability (O.R. 95%C.I., age and gender adjusted) of having elevated sgp130 plasma levels, in subjects with MS, according to different phenotypes of MS. For each MS criteria a new variable was created by selecting the diagnosis of MS plus the absence (−) or the presence (+) of the single criterion. In this way, all subjects included into the analysis qualified as affected by MS; nevertheless, each group included MS individuals which necessarily bear (+) or not (−) a specific criterion for MS. The criterion “arterial hypertension” was excluded from analysis due to its very high prevalence among individuals with MS (98%).
In general, MS was significantly associated with the risk of having elevated sgp130 levels (O.R.: 1.77, 95%C.I.: 1.39-2.25). The absence or presence of MS criteria “increased waist circumference” (−Waist: O.R.: 1.87; 95%C.I.: 1.18-2.96; +Waist: O.R.: 1.61; 95%C.I.: 1.23-2.10), and “low HDL-C” (−HDL-C: O.R.:1.75; 95%C.I.:1.25-2.45; +HDL-C: O.R.:1.51; 95%C.I.:1.12-2.03) did not change significantly the risk. Similarly, in MS phenotypes bearing the criteria “elevated glucose” (+ Gluc: O.R.: 2.08; 95%C.I.: 1.53-2.82) or “elevated triglycerides” (+ TG: O.R.: 1.93; 95%C.I.: 1.45-2.58), only a slight increase in the risk was observed. On the contrary, in the MS phenotypes excluding the criteria “elevated glucose” (−Gluc: O.R.: 1.21; 95%C.I.: 0.87-1.68) or “elevated triglycerides” (−TG: O.R.: 1.23; 95%C.I.: 0.86-1.75), the risk for high sgp130 was substantially normalized and no more significant.
Afterwards, we analysed thoroughly the relationship between MS, insulin resistance, and sgp130 levels. We found that HOMA was associated with high sgp130 levels (O.R.: 1.10; 95%C.I.: 1.02-1.17), also after inclusion of MS in the model (O.R.: 1.08; 95%C.I.: 1.009-1.16), while the association between high sgp130 levels and MS disappeared after adjustment for HOMA (O.R.: 1.21; 95%C.I.: 0.91-1.61). Moreover, while MS was significantly associated with high sgp130 levels in individuals with high HOMA (above the median value: 2.26) (O.R.: 1.48; 95%C.I.: 1.04-2.11), it was not in subjects with low HOMA (below the median value) (O.R.: 1.02; 95%C.I.: 0.65-1.58). Finally, after exclusion of individuals with a current diagnosis of diabetes (n° 117; 70% treated with anti-diabetics, 15% treated with insulin) HOMA was still associated with high sgp130 levels (O.R.: 1.10; 95%C.I.: 0.008-1.19), while MS was not (O.R.: 1.26; 95%C.I.: 0.94-1.69). When we reversed the analysis, and evaluated the association between elevated sgp130 levels and the fulfilment of the NCEP ATP III criteria for MS, we found that high sgp130 levels were significantly associated with “elevated blood glucose” (O.R.: 1.81; 95%C.I.: 1.38-2.38), and “hypertension” (O.R.: 3.15; 95%C.I.: 2.02-4.90).
DISCUSSION
We investigated the plasma levels of IL-6, sIL-6r, and sgp130 in a large sample of older individuals with and without MS, to test the hypothesis of a possible involvement of the IL-6 trans-signalling pathway in this medical condition.
The first finding of our study is that, compared to normal, subjects with MS had higher plasma levels of sgp130, while only a trend toward higher levels of sIL-6R was observed. The association between MS and sgp130 levels was independent from age, gender, and other inflammatory mediators related to MS. To our knowledge, this is the first study reporting an increase of sgp130 levels in subjects with MS.
We successively analysed the relationship between the components of the IL-6 trans-signalling pathway and MS (see FIGURE 1). The risk for MS progressively increased from subjects with high sIL-6r levels, to subjects with high sgp130, to subjects with elevated levels of both sIL-6r and sgp130. The risk for MS was significant and independent of age, gender, and also IL-6 plasma levels; this observation suggest that high sIL-6r, or better still high sgp130 plasma levels might give important information about the risk of being affected by MS, no matter if plasma IL-6 levels are low or elevated.
Soluble gp130 is considered the natural inhibitor of the IL-6/sIL-6r complex (14). We found that sgp130 levels were significantly correlated only with IL-18 levels (r:0.11, p:0.001), which in turn were elevated in subjects with MS, both in our study and in a previous report by Hung et al. (15). Interestingly, it was demonstrated in vitro that IL-18 enhances IL-6 production by neutrophils (16) and, together with IL-15, indirectly exerts an anti-inflammatory action exactly by inducing the secretion of sgp130 by neutrophils (17). In individuals with MS, sgp130 also correlated with IL-1ra levels (r: 0.13, p: 0.001), another anti-inflammatory cytokine that was elevated in subjects with MS, and whose expression is induced by IL-6 (18). On the whole these findings suggest that, in MS, increased sgp130 levels might be related with the activation of inflammatory processes involving both IL-18 and the IL-6.
A second important finding of our study is the observation that in older individuals with MS, high levels of sgp130 were associated with higher fasting glucose, triglycerides, HOMA, and with diabetes. Since a positive correlation between sgp130 levels and fasting glucose, HOMA score, fasting insulin, and triglycerides levels was also observed, both in subjects with and in subjects without MS, the existence of a relationship between sgp130 and insulin resistance was assumed.
We indirectly confirmed this hypothesis by evaluating the probability of having high sgp130 in different MS phenotypes. Actually, the risk of high sgp130 was increased in MS, but not in phenotypes specifically excluding the two criteria “increased fasting glucose” or “hypertriglyceridemia” (Figure 2).
Interestingly, we have previously demonstrated in this population that the risk of having high CRP levels was increased in subjects with MS, but not in those with “normal” waist circumference (7).
By analysing thoroughly the relationship between plasma cytokines, abdominal fat deposition, and insulin resistance in subjects with MS, we found that CRP, IL-6, and IL-18 were independently correlated with both waist circumference and HOMA, while sgp130 and sIL-6r correlated only with HOMA, independent of waist circumference. Moreover, unlike CRP, IL-6 and IL-18, the association between high sgp130 levels and MS was no longer significant after adjustment for HOMA score. Thus, it appears that the relationship between MS and elevated sgp130 levels might be supported by insulin resistance.
On one hand, LGSI associated with MS (i.e. increased CRP) seems to be largely dependent on the expansion of the visceral fat compartment (7,8), and over-production of pro-inflammatory cytokines such as IL-6 (19) and IL-18 (20). Indeed, hepatic synthesis of CRP is largely regulated by IL-6 (21) and by direct production by visceral fat (22). IL-6 and IL-18 have been also associated with insulin resistance (23,24); nevertheless, the relationship between these cytokines and MS might not be totally mediated by insulin resistance, since they were associated with MS independent of HOMA score.
On the other hand, sgp130 (i.e. the IL-6 trans-signalling inhibitor) was also elevated in MS. However, this phenomenon was strongly related to insulin resistance, as the relationship between sgp130 and MS disappeared after adjustment for HOMA. As regards this topic, an inverse relationship between sgp130 levels and insulin sensitivity, evaluated by euglycemic-hyperinsulinemic clamp, has been recently reported by Nikolajuk et al. (25) in a sample of women affected by polycystic ovary syndrome. The relationship was independent of important confounders including BMI, body fat percentage, post-load glucose/insulin, and triglycerides. Since it has been proposed that the gp130 cytokine family might exert beneficial effects on insulin resistance and obesity (26), these Authors suggested that the negative correlation between sgp130 and insulin sensitivity might reflect its inhibitory effect on intracellular sgp130 signalling (25).
Actually, the direction of the relationship between IL-6 and insulin resistance is still uncertain. In the long-term period, IL-6 has been shown to induce insulin resistance in humans. Nevertheless, it is also known that insulin resistance might prevent the anti-inflammatory effect of insulin; moreover, it is possible that in some circumstances IL-6 might activate pathways that promote insulin sensitivity (27).
Likewise, the direction of the relationship between insulin resistance and sgp130 is not known. IL-6 trans-signalling is known to contribute to the perpetuation of inflammation, at least in some diseases (28); of consequence, high sgp130 might represent an indirect marker of IL-6 mediated inflammation becoming a chronic process. Alternatively, high sgp130 might be considered a strategy to avoid IL-6 trans-signalling; anyhow, the inhibition of gp130 intracellular signalling could promote insulin resistance as suggested by Nikolajuk et al. (25)
Finally, some limitations of the study must be acknowledged. First, because of the cross-sectional study, we cannot define cause–effect relationships. In particular, we cannot distinguish whether chronically increased sgp130 levels cause insulin resistance, insulin resistance causes high sgp130 levels or a third factor (e.g. elevated IL-18 levels) causes both insulin resistance and an increase in sgp130. Second, we did not measure the plasma levels of the IL-6/sIL-6-R complex, and this would add important information about the real regulation of the IL-6 “trans-signalling” pathway in MS. Third, we used an indirect measure of insulin resistance (HOMA index), while a direct measure such as the hyperinsulinemic euglycemic clamp would be much more precise. However, HOMA has been validated with the hyperinsulinemic euglycemic clamp and has shown a strong correlation with it (28).
We also would like to underline the strengths of the study. First, complete data on the IL-6 system including sIL-6r and sgp130 are not easily found in large population studies. Second, to our knowledge, this is the first attempt to provide novel insight in the physiopathology of MS, focusing on the role of different components of the IL-6 pathway in the relationship between MS, insulin resistance, and abdominal fat deposition.
In conclusion, we found that older individuals with MS have increased sgp130 plasma levels compared with controls. Nevertheless, our data suggest that the association between MS and elevated sgp130 might be mediated by the presence of insulin resistance.
Acknowledgments
Grants: The InCHIANTI study baseline (1998-2000) was supported as a “targeted project” [ICS110.1/RF97.71] by the Italian Ministry of Health and in part by the U.S. National Institute on Aging [Contracts: 263 MD 9164 and 263 MD 821336]. The InCHIANTI Follow-up 1 (2001-2003) was funded by the U.S. National Institute on Aging [Contracts: N.1-AG-1-1 and N.1-AG-1-2111].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Reaven GM. Banting lecture 1988: Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Ferrannini E, Haffner SM, Mitchell BD, Stern MP. Hyperinsulinaemia: The key feature of a cardiovascular and metabolic syndrome. Diabetologia. 1991;34:416–422. doi: 10.1007/BF00403180. [DOI] [PubMed] [Google Scholar]
- 3.Pickup JC, Mattock MB, Chusney GD, et al. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–1292. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 4.Yudkin JS, Stehouwer CDA, Emeis JJ, et al. C-reactive protein in healthy subjects: association with obesity, insulin resistance, and endothelial dysfunction. Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 5.Festa A, D’Agostino R, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the insulin resistance atherosclerosis study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 6.Hak AE, Pols HA, Stehouwer CD, Meijer HJ, Kiliaan AJ, Hofman A, Breteler MM, Witteman JC. Markers of inflammation and cellulare adhesion molecules in relation to insulin resistance in nondiabetic subjects. J Clin Endocrinol Metabol. 1991;86:4398–4405. doi: 10.1210/jcem.86.9.7873. [DOI] [PubMed] [Google Scholar]
- 7.Zuliani G, Volpato S, Galvani M, Blè A, Bandinelli S, Corsi AM, Lauretani F, Maggio M, Guralnik JM, Fellin R, Ferrucci L. Elevated C-reactive protein levels and metabolic syndrome in the elderly: The role of central obesity. Data from the InChianti study. Atherosclerosis. 2009;203:626–632. doi: 10.1016/j.atherosclerosis.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Despres J-P, Prud’homme D, Pouliot MC, Tremblay A, Bouchard C. Estimation of deep abdominal adipose tissue accumulation from simple anthropometric measurements in men. Am J Clin Nutr. 1991;54:471–477. doi: 10.1093/ajcn/54.3.471. [DOI] [PubMed] [Google Scholar]
- 9.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Geront Med Sci. 2006;61A:575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- 11.Hurst SM, Wilkinson TS, McLoughlin RM, et al. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 12.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM, InCHIANTI Group Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. for the. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Frankin BA, et al. Diagnosis and management of metabolic syndrome: an American Heart Association / National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 14.Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Volts N, Fischer M, Neurath MF, Rose-John S. Soluble gp130 in the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268:160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 15.Hung J, McQuillan BM, Chapman CML, Thompson PL, Beilby JP. Elevated interleukin 18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:1268–1273. doi: 10.1161/01.ATV.0000163843.70369.12. [DOI] [PubMed] [Google Scholar]
- 16.Jablonska E, Jablonski J. Effect of IL-18 on the release of IL-6 and its soluble receptors: sIL-6r and sgp130 by human neutrophils. Immunol Invest. 2002;31:159–167. doi: 10.1081/imm-120016237. [DOI] [PubMed] [Google Scholar]
- 17.Jablonska E, Marcinczyk M. Role of interleukin-15 and interleukin-18 in the secretion of sIL6r and sgp130 by human neutrophils. Mediators of Inflammation. 2003;12:179–183. doi: 10.1080/0962935031000134905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. [PubMed] [Google Scholar]
- 19.Bastard J-P, Jardel C, Delattre J, Hainque B, Bruckert E, Oberlin F. Evidence for a link between adipose tissue IL-6 content and serum C-reactive protein concentrations in obese subjects. Circulation. 1999;99:2219c–2222c. [PubMed] [Google Scholar]
- 20.Esposito K, Pontillo A, Ciotola M, Di palo C, Grella E, Nicoletti G, Giugliano D. Weight loss reduces IL-18 levels in obese women. J Clin Endocrinol Metab. 2002;87:3864–3866. doi: 10.1210/jcem.87.8.8781. [DOI] [PubMed] [Google Scholar]
- 21.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S. Subcutaneous adipose tissue releases IL-6 but not tumor necrosis factor-alpha in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 22.Lau D, Yan H, Abdel-Hafez M, Kermouni A. Adipokines and the paracrine control of their production in obesity and diabetes. Int J Obes Relat Metab Disord. 2002;26:S111. Abstract. [Google Scholar]
- 23.Rotter V, Nagaev I, Smith U. Interleukin-6 induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 24.Escobar-Morreale HF, Botella-carrettero JL, Villuendas G, Sancho J, San Millan JL. Serum IL-18 concentrations are increased in the polycistic ovary sindrome: relationship to insulin resistance and to obesità. J Clin Endocrinol Metab. 2004;89:806–811. doi: 10.1210/jc.2003-031365. [DOI] [PubMed] [Google Scholar]
- 25.Nikolajuk A, Kowalska I, Karczewska-Kupczewska M, Adamska A, Otziomek E, Wolczynski S, Kinalska I, Gorska M, Straczowski M. Serum soluble glycoprotein 130 consentration is inversely related to insulin sensitivity in women with polycystic ovary syndrome. Diabetes. 2010;50:1026–29. doi: 10.2337/db09-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Febbraio MA. gp130 receptor ligands as potential therapeutic targets for obesity. J Clin Invest. 2007;117:841–849. doi: 10.1172/JCI30453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey AL, Febbraio MA. Interleukin-6 and insulin sensitivity: friend or foe ? Diabetologia. 2004;47:1135–1142. doi: 10.1007/s00125-004-1447-y. [DOI] [PubMed] [Google Scholar]
- 28.Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schürmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blokade of interleukin 6 trans-signalling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 29.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]