SUMMARY
The phage shock protein (Psp) system is induced by extracytoplasmic stress and thought to be important for the maintenance of proton motive force (PMF). We investigated the contribution of PspA to Salmonella virulence. A pspA deletion mutation significantly attenuates the virulence of S. Typhimurium following intraperitoneal inoculation of C3H/HeN (Ityr) mice. PspA was found to be specifically required for virulence in mice expressing the Nramp1 (Slc11a1) divalent metal transporter, which restricts microbial growth by limiting the availability of essential divalent metals within the phagosome. Salmonella competes with Nramp1 by expressing multiple metal uptake systems including the Nramp-homolog MntH, the ABC transporter SitABCD, and the ZIP family transporter ZupT. PspA was found to facilitate Mn2+ transport by MntH and SitABCD, as well as Zn2+ and Mn2+ transport by ZupT. In vitro uptake of 54Mn2+ by MntH and ZupT was reduced in the absence of PspA. Transport-deficient mutants exhibit reduced viability in the absence of PspA when grown under metal-limited conditions. Moreover, the ZupT transporter is required for S. Typhimurium virulence in Nramp1-expressing mice. We propose that PspA promotes Salmonella virulence by maintaining PMF, which is required for the function of multiple transporters mediating bacterial divalent metal acquisition during infection.
INTRODUCTION
Integrity of the bacterial cell envelope is essential for fundamental physiological processes including energy generation, nutrient uptake, protein folding and secretion, cell wall synthesis, motility and signal transduction. Extracytoplasmic stress in gram-negative bacteria is sensed by multiple regulatory systems that serve to maintain cell envelope and periplasmic homeostasis, including the alternative sigma factor σE (RpoE), the CpxA-CpxR and BaeS-BaeR two-component regulatory systems, and the phage shock protein (Psp) system (Ades, 2004; Darwin, 2005; Duguay and Silhavy, 2004; Raivio and Silhavy, 2001; Rowley et al., 2006; Ruiz and Silhavy, 2005).
CpxA-CpxR and σE control overlapping but distinct stress responses (Raivio, 2005; Raivio and Silhavy, 1999). The importance of σE for the virulence of Salmonella enterica serovar Typhimurium (S. Typhimurium) has been amply demonstrated. The σE regulon is required for S. Typhimurium to withstand multiple stresses encountered within mammalian hosts, including acid and oxidative stress, and exposure to antimicrobial peptides (Bang et al., 2005; Crouch et al., 2005; Humphreys et al., 1999; Muller et al., 2009; Testerman et al., 2002). As a result, σE-deficient S. Typhimurium strains are impaired in their ability to survive within macrophages and cause progressive systemic infection in mice (Cano et al., 2001; Humphreys et al., 1999; Rowley et al., 2005). In addition to activation by the presence of misfolded outer membrane proteins (Alba and Gross, 2004), recent work has shown that σE can be activated by acid stress encountered within the macrophage phagosome (Muller et al., 2009). The Cpx system is also required for Salmonella virulence. A cpxA mutation reduces S. Typhimurium adherence to and invasion of cultured epithelial cells, and impairs the ability of bacteria to replicate in mice (Humphreys et al., 2004). This may be because the Cpx system is required for the assembly and expression of surface organelles such as pili. In addition, studies in a related enteric pathogen, Yersinia pseudotuberculosis, indicate that both σE and CpxA-CpxR are important for the function of Type III Secretory Systems (T3SS) (Carlsson et al., 2007), which play a vital role in Salmonella pathogenesis as well (Carlsson et al., 2007; Humphreys et al., 2004; Humphreys et al., 1999). The BaeS-BaeR system has not yet been characterized with respect to Salmonella virulence, but this regulatory system is also induced by envelope stress and may provide some compensation for the loss of Cpx function (Raffa and Raivio, 2002).
The Psp system was first discovered by Model as a response of E. coli to infection with filamentous bacteriophages (Brissette et al., 1990). The six phage shock proteins are encoded by an operon (pspABCDE) and an unlinked gene (pspG) (Brissette et al., 1991; Lloyd et al., 2004). A divergently transcribed locus called pspF encodes a transcriptional activator that is required along with the sigma factor σN for psp operon transcription (Jovanovic et al., 1996). PspA is abundantly expressed during filamentous bacteriophage infection of E. coli (Brissette et al., 1990) and is believed to have both regulatory and effector roles. PspA negatively regulates psp operon expression by binding to PspF (Dworkin et al., 2000), but PspB and PspC bind to PspA to promote psp gene expression (Adams et al., 2003).
Since Model’s original observations, the phage shock response has been shown to be induced by conditions in addition to bacteriophage infection that perturb the integrity of the cytoplasmic membrane, including the protonophore CCCP (carbonylcyanide m-chlorphenylhydrazone) and overproduction of the secretin component of a Type II or Type III Secretory System (Becker et al., 2005; Brissette et al., 1991; Guilvout et al., 2006; Kleerebezem et al., 1996; Maxson and Darwin, 2004; Weiner and Model, 1994). Defects in secretion can also induce expression of the psp genes (Jones et al., 2003), as can ethanol, organic solvents, alkaline pH, osmotic or heat shock, and stationary phase (Brissette et al., 1991; Kobayashi et al., 1998; Weiner and Model, 1994). Mutations in rpoE strongly induce expression of the CpxA-CpxR and Psp regulons (Bang et al., 2005; Becker et al., 2005; Connolly et al., 1997), suggesting complementarity of these extracytoplasmic stress responses. Accordingly, mutations in both rpoE and pspA have a greater effect on stress-related phenotypes than single mutations in either locus alone (Becker et al., 2005). Psp-inducing conditions appear to share the common feature of membrane damage resulting in the impairment of proton motive force (PMF).
Model suggested that the primary function of the phage shock response is to maintain PMF during extracytoplasmic stress (Model et al., 1997). As a function of membrane potential (Δψ) and the transmembrane pH gradient (ΔpH), PMF (Δp) is sensitive to alterations in membrane permeability (Harold, 1996). PspA has been shown to play a role in the maintenance of PMF when protein export is impaired (Kleerebezem et al., 1996). More recently, Kobayashi et al. have shown that PspA can oligomerize and prevent proton leakage across damaged membrane vesicles in vitro (Kobayashi et al., 2007). The formation of extensive scaffold structures has been proposed as a mechanism by which PspA might stabilize membrane integrity (Standar et al., 2008).
Activation of the phage shock response has been observed under a variety of complex biological conditions of potential importance in bacterial virulence, including swarming motility, biofilm formation, activation of type III secretion, epithelial cell invasion, and bacterial internalization by macrophages (Beloin et al., 2004; Darwin and Miller, 1999; Darwin and Miller, 2001; Eriksson et al., 2003; Lucchini et al., 2005; Wang et al., 2004). Given the putative role of the phage shock response in the maintenance of PMF and the importance of PMF for a number of processes important for pathogenesis, including motility, resistance to host-derived antimicrobial mediators, secretion and nutrient acquisition, we investigated the contribution of the phage shock response to Salmonella virulence.
RESULTS
PspA is required for Salmonella virulence
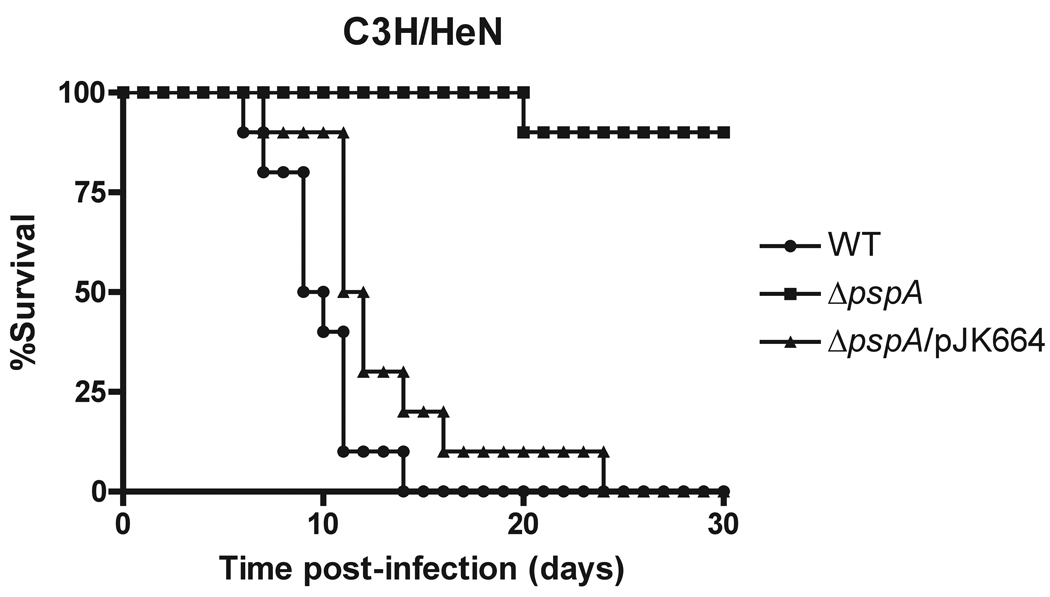
To determine whether PspA is essential for Salmonella virulence, six week-old C3H/HeN (Ityr, Nramp1/Slc11a1+) mice were injected intraperitoneally with 3000 CFU wild-type or isogenic mutant S. Typhimurium strains carrying an in-frame pspA deletion mutation (ΔpspA) and monitored for signs of illness (see Experimental procedures). The S. Typhimurium strain lacking pspA was severely attenuated for virulence in C3H/HeN mice (Fig. 1). All mice infected with wild-type Salmonella succumbed to infection within 15 days, whereas no mouse infected with the ΔpspA mutant died during this time period, and most animals appeared completely healthy throughout the course of the experiment. The promoter and coding region of pspA were cloned into plasmid vector pRB3 (see Experimental procedures), and this construct, pJK664, was introduced into the S. Typhimurium ΔpspA strain. PspA levels of the ΔpspA strain carrying pJK664 were comparable to wild-type by Western blot analysis (data not shown), indicating that pJK664 restored PspA production. Salmonella virulence in C3H/HeN mice was fully complemented by introduction of pJK664 into the ΔpspA strain, demonstrating that the virulence defect in the ΔpspA mutant resulted from the loss of pspA (Fig. 1).
Figure 1. PspA is required for virulence in C3H/HeN (Nramp1+) mice.
Six-week-old C3H/HeN mice were inoculated i.p. with 3 × 103 CFU wild-type (●), ΔpspA (□), or ΔpspA/pJK664 (▲) strains of S. Typhimurium 14028s. Two groups of five mice were assayed independently. A statistical test of mouse survival indicated that the ΔpspA mutant is attenuated for virulence (P<0.0001 by Wilcoxon Rank Sum) compared to wild-type. Virulence was fully restored by the pspA-expressing plasmid pJK664 (P<0.0001 compared to the uncomplemented ΔpspA strain).
PspA is not required for Type III Secretion in Salmonella
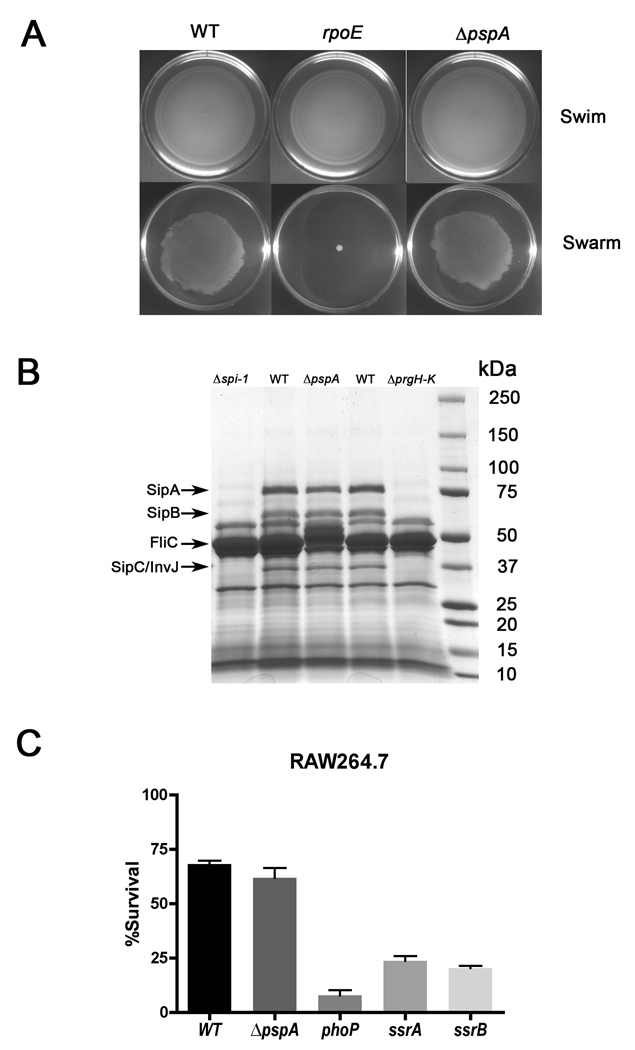
Expression of the phage shock operon is induced when Type III secretion is activated in Y. enterocolitica (Darwin and Miller, 2001) or Salmonella (Wang et al., 2004). Additional studies have shown the importance of PMF for T3SS function (Minamino and Namba, 2008; Paul et al., 2008; Wilharm et al., 2004). As T3SSs play an essential role in Salmonella motility, host cell invasion, intracellular survival and virulence, we investigated whether a pspA mutation affected T3SS-dependent phenotypes. As shown in Fig. 2A, the loss of pspA affected neither motility nor swarming behavior under conventional laboratory conditions. Thus, flagellar Type III secretion appears to be unaffected by the loss of PspA.
Figure 2. Salmonella type III secretion is unaffected by the loss of PspA.
A. Flagellar type III secretion was assayed in S. Typhimurium 14028s wild type and mutant strains by motility on swim medium (0.3% agar) and swarming on swarm medium (0.6% agar) at 37°C. B. SPI1 type III secretion system-dependent protein secretion was assayed in culture supernatants of wild-type S. Typhimurium 14028s and isogenic ΔSPI1, ΔpspA and ΔprgH-K mutant strains. Fifty ml of overnight culture supernatants were TCA-precipitated and fractionated on a 4–15% SDS polyacrylamide gel with protein detection by Coomassie blue staining. SPI1 secreted proteins SipA (74kDa), SipB (62kDa), SipC (43kDa) and InvJ (36kDa), as well as flagellar type III secreted protein FliC (52kDa,) are indicated on the left. C. SPI2 type III secretion-dependent intracellular survival of S. Typhimurium was assayed in RAW264.7 macrophages. The RAW264.7 cells were infected in parallel with wild-type S. Typhimurium 14028s along with isogenic ΔpspA, phoP, ssrA and ssrB mutant strains at an MOI of 5:1. CFU were quantified at 0h and 18h post-infection.
Salmonella invasion of host epithelial cells requires effector proteins secreted by the T3SS encoded on Salmonella Pathogenicity Island 1 (SPI1) (Hansen-Wester and Hensel, 2001; Lostroh and Lee, 2001). As SPI1 and flagellar secreted proteins can be detected in culture supernatants (Kimbrough and Miller, 2000; Lee and Galan, 2004), culture supernatants were obtained from wild type and ΔpspA strains under conditions favoring SPI1 expression, subjected to SDS-PAGE, and stained with Coomassie blue (see Experimental procedures). No differences in SPI1 or flagellar T3SS secreted proteins were observed between wild-type and ΔpspA bacteria (Fig. 2B). Further, the loss of PspA did not abrogate the ability of Salmonella to invade a non-phagocytic epithelial cell line (HeLa) compared to wild-type (Supplemental Fig. S1).
Effector proteins secreted by the T3SS encoded on Salmonella Pathogenicity Island 2 (SPI2) are required for bacterial survival in macrophages (Hansen-Wester and Hensel, 2001; Kuhle and Hensel, 2004). Expression of SPI2 genes is dependent on the SsrA-SsrB two-component regulatory system (Feng et al., 2003; Feng et al., 2004; Garmendia et al., 2003; Walthers et al., 2007). RAW264.7 macrophages were infected with wild-type Salmonella or isogenic ΔpspA, phoP, ssrA or ssrB mutant strains, and the numbers of surviving bacteria after 18h of infection were determined by lysis, serial dilution and plating (see Experimental procedures). Survival of the ΔpspA mutant strain was comparable to wild-type, whereas the SPI2-deficient ssrA and ssrB mutant strains, as well as the attenuated phoP mutant control strain, exhibited intracellular survival defects (Fig. 2C). Thus, SPI2-dependent intracellular bacterial survival does not require an intact phage shock response. In addition, a ΔpspA mutant was no more sensitive than wild-type Salmonella to host-derived antimicrobial mediators including hydrogen peroxide, nitric oxide, and the antimicrobial peptides cryptdin-4 and P2 (data not shown). Collectively, these data indicate that the attenuated virulence of ΔpspA mutant S. Typhimurium does not result from defective Type III secretion, nor to increased sensitivity to phagocyte killing, oxidative stress, nitrosative stress, or antimicrobial peptides encountered in the host environment.
The contribution of PspA to Salmonella virulence is Nramp1-dependent
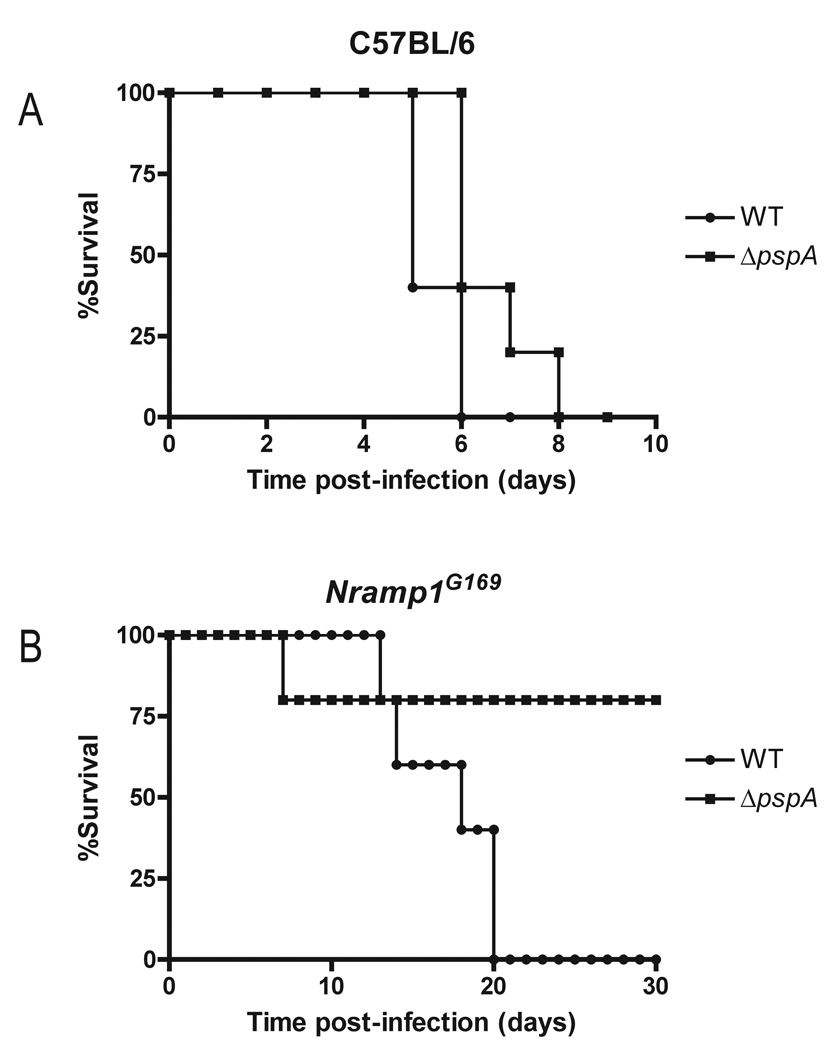
In contrast to our observations in C3H/HeN mice (Fig. 1), a ΔpspA mutant S. Typhimurium strain was unexpectedly found to be as virulent as wild-type in C57BL/6 (Itys) mice, which lack a functional Nramp1/Slc11a1 locus (Fig. 3A). We hypothesized that PspA might only be able to promote Salmonella virulence in mice possessing an intact Nramp1 locus (also designated ity/lsh/bcg) on chromosome 1. This locus, encoding natural-resistance-associated macrophage protein 1 (Nramp1), is associated with enhanced susceptibility to intracellular pathogens including S. Typhimurium, Leishmania major and Mycobacterium bovis BCG (Bradley, 1977; Gros et al., 1981; Plant and Glynn, 1976). Nramp1, also called Slc11a1, is now established to be a proton-dependent divalent metal transporter expressed by phagocytic cells and localized to the phagosomal membrane (Forbes and Gros, 2003; Goswami et al., 2001; Gruenheid et al., 1997). Nramp1 enhances host resistance to intracellular pathogens by limiting essential metal availability within the phagosome (Cellier et al., 2007; Forbes and Gros, 2001; Mackenzie and Hediger, 2004). C57BL/6 and many other strains of inbred mice are phenotypically Nramp1− due to a G169D mutation (Malo et al., 1994). To investigate the importance of Nramp1 for the attenuated virulence phenotype of the ΔpspA S. Typhimurium mutant strain, we compared the virulence of wild-type and ΔpspA mutant S. Typhimurium in C57BL/6 mice and transgenic C57BL/6 Nramp1G169 mice that are phenotypically Nramp1+ (Govoni et al., 1996). The ΔpspA mutant bacteria were avirulent in mice expressing the Nramp1G169 transgene (Fig. 3B), indicating that the requirement for PspA in Salmonella pathogenesis is dependent on the presence of a functional Nramp1 locus in the host. In addition, we investigated the survival of isogenic wild-type and ΔpspA S. Typhimurium strains in primary macrophages isolated from C57BL/6 (Nramp1−) and C57BL/6 Nramp1G169 (Nramp1+). S. Typhimurium lacking PspA survived similarly to wild-type in C56BL/6 (Nramp1−) macrophages (Supplemental Fig. S2), whereas a ΔpspA S. Typhimurium mutant was defective for survival in C57BL/6 Nramp1G169 (Nramp1+) macrophages in comparison to wild-type (Supplemental Fig. S2). These results support the in vivo observation that PspA promotes Salmonella virulence in mice expressing the Nramp1 (Slc11a1) divalent metal transporter.
Figure 3. PspA promotion of S. Typhimurium virulence is Nramp1-dependent.
A. Six week-old C57BL/6 mice (Nramp1−) were inoculated i.p. with 1 × 102 CFU wild-type (●) or ΔpspA mutant (□) S. Typhimurium 14028s. Two groups of five mice each were assayed independently. Survival of the ΔpspA mutant was comparable to wild type (P=0.3783 by Wilcoxon Rank Sum). B. Six week-old congenic C57BL/6 Nramp1G169 (Nramp1+) mice were inoculated i.p. with 1 × 105 CFU of wild-type (●) or ΔpspA mutant (□) S. Typhimurium 14028s. Two groups of five mice each were assayed independently. The ΔpspA mutant was attenuated for virulence compared to wild-type (P=0.0034 by Wilcoxon Rank Sum).
PspA promotes Mn2+ and Fe2+ transport-dependent growth
Nramp1 enhances host resistance presumably by limiting the availability of essential metals required for microbial growth in the phagosomal compartment (Cellier et al., 2007; Forbes and Gros, 2001). Previous studies have shown that murine Nramp1 facilitates the transport of divalent cations including Mn2+, Fe2+ and Zn2+ along a proton gradient (Forbes and Gros, 2003; Goswami et al., 2001). Salmonella possesses a high-affinity manganese transporter called MntH, which is a homolog of Nramp1, as well as an ATP-Binding Cassette transporter known as SitABCD, which can mediate both high affinity manganese and low affinity iron uptake at alkaline pH (Kehres et al., 2002; Kehres et al., 2000; Zhou et al., 1999). Cation uptake by MntH requires a proton gradient, whereas ABC-type transport systems such as SitABCD utilize ATP hydrolysis (Courville et al., 2004; Davidson and Chen, 2004; Higgins, 1992; Makui et al., 2000). The MntH and SitABCD transporters are required for S. Typhimurium virulence in Nramp1+ but not in Nramp1-deficient mice (Zaharik et al., 2004), suggesting that metal acquisition becomes of vital importance to intracellular pathogens when the host is able to actively evacuate divalent metals from the phagosome via Nramp1. The putative role of PspA in the maintenance of PMF, the proton-dependence of MntH (Makui et al., 2000), and the Nramp1-dependence of the pspA virulence phenotype (Fig. 3), as well as the reduced survival of a ΔpspA Salmonella mutant in C57BL/6 Nramp1G169 (Nramp1+) primary macrophages (Supplemental Fig. S2), led us to investigate a possible role of PspA in Salmonella metal transport. PspA induction during Salmonella growth in chelated media (LB+dipyridyl, Supplemental Fig. S3) further suggested a possible role of PspA under metal-limited conditions.
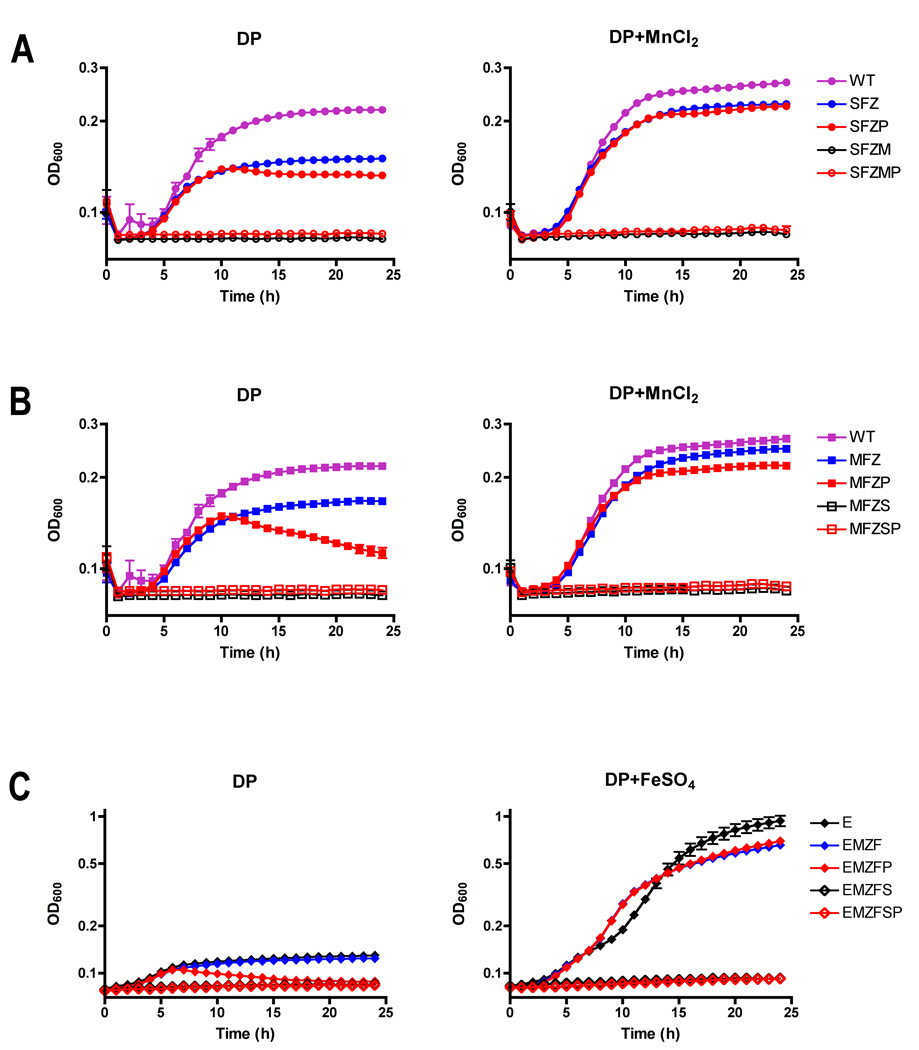
Salmonella strains defective for various divalent metal transporters were constructed to determine the effect of a pspA mutation on the MntH and SitABCD manganese transport systems (see Experimental procedures). To assess the individual contribution of each manganese transport system, the growth of wild-type and transport-deficient mutant strains was examined in chelated medium (LB+dipyridyl) to which Mn2+ was or was not added back. The contribution of MntH transporter for growth in the same chelated medium was assessed in an SFZ (sitA feoB ΔzupT) triple mutant strain lacking the ABC transporter SitABCD, the major Fe2+ transporter FeoB, and the ZIP family metal permease ZupT. The SFZ mutant grew poorly in chelated medium, but growth was restored close to wild-type levels by the addition of MnCl2 (Fig. 4A), consistent with MntH-dependent acquisition of Mn2+. MntH dependence was confirmed in a SFZM (sitA feoB ΔzupT mntH) mutant strain, for which growth in chelated medium was not restored by the addition of MnCl2 (Fig. 4A). An SFZP (sitA feoB ΔzupT ΔpspA) mutant strain exhibited an even greater impairment of growth in chelated medium (Fig. 4A), but growth was restored by complementation with the pspA-expressing plasmid pJK664, indicating that PspA supports MntH-dependent Mn2+ uptake (Supplemental Fig. S4A). The ABC-type metal transporter SitABCD was similarly assessed in an MFZ (mntH feoB ΔzupT) triple mutant strain. The MFZ mutant strain grew poorly in chelated medium, but growth was restored by the addition of MnCl2, consistent with SitABCD-mediated uptake of Mn2+ (Fig. 4B). Somewhat unexpectedly, PspA was also found to facilitate SitABCD-mediated Mn2+ transport. Like the SFZP (sitA feoB ΔzupT ΔpspA) mutant, an MFZP (mntH feoB ΔzupT ΔpspA) mutant strain grew poorly in chelated medium (Fig. 4B), and growth was restored by the pspA-expressing plasmid pJK664 (Supplemental Fig. S4B). Acquisition of Mn2+ by MntH and Mn2+ and possibly Fe2+ by SitABCD is important for survival in macrophages as well as virulence in S. Typhimurium (Boyer et al., 2002). To assess SitABCD-mediated Fe2+ uptake, an ΔentC mutation was introduced into transport-deficient mutant strains to eliminate siderophore-mediated iron uptake (Crouch et al., 2008). SitABCD-dependent growth of an EMZF mutant (ΔentC mntH ΔzupT feoB) in chelated medium was enhanced by the addition of FeSO4 (Fig. 4C). The addition of a ΔpspA mutation significantly impaired growth of the EMZF strain in chelated medium (Fig. 4C).
Figure 4. PspA facilitates MntH- and SitABCD-dependent growth of Salmonella in Mn2+- and Fe2+-chelated medium.
Overnight cultures of S. Typhimurium 14028s were inoculated to 4×105 CFU/ml in LB supplemented with the chelator dipyridyl (DP), with or without MnCl2, or FeSO4, and growth was monitored for 24h at 37°C. Wild-type (WT) S. Typhimurium was compared with isogenic mutants containing combinations of the following mutations: entC (E), sitA (S), mntH (M), feoB (F), ΔzupT (Z), and ΔpspA (P). A. To assess MntH-dependent Mn2+ transport, growth of wild-type S. Typhimurium (purple circle) was compared with mutant strains SFZ (blue circle), SFZP (red circle), SFZM (open black circle) and SFZMP (open red circle) grown in LB with 600µM dipyridyl alone or with the addition of 10µM MnCl2. B. To assess SitABCD-dependent Mn2+ transport, growth of wild-type S. Typhimurium (purple square) was compared with mutant strains MFZ (blue square), MFZP (red square), MFZS (open black square) and MFZSP (open red square) grown in LB with 600µM dipyridyl alone or with the addition of 10µM MnCl2. C. To assess SitABCD-dependent Fe2+ transport, an entC mutation was first introduced to inhibit siderophore dependent iron uptake. Then transport mutants EMZF (blue triangle), EMZFP (red triangle), EMZFS (open black triangle) and EMZFSP (open red triangle) were compared to an entC mutant (black triangle) for growth in LB with 200µM dipyridyl alone or with the addition of 18µM FeSO4.
Collectively, these observations suggest that PspA can facilitate divalent metal transport by both Nramp-type (MntH) and ABC-type (SitABCD) transport systems. Furthermore, at high dipyridyl concentrations, a pspA mutation alone results in growth impairment (Supplemental Fig. S5), supporting the notion that PspA plays an integral role in metal acquisition by Salmonella. In the growth assays, we noted that transport-deficient mutant strains lacking PspA may exhibit a decline in OD600 following initial growth (Figs. 4B, 4C and Supplemental Figs. S4 and S5), suggesting that PspA is required for cell viability during essential metal deprivation. We therefore determined the viability of MntH-deficient mutants under metal-limiting conditions. Salmonella strains WT, P (ΔpspA), SFZ (sitA feoB ΔzupT), and SFZP (sitA feoB ΔzupT ΔpspA), were grown in chelated medium and monitored for 24h by OD600, with aliquots obtained in parallel to determine colony forming units (CFU). After 24h growth in metal-depleted conditions, cultures of WT and an isogenic SFZ mutant were found to contain ~40-fold more CFU than at T=0 (Supplemental Fig. S6), and a ΔpspA mutant showed only a two-fold reduction in survival compared to wild-type (Supplemental Fig. S6). However, the transport-deficient SFZ mutant strain lacking PspA (SFZP) was essentially non-viable after 24h (Supplemental Fig. S6). These data suggest that PspA not only facilitates metal transport, but is also required for Salmonella viability during metal deprivation.
PspA promotes growth in Zn2+-limited medium that is dependent on a ZIP family metal permease
The ZIP metal transporter family was first described in Arabidopsis, but members have subsequently been identified in all eukaryotic kingdoms (Eng et al., 1998; Guerinot, 2000). ZupT is the first bacterial ZIP homolog to be discovered and was recently shown to transport a broad range of divalent cations in Escherichia coli (Grass et al., 2005; Grass et al., 2002). Although the energy requirements of ZIP transporters are not known, family members share some sequence similarity to a cation diffusion facilitator family transporter that is driven by PMF (Guerinot, 2000). The S. Typhimurium genome sequence is predicted to encode a ZupT protein that is 86% identical to ZupT of E. coli W3110. We investigated whether ZupT in S. Typhimurium is also a divalent metal permease and whether PspA supports ZupT-dependent transport.
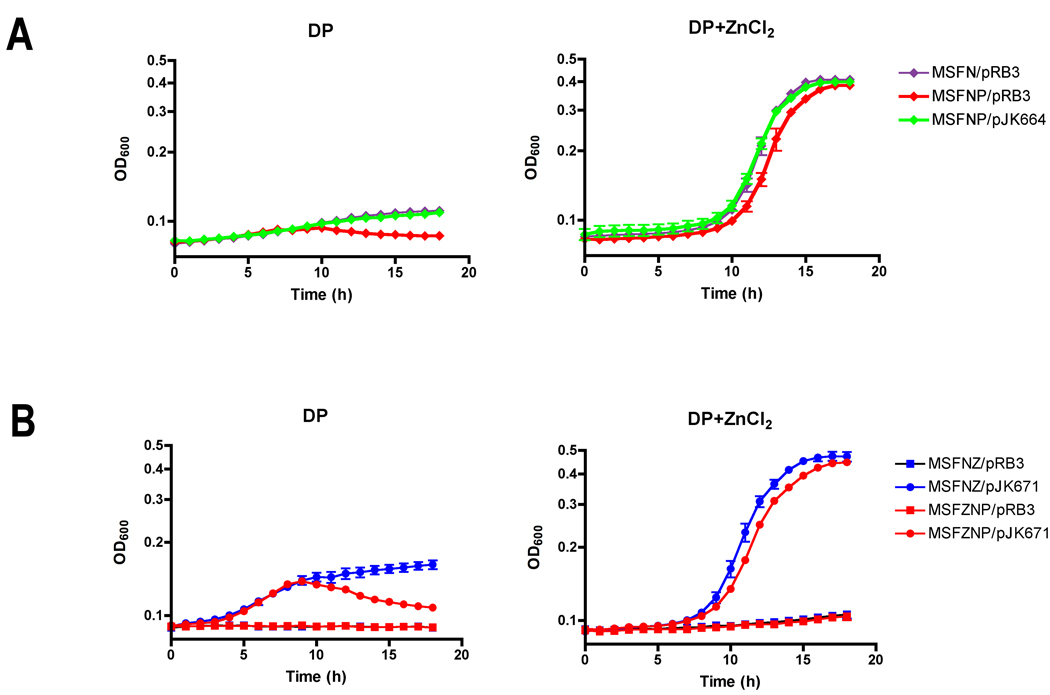
The contribution of ZupT to S. Typhimurium growth in chelated medium was examined in an MSFN (mntH sitA feoB ΔznuABC) strain background. The MSFN mutant was unable to grow in chelated medium, but growth was restored when the medium was supplemented with ZnCl2 (Fig. 5A). In addition, an MSFNZ (mntH sitA feoB ΔznuABC ΔzupT) mutant was unable to grow in chelated medium, even with ZnCl2 supplementation, unless the zupT gene was provided on plasmid pJK671 in trans (Fig. 5B). Thus, ZupT mediates Zn2+ transport in Salmonella. MSFN and MSFNZ/pJK671 mutant strains lacking PspA exhibited impaired growth in chelated medium (Figs. 5A, 5B). Growth of the MSFN mutant in chelated medium was restored by the introduction of the pspA-expressing plasmid pJK664 (Fig. 5A). Parallel studies performed with MnCl2 instead of ZnCl2 supplementation suggest that ZupT may mediate transport of Mn2+ as well (data not shown). Thus, ZupT exhibits broad cation substrate specificity in S. Typhimurium as shown previously in E. coli (Grass et al., 2005). Together, these observations demonstrate that PspA facilitates the ZupT-mediated transport of Zn2+ and Mn2+ in Salmonella.
Figure 5. PspA facilitates ZupT-dependent growth of Salmonella in Zn2+-chelated medium.
Overnight cultures of S. Typhimurium 14028s were inoculated to 4×105 CFU/ml in LB supplemented with dipyridyl (DP), with or without 500µM ZnCl2, and growth monitored for 18h at 37°C. Analysis was performed on strains containing combinations of the following mutations: mntH (M), sitA (S), feoB (F), ΔzupT (Z) ΔznuABC (N), and ΔpspA (P). A. To assess ZupT-dependent Zn2+ transport, growth of S. Typhimurium MSFN with the pRB3 vector (purple diamond) was compared with strains MSFNP/pRB3 (red diamond) and MSFNP/pspA plasmid pJK664 (green diamond) in LB with 400µM dipyridyl alone or with the addition of 500µM ZnCl2. B. Growth of S. Typhimurium MSFNZ with the pRB3 vector (blue square) was compared with strains MSFNZ/zupT plasmid pJK671 (blue circle), MSFNZP/pRB3 (red square), and MSFNZP/pJK671 (red circle) grown in LB with 525µM dipyridyl alone or with the addition of 500µM ZnCl2.
PspA promotes 54Mn2+ uptake by MntH and ZupT transporters
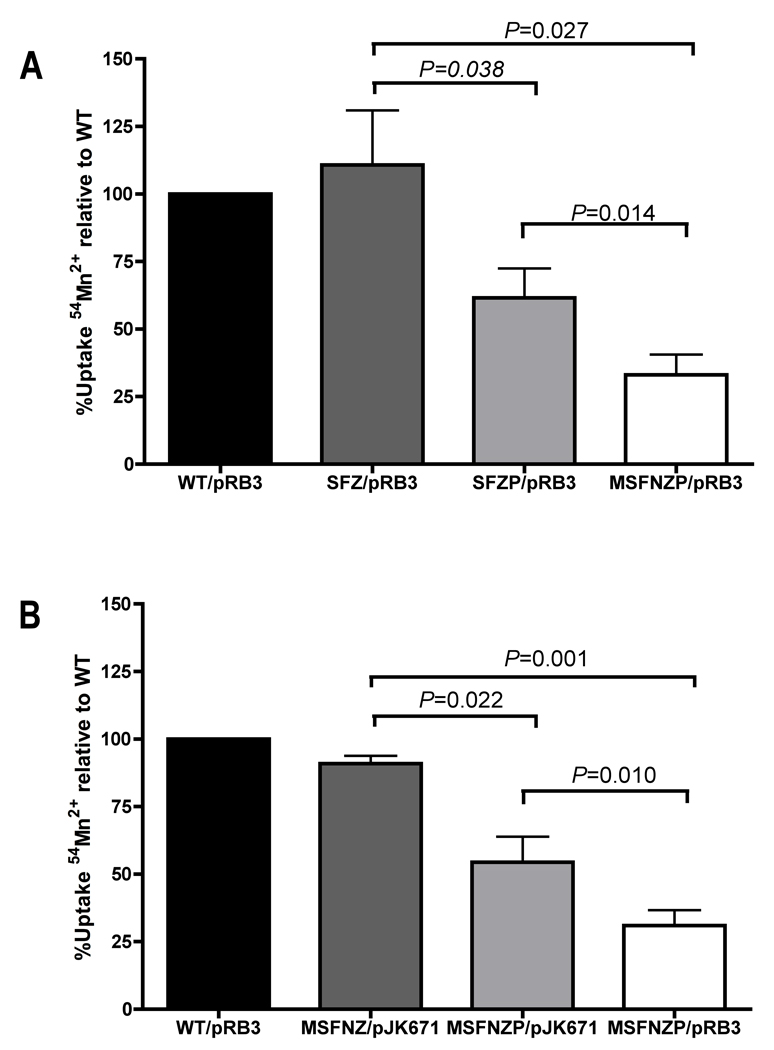
Transport assays were performed to determine the dependence of MntH and ZupT transport function on PspA by measuring uptake of 54Mn2+ in wild-type and transport-deficient mutant strains (see Experimental procedures). The activity of the MntH transporter was assessed in an SFZ (sitA feoB ΔzupT) triple mutant background in which uptake of 54Mn2+ is primarily mntH-dependent (Fig. 6A). In the absence of PspA, MntH-mediated uptake of 54Mn2+ was reduced by 45% (Fig. 6A). The ZupT transporter was assessed in an MSFNZ (mntH sitA feoB ΔznuABC ΔzupT) mutant strain carrying the ZupT plasmid pJK671, in which 54Mn2+ uptake is primarily ZupT-dependent (Fig. 6B). The absence of PspA reduced ZupT-mediated uptake of 54Mn2+ by 40% (Fig. 6B). These data provide direct evidence that PspA facilitates the uptake of divalent cations by metal transport systems in Salmonella.
Figure 6. PspA facilitates 54Mn+2 uptake by the MntH and ZupT transport systems.
Uptake of 54Mn+2 by S. Typhimurium 14028s was measured as described in the Experimental procedures. Wild-type (WT) S. Typhimurium was compared with isogenic mutants containing combinations of the following mutations: sitA (S), mntH (M), feoB (F), ΔzupT (Z), ΔznuABC (N) and ΔpspA (P). A. To assess MntH-dependent uptake of 54Mn2+, S. Typhimurium WT with vector pRB3 (black) was compared with mutant S. Typhimurium strains SFZ/pRB3 (dark gray), SFZP/pRB3 (light gray) and MSFNZP/pRB3 (white). B. To assess ZupT-dependent uptake of 54Mn2+, S. Typhimurium WT with vector pRB3 (black) was compared with mutant S. Typhimurium strains MSFNZ with pZupT plasmid pJK671 (dark gray), MSFNZP/pJK671 (light gray) and MSFNZP/pRB3 (white). WT uptake of 54Mn2+ was ~12,000 cpm over a background of 400 cpm. Significant differences in 54Mn2+ uptake between strains were determined using a paired t-test and are indicated above the horizontal bars.
The ZupT metal transporter is required for Salmonella virulence in Nramp+ mice
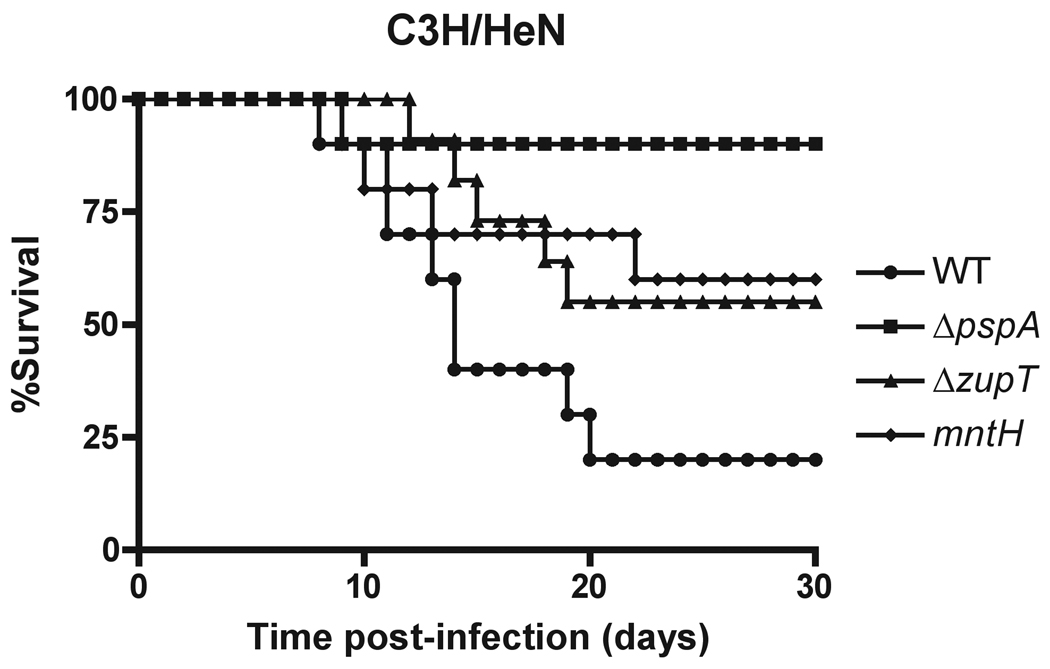
Uropathogenic E. coli carrying a zupT mutation was found to be defective for competitive infections in CBA/J mice only when the high-affinity ZnuABC zinc transporter was absent, suggesting a supportive role for ZupT in E. coli pathogenesis (Sabri et al., 2009). We compared the contributions of ZupT and MntH to Salmonella virulence. Previous studies showed that an mntH S. Typhimurium mutant is moderately attenuated for virulence in Nramp+ mice (Zaharik et al., 2004). Therefore, we compared the ability of zupT and mntH mutant strains to cause progressive infection in Nramp+ C3H/HeN (Ityr) mice following intraperitoneal inoculation. The S. Typhimurium ΔzupT mutant exhibited an attenuated virulence phenotype similar to that of an isogenic mntH Nramp+ mutant in C3H/HeN mice, with 50% of mice infected with the ΔzupT mutant surviving at 25 days post-infection in comparison to only 20% of mice infected with wild-type S. Typhimurium (Fig. 7). Attenuated virulence was not observed in Nramp− C57BL/6 mice infected with the S. Typhimurium ΔzupT mutant, and animals infected with either wild-type or the ΔzupT mutant succumbed equally to infection (Supplemental Fig. S7). These results demonstrate that ZupT is required for full Salmonella virulence in Nramp+ mice.
Figure 7. The ZupT transporter is required for S. Typhimurium virulence in C3H/HeN (Nramp1+) mice.
Six week-old C3H/HeN (Nramp1+) mice were inoculated i.p. with 3 × 103 CFU S. Typhimurium 14028s wild type (●) or isogenic ΔpspA (□), ΔzupT (▴) or mntH (◆) mutants. Two groups of five mice each were assayed independently. Significant differences in murine survival in comparison to wild-type were observed for the ΔpspA (P<0.0001), ΔzupT (P=0.0005) and mntH (P=0.0011) mutant strains, as analyzed using a Wilcoxon Rank Sum test.
DISCUSSION
The phage shock response is a regulated response to extracytoplasmic stress that serves to maintain PMF and preserve essential cellular functions. PspA, the most abundant phage shock protein, is required for most of the phenotypes associated with the phage shock response (Darwin, 2005). The goal of this study was to investigate the contribution of the phage shock response to Salmonella virulence. We have demonstrated that PspA-deficient mutant S. Typhimurium is profoundly attenuated for virulence following intraperitoneal inoculation of C3H/HeN mice. However, although the phage shock response is strongly induced by the activation of type III secretion, Salmonella motility and the SPI1 and SPI2 T3SSs appear to be unimpaired in the absence of PspA. Moreover, a pspA mutant strain appears no more susceptible than wild-type S. Typhimurium to extracellular stresses encountered in the host environment, including exposure to reactive oxygen or nitrogen species, and antimicrobial peptides.
An important clue to the role of PspA in Salmonella pathogenesis came from the observation that a pspA mutation only attenuates virulence in mice expressing Nramp1 (Fig. 3). Nramp1 (also called Slc11a1) was originally discovered as a genetic locus (ity/lsh/bcg) of inbred mice that is associated with increased susceptibility to phylogenetically diverse intracellular pathogens including S. Typhimurium, Leishmania major and Mycobacterium bovis BCG (Bradley, 1977; Gros et al., 1981; Plant and Glynn, 1976). The Nramp1 locus was shown to encode a 56kDa membrane protein that contains a nonconservative glycine to aspartic acid substitution at residue 169 in animals with deficient Nramp1 function (Malo et al., 1994). Subsequent studies showed that Nramp1 is a metal ion transporter that can mediate the transport of divalent cations including Mn2+, Fe2+, Zn2+ and Co2+ by a proton-dependent mechanism. (Forbes and Gros, 2003; Goswami et al., 2001). Although some investigators have suggested that Nramp1 might transport metals into the phagosome (Blackwell et al., 2001; Goswami et al., 2001; Kuhn et al., 1999; Kuhn et al., 2001; Techau et al., 2007; Zwilling et al., 1999), the preponderance of evidence indicates that Nramp1 leads to the depletion of divalent metal ions in the phagosome (Barton et al., 1999; Cellier et al., 2007; Forbes and Gros, 2001; Forbes and Gros, 2003; Fritsche et al., 2007; Jabado et al., 2000; Mulero et al., 2002; Nevo and Nelson, 2006), and a recent study directly measuring total cellular iron content by atomic absorption spectrometry has demonstrated that Nramp1-expressing macrophages contain lower quantities of iron (Nairz et al., 2009). Depletion of metal ions serves as a defense mechanism against intracellular pathogens, as microbes require metals such as iron, zinc and manganese for a variety of essential metabolic processes (Cellier et al., 2007; Forbes and Gros, 2001; Mackenzie and Hediger, 2004). The selective PspA requirement of S. Typhimurium in Nramp1-expressing mice and peritoneal macrophages (Fig. 3 and Supplemental Fig. S2) suggested a possible role of PspA in Salmonella metal acquisition.
Bacteria utilize multiple types of transporter with overlapping specificities to acquire metal ions within the host environment. S. Typhimurium utilizes the Nramp-like transporter MntH, the ATP-binding cassette SitABCD transport system, the ZIP family transporter ZupT, and the Feo transporter to mediate the uptake of divalent cations such as Mn2+, Fe2+ and Zn2+. These transporters have been shown in this and earlier studies to play in important role in Salmonella pathogenesis (Boyer et al., 2002; Campoy et al., 2002; Janakiraman and Slauch, 2000; Zaharik et al., 2004). Here we have also shown that the phage shock protein PspA facilitates Mn2+ transport by MntH, Mn2+ and Fe2+ transport by SitABCD (Fig. 4 and Supplemental Fig. S4), and Zn2+ transport by the ZIP family transporter ZupT (Fig. 5). Like MntH and SitABCD (Zaharik et al., 2004), we have found that ZupT and PspA are only required for Salmonella virulence in mice possessing a functional Nramp1 locus, consistent with the idea that host Nramp1 and bacterial MntH, SitABCD and ZupT transporters compete for metals within the phagosomal vacuole.
Accumulating evidence continues to support Model’s original hypothesis that the phage shock response preserves PMF under extracytoplasmic stress conditions (Becker et al., 2005; Kleerebezem et al., 1996; Kobayashi et al., 2007; Standar et al., 2008). Our observations further reinforce this model, showing that PspA promotes Mn2+ uptake by the MntH transporter during growth in chelated medium (Fig. 4 and Supplemental Fig. S4A) as well as 54Mn2+ uptake in transport assays (Fig. 6A). As MntH is a proton symporter in the Nramp family (Papp-Wallace and Maguire, 2006), MntH function would be predicted to be highly dependent on PMF (Fig. 8). Although PspA is most likely to promote MntH-mediated transport by facilitating the maintenance of PMF, an effect of PspA on transport protein expression or activity cannot be excluded. Somewhat less anticipated was our discovery that PspA also facilitates metal uptake by the ABC-type SitABCD transporter (Fig. 4 and Supplemental Fig. S4B). However, although ABC-type transporters are energized by ATP hydrolysis (Davidson and Chen, 2004; Higgins, 1992), PMF can drive the generation of ATP via oxidative phosphorylation by the F0F1 ATPase (Harold, 1996; Senior, 1990). The reverse reaction can occur under anaerobic conditions when the proton gradient is low, allowing the F0F1 ATPase to support PMF as well as utilize it for ATP generation (Senior, 1990). Interestingly, mutations in atpB encoding the a subunit of the proton-translocating F0 complex increase the expression of pspA (Becker et al., 2005; Maxson and Darwin, 2004), suggesting a complex interplay between the F0F1 ATPase, PspA and PMF. Furthermore, overexpression of a thermophilic F0F1 ATPase has been shown to cause transmembrane proton leakage and the induction of pspA expression in E. coli (Kobayashi et al., 2007). PspA can oligomerize and inhibit proton leakage across damaged membranes in vitro (Kobayashi et al., 2007). In addition, the formation of extensive scaffold structures by PspA is proposed to stabilized membrane integrity (Standar et al., 2008). Although the precise mechanism by which PspA maintains PMF is uncertain, we suggest that a reduction in PMF resulting from the loss of PspA may limit ATP generation by the F0F1 ATPase, thereby limiting transport by SitABCD (Figs. 4 and 8). Defective survival of strains lacking PspA in chelated medium (Supplemental Fig. S6) may reflect reduced activity of other ATP-dependent processes and could suggest a possible role of PspA in maintaining the overall energy status of the cell.
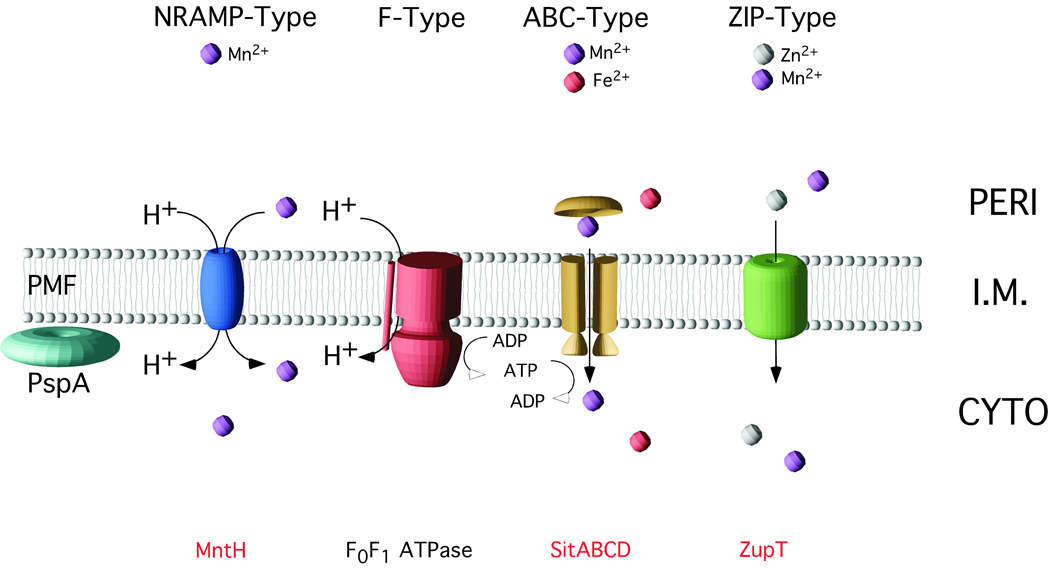
Figure 8. Model of PspA facilitated divalent metal transport in Salmonella.
PspA is important for the maintenance of PMF under extracytoplasmic stress. Nramp-Type and F-Type ATPase systems are depended on PMF for the transport of metal ions and ATP synthesis, respectively. ABC-Type transporters require ATP for metal import. Transport of divalent metals by Nramp-Type MntH, ABC-Type SitABCD and ZIP-Type ZupT (in red type) is facilitated by PspA in Salmonella. The Nramp family proton symporter MntH is predicted to be dependent upon PMF for transport function. The facilitation of ABC-Type SitABCD transport by PspA may be due to decreased ATP generation by the F0F1 ATPase in the absence of PspA. Additional studies are required to determine the specific energy requirements of ZIP-Type ZupT-mediated transport.
In this work, we have also shown for the first time that a bacterial ZIP family transporter plays an essential role in pathogenesis. S. Typhimurium lacking the ZIP transporter ZupT is attenuated for virulence in Nramp1+ mice following intraperitoneal inoculation (Fig. 7). It is interesting to note that LIT1, a ZIP family Fe2+ transporter, is required for the growth of the protozoan Leishmania amazonensis in macrophages (Huynh et al., 2006), providing an example of a common mechanism utilized by a bacterial and a eukaryotic pathogen to promote growth within the host environment. Little is currently understood regarding the energy requirements for metal transport by the ZIP protein family. However, ZIP transporters share sequence homology with the cation diffusion facilitator family, which mediate metal efflux from eukaryotic cells (Kambe et al., 2006). Transporters in the cation diffusion facilitator family appear to function as proton antiporters, thus requiring a proton gradient for metal efflux (Haney et al., 2005). We have shown that PspA facilitates Zn2+ uptake via ZupT in chelated medium (Fig. 5) as well as uptake of 54Mn2+ by transport assays (Fig. 6B), although additional studies will be required to establish whether ZupT-mediated transport is directly PMF-dependent. Collectively, our observations demonstrate that the phage shock response plays an essential role in Salmonella pathogenesis by facilitating high-affinity metal acquisition within the nutrient-limited host cell environment.
EXPERIMENTAL PROCEDURES
Strains, culture conditions, mutant strains and plasmid constructions
Salmonella enterica serovar Typhimurium strains, plasmids and primers used in this study are listed in Table 1. Strains were routinely grown in LB at 37°C. Antibiotics were used at the following concentrations as required: ampicillin (100µg·ml−1), kanamycin (50µg·ml−1), chloramphenicol (20µg·ml−1), and tetracycline (25µg·ml−1). Deletion mutants were constructed by λ-Red-mediated methods as described (Datsenko and Wanner, 2000; Karlinsey, 2007). In-frame deletion of pspA was constructed with primers JP18, JP21, JP61 and JP62 using the λ-Red tetRA replacement method (Karlinsey, 2007). An additional in-frame deletion of pspA (pspA::FRT) was constructed with primers MLC165 and MLC166 using the λ-Red method as described (Datsenko and Wanner, 2000). Both pspA deletions were phenotypically identical as determined by susceptibility to hydrogen peroxide and Sper/NO, growth in chelated medium with divalent metal add-back, and mouse virulence assays. In-frame deletions of zupT and znuABC were constructed using primers JP272/JP273 and JP316/JP317, respectively, using the λ-Red method (Datsenko and Wanner, 2000). A SPI1 deletion was constructed using primers KMp161 and KMp162 (Main-Hester et al., 2008), except that the FRTkanFRT cassette was not flipped out. All mutations were transduced into wild-type S. Typhimurium using bacteriophage P22HT105/int (Davis, 1980). Plasmids with pspA or zupT used for complementation in mouse virulence assays and for assays of growth in chelated medium were constructed as follows. Primers JP5/JP62 and JP279/JP280 were used to PCR-amplify the promoter and coding regions of pspA and zupT, respectively. The genes were cloned into the stable low-copy cloning vector pRB3 (Berggren et al., 1995) to create pJK664 (pRB3::pspA) and pJK671 (pRB3::zupT). To purify His8-PspA protein for subsequent anti-PspA antibody production, plasmid pJK654 (pBAD24-His8-PspA) was made using primers JP143 and JP144 to PCR-amplify and engineer the addition of eight histidines to the N-terminal coding region of pspA before cloning into pBAD24 (Galan and Curtiss, 1989).
Table 1.
Bacterial strains, plasmids and primers.
| Strain or plasmid |
Genotype or relevant characteristics | Source, Reference or Construction |
|---|---|---|
| Strain | ||
| 14028s |
Salmonella enterica serovar Typhimurium wild type |
ATCC |
| CS051 | 14028s phoP102::Tn10dCm | S. Miller |
| CS735 | 14028s ΔprgH-K | S. Miller |
| KM293 | 14028s Δspi-1::FRTkanFRT | This study |
| MLC162 | 14028s feoB::Tn10 | Y. Xu |
| MLC375 | 14028s ΔpspA:: FRTcatFRT | This study |
| MLC619 | 14028s ΔentC:: FRTkanFRT | (Crouch et al., 2008) |
| MM2165 | SL1344 mntH11::kan | (Kehres et al., 2000) |
| MM2562 | SL1344 sitA::MudCm | (Kehres et al., 2000) |
| SL2926 | 14028s invA::kan | (Galan and Curtiss, 1989) |
| SL3627 | 14028s ssrA::aph | S. Libby |
| SL3628 | 14028s ssrB::aph | S. Libby |
| TF951 | 14028s rpoE::cat | (Testerman et al., 2002) |
| JK224 | 14028s ΔpspA | This study |
| JK339 | 14028s ΔSPI-1:: FRTkanFRT | P22(KM293) × 14028s |
| JK451 | 14028s / pJK645 | This study |
| JK560 | 14028s phoP102::Tn10dCm | P22(CS051) × 14028s |
| JK601 | 14028s mntH11::kan | P22(MM2165) × 14028s |
| JK603 | 14028s sitA::MudCm | P22(MM2562) × 14028s |
| JK610 | 14028s ΔpspA::FRT | This study |
| JK620 | 14028s ΔpspA::FRT/pJK664 | This study |
| JK695 | 14028s feoB::Tn10 | P22(MLC162) × 14028s |
| JK713 | 14028s mntH11::kan feoB::Tn10 ΔzupT::FRT | This study |
| JK715 | 14028s ΔzupT:: FRTkanFRT | This study |
| JK725 | 14028s sitA::MudCm feoB::Tn10 ΔzupT:: FRTkanFRT |
This study |
| JK730 | 14028s mntH11::kan sitA::MudCm feoB::Tn10 ΔzupT::FRT |
This study |
| JK752 | 14028s sitA::MudCm feoB::Tn10 ΔzupT:: FRTkanFRT ΔpspA::FRT |
This study |
| JK753 | 14028s mntH11::kan feoB::Tn10 ΔzupT::FRT ΔpspA::FRT |
This study |
| JK755 | 14028s mntH11::kan sitA:: MudCm feoB::Tn10 ΔzupT::FRT ΔpspA::FRT |
This study |
| JK768 | 14028s/pRB3 | This study |
| JK770 | 14028s mntH11::kan feoB::Tn10 ΔzupT::FRT/pRB3 |
This study |
| JK771 | 14028s sitA:: MudCm feoB::Tn10 ΔzupT:: FRTkanFRT pRB3 |
This study |
| JK774 | 14028s sitA::MudCm feoB::Tn10 ΔzupT:: FRTkanFRT ΔpspA::FRT/pRB3 |
This study |
| JK775 | 14028s sitA::MudCm feoB::Tn10 ΔzupT:: FRTkanFRT ΔpspA::FRT/pJK664 |
This study |
| JK776 | 14028s mntH11::kan feoB::Tn10 ΔzupT::FRT ΔpspA::FRT/pRB3 |
This study |
| JK777 | 14028s mntH11::kan feoB::Tn10 ΔzupT::FRT ΔpspA::FRT/pJK664 |
This study |
| JK778 | 14028s mntH11::kan sitA:: MudCm feoB::Tn10 ΔzupT:: FRT/pRB3 |
This study |
| JK780 | 14028s mntH11::kan sitA::MudCm feoB::Tn10 ΔzupT::FRT ΔpspA::FRT/pRB3 |
This study |
| JK823 | 14028s ΔznuABC:: FRTkanFRT | This study |
| JK841 | 14028s ΔentC::FRT | P22(MLC619) × 14028s |
| JK854 | 14028s ΔentC::FRT mntH11::kan sitA::MudCm feoB::Tn10 ΔzupT::FRT |
This study |
| JK859 | 14028s ΔentC::FRT mntH11::kan sitA::MudCm feoB::Tn10 ΔzupT::FRT ΔpspA::FRT |
This study |
| JK860 | 14028s mntH11::kan sitA::MudCm feoB::Tn10 ΔznuABC::FRT/pRB3 |
This study |
| JK862 | 14028s mntH11::kan sitA::MudCm feoB::Tn10 ΔznuABC::FRT ΔpspA::FRT/pRB3 |
This study |
| JK863 | 14028s mntH11::kan sitA::MudCm feoB::Tn10 ΔznuABC::FRT ΔpspA::FRT/pJK664 |
This study |
| JK865 | 14028s mntH11::kan sitA::MudCm feoB::Tn10 ΔznuABC::FRT ΔzupT::FRT/pRB3 |
This study |
| JK866 | 14028s mntH11::kan sitA::MudCm feoB::Tn10 ΔznuABC::FRT ΔzupT::FRT/pJK671 |
This study |
| JK867 | 14028s mntH11::kan sitA::MudCm feoB::Tn10 ΔznuABC::FRT ΔzupT::FRT ΔpspA::FRT/pRB3 |
This study |
| JK869 | 14028s mntH11::kan sitA::MudCm feoB::Tn10 ΔznuABC::FRT ΔzupT::FRT ΔpspA::FRT/pJK671 |
This study |
| JK878 | 14028s ΔentC::FRT mntH11::kan feoB::Tn10 ΔzupT::FRT |
This study |
| JK879 | 14028s ΔentC::FRT mntH11::kan feoB::Tn10 ΔzupT::FRT ΔpspA::FRT |
This study |
| JK937 | 14028s invA::kan | P22(SL2926) × 14028s |
| Plasmid | ||
| pKD3 | bla FRTcatFRT PS1 PS2 ori6K | (Datsenko and Wanner, 2000) |
| pKD4 | bla FRTkanFRT PS1 PS2 ori6K | (Datsenko and Wanner, 2000) |
| pKD46 | bla araC-ParaB γ β exo oriR101 repA101ts | |
| pCP20 | bla cat cI857 λPr flp PSC101 oriTS | (Datsenko and Wanner, 2000) |
| pRB3 | par RK2 bla stable low copy cloning vector | (Berggren et al., 1995) |
| pJK645 | pBAD24-His8-PspA | This study |
| pJK664 | pRB3::pspA | This study |
| pJK671 | pRB3::zupT | This study |
| Primer | Primer sequence (5’-3’) | |
| JP5 | GGGCGGTACCTCGCCACTTGTTAGTGTT | |
| JP18 | CAGAACATTATGTGAGGATTGAATTATGGG TATTTTTTCTTTAAGACCCACTTTCACATT |
|
| JP21 | TGATGAGCGACGGCGCGTATCGGCGCCGCC ATTGTCATTACTAAGCACTTGTCTCCTG |
|
| JP61 | CAGAACATTATGTGAGGATTGAATTATGGG TATTTTTTCTTAATGACAATGGCGGCGCCGA TACGCGCCGTCGCTCATCA |
|
| JP62 | TGATGAGCGACGGCGCGT | |
| JP143 | CCCCTATCATGAGCCATCATCATCATCATCA TCATCATGGTATTTTTTCTCGTTTTGC |
|
| JP144 | CCCCATAAGCTTATTGATTATCTTGCTTCAT | |
| JP272 | ACCACTTATTCTGACCTTACTGGCGGGCGC CGCCACCTTTGTGTAGGCTGGAGCTGCTTC |
|
| JP273 | CTATCGTCTGCAAAATGACGAGACTGAGCC CCATGATGGACATATGAATATCCTCCTTAG |
|
| JP279 | AACCGGATCCAATCGTTATCGTCCAGCACG | |
| JP280 | AACCAAGCTTAACCGATACCTATCGTCT | |
| JP316 | CGAACGGATGTCCCTCTCGCCACGGCTTCC ACGACCGCTGGTGTAGGCTGGAGCTGCTTC |
|
| JP317 | AAAGCGACGCGCGGTTGCGGCGGGGATAA TCAGCAGCGACCATATGAATATCCTCCTTA G |
|
| MLC165 | CTTGCTTCATTTTGGCTTTCAACTGCGCCAG CTGCTCGCTGATTTCATCATCGGCTTTCAGT GTAGGCTGGAGCTGCTTG |
|
| MLC166 | AGATCCGCAGAAGCTGGTGCGCCTGATGAT TCAGGAGATGGAAGATACGCTGGTGGAGGT CATATGAATATCCTCCTTAG |
Mouse virulence assay
Moribund animals were euthanized. The following inocula were used for infection in various mouse backgrounds: 3×103 CFU in six to eight week-old C3H/HeN (Nramp+, Ityr) mice (Charles River Laboratories), 1×102 CFU in six to eight week-old C57/BL6 (Nramp−) mice (Charles River Laboratories), and 105 CFU in six to eight week-old congenic C57/BL6 Nramp1G169 mice (bred and housed in Modified Specific Pathogen Free facilities at the University of Washington animal facilities).
Motility, swarming and SPI secretion assays
Motility assays were performed at 37°C for 8.5 h in motility medium as described (Silverman and Simon, 1973), except the agar concentration was reduced to 0.3%. Swarming assays were performed on swarm medium (Wang et al., 2004) at 37°C for 9h. SPI1-secreted proteins were purified from overnight cultures essentially as described (Pegues et al., 1995). Fifty ml of overnight culture supernatants were TCA-precipitated and fractionated on a 4–15% polyacrylamide gel in Tris-Glycine-SDS buffer (Biorad, Hercules, CA) and the proteins detected by Coomassie blue staining.
Invasion Assay
HeLa cells were grown in RPMIc (RPMI 1640 with L-glutamine, 25mM HEPES (Mediatech Inc.), 10% heat-inactivated fetal calf serum (Hyclone) and 1:100 Pen/Strep (Mediatech Inc)). Cells were seeded at 2×105 cells per well in a 48 well microtiter dish. The cells were changed into fresh RPMIc without antibiotic 24h before the assay. S. Typhimurium strains were grown in LB without shaking at 37°C for 18–24h. HeLa cells were infected with an MOI of ~25:1 and centrifuged at 215g for 10min at 25°C and incubated for 20min at 37°C in 5% CO2. Cells were washed 3× in RPMIc (without antibiotics) then incubated in RPMIc + 100µg/ml gentamicin sulfate (MP Biochemicals) for 1h at 37°C in 5% CO2. At indicated times the cells were lysed in 1% Triton X-100 for 5min and bacteria quantified by plating on LB agar. % invasion was calculated as follows: (CFU T1h/CFU Tinitial) × 100.
Macrophage killing assay
S. Typhimurium survival within primary Nramp1− and Nramp1+ macrophages and RAW264.7 cells lines was determined as previously described (Zaharik et al., 2004). Cultures were grown with shaking in LB at 37°C for 18–24h. Salmonella strains were opsonized with mouse serum, infected at a multiplicity of infection of 5:1 and incubated with macrophages for 15 min. After phagocytosis, the cells were washed in RPMI with 12 µg·ml−1 gentamicin to kill any remaining bacterial that were not internalized. At designated time intervals, the cells were lysed in 1% Triton X-100 and surviving bacteria quantified by plating on LB agar.
Antimicrobial peptide, hydrogen peroxide and Sper/NO sensitivity assays
Peptide killing of cryptdin-4 and P2 were performed as described (Crouch et al., 2005). Sensitivity to hydrogen peroxide (Fisher Chemical, Fairlawn, NJ) or nitric oxide donor spermine NONOate (Sper/NO, Calbiochem, La Jolla, CA) were performed as follows. Overnight cultures were diluted to 2×106 CFU/ml in LB with the addition of either hydrogen peroxide (ranging up to 1mM) or spermine NONOate (ranging up to 2mM) in a final volume of 300µl. Growth kinetics were determined by measuring the optical density at 600nm at 37°C on a Bioscreen C Microbiology Microplate reader (Growth Curves USA, Piscataway, NJ).
Immunoblotting
His-PspA was purified from strain JK451 using His-Bind resin (Novagen, Gibbstown, NJ) according to the manufacturer’s instructions. Rabbit anti-PspA antisera were produced at Scantibodies Laboratories, Inc. (Santee, CA) and non-specific antibodies adsorbed as described (de Wet et al., 1984). Goat peroxidase-conjugated anti-rabbit HRP was obtained through Bio-Rad (Hercules, CA). Culture sample preparation, SDS electrophoresis, and western blot hybridization and detection were performed as described (Muller et al., 2009).
Assays of growth in chelated media with divalent metal add-back
Overnight cultures were diluted to 4×105 CFU/ml in LB chelated with 2,2’-dipyridyl (Sigma-Aldrich, St. Louis, MO). Divalent metals (all acquired from Sigma-Aldrich, St. Louis, MO) were added back to chelated LB medium at concentrations indicated in the figure legends. Growth kinetics were determined by measuring the optical density at 600nm at 37°C on a Bioscreen C Microbiology Microplate reader (Growth Curves USA, Piscataway, NJ). Three to five biological replicates were performed on each strain, and the averages and standard deviations of the optical density measurements were calculated for each time point.
Transport Assays of 54Mn2+
Uptake of 54Mn2+ by whole cells was measured essentially as described (Maguire, 2007). Overnight cultures were diluted in fresh LB broth and grown to OD600 0.6 to 0.7. The cells were spun and washed in 1× cold N-minimal medium (Maguire, 2007) and resuspended at OD600 2.0 in cold N-minimal medium with no additions. Cells (100 µl) were added to 900 µl pre-warmed N-minimal medium containing 0.5 to 1 µCi 54Mn2+ at a final total Mn2+ concentration of 0.1 µM (equal to the K0.5 for Mn2+ uptake by MntH and SitABCD (Kehres et al., 2000), and uptake allowed to proceed for 10 min. Four ml of cold wash buffer (1× N-minimal medium, 0.5 mM EDTA and 10 mM MgSO4) were added and immediately filtered onto a BA85 filter (Schleicher and Schuell, Keene, NH) before washing once with cold wash buffer. Filters were placed in vials and counted in a Beckman scintillation counter at any efficiency of ~80%. At least three biological replicates were assayed for each strain.
Supplementary Material
ACKNOWLEGMENTS
We are grateful to Stephen Libby, Kara Main-Hester, Samuel Miller and Barry Wanner for strains used in this study. This work was supported by research grants (AI44486, AI77629) to F.C.F. and a training grant (AI54052) to M.L.C. from the National Institutes of Health.
REFERENCES
- Adams H, Teertstra W, Demmers J, Boesten R, Tommassen J. Interactions between phage-shock proteins in Escherichia coli. J Bacteriol. 2003;185:1174–1180. doi: 10.1128/JB.185.4.1174-1180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ades SE. Control of the alternative sigma factor sigmaE in Escherichia coli. Curr Opin Microbiol. 2004;7:157–162. doi: 10.1016/j.mib.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Alba BM, Gross CA. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol Microbiol. 2004;52:613–619. doi: 10.1111/j.1365-2958.2003.03982.x. [DOI] [PubMed] [Google Scholar]
- Bang IS, Frye JG, McClelland M, Velayudhan J, Fang FC. Alternative sigma factor interactions in Salmonella: sigma and sigma promote antioxidant defences by enhancing sigma levels. Mol Microbiol. 2005;56:811–823. doi: 10.1111/j.1365-2958.2005.04580.x. [DOI] [PubMed] [Google Scholar]
- Barton CH, Biggs TE, Baker ST, Bowen H, Atkinson PG. Nramp1: a link between intracellular iron transport and innate resistance to intracellular pathogens. J Leukoc Biol. 1999;66:757–762. doi: 10.1002/jlb.66.5.757. [DOI] [PubMed] [Google Scholar]
- Becker LA, Bang IS, Crouch ML, Fang FC. Compensatory role of PspA, a member of the phage shock protein operon, in rpoE mutant Salmonella enterica serovar Typhimurium. Mol Microbiol. 2005;56:1004–1016. doi: 10.1111/j.1365-2958.2005.04604.x. [DOI] [PubMed] [Google Scholar]
- Beloin C, Valle J, Latour-Lambert P, Faure P, Kzreminski M, Balestrino D, Haagensen JA, Molin S, Prensier G, Arbeille B, Ghigo JM. Global impact of mature biofilm lifestyle on Escherichia coli K-12 gene expression. Mol Microbiol. 2004;51:659–674. doi: 10.1046/j.1365-2958.2003.03865.x. [DOI] [PubMed] [Google Scholar]
- Berggren RE, Wunderlich A, Ziegler E, Schleicher M, Duke RC, Looney D, Fang FC. HIV gp120-specific cell-mediated immune responses in mice after oral immunization with recombinant Salmonella. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:489–495. [PubMed] [Google Scholar]
- Blackwell JM, Goswami T, Evans CA, Sibthorpe D, Papo N, White JK, Searle S, Miller EN, Peacock CS, Mohammed H, Ibrahim M. SLC11A1 (formerly NRAMP1) and disease resistance. Cell Microbiol. 2001;3:773–784. doi: 10.1046/j.1462-5822.2001.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer E, Bergevin I, Malo D, Gros P, Cellier MF. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2002;70:6032–6042. doi: 10.1128/IAI.70.11.6032-6042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley DJ. Regulation of Leishmania populations within the host. II. genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin Exp Immunol. 1977;30:130–140. [PMC free article] [PubMed] [Google Scholar]
- Brissette JL, Russel M, Weiner L, Model P. Phage shock protein, a stress protein of Escherichia coli. Proc Natl Acad Sci U S A. 1990;87:862–866. doi: 10.1073/pnas.87.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette JL, Weiner L, Ripmaster TL, Model P. Characterization and sequence of the Escherichia coli stress-induced psp operon. J Mol Biol. 1991;220:35–48. doi: 10.1016/0022-2836(91)90379-k. [DOI] [PubMed] [Google Scholar]
- Campoy S, Jara M, Busquets N, Perez De Rozas AM, Badiola I, Barbe J. Role of the high-affinity zinc uptake ZnuABC system in Salmonella enterica serovar Typhimurium virulence. Infect Immun. 2002;70:4721–4725. doi: 10.1128/IAI.70.8.4721-4725.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano DA, Martinez-Moya M, Pucciarelli MG, Groisman EA, Casadesus J, Garcia-Del Portillo F. Salmonella enterica serovar Typhimurium response involved in attenuation of pathogen intracellular proliferation. Infect Immun. 2001;69:6463–6474. doi: 10.1128/IAI.69.10.6463-6474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson KE, Liu J, Edqvist PJ, Francis MS. Extracytoplasmic-stress-responsive pathways modulate type III secretion in Yersinia pseudotuberculosis. Infect Immun. 2007;75:3913–3924. doi: 10.1128/IAI.01346-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellier MF, Courville P, Campion C. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect. 2007;9:1662–1670. doi: 10.1016/j.micinf.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Connolly L, De Las Penas A, Alba BM, Gross CA. The response to extracytoplasmic stress in Escherichia coli is controlled by partially overlapping pathways. Genes Dev. 1997;11:2012–2021. doi: 10.1101/gad.11.15.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courville P, Chaloupka R, Veyrier F, Cellier MF. Determination of transmembrane topology of the Escherichia coli natural resistance-associated macrophage protein (Nramp) ortholog. J Biol Chem. 2004;279:3318–3326. doi: 10.1074/jbc.M309913200. [DOI] [PubMed] [Google Scholar]
- Crouch ML, Becker LA, Bang IS, Tanabe H, Ouellette AJ, Fang FC. The alternative sigma factor sigma is required for resistance of Salmonella enterica serovar Typhimurium to anti-microbial peptides. Mol Microbiol. 2005;56:789–799. doi: 10.1111/j.1365-2958.2005.04578.x. [DOI] [PubMed] [Google Scholar]
- Crouch ML, Castor M, Karlinsey JE, Kalhorn T, Fang FC. Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Mol Microbiol. 2008;67:971–983. doi: 10.1111/j.1365-2958.2007.06089.x. [DOI] [PubMed] [Google Scholar]
- Darwin AJ. The phage-shock-protein response. Mol Microbiol. 2005;57:621–628. doi: 10.1111/j.1365-2958.2005.04694.x. [DOI] [PubMed] [Google Scholar]
- Darwin AJ, Miller VL. Identification of Yersinia enterocolitica genes affecting survival in an animal host using signature-tagged transposon mutagenesis. Mol Microbiol. 1999;32:51–62. doi: 10.1046/j.1365-2958.1999.01324.x. [DOI] [PubMed] [Google Scholar]
- Darwin AJ, Miller VL. The psp locus of Yersinia enterocolitica is required for virulence and for growth in vitro when the Ysc type III secretion system is produced. Mol Microbiol. 2001;39:429–444. doi: 10.1046/j.1365-2958.2001.02235.x. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AL, Chen J. ATP-binding cassette transporters in bacteria. Annu Rev Biochem. 2004;73:241–268. doi: 10.1146/annurev.biochem.73.011303.073626. [DOI] [PubMed] [Google Scholar]
- Davis RW, Botstein D, Roth JR. A manual for genetic engineering:advanced bacterial genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- de Wet JR, Fukushima H, Dewji NN, Wilcox E, O'Brien JS, Helinski DR. Chromogenic immunodetection of human serum albumin and alpha-L-fucosidase clones in a human hepatoma cDNA expression library. DNA. 1984;3:437–447. doi: 10.1089/dna.1.1984.3.437. [DOI] [PubMed] [Google Scholar]
- Duguay AR, Silhavy TJ. Quality control in the bacterial periplasm. Biochim Biophys Acta. 2004;1694:121–134. doi: 10.1016/j.bbamcr.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Dworkin J, Jovanovic G, Model P. The PspA protein of Escherichia coli is a negative regulator of sigma(54)-dependent transcription. J Bacteriol. 2000;182:311–319. doi: 10.1128/jb.182.2.311-319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng BH, Guerinot ML, Eide D, Saier MHJ. Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins. J Membr Biol. 1998;166:1–7. doi: 10.1007/s002329900442. [DOI] [PubMed] [Google Scholar]
- Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JC. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol. 2003;47:103–118. doi: 10.1046/j.1365-2958.2003.03313.x. [DOI] [PubMed] [Google Scholar]
- Feng X, Oropeza R, Kenney LJ. Dual regulation by phospho-OmpR of ssrA/B gene expression in Salmonella pathogenicity island 2. Mol Microbiol. 2003;48:1131–1143. doi: 10.1046/j.1365-2958.2003.03502.x. [DOI] [PubMed] [Google Scholar]
- Feng X, Walthers D, Oropeza R, Kenney LJ. The response regulator SsrB activates transcription and binds to a region overlapping OmpR binding sites at Salmonella pathogenicity island 2. Mol Microbiol. 2004;54:823–835. doi: 10.1111/j.1365-2958.2004.04317.x. [DOI] [PubMed] [Google Scholar]
- Forbes JR, Gros P. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 2001;9:397–403. doi: 10.1016/s0966-842x(01)02098-4. [DOI] [PubMed] [Google Scholar]
- Forbes JR, Gros P. Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood. 2003;102:1884–1892. doi: 10.1182/blood-2003-02-0425. [DOI] [PubMed] [Google Scholar]
- Fritsche G, Nairz M, Theurl I, Mair S, Bellmann-Weiler R, Barton HC, Weiss G. Modulation of macrophage iron transport by Nramp1 (Slc11a1) Immunobiology. 2007;212:751–757. doi: 10.1016/j.imbio.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Galan JE, Curtiss R., 3d Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmendia J, Beuzon CR, Ruiz-Albert J, Holden DW. The roles of SsrA-SsrB and OmpR-EnvZ in the regulation of genes encoding the Salmonella typhimurium SPI-2 type III secretion system. Microbiology. 2003;149:2385–2396. doi: 10.1099/mic.0.26397-0. [DOI] [PubMed] [Google Scholar]
- Goswami T, Bhattacharjee A, Babal P, Searle S, Moore E, Li M, Blackwell JM. Natural-resistance-associated macrophage protein 1 is an H+/bivalent cation antiporter. Biochem J. 2001;354:511–519. doi: 10.1042/0264-6021:3540511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni G, Vidal S, Gauthier S, Skamene E, Malo D, Gros P. The Bcg/Ity/Lsh locus: genetic transfer of resistance to infections in C57BL/6J mice transgenic for the Nramp1 Gly169 allele. Infect Immun. 1996;64:2923–2929. doi: 10.1128/iai.64.8.2923-2929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass G, Franke S, Taudte N, Nies DH, Kucharski LM, Maguire ME, Rensing C. The metal permease ZupT from Escherichia coli is a transporter with a broad substrate spectrum. J Bacteriol. 2005;187:1604–1611. doi: 10.1128/JB.187.5.1604-1611.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass G, Wong MD, Rosen BP, Smith RL, Rensing C. ZupT is a Zn(II) uptake system in Escherichia coli. J Bacteriol. 2002;184:864–866. doi: 10.1128/JB.184.3.864-866.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros P, Skamene E, Forget A. Genetic control of natural resistance to Mycobacterium bovis (BCG) in mice. J Immunol. 1981;127:2417–2421. [PubMed] [Google Scholar]
- Gruenheid S, Pinner E, Desjardins M, Gros P. Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J Exp Med. 1997;185:717–730. doi: 10.1084/jem.185.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML. The ZIP family of metal transporters. Biochim Biophys Acta. 2000;1465:190–198. doi: 10.1016/s0005-2736(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Guilvout I, Chami M, Engel A, Pugsley AP, Bayan N. Bacterial outer membrane secretin PulD assembles and inserts into the inner membrane in the absence of its pilotin. EMBO J. 2006;25:5241–5249. doi: 10.1038/sj.emboj.7601402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney CJ, Grass G, Franke S, Rensing C. New developments in the understanding of the cation diffusion facilitator family. J Ind Microbiol Biotechnol. 2005;32:215–226. doi: 10.1007/s10295-005-0224-3. [DOI] [PubMed] [Google Scholar]
- Hansen-Wester I, Hensel M. Salmonella pathogenicity islands encoding type III secretion systems. Microbes Infect. 2001;3:549–559. doi: 10.1016/s1286-4579(01)01411-3. [DOI] [PubMed] [Google Scholar]
- Harold FMaPCM. Energy Transduction by Ion Currents. In: Neidhardt FC, editor. In Energy Transduction by Ion Currents. Washington, D.C: ASM Press; 1996. pp. 283–306. [Google Scholar]
- Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Humphreys S, Rowley G, Stevenson A, Anjum MF, Woodward MJ, Gilbert S, Kormanec J, Roberts M. Role of the two-component regulator CpxAR in the virulence of Salmonella enterica serotype Typhimurium. Infect Immun. 2004;72:4654–4661. doi: 10.1128/IAI.72.8.4654-4661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys S, Stevenson A, Bacon A, Weinhardt AB, Roberts M. The alternative sigma factor, sigmaE, is critically important for the virulence of Salmonella typhimurium. Infect Immun. 1999;67:1560–1568. doi: 10.1128/iai.67.4.1560-1568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh C, Sacks DL, Andrews NW. A Leishmania amazonensis ZIP family iron transporter is essential for parasite replication within macrophage phagolysosomes. J Exp Med. 2006;203:2363–2375. doi: 10.1084/jem.20060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med. 2000;192:1237–1248. doi: 10.1084/jem.192.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janakiraman A, Slauch JM. The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella typhimurium. Mol Microbiol. 2000;35:1146–1155. doi: 10.1046/j.1365-2958.2000.01783.x. [DOI] [PubMed] [Google Scholar]
- Jones SE, Lloyd LJ, Tan KK, Buck M. Secretion defects that activate the phage shock response of Escherichia coli. J Bacteriol. 2003;185:6707–6711. doi: 10.1128/JB.185.22.6707-6711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic G, Weiner L, Model P. Identification, nucleotide sequence, and characterization of PspF, the transcriptional activator of the Escherichia coli stress-induced psp operon. J Bacteriol. 1996;178:1936–1945. doi: 10.1128/jb.178.7.1936-1945.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambe T, Suzuki T, Nagao M, Yamaguchi-Iwai Y. Sequence similarity and functional relationship among eukaryotic ZIP and CDF transporters. Genomics Proteomics Bioinformatics. 2006;4:1–9. doi: 10.1016/S1672-0229(06)60010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlinsey JE. lambda-Red genetic engineering in Salmonella enterica serovar Typhimurium. Methods Enzymol. 2007;421:199–209. doi: 10.1016/S0076-6879(06)21016-4. [DOI] [PubMed] [Google Scholar]
- Kehres DG, Janakiraman A, Slauch JM, Maguire ME. SitABCD is the alkaline Mn(2+) transporter of Salmonella enterica serovar Typhimurium. J Bacteriol. 2002;184:3159–3166. doi: 10.1128/JB.184.12.3159-3166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres DG, Zaharik ML, Finlay BB, Maguire ME. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol Microbiol. 2000;36:1085–1100. doi: 10.1046/j.1365-2958.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- Kimbrough TG, Miller SI. Contribution of Salmonella typhimurium type III secretion components to needle complex formation. Proc Natl Acad Sci U S A. 2000;97:11008–11013. doi: 10.1073/pnas.200209497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleerebezem M, Crielaard W, Tommassen J. Involvement of stress protein PspA (phage shock protein A) of Escherichia coli in maintenance of the protonmotive force under stress conditions. EMBO J. 1996;15:162–171. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Yamamoto M, Aono R. Appearance of a stress-response protein, phage-shock protein A, in Escherichia coli exposed to hydrophobic organic solvents. Microbiology. 1998;144:353–359. doi: 10.1099/00221287-144-2-353. [DOI] [PubMed] [Google Scholar]
- Kobayashi R, Suzuki T, Yoshida M. Escherichia coli phage-shock protein A (PspA) binds to membrane phospholipids and repairs proton leakage of the damaged membranes. Mol Microbiol. 2007;66:100–109. doi: 10.1111/j.1365-2958.2007.05893.x. [DOI] [PubMed] [Google Scholar]
- Kuhle V, Hensel M. Cellular microbiology of intracellular Salmonella enterica: functions of the type III secretion system encoded by Salmonella pathogenicity island 2. Cell Mol Life Sci. 2004;61:2812–2826. doi: 10.1007/s00018-004-4248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DE, Baker BD, Lafuse WP, Zwilling BS. Differential iron transport into phagosomes isolated from the RAW264.7 macrophage cell lines transfected with Nramp1Gly169 or Nramp1Asp169. J Leukoc Biol. 1999;66:113–119. doi: 10.1002/jlb.66.1.113. [DOI] [PubMed] [Google Scholar]
- Kuhn DE, Lafuse WP, Zwilling BS. Iron transport into Mycobacterium avium-containing phagosomes from an Nramp1(Gly169)-transfected RAW264.7 macrophage cell line. J Leukoc Biol. 2001;69:43–49. [PubMed] [Google Scholar]
- Lee SH, Galan JE. Salmonella type III secretion-associated chaperones confer secretion-pathway specificity. Mol Microbiol. 2004;51:483–495. doi: 10.1046/j.1365-2958.2003.03840.x. [DOI] [PubMed] [Google Scholar]
- Lloyd LJ, Jones SE, Jovanovic G, Gyaneshwar P, Rolfe MD, Thompson A, Hinton JC, Buck M. Identification of a new member of the phage shock protein response in Escherichia coli, the phage shock protein G (PspG) J Biol Chem. 2004;279:55707–55714. doi: 10.1074/jbc.M408994200. [DOI] [PubMed] [Google Scholar]
- Lostroh CP, Lee CA. The Salmonella pathogenicity island-1 type III secretion system. Microbes Infect. 2001;3:1281–1291. doi: 10.1016/s1286-4579(01)01488-5. [DOI] [PubMed] [Google Scholar]
- Lucchini S, Liu H, Jin Q, Hinton JC, Yu J. Transcriptional adaptation of Shigella flexneri during infection of macrophages and epithelial cells: insights into the strategies of a cytosolic bacterial pathogen. Infect Immun. 2005;73:88–102. doi: 10.1128/IAI.73.1.88-102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie B, Hediger MA. SLC11 family of H+-coupled metal-ion transporters NRAMP1 and DMT1. Pflugers Arch. 2004;447:571–579. doi: 10.1007/s00424-003-1141-9. [DOI] [PubMed] [Google Scholar]
- Maguire ME. Magnesium, manganese, and divalent cation transport assays in intact cells. Methods Mol Biol. 2007;394:289–305. doi: 10.1007/978-1-59745-512-1_14. [DOI] [PubMed] [Google Scholar]
- Main-Hester KL, Colpitts KM, Thomas GA, Fang FC, Libby SJ. Coordinate regulation of Salmonella pathogenicity island 1 (SPI1) and SPI4 in Salmonella enterica serovar Typhimurium. Infect Immun. 2008;76:1024–1035. doi: 10.1128/IAI.01224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makui H, Roig E, Cole ST, Helmann JD, Gros P, Cellier MF. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol Microbiol. 2000;35:1065–1078. doi: 10.1046/j.1365-2958.2000.01774.x. [DOI] [PubMed] [Google Scholar]
- Malo D, Vogan K, Vidal S, Hu J, Cellier M, Schurr E, Fuks A, Bumstead N, Morgan K, Gros P. Haplotype mapping and sequence analysis of the mouse Nramp gene predict susceptibility to infection with intracellular parasites. Genomics. 1994;23:51–61. doi: 10.1006/geno.1994.1458. [DOI] [PubMed] [Google Scholar]
- Maxson ME, Darwin AJ. Identification of inducers of the Yersinia enterocolitica phage shock protein system and comparison to the regulation of the RpoE and Cpx extracytoplasmic stress responses. J Bacteriol. 2004;186:4199–4208. doi: 10.1128/JB.186.13.4199-4208.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Namba K. Distinct roles of the FliI ATPase and proton motive force in bacterial flagellar protein export. Nature. 2008;451:485–488. doi: 10.1038/nature06449. [DOI] [PubMed] [Google Scholar]
- Model P, Jovanovic G, Dworkin J. The Escherichia coli phage-shock-protein (psp) operon. Mol Microbiol. 1997;24:255–261. doi: 10.1046/j.1365-2958.1997.3481712.x. [DOI] [PubMed] [Google Scholar]
- Mulero V, Searle S, Blackwell JM, Brock JH. Solute carrier 11a1 (Slc11a1; formerly Nramp1) regulates metabolism and release of iron acquired by phagocytic, but not transferrin-receptor-mediated, iron uptake. Biochem J. 2002;363:89–94. doi: 10.1042/0264-6021:3630089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Bang IS, Velayudhan J, Karlinsey J, Papenfort K, Vogel J, Fang FC. Acid stress activation of the sigma(E) stress response in Salmonella enterica serovar Typhimurium. Mol Microbiol. 2009;71:1228–1238. doi: 10.1111/j.1365-2958.2009.06597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M, Fritsche G, Crouch ML, Barton HC, Fang FC, Weiss G. Slc11a1 limits intracellular growth of Salmonella enterica sv. Typhimurium by promoting macrophage immune effector functions and impairing bacterial iron acquisition. Cell Microbiol. 2009 doi: 10.1111/j.1462-5822.2009.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo Y, Nelson N. The NRAMP family of metal-ion transporters. Biochim Biophys Acta. 2006;1763:609–620. doi: 10.1016/j.bbamcr.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Papp-Wallace KM, Maguire ME. Manganese transport and the role of manganese in virulence. Annu Rev Microbiol. 2006;60:187–209. doi: 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- Paul K, Erhardt M, Hirano T, Blair DF, Hughes KT. Energy source of flagellar type III secretion. Nature. 2008;451:489–492. doi: 10.1038/nature06497. [DOI] [PubMed] [Google Scholar]
- Pegues DA, Hantman MJ, Behlau I, Miller SI. PhoP/PhoQ transcriptional repression of Salmonella typhimurium invasion genes: evidence for a role in protein secretion. Mol Microbiol. 1995;17:169–181. doi: 10.1111/j.1365-2958.1995.mmi_17010169.x. [DOI] [PubMed] [Google Scholar]
- Plant J, Glynn AA. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976;133:72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Raffa RG, Raivio TL. A third envelope stress signal transduction pathway in Escherichia coli. Mol Microbiol. 2002;45:1599–1611. doi: 10.1046/j.1365-2958.2002.03112.x. [DOI] [PubMed] [Google Scholar]
- Raivio TL. Envelope stress responses and Gram-negative bacterial pathogenesis. Mol Microbiol. 2005;56:1119–1128. doi: 10.1111/j.1365-2958.2005.04625.x. [DOI] [PubMed] [Google Scholar]
- Raivio TL, Silhavy TJ. The sigmaE and Cpx regulatory pathways: overlapping but distinct envelope stress responses. Curr Opin Microbiol. 1999;2:159–165. doi: 10.1016/S1369-5274(99)80028-9. [DOI] [PubMed] [Google Scholar]
- Raivio TL, Silhavy TJ. Periplasmic stress and ECF sigma factors. Annu Rev Microbiol. 2001;55:591–624. doi: 10.1146/annurev.micro.55.1.591. [DOI] [PubMed] [Google Scholar]
- Rowley G, Spector M, Kormanec J, Roberts M. Pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nat Rev Microbiol. 2006;4:383–394. doi: 10.1038/nrmicro1394. [DOI] [PubMed] [Google Scholar]
- Rowley G, Stevenson A, Kormanec J, Roberts M. Effect of inactivation of degS on Salmonella enterica serovar Typhimurium in vitro and in vivo. Infect Immun. 2005;73:459–463. doi: 10.1128/IAI.73.1.459-463.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz N, Silhavy TJ. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr Opin Microbiol. 2005;8:122–126. doi: 10.1016/j.mib.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Sabri M, Houle S, Dozois CM. Roles of the extraintestinal pathogenic Escherichia coli ZnuACB and ZupT zinc transporters during urinary tract infection. Infect Immun. 2009;77:1155–1164. doi: 10.1128/IAI.01082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior AE. The proton-translocating ATPase of Escherichia coli. Annu Rev Biophys Biophys Chem. 1990;19:7–41. doi: 10.1146/annurev.bb.19.060190.000255. [DOI] [PubMed] [Google Scholar]
- Silverman M, Simon M. Genetic analysis of flagellar mutants in Escherichia coli. J Bacteriol. 1973;113:105–113. doi: 10.1128/jb.113.1.105-113.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standar K, Mehner D, Osadnik H, Berthelmann F, Hause G, Lunsdorf H, Bruser T. PspA can form large scaffolds in Escherichia coli. FEBS Lett. 2008;582:3585–3589. doi: 10.1016/j.febslet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Techau ME, Valdez-Taubas J, Popoff JF, Francis R, Seaman M, Blackwell JM. Evolution of differences in transport function in Slc11a family members. J Biol Chem. 2007;282:35646–35656. doi: 10.1074/jbc.M707057200. [DOI] [PubMed] [Google Scholar]
- Testerman TL, Vazquez-Torres A, Xu Y, Jones-Carson J, Libby SJ, Fang FC. The alternative sigma factor sigmaE controls antioxidant defences required for Salmonella virulence and stationary-phase survival. Mol Microbiol. 2002;43:771–782. doi: 10.1046/j.1365-2958.2002.02787.x. [DOI] [PubMed] [Google Scholar]
- Walthers D, Carroll RK, Navarre WW, Libby SJ, Fang FC, Kenney LJ. The response regulator SsrB activates expression of diverse Salmonella pathogenicity island 2 promoters and counters silencing by the nucleoid-associated protein H-NS. Mol Microbiol. 2007;65:477–493. doi: 10.1111/j.1365-2958.2007.05800.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Frye JG, McClelland M, Harshey RM. Gene expression patterns during swarming in Salmonella typhimurium: genes specific to surface growth and putative new motility and pathogenicity genes. Mol Microbiol. 2004;52:169–187. doi: 10.1111/j.1365-2958.2003.03977.x. [DOI] [PubMed] [Google Scholar]
- Weiner L, Model P. Role of an Escherichia coli stress-response operon in stationary-phase survival. Proc Natl Acad Sci U S A. 1994;91:2191–2195. doi: 10.1073/pnas.91.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilharm G, Lehmann V, Krauss K, Lehnert B, Richter S, Ruckdeschel K, Heesemann J, Trulzsch K. Yersinia enterocolitica type III secretion depends on the proton motive force but not on the flagellar motor components MotA and MotB. Infect Immun. 2004;72:4004–4009. doi: 10.1128/IAI.72.7.4004-4009.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharik ML, Cullen VL, Fung AM, Libby SJ, Kujat Choy SL, Coburn B, Kehres DG, Maguire ME, Fang FC, Finlay BB. The Salmonella enterica serovar Typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1G169 murine typhoid model. Infect Immun. 2004;72:5522–5525. doi: 10.1128/IAI.72.9.5522-5525.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Hardt WD, Galan JE. Salmonella typhimurium encodes a putative iron transport system within the centisome 63 pathogenicity island. Infect Immun. 1999;67:1974–1981. doi: 10.1128/iai.67.4.1974-1981.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwilling BS, Kuhn DE, Wikoff L, Brown D, Lafuse W. Role of iron in Nramp1-mediated inhibition of mycobacterial growth. Infect Immun. 1999;67:1386–1392. doi: 10.1128/iai.67.3.1386-1392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.