Abstract
Our previous studies have found that intracerebral pretreatment with a low dose of thrombin (thrombin preconditioning, TPC) reduces infarct volume and attenuates brain edema after focal cerebral ischemia. In this study, we examined whether TPC protects against the neuronal death induced by oxygen glucose deprivation (OGD), and whether the protection is through thrombin receptors and the p44/42 mitogen activated protein kinases (MAPK)/ribosomal protein S6 kinases (p70 S6K) pathway.
Expression of protease-activated receptors (PARs) mRNA was detected in cultured primary rat neurons and thrombin upregulated PAR-1 and PAR-4 mRNA expression. TPC reduced OGD-induced neuronal death (e.g. dead cells: 52.5±5.4% vs. 72.3±7.2% in the control group, n=6, p<0.01). Agonists of PAR-1 and PAR-4 mimicked the effects of thrombin and reduced OGD-induced neuronal death. Pretreatment with thrombin or PAR agonists induced the upregulation of activated p44/42 MAPK and p70S6K (Thr 421/Ser 424). PD98059, an inhibitor of p44/42 MAPK kinase, blocked thrombin-induced upregulation of activated p44/42 MAPK and p70S6K. It also reduced TPC-induced neuronal protection (e.g. dead cells: 68.2±5.2% vs. 56.9±4.6% in vehicle+TPC group, n=6, p<0.05).
These results suggest that TPC-induced ischemic tolerance is through activation of thrombin receptors and the p44/42 MAPK/p70S6K pathway.
Keywords: Thrombin, protease activated receptor, preconditioning, oxygen glucose deprivation, p44/42 MAPK
1. Introduction
Thrombin, a serine protease, has a role in brain injury and recovery after hemorrhagic and ischemic stroke. High concentrations of thrombin cause brain edema and cell death, but low concentrations are neuroprotective (Choi et al., 2005; Henrich-Noack et al., 2006; Hua et al., 2003; Jiang et al., 2002; Masada et al., 2000; Striggow et al., 2000). Thus, we have found that prior treatment with a low dose of thrombin attenuates the brain edema induced by thrombin, iron or hemorrhage (Hua et al., 2003; Xi et al., 1999), significantly reduces infarct size in a rat middle cerebral artery occlusion model (Masada et al., 2000) and reduces brain damage in a rat model of Parkinson’s disease (Cannon et al., 2005; Cannon et al., 2006). We have termed this phenomena thrombin preconditioning (TPC), or thrombin-induced brain tolerance.
The precise mechanisms of thrombin-induced brain tolerance to hemorrhagic and ischemic stroke are not known. However, activation of thrombin receptors and upregulation of thrombin inhibitors, iron handling proteins, aquaporin 4 and heat shock proteins in the brain may be associated with the induced tolerance (Hirt et al., 2009; Hua et al., 2002; Hua et al., 2003; Xi et al., 1999; Yang et al., 2006). Thrombin receptors are seven transmembrane G protein-coupled receptors that are activated by proteolytic cleavage rather than by ligand binding. Three protease-activated receptors (PARs), PAR-1, PAR-3 and PAR-4, can be activated by thrombin whereas PAR-2 is activated by trypsin and mast cell tryptase (Coughlin, 1999; Coughlin, 2000). PAR-1 expression is found in neurons, astrocytes, oligodendroglial cells and microglia and there is functional evidence for the presence of PAR-1 on all cell types (Gingrich and Traynelis, 2000; Junge et al., 2004; Suo et al., 2003; Ubl et al., 2000; Wang et al., 2006; Weinstein et al., 1995).
Cerebral preconditioning-induced neuronal protection may be associated with new protein synthesis (Dirnagl et al., 2009; Gidday, 2006). Activation of p44/42 mitogen activated protein kinases(MAPK)/ribosomal protein S6 kinases (p70 S6K) pathway is related to new protein synthesis and has been associated with ischemic tolerance in heart (Hausenloy et al., 2004; Hausenloy et al., 2005).
In the present study, we investigated the effects of TPC on ischemic neuronal death and the role of the p44/42 MAPK/p70 S6K pathway in thrombin-induced neuroprotection in vitro using rat primary neuronal cultures.
2. RESULTS
Effects of thrombin preconditioning on neuronal death induced by OGD
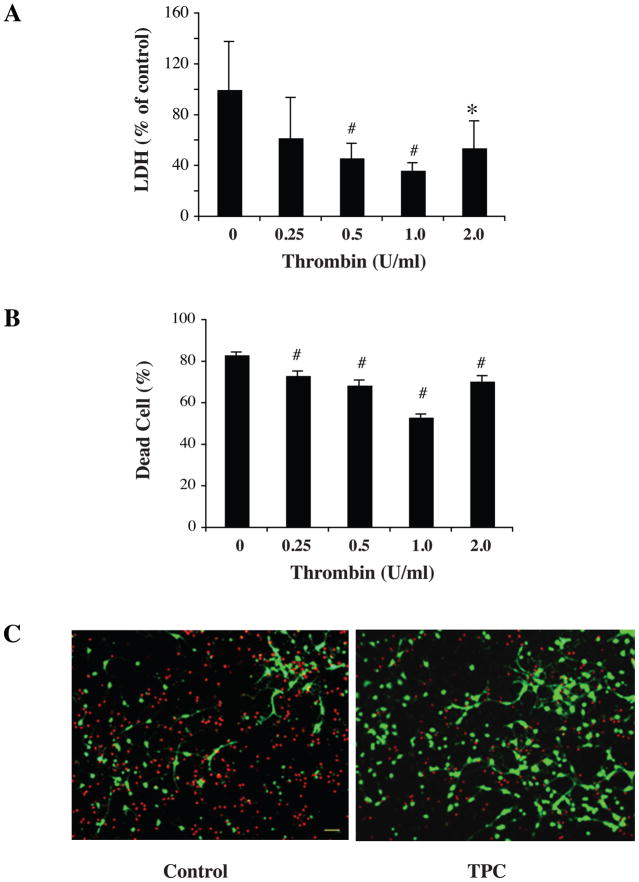
To determine which dose of thrombin has the best protective effect, cultured rat neurons were pretreated with different thrombin doses (0.25, 0.5, 1.0 and 2.0 U/ml) for 24 hours. Neurons then underwent 2 hours OGD, and both lactate dehydrogenase (LDH) and live/dead cell assays were performed at 22 hours after OGD. LDH assay showed that neurons pretreated with 0.5, 1.0 and 2.0 U/ml thrombin had significantly lower LDH release compared with neurons without preconditioning (p< 0.01, Fig. 1A). Live/dead cell staining directly also showed less dead cells in the TPC groups (p< 0.05, Fig. 1B). Based upon these experiments, we chose 1.0 U/ml thrombin as the dose for TPC in the experiments below.
Figure 1.
Primary cultured neurons were pretreated with different doses of thrombin for 24 hours and then exposed to oxygen glucose deprivation (OGD) for 2 hours. The levels of lactate dehydrogenase (LDH) released into the medium (A) and the percent of dead cells by live/dead cell staining (B and C) were measured after 22 hours of reoxygenation. Values are expressed as means ± SD. *p< 0.05, # p< 0.01 vs. control (0 U/ml thrombin). In (C), neurons stained red indicate dead neurons and those green are live. TPC = thrombin preconditioning with 1U/ml. Scale bar = 20 μM.
TPC-induced PAR-1 and PAR-4 mRNA upregulation in cultured neurons
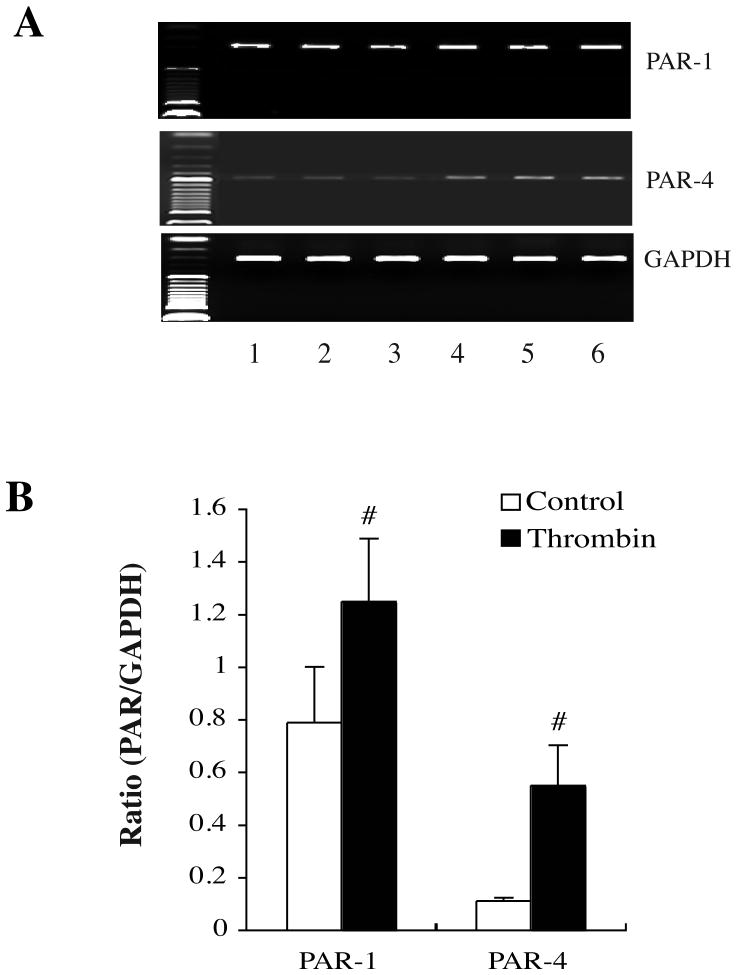
Primary cultured neurons were treated with vehicle or thrombin 1.0 U/ml for 24 hours. The levels of PARs mRNAs in neurons after TPC were determined by a semiquantitative RT-PCR. In the vehicle-treated neurons, PAR-1 mRNA expression was strong and PAR-4 mRNA expression was weak. After thrombin treatment, both PAR-1 (PAR-1/GAPDH: 1.25±0.24 vs. 0.79±0.21, in the vehicle-treated group, p<0.01, Fig. 2) and PAR-4 mRNA (PAR-4/GAPDH: 0.55±0.15 vs. 0.11±0.01 in the vehicle-treated group, p<0.01, Fig. 2) were upregulated after 24 hours.
Figure 2.
PAR-1 and PAR-4 mRNA levels in cultured neurons 24 hours after thrombin (1 U/ml) stimulation. (A) Lanes 1–3: vehicle control-treated neurons; Lanes: thrombin-treated neurons. (B) Bar graphs showing neuronal PAR-1 and PAR-4 mRNA levels. Values are means ± S.D., #p<0.01 vs. control.
PAR agonists-induced neuronal protection
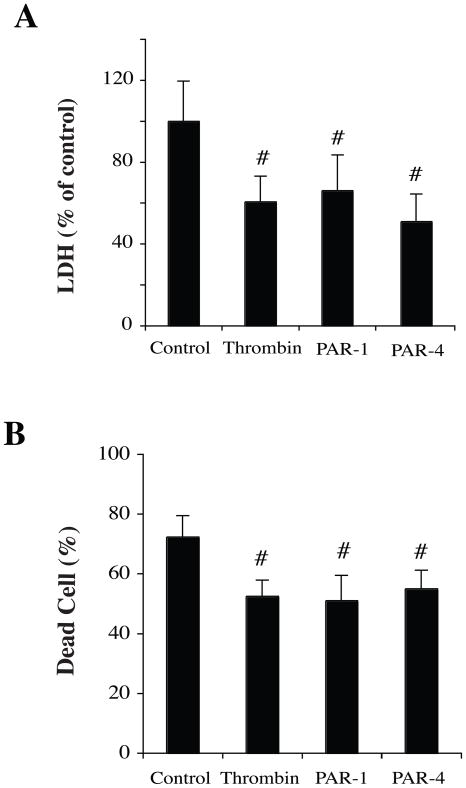
To examine whether or not thrombin-induced neuroprotection is via PARs, PAR-1, −4 agonist peptides were used. Neurons were pretreated with PAR agonists for 24 hours and then exposed to OGD for 2 hours. LDH release and live/dead cell counting were examined at 24 hours. Results showed significantly lower LDH levels in the groups preconditioned with thrombin, PAR-1 agonist or PAR-4 agonist compared to vehicle treatment (p<0.01, Fig 3A). Preconditioning with thrombin and agonists of PAR-1 and PAR-4 also markedly reduced neuronal death caused by 2 hours OGD (Fig 3B).
Figure 3.
Neurons pretreated with agonist peptides of PAR-1 and PAR-4 mimicked the effects of thrombin. Neurons were pretreated with vehicle, thrombin (1 U/ml) or agonists of PAR-1 and PAR-4 (50 nM) for 24 hours and then exposed to OGD for 2 hours. Levels of LDH release in the culture medium (A) and percent of dead cells (B) were measured after 22 hours of reoxygenation. Values are expressed as means ± SD. #p< 0.01, compared with control group.
Activation of p44/42 MAPK and p70S6K by thrombin and PAR agonists
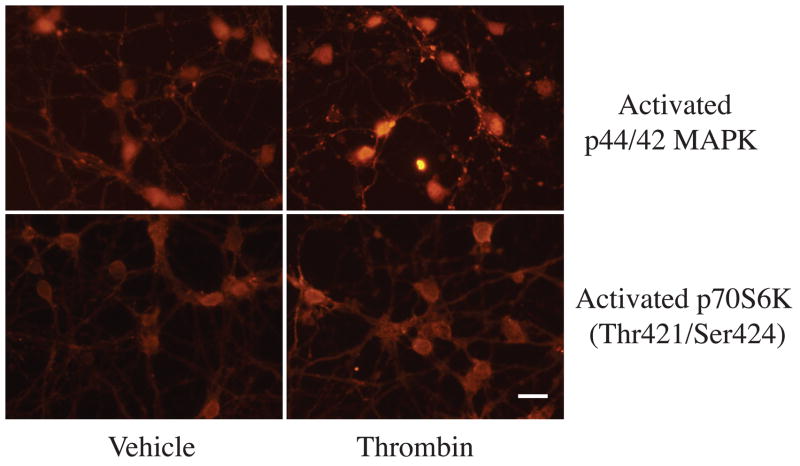
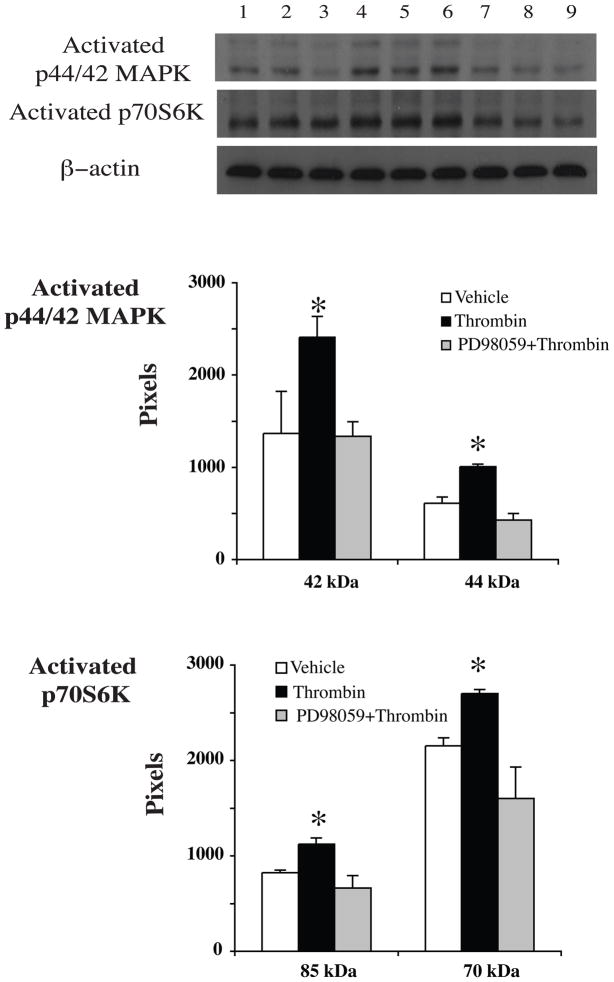
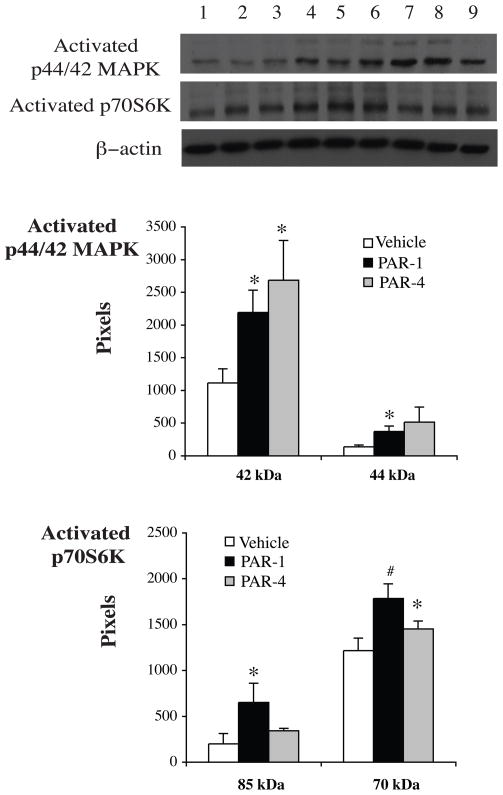
To determine whether or not TPC-induced protection through activating the p44/42 MAPK/p70S6K (Thr421/Ser424) pathway, we measured levels of activated p44/42 MAPK and downstream protein p70S6K (Thr421/Ser424) by Western blots and immunocytochemistry. The latter showed that activated p44/42 MAPK and p70S6K (Thr421/Ser424) were mainly localized in the cytosol of neurons and the location was not changed after thrombin stimulation (Fig 4). Western blot analysis revealed that levels of activated p44/42 MAPK and p70S6K (Thr421/Ser424) were upregulated in neurons after TPC. PD098059, an inhibitor of p44/42 MAPK kinase, blocked TPC-induced upregulation of activated p44/42 MAPK and p70S6K (Fig 5). PAR-1 and PAR-4 agonists mimicked the effects of thrombin and increased activated p44/42 MAPK and p70S6K (Thr421/Ser424) protein levels (Fig 6).
Figure 4.
Immunoreactivity of activated p44/42 MAPK and p70 S6K (Thr421/Ser424) in neurons 24 hours after vehicle or thrombin (1 U/ml) treatment. Scale bar=10 μm.
Figure 5.
Activated p44/42 MAPK and p70S6K (Thr421/Ser424) protein levels in neurons after vehicle (lanes 1–3), thrombin (1 U/ml, lanes 4–6) or thrombin (1U/ml) plus PD098059 (20 μM, lanes 7–9) treatment for 24 hours. Values are mean ± SD, *p< 0.05 vs. the other groups.
Figure 6.
Western blot showing activated p44/42 MAPK and p70S6K (Thr421/Ser424) protein levels in neurons 24 hours after vehicle (lanes 1–3), PAR-1 agonist (50nM, lanes 4–6) or PAR-4 (50nM, lanes 7–9) agonist treatment. Values are mean ± SD, *p< 0.05, #p < 0.01 vs. vehicle-treated group.
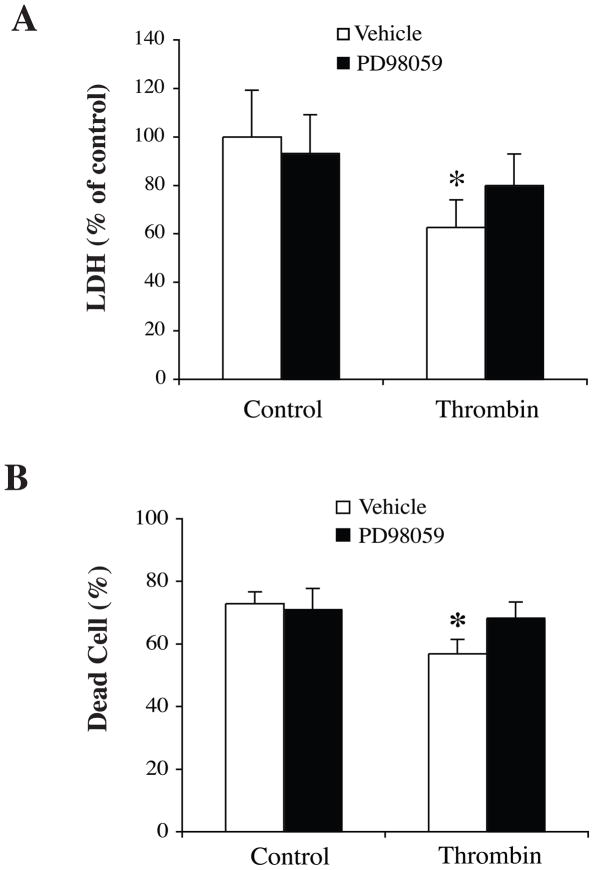
Effects of PD098059 on TPC-induced neuroprotection against OGD
We hypothesized that the protective effect of TPC was mediated by activating the p44/42 MAPK pathway. PD98059 was added 60 minutes before TPC. Neurons were exposed to OGD for 2 hours at 24 hours after TPC. LDH levels and live/dead cells were examined after a further 24 hours. We found that PD98059 reduced TPC-induced neuroprotection against OGD (Fig. 7).
Figure 7.
Neurons were pretreated with PD098059 (20 μM), an inhibitor of p44/42 MAPK kinase, or vehicle 60 minutes before thrombin (1 U/ml) treatment. Neurons were then exposed to OGD for 2 hours and LDH levels (A) and number of dead cells (B) were measured after 22 hours of reoxygenation. Values are mean±SD, *p<0.05, vs. the other groups.
3. DISCUSSION
This study reports several important findings. 1) Thrombin preconditioning at different doses (0.5~2.0 U/ml) can protect against neuronal death induced by OGD (in vitro ‘ischemia’). This is the first report of a direct neuronal effect of TPC. 2) The activating peptides of thrombin receptors can mimic the protective effects of thrombin. 3) Preconditioning with thrombin upregulated activated p44/42 MAPK and p70S6K (Thr421/Ser424) levels in neurons and these effects of thrombin were blocked by an inhibitor of p44/42 MAPK kinase, PD098059. 4) PD098059 also blocked TPC-induced neuronal protection. In total, these results suggest that PARs and p44/42 MAPK/p70S6K pathway have a role in thrombin-induced neuronal protection.
Preconditioning-induced brain tolerance refers to an endogenous mechanism for brain protection against different kinds of brain injury (Dirnagl et al., 2003; Gidday, 2006). Preconditioning has been used for heart and brain surgery and is now a clinical reality (Keep et al., 2010; Steiger and Hanggi, 2007; Yellon and Dana, 2000). In the brain, ischemic preconditioning has been used during clipping of cerebral aneurysm and it attenuates tissue hypoxia during clipping (Chan et al., 2005). It is well known that neuroprotection is a major issue in neurosurgery since neurosurgical procedures may result in edema, hemorrhage and/or ischemia. We have demonstrated that TPC can reduce hemorrhagic and ischemic brain edema. Therefore, TPC, or manipulation of the downstream mechanisms involved in TPC, may also reduce surgery-related brain edema or brain damage.
The current study indicates that thrombin-induced ischemic tolerance in neurons is associated with p44/42 MAPK activation. MAPKs are well-known cytoplasmic signal transducers with an important role in both thrombin- and ischemia-induced brain tolerance (Irving and Bamford, 2002; Xi et al., 2001). In vivo, p44/42 MAPKs are activated in the brain after intracerebral injection of thrombin and PD 098059 abolishes thrombin-induced activation of p44/42 MAPKs and also blocks thrombin-induced brain tolerance (Xi et al., 2001). In addition, thrombin increases brain hypoxia inducible factor-1alpha levels through a p44/42 MAPKs pathway (Hua et al., 2003). In vitro, thrombin treatment also activates p44/42 MAPKs in astrocytes (Wang et al., 2002) and PD98059 blocks the cytoprotective effect of thrombin pretreatment in mixed glial/neuronal cultures (Jiang et al., 2002). In combination with the current results, these findings indicate that TPC has effects on multiple cell types (at least neurons and astrocytes), utilizing a common intracellular pathway, p44/42 MAPK system, to induce protection.
New protein synthesis is crucial for preconditioning-induced neuroprotection (Dirnagl et al., 2009). The major target of activated p70 S6K is the 40S ribosomal protein S6, a major regulator of protein synthesis (Berven and Crouch, 2000). p70 S6K is activated by phosphorylation on multiple sites. Thus, as well as the PI3K-Akt pathway that phosphorylates thr389, the p44/42 MAPK pathway phosphorylates p70 S6K on thr421/ser424 (Hausenloy et al., 2004). In heart, there is evidence that activation of p70 S6K is essential for preconditioning (Hausenloy et al., 2004; Hausenloy et al., 2005). In this study we found that thrombin activates p70 S6K suggesting a role in thrombin-induced neuronal protection.
In summary, our results show that thrombin preconditioning can have direct effects on neurons to induce protection against ischemia. They also indicate that activation of PARs and the p44/42 MAPK/p70 S6K pathway plays an important role in this thrombin-induced neuronal protection.
4. EXPERIMENTAL PROCEDURES
Primary neuronal culture
Primary neuronal cultures were prepared from embryonic day-17 Sprague-Dawley rats (Charls River Laboratories, MI, U.S.A.) as described previously (He et al., 2010). In brief, the cerebral cortex was dissected and placed in the culture medium (DMEM medium plus 10% FBS, 1% glutamine and 2% Antibiotic-Antimycotic). Tissues were triturated in modified Hanks’ Balanced Solution (25mM HEPES+1% Antibiotic-Antimycotic, Invitrogen, Carlsbad, CA). After centrifuging at 800g ×5 minutes, the supernatant was removed, and the neurons were digested for 20 min in 0.5% trypsin at 37°C. The neurons were then re-suspended in Buffer T (23.5ml of modified HBSS+200 units Dnase+0.5ml 3.8% MgSO4) for 5min at room temperature and were centrifuged. The neurons were plated at a density of 3×100,000/cm2 on the culture slides, 6-well, or 24-well plates coated previously with poly-L-lysine (100 μg/ml). The neurons were incubated at 37°C in an atmosphere of 5% CO2/95% air with the medium replaced every 3 days. The cultured neurons were used for oxygen-glucose deprivation (OGD) studies after 7–10 days.
Experimental groups
There were 5 parts to this study. In the first part, experiments were performed to find the most effective dose of thrombin for preconditioning against OGD. Neurons were pretreated with either vehicle or different doses (0.25, 0.5, 1.0 and 2.0 U/ml, n=6) of human thrombin (Sigma, St. Louis, MO, U.S.A.) and then exposed to OGD for 2 hours. Neuronal injury was quantified by LDH release assay and live/dead cell staining methods 22 hours after OGD. In the second part, neurons were treated with vehicle or thrombin (1 U/ml, n=6) for 24 hours and collected for RNA isolation and RT-PCR assay to examine the mRNA expression of protease activated receptor-1 (PAR-1) and -4 (PAR-4). In the third part, neurons were pretreated with agonists (50 nM) of PAR-1 (SC989E; Ala-pFluoro-Phe-Arg-Cha-HomoArg-Tyr-NH2, NeoMPS, San Diego, CA, U.S.A.) or PAR-4 (H-4404; H-Gly-Tyr-Pro-Gly-Lys-Phe-OH, Bachem, Bachem California Inc., Torrance, CA, USA) for 24 hours and exposed to OGD for 2 hours. LDH assay and live/dead cell staining (n=6) were performed 22 hours after OGD. In the fourth part, expressions of activated p44/42 MAPK and p70S6K (Thr421/Ser424) were examined in neurons after thrombin and PAR agonist preconditioning. Neurons were treated with thrombin (1 U/ml, n=6) with or without the pretreatment of PD098059 (20 μM, Calbiochem-Novabiochem, La Jolla, CA, U.S.A.), an inhibitor of p44/42 MAPK kinase. Some neurons were treated with PAR-1 or PAR-4 agonists (50 nM, n=6). All neurons were collected at 24 hours for Western blot analysis or immunocytochemistry to measure phosphorylated p44/42 MAPK and p70S6K levels. In the last part, whether or not thrombin preconditioning against OGD is mediated by p44/42 MAPK pathway was tested. Neurons were pretreated with vehicle or PD098059 (20 μM) for 60 minutes before thrombin (1 U/ml, n=6) treatment. After 24 hours thrombin preconditioning, the neurons were exposed to OGD for 2 hours. Neuronal death was quantified using LDH assay and live/dead cell staining at 24 hours after OGD.
Oxygen-glucose deprivation
Neurons were transferred into a temperature-controlled (37°±1°C) anaerobic chamber (Coy Laboratory, MI, U.S.A.) containing a gas mixture composed of 5% CO2, 10% H2, 85% N2. This chamber maintains a strict 0–5ppm oxygen atmosphere through the hydrogen gas reacting with palladium catalyst to remove oxygen. The culture medium was replaced with deoxygenated glucose-free DMEM solution, and neurons were maintained in the anaerobic chamber for 2 hours. After that, medium was replaced by serum-free medium and returned to a normoxic incubator under 5% CO2/95% air.
Measurement of lactate dehydrogenase
Neuronal death was assessed by measurement of released LDH using a CytoTox-96 kit (Promega, Madison, WI, U.S.A.). Results were analyzed with microplate data analysis software, KC junior (Bio-Tek Instruments, Inc.).
Live/dead cell determination
Cell viability was also measured by using a LIVE/DEAD Viability/Cytotoxicity assay kit (Molecular Probes, Eugene, OR, U.S.A.). Calcein-AM was used to dye living cells with bright-green fluorescence at excitation/emission wavelengths of 495/515 nm. The assay for dead cells used ethidium homodimer-1 (EthD-1), a molecule that binds to DNA and then fluoresces red at excitation/emission wavelength of 495/635 nm. Briefly, the samples were washed 3 times in phosphate-buffered saline (PBS), and then were incubated with 2 μM calcein-AM and 4 μM EthD-1 in PBS for 30 minutes at room temperature. Quantification of dead cells (percent of red cells/red+ green cells) was performed by NIH ImageJ software.
Reverse transcription and polymerase chain reaction
Total RNA was extracted from neurons in 6-well plates using Trizol reagent (Gibco BRL, Grand Island, NY, U.S.A.). One microgram RNA was digested with amplification-grade deoxyribonuclease I (Gibco BRL). Complementary DNA was synthesized by reverse transcription, using the digested 1μg RNA (11μl) with 14μl reaction buffer (Perkin Elmer, Foster City, CA, U.S.A.) containing dNTP (dATP, dCTP, dGTP and dTTP), 25mmol/L magnesium chloride, 10× polymerase chain reaction buffer II, Random Hexamer Primer, ribonuclease inhibitor, and murine leukemia virus reverse transcriptase. The reaction was performed at 42°C for 30 minutes and terminated at 99°C after 5 minutes. Diethylpyrocarbonate water (75μl) was added to dilute the complimentary DNA to 100 μl. Polymerase chain reaction was performed with 12 μl reverse transcriptase reaction mixture (Perkin Elmer) containing 25 mmol/L magnesium chloride, 10×polymerase chain reaction buffer II, dNTP, and AmpliTaq DNA polymerase in a final volume of 50μl. Intronflanking primer pairs were designed from available rat receptor-specific sequences in GenBank. The following primers were used ([NN]indicates position of first nucleotide in coding sequence): PAR-1, [900]5′-ACTATTTCTCCGCCTTCTCCG-CCAT-3′ sense, [1172] 5′-TCACGCAGACGCAGAGGAGGTAAGC-3′, antisense (M81642), giving a 273 base pair (bp) product; PAR-4, [54] 5′-GGAATGCCAGACGCCCAGCATC-3′ sense, [612] 5′-GGTGAGGCGTTGACCACGCA-3′, antisense (AF310216), giving a 559-bp product. Rat GAPDH primers (sense, 5′-CATGCCGCCTGGAGAAACCTGCCA-3′, antisense, 5′-TGGGCTGGGTGGTCCAGGGGTTTC-3′) were used as a control. Amplification was performed in a DNA thermal cycler (MJ Research, Watertown, MA, U.S.A.). Polymerase chain reaction production was analyzed by electrophoresis on 1% agarose gel. Gels were prestained with ethidium bromide and observed by ultraviolet transillumination. Photographs were taken by using ChemiDoc XRS Imager and modified with Quantitiy One 4.6 (BIO-RAD, Hercules, CA, U.S.A.). The relative densities of the bands were analyzed with the NIH Image method.
Western blot analysis
Western blots were performed as described previously (Xi et al., 1999). Neurons were collected and immersed in Western sample buffer (62.5 mM Tris-HCl, pH 6.8, 2.3% sodium dodecyl sulfate, 10% glycerol, and 5% β-mercaptoethanol) and were then sonicated for 10 seconds. Protein concentrations were measured using Bio-Rad Protein AssayReagents (Bio-Rad, Hercules, CA). Samples were loaded after 5 minutes boiling at 95 °C. The protein was transferred to a hybond-C pure nitrocellulose membrane (Amersham). The membranes were blocked in 5% Carnation non-fat dry milk for 1 hour at 37 °C. Blots were washed and membranes were incubated with the following primary antibodies: polyclonal rabbit phospho-p44/42 MAPK antibody (1:2000; Cell Signaling, MA, U.S.A.), polyclonal rabbit phospho-p70S6K (Thr421/Ser424) antibody (1:1000; Cell Signaling, MA, U.S.A.). The appropriate second antibodies were applied for 1 hour at room temperature. Proteins were visualized with the ECL chemiluminescence system (Amersham) and exposed to Kodak X-OMAT film. The relative densities of the protein bands are analyzed with a public domain NIH Image program (Version 1.63, Bethesda, MD, U.S.A.).
Immunocytochemistry
Neurons were grown on culture slides and fixed with formalin. Slides were rinsed and the cells permeabilized with 100% methanol at −20°C for 5 minutes. Polyclonal rabbit phospho-p44/42 MAPK antibody (1:500) and polyclonal rabbit phospho-p70S6K (Thr421/Ser424) antibody (1:250) were applied and the slides incubated overnight at 4°C in a moist chamber after 5% normal goat serum blocking for 1 hour at room temperature. After washing three times, the slides were incubated with rhodamine goat anti rabbit IgG (1:500, Chemico, Temecula, CA, U.S.A.) for 1 hour at room temperature and neurons were then photographed using a fluorescence microscope.
Statistical analysis
All data in this study are presented as mean ± standard deviation. Data were analyzed with Student’s t test or ANOVA test. Significance levels were set at p<0.05.
Acknowledgments
This study was supported by grants NS-039866 and NS-057539 from the National Institutes of Health (NIH) and 0840016N from American Heart Association (AHA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and AHA.
Abbreviations
- TPC
thrombin preconditioning
- OGD
oxygen glucose deprivation
- PARs
protease-activated receptors
- MAPK
mitogen activated protein kinases
- p70 S6K
ribosomal protein S6 kinases
References
- Berven LA, Crouch MF. Cellular function of p70S6K: a role in regulating cell motility. Immunol Cell Biol. 2000;78:447–51. doi: 10.1046/j.1440-1711.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Keep RF, Hua Y, Richardson RJ, Schallert T, Xi G. Thrombin preconditioning provides protection in a 6-hydroxydopamine Parkinson’s disease model. Neurosci Lett. 2005;373:189–94. doi: 10.1016/j.neulet.2004.10.089. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Keep RF, Schallert T, Hua Y, Richardson RJ, Xi G. Protease-activated receptor-1 mediates protection elicited by thrombin preconditioning in a rat 6-hydroxydopamine model of Parkinson’s disease. Brain Res. 2006;1116:177–86. doi: 10.1016/j.brainres.2006.07.094. [DOI] [PubMed] [Google Scholar]
- Chan MT, Boet R, Ng SC, Poon WS, Gin T. Effect of ischemic preconditioning on brain tissue gases and pH during temporary cerebral artery occlusion. Acta Neurochir Suppl. 2005;95:93–6. doi: 10.1007/3-211-32318-x_20. [DOI] [PubMed] [Google Scholar]
- Choi SH, Lee da Y, Kim SU, Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: role of microglial NADPH oxidase. J Neurosci. 2005;25:4082–90. doi: 10.1523/JNEUROSCI.4306-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin SR. How the protease thrombin talks to cells. Proc Natl Acad Sci USA. 1999;96:11023–7. doi: 10.1073/pnas.96.20.11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–64. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends in Neurosciences. 2003;26:248–54. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–48. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Gingrich MB, Traynelis SF. Serine proteases and brain damage - is there a link? Trends in Neurosciences. 2000;23:399–407. doi: 10.1016/s0166-2236(00)01617-9. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Mocanu MM, Yellon DM. Cross-talk between the survival kinases during early reperfusion: its contribution to ischemic preconditioning. Cardiovasc Res. 2004;63:305–12. doi: 10.1016/j.cardiores.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–6. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- He Y, Hua Y, Lee J, Liu W, Keep RF, Wang MM, Xi G. Brain Alpha- and Beta-Globin Expression after Intracerebral Hemorrhage. Translational Stroke Research. 2010;1:48–56. doi: 10.1007/s12975-009-0004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich-Noack P, Striggow F, Reiser G, Reymann KG. Preconditioning with thrombin can be protective or worsen damage after endothelin-1-induced focal ischemia in rats. J Neurosci Res. 2006;83:469–75. doi: 10.1002/jnr.20746. [DOI] [PubMed] [Google Scholar]
- Hirt L, Ternon B, Price M, Mastour N, Brunet JF, Badaut J. Protective role of early aquaporin 4 induction against postischemic edema formation. J Cereb Blood Flow Metab. 2009;29:423–33. doi: 10.1038/jcbfm.2008.133. [DOI] [PubMed] [Google Scholar]
- Hua Y, Xi G, Keep RF, Wu J, Jiang Y, Hoff JT. Plasminogen activator inhibitor-1 induction after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. 2002;22:55–61. doi: 10.1097/00004647-200201000-00007. [DOI] [PubMed] [Google Scholar]
- Hua Y, Keep RF, Hoff JT, Xi G. Thrombin preconditioning attenuates brain edema induced by erythrocytes and iron. J Cereb Blood Flow Metab. 2003;23:1448–54. doi: 10.1097/01.WCB.0000090621.86921.D5. [DOI] [PubMed] [Google Scholar]
- Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. Journal of Cerebral Blood Flow & Metabolism. 2002;22:631–47. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Wu J, Hua Y, Keep RF, Xiang J, Hoff JT, Xi G. Thrombin-receptor activation and thrombin-induced brain tolerance. J Cereb Blood Flow Metab. 2002;22:404–410. doi: 10.1097/00004647-200204000-00004. [DOI] [PubMed] [Google Scholar]
- Junge CE, Lee CJ, Hubbard KB, Zhang Z, Olson JJ, Hepler JR, Brat DJ, Traynelis SF. Protease-activated receptor-1 in human brain: localization and functional expression in astrocytes. Exp Neurol. 2004;188:94–103. doi: 10.1016/j.expneurol.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Keep RF, Wang MM, Xiang J, Hua Y, Xi G. Is there a place for cerebral preconditioning in the clinic? Translational Stroke Research. 2010;1:4–18. doi: 10.1007/s12975-009-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masada T, Xi G, Hua Y, Keep RF. The effects of thrombin preconditioning on focal cerebral ischemia in rats. Brain Res. 2000;867:173–179. doi: 10.1016/s0006-8993(00)02302-7. [DOI] [PubMed] [Google Scholar]
- Steiger HJ, Hanggi D. Ischaemic preconditioning of the brain, mechanisms and applications. Acta Neurochirurgica. 2007;149:1–10. doi: 10.1007/s00701-006-1057-1. [DOI] [PubMed] [Google Scholar]
- Striggow F, Riek M, Breder J, Henrich-Noack P, Reymann KG, Reiser G. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proc Natl Acad Sci USA. 2000;97:2264–9. doi: 10.1073/pnas.040552897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo Z, Wu M, Citron BA, Gao C, Festoff BW. Persistent protease-activated receptor 4 signaling mediates thrombin-induced microglial activation. Journal of Biological Chemistry. 2003;278:31177–83. doi: 10.1074/jbc.M302137200. [DOI] [PubMed] [Google Scholar]
- Ubl JJ, Sergeeva M, Reiser G. Desensitisation of protease-activated receptor-1 (PAR-1) in rat astrocytes: evidence for a novel mechanism for terminating Ca2+ signalling evoked by the tethered ligand. Journal of Physiology. 2000;2:319–30. doi: 10.1111/j.1469-7793.2000.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ubl JJ, Stricker R, Reiser G. Thrombin (PAR-1)-induced proliferation in astrocytes via MAPK involves multiple signaling pathways. Am J Physiol Cell Physiol. 2002;283:C1351–1364. doi: 10.1152/ajpcell.00001.2002. [DOI] [PubMed] [Google Scholar]
- Wang Y, Luo W, Stricker R, Reiser G. Protease-activated receptor-1 protects rat astrocytes from apoptotic cell death via JNK-mediated release of the chemokine GRO/CINC-1. J Neurochem. 2006 doi: 10.1111/j.1471-4159.2006.03950.x. in press. [DOI] [PubMed] [Google Scholar]
- Weinstein JR, Gold SJ, Cunningham DD, Gall CM. Cellular localization of thrombin receptor mRNA in rat brain: expression by mesencephalic dopaminergic neurons and codistribution with prothrombin mRNA. Journal of Neuroscience. 1995;15:2906–19. doi: 10.1523/JNEUROSCI.15-04-02906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hua Y, Xiang JM, Hoff JT. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke. 1999;30:1247–1255. doi: 10.1161/01.str.30.6.1247. [DOI] [PubMed] [Google Scholar]
- Xi G, Hua Y, Keep RF, Duong HK, Hoff JT. Activation of p44/42 mitogen activated protein kinases in thrombin-induced brain tolerance. Brain Research. 2001;895:153–9. doi: 10.1016/s0006-8993(01)02064-9. [DOI] [PubMed] [Google Scholar]
- Yang S, Hua Y, Nakamura T, Keep RF, Xi G. Up-regulation of brain ceruloplasmin in thrombin preconditioning. Acta Neurochir Suppl. 2006;96:203–6. doi: 10.1007/3-211-30714-1_44. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Dana A. The preconditioning phenomenon: A tool for the scientist or a clinical reality? Circ Res. 2000;87:543–50. doi: 10.1161/01.res.87.7.543. [DOI] [PubMed] [Google Scholar]