Abstract
Purpose
To determine if patients with parkinsonism and fragile X mental retardation 1 (FMR1) gene expansions have a striatal dopamine deficit similar to Parkinson disease (PD) patients.
Scope
The authors studied three patients with parkinsonism carrying small expansions in the FMR1 gene (41–60 CGG) with [123I] -CIT SPECT imaging. The patients responded to dopaminergic medications, but had preserved dopamine transporter density.
Conclusions
These results suggest that parkinsonism associated with smaller FMR1 expansions may be related to mechanisms other than presynaptic dopaminergic changes and may represent a potential explanation for at least some parkinsonian cases with scans without evidence of dopaminergic deficits (SWEDD).
Keywords: FMR1, SPECT, FXTAS, parkinsonism, dopamine
1. Introduction
The FMR1 gene contains a CGG repeat in the untranslated portion of the gene. Individuals with FMR1 premutation range expansions (55–200 CGG repeats) are at risk to develop the fragile X-associated tremor/ataxia syndrome (FXTAS), which is characterized by kinetic tremor, ataxia, parkinsonism, autonomic dysfunction, peripheral neuropathy, and cognitive decline [1]. In addition, some premutation carriers have a PD phenotype [2]. Gray zone expansions (41–54 CGG repeats) in the FMR1 gene have recently been associated with parkinsonism in females [3]. PD patients with scans without evidence of dopaminergic deficits (SWEDD) were identified in several clinical trials enrolling early PD subjects, including the Elldopa, REAL-PET, and PRECEPT studies [4–5]. In some of these parkinsonism cases, there is no explanation to account for the lack of dopamine transporter (DAT) deficit on imaging [6]. This paper describes phenotypic features of three parkinsonism patients with gray or premutation size FMR1 expansions (without classic FXTAS) who have normal dopamine transporter SPECT imaging results compared to a pre-existing healthy subject data base [7].
2. Report of Cases
2.1. Case 1
A 66-year-old woman had a 10-year history of head tremor and leg tremor, when standing in one position for too long (Table 1). The tremor improved on carbidopa/levodopa. On exam, the patient had a constant no-no tremor in the head and neck, bilateral kinetic and intention tremor in the upper extremities, and a faster frequency tremor in the lower extremities after a latency period. Eighteen months later; she had rest tremor and cogwheel rigidity, bradykinesia bilaterally, stooped posture, en bloc turns, and decreased arm swing on the right. She was a carrier of a normal and a gray zone FMR1 allele of 23 and 41 CGG repeats.
Table 1.
Clinical and neuroimaging findings in the study population
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age, y | 66 | 68 | 46 |
| Age at onset, y | 56 | 64 | 45 |
| Gender | F | F | M |
| Clinical features | Head tremor, right arm rest tremor, kinetic arm tremor, orthostatic tremor, rigidity, bradykinesia | Head tremor, left arm rest tremor, bilateral kinetic tremor, bradykinesia, retropulsion, tandem difficulties | Head tremor, rest tremor, kinetic tremor, blepharospasm, bradykinesia and rigidity on the right, facial masking, slowed gait initiation |
| Family history | Cousin with hand tremor | 2 siblings with PD | 5 relatives with tremor and/or balance problems, niece and nephew with learning difficulties |
| Response to dopaminergic meds | Positive | Positive with motor fluctuations | Positive |
| UPDRS* (motor) score | 11 | 13 | 11 |
| UPSIT** | 22 | 30 | 36 |
| FMR1 repeat | 41, 23 | 51, 21 | 60 |
| MRI | Nonspecific white matter hyperintensities | Nonspecific white matter hyperintensities | Normal |
| [123I]β-CIT SPECT | Normal | Normal | Normal |
UPDRS, Unified Parkinson’s Disease Rating Scale;
UPSIT, University of Pennsylvania Smell Identification Test
2.2. Case 2
A 68-year-old woman had a 2-year history of tremor, falls, and short term memory problems. She had two siblings with PD. She was started on carbidopa/levodopa, with marked improvement in her symptoms and energy level. Five years later, she developed difficulty with turns and wearing off of her medications. On exam, she had masked facies, head tremor, rest tremor in the left hand, and kinetic tremor symmetrically in both hands. She had increased tone on the right, bradykinesia with finger tapping and hand grasping, decreased arm swing when ambulating, en bloc turns, complete retropulsion, and difficulty with tandem. Neuropsychological testing showed severely impaired memory, moderately impaired cognitive processing speed, and mildly impaired executive function. She was a carrier of a normal and a gray zone FMR1 allele of 21 and 51 CGG repeats.
2.3. Case 3
A 46-year old right handed man had one year of rest tremor in the right hand, with pain and stiffness. On exam, he had rigidity and rest tremor on the right, bradykinesia with finger tapping and decreased arm swing on the right. One year later, the patient reported worsening of his speech and gait freezing. Exam showed masked facies, increased tone in the head and neck, slowed gait initiation, decreased stride length, and he took three steps to turn. Pramipexole was started, with tremor resolution and improved balance. The following year, he developed head tremor, intention tremor, and blepharospasm. He was a carrier of a premutation FMR1 allele of 60 CGG repeats.
[123I] -CIT SPECT imaging
Subjects underwent [123I] -CIT SPECT imaging, as previously described [8]. High specific activity [123I] β-CIT was prepared from the corresponding trimethylstannyl precursor, and subjects were injected with up to a 6 mCi dosage of [123I] β-CIT [9]. Manual regions of interest (ROI) analysis of the [123I] -CIT/SPECT scans was performed by a nuclear medicine technologist, who was masked to the clinical data, using methods described in previous studies. The primary quantitative imaging outcome measure, the specific non-displaceable putamen uptake (V3”), was determined through a standardized analysis method [9]. Based on a previously acquired database of 100 healthy subjects (aged 19–88), scans were categorized as DAT deficient (< 70% age-expected lowest putamen [123I] β-CIT), or not DAT deficient (> 70% age-expected lowest putamen [123I] β-CIT)[7]. Informed consent was obtained from each subject and the study was approved by Western Institutional Review Board. None of our cases met criteria for FXTAS. The uptake of [123I] β-CIT was normal in all three patients (Figure 1).
Figure 1.
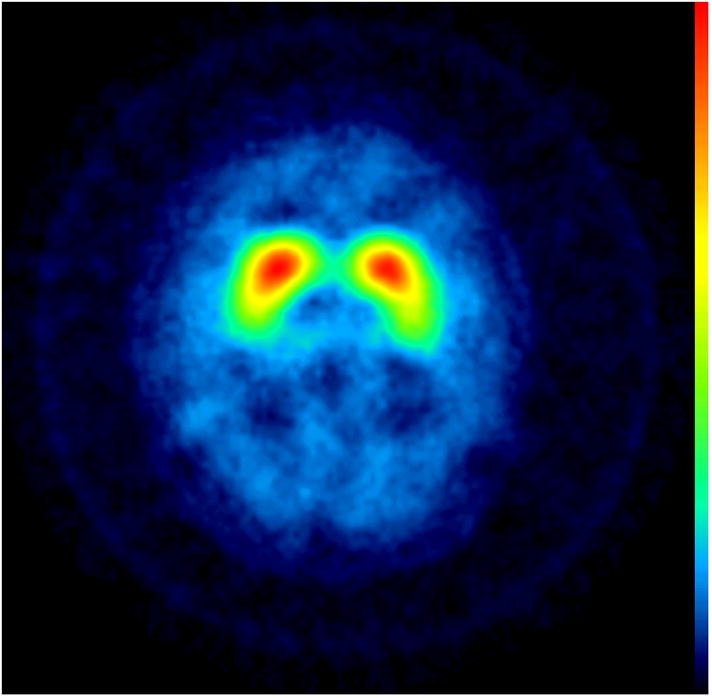
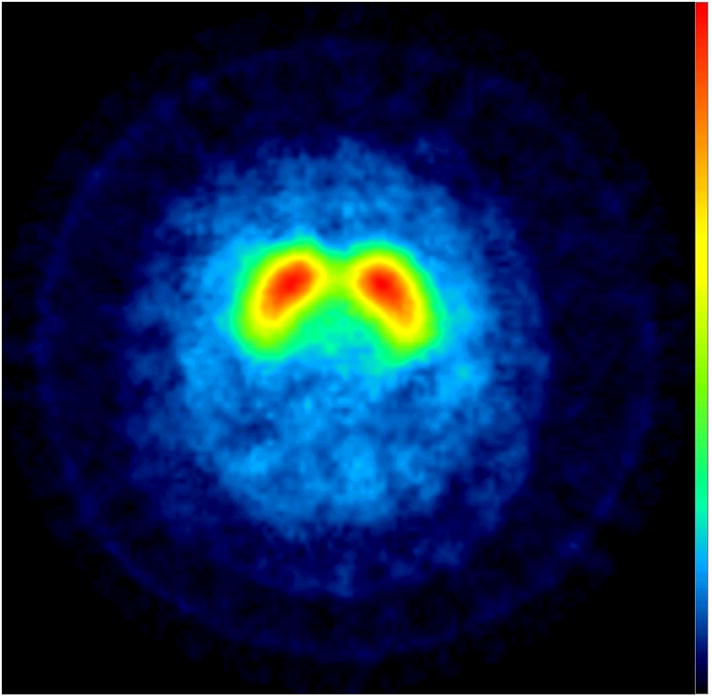
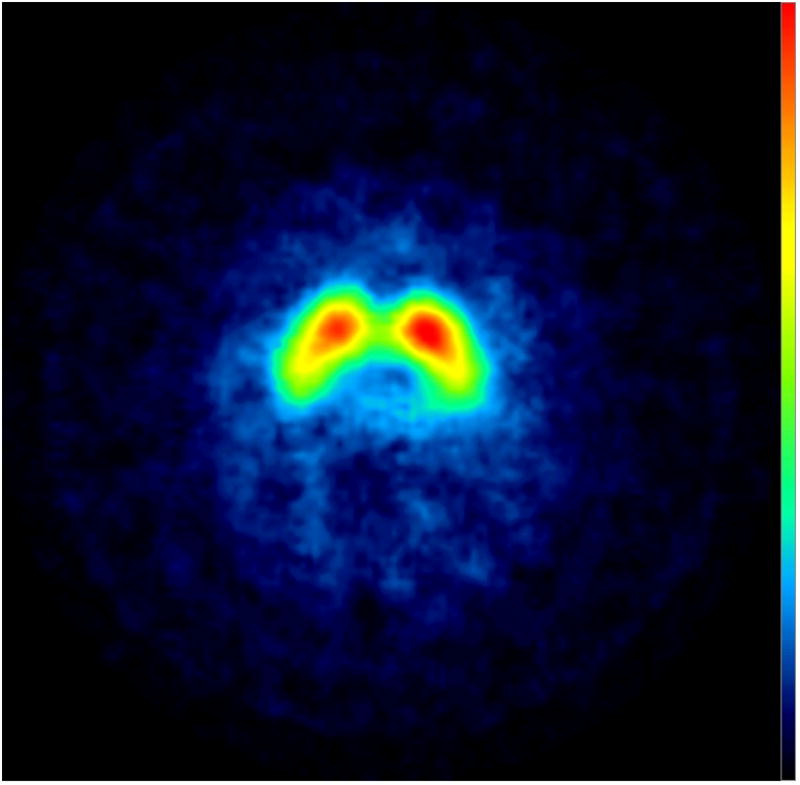
[123I] β-CIT SPECT Images: transverse slices at the level of the striatum show normal uptake in the three subjects.
1. Discussion
In our patients with small FMR1 repeat expansions (41–60 CGG repeats), parkinsonism was mild to moderate. The patients had been diagnosed with PD and had a positive response to dopaminergic medications, but some of the patients had or developed features atypical for PD, to include kinetic tremor or tandem difficulties.
These results provide evidence that some patients with parkinsonism who are carriers of FMR1 gray or premutation repeat expansions have a normal [123I] -CIT SPECT scan. It is uncertain if patients lack a presynaptic dopamine receptor deficit like that commonly seen in PD or whether the cut-off value used to define normal [123I] -CIT SPECT imaging caused us to miss smaller changes on the scans. Nigrostriatal dopaminergic function has been investigated in FXTAS [8]. [123I]FP-CIT SPECT imaging was done in four FMR1 premutation carriers with clinical parkinsonism. Repeat sizes in these patients ranged from 73–105 CGG repeats and clinical features included either action tremor or cerebellar gait ataxia, in addition to non-dopamine responsive parkinsonism. Imaging showed no difference in [123I]FP-CIT uptake in the FXTAS patients compared to healthy subjects. Our patients were different than those in this study due to lower repeat sizes and better response to dopaminergic medications.
[123I] -CIT SPECT imaging has been shown to be a useful tool in evaluating patients presenting with parkinsonism. In fact, it can improve diagnostic accuracy in early parkinsonism syndromes with patients in whom a diagnosis of parkinsonism is suspected but showing no dopamine transporter deficit on imaging [7]. While it appears that SWEDD subjects do not have a pre-synaptic dopaminergic deficit, the etiology of parkinsonism symptoms in these subjects is uncertain. Possible explanations include alternative neurological syndromes, such as dystonic tremor, drug induced or vascular parkinsonism, or simply non-specific mild neurologic complaints. The subjects in this study raise the possibility as well of a genetic variant different from idiopathic PD, without or with milder pre-synaptic dopaminergic loss. Of note, we identified a fourth patient with a FMR1 gray zone expansion who was scanned as part of a different research study and did have a dopaminergic transporter deficit that worsened over time. This may suggest that not all patient with FMR1 expansions will have normal [123I] -CIT SPECT imaging or that some subjects with FMR1 expansions may also have Parkinson disease.
It is unclear why patients who have responded to dopminergic medications would have preserved striatal dopamine transporter densities. It may be related to the cut-off threshold missing milder striatal involvement. It may be that the patients had improvement on dopaminergic therapy due to a placebo effect.
In individuals in the FMR1 premutation range, there is elevation of FMR1 mRNA levels and a slight depletion in fragile X mental retardation protein levels [11]. Elevated mRNA has been described as a molecular phenotype for males with the premutation and may reflect a defect in the translation of the mRNA into FMR1 protein [11]. In gray zone FMR1 repeat expansion carriers, there is also an increase in mRNA levels starting at 39 CGG repeats [12]. Thus, it is possible that the mechanism leading to neurological signs in FXTAS, likely RNA toxicity due to elevated levels of FMR1 mRNA, may also lead to neurological signs in gray zone (41–54 CGG repeats) carriers.
This study is interesting in two ways. First, it suggests that individuals with FMR1 repeat expansions and parkinsonism responsive to dopaminergic medications and diagnosed with PD may have normal [123I] -CIT SPECT imaging based on established cut-off values in PD, possibly accounting for some cases of SWEDD. Second, one of these patients developed features atypical for PD specifically kinetic tremor suggesting that this genetic abnormality may represent a subtype of parkinsonism with characteristic clinical features and imaging findings. Repeat expansions in this gene are important to recognize not only because of the association to movement disorders but also due to the possibility of expansion of the trinucleotide in later generations leading to inherited intellectual disability or fragile X syndrome (FXS). Genetic counseling for patients should be considered, especially in those patients testing positive for an expansion. The next step will be to investigate the findings of this study by imaging a larger sample size of FMR1 expansion carriers and by screening subjects identified as SWEDD for the genetic abnormality.
Acknowledgments
This work was funded by a grant from Anthony H. Kruse Foundation on behalf of Julie Berardi and NS052487 (D.A.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291:460–9. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- 2.Hall DA, Howard K, Hagerman RJ, Leehey MA. Parkinsonism in FMR1 premutation carriers may be indistinguishable from Parkinson disease. Parkinsonism Relat Disord. 2009;15:156–9. doi: 10.1016/j.parkreldis.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall DA, Howard K, Byers T, Zerbe G, Zhang W, Tassone F, et al. Prevalence of FMR1 Gray Zone Alleles Is Increased in Female Patients with Parkinsonism. Neurology. 2009;72(suppl 3):A388. [Google Scholar]
- 4.Fahn S the Parkinson Study Group. Does levodopa slow or hasten the rate of progression of Parkinson’s disease? J Neurol. 2005;252(Suppl 4):IV37–IV42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- 5.Parkinson Study Group PRECEPT Investigators. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology. 2007;69(15):1480–90. doi: 10.1212/01.wnl.0000277648.63931.c0. [DOI] [PubMed] [Google Scholar]
- 6.Marek K, Jennings D, Seibyl J. Long-term follow-up of patients with scans without evidence of dopaminergic deficit (SWEDD) in the ELLDOPA study. Neurology. 2005;64 (Suppl 1):A274. [Google Scholar]
- 7.Jennings DL, Seibyl JP, Oakes D, Eberly S, Murphy J, Marek K. (123I) β-CIT and single-photon emission computed tomographic imaging vs clinical evaluation in Parkinsonian syndrome: unmasking an early diagnosis. Arch Neurol. 2004;61:1224–9. doi: 10.1001/archneur.61.8.1224. [DOI] [PubMed] [Google Scholar]
- 8.Laruelle M, Wallace E, Seibyl JP, Baldwin RM, Zea-Ponce Y, Zoghbi SS, et al. Graphical, kinetic, and equilibrium analyses of in vivo [123I] beta-CIT binding to dopamine transporters in healthy human subjects. J Cereb Blood Flow Metab. 1994;14:982–994. doi: 10.1038/jcbfm.1994.131. [DOI] [PubMed] [Google Scholar]
- 9.Innis RB, Seibyl JP, Scanley BE, Laruelle M, Abi-Dargham A, Wallace E, et al. Single photon emission computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson disease. Proc Natl Acad Sci U S A. 1993;90:11965–9. doi: 10.1073/pnas.90.24.11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceravolo R, Antonini A, Volterrani D, Rossi C, Goldwurm S, Di Maria E, et al. Dopamine transporter imaging study in parkinsonism occurring in fragile X premutation carriers. Neurology. 2005;65:1971–3. doi: 10.1212/01.wnl.0000188821.51055.52. [DOI] [PubMed] [Google Scholar]
- 11.Tassone F, Hagerman RJ, Taylor AK, Mills JB, Harris SW, Gane LW, et al. Clinical involvement and protein expression in individuals with the FMR1 premutation. Am J Med Genet. 2000;91:144–152. doi: 10.1002/(sici)1096-8628(20000313)91:2<144::aid-ajmg14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 12.Loesch DZ, Bui QM, Huggins RM, Mitchell RJ, Hagerman RJ, Tassone F. Transcript levels of the intermediate size or grey zone fragile X mental retardation 1 alleles are raised, and correlate with the number of CGG repeats. J Med Genet. 2007;44:200–4. doi: 10.1136/jmg.2006.043950. [DOI] [PMC free article] [PubMed] [Google Scholar]


