Abstract
Background
The purpose of this study was to test the discriminant validity of ISHLT PGD grades in terms of lung injury biomarker profiles and survival.
Methods
The study samples consisted of a multicenter prospective cohort study for the biomarker analysis and a 450 patient cohort study for the mortality analyses. PGD was defined according to ISHLT consensus at 24, 48 and 72 hours after transplantation. We compared the changes in plasma markers of acute lung injury between PGD grades using longitudinal data models. To test predictive validity, we compared differences in the 30-day mortality and long-term survival according to PGD grade.
Results
PGD grade 3 demonstrated greater differences between plasma ICAM-1, protein C, and PAI-1 levels than did PGD grades 0-2 at 24, 48, and 72 hours after lung transplantation (p<0.05 for each). Grade 3 had the highest 30-day (test for trend p<0.001) and overall mortality (log rank p<0.001), with PGD grades 1 and 2 demonstrating intermediate risks of mortality. The ability to discriminate both 30 day and overall mortality improved as the time of grading moved away from the time of transplantation (test for trend p<0.001).
Conclusions
The ISHLT grading system has good discriminant validity, based on plasma markers of lung injury and mortality. Grade 3 PGD was associated with the most severely altered plasma biomarker profile and the worst outcomes, regardless of the time point of grading. PGD grade at 48 and 72 hours discriminated mortality better than PGD grade at 24 hours.
MeSH Headings: Lung Transplantation, complications, Acute lung injury, Primary graft dysfunction, reperfusion injury
Introduction
Primary graft dysfunction (PGD) is an acute lung injury syndrome that occurs in the post-transplant period and is characterized by radiographic pulmonary infiltrates and hypoxemia. Clinically and pathologically, the syndrome is similar to the Acute Respiratory Distress Syndrome (ARDS). PGD represents a spectrum of injury from mild to more severe hypoxemia and lung injury. The more severe forms of PGD have been associated with worse morbidity, and PGD is the leading cause of mortality in the early post-transplant period.1-5
PGD research has been hampered by the lack of a consistent definition or gold standard.6-9 For example, different criteria have led to mortality estimates for PGD ranging from 21% to 63%, as well as inconsistent reporting of clinical risk factors.1,10-12 In 2005, the International Society of Heart and Lung Transplantation (ISHLT) published a consensus statement with the aim of standardizing the definition and grading of PGD.1,6,7,13,14 Similar to that of ARDS, the grading scheme for PGD considers two factors: the appearance of post-transplant chest radiographs and the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) at multiple time points within the first 72 hours after transplantation.6,15
This study proposed to assess the optimal grading and timing of the definition of PGD for use in clinical and translational studies aimed at elucidating mechanisms of and risks for PGD. 6,9 We compared the association between established plasma biomarkers of acute lung injury with PGD variously defined, and we tested the association of PGD definitions with mortality risk.
Methods
Study Design and Populations
Biomarkers
We performed a prospective cohort study of patients undergoing first lung transplantation at six centers in the United States participating in the Lung Transplant Outcomes Group (LTOG - see Appendix for institutions and investigators.) The study sample consisted of 128 adult lung transplant recipients enrolled between June 2003 and November 2004 at these centers, with biomarker results published previously.16,17
Survival
We performed a retrospective cohort study of 450 consecutive adult lung transplant procedures at the University of Pennsylvania between October 1991 and October 2005. The follow-up period for survival analysis extended to October 2009. We excluded two heart-lung recipients and two lung-liver recipients. Thus, the study sample was comprised of 446 patients.
Definition of Primary Graft Dysfunction
We used the ISHLT consensus definition of PGD.18 Specifically, PGD grade was based on the following criteria: (1) the presence or absence of a diffuse pulmonary radiographic infiltrates involving the lung allograft(s) and, in the case of single lung transplant, sparing the native lung; (2) PaO2/FiO2 ratio in mmHg; and (3) no other secondary cause of graft dysfunction readily identified, including: a) cardiogenic pulmonary edema, defined as a pulmonary artery occlusion pressure of greater than 18 mmHg or resolution of infiltrates with effective diuresis, b) pathologic evidence of rejection, c) pneumonia, as evidenced by the presence of fever, leukocytosis, and purulent secretions with positive cultures on bronchoscopy, or d) pulmonary venous outflow obstruction, as demonstrated by transesophageal echocardiogram, surgical re-exploration, or postmortem examination. All patients on extracorporeal membrane oxygenation were classified as grade 3 PGD. All subjects receiving oxygen via nasal cannula with FiO2 estimated as less than 0.3 were graded as 0 or 1 based on chest radiograph findings. These criteria were applied at the 24, 48, and 72 hour time points (T24, T48, and T72) following transplantation according to ISHLT guidelines.18
Analysis of varying definitions of PGD on association of biomarkers of acute lung injury
Our hypothesis was that higher grades of PGD would have different levels of biomarkers of acute lung injury than lower grades. To address this hypothesis, we chose established biomarkers of lung injury from prior studies: protein C, type 1 plasminogen activator inhibitor (PAI-1), and Type 1 Intracellular adhesion molecule (ICAM-1).16,17,19,20 We measured plasma levels of these biomarkers at 6, 24, 48, and 72 hours following reperfusion in 128 lung transplant patients enrolled in the LTOG, and the main results have been presented in prior publications.16,21 Standard ELISA methods were employed and are described elsewhere.16,21
Logistic regression models were fit, and were stratified by biomarker measurement time and PGD grading day for each biomarker separately. Because there were two time dimensions to consider in these analyses (e.g., biomarker assessment time and PGD grading time), the stratified analyses fixed both time dimensions, and summaries were presented for each biomarker measurement time (baseline, time 1, time 2 and time 3) and PGD grading day combination (grading on day 1, day 2 and day 3). These models were fit to estimate the association of each biomarker on PGD and non-PGD subjects using a range of grading criteria to define PGD status (e.g., grade = 3 versus grade = 2 or 1 or 0; grade = 2 or 3 versus grade = 1 or 0; grade = 3 or 2 or 1 versus grade = 0). Adjusted for biomarker, the predicted probability of PGD status and estimated risk differences (estimated probability of PGD case minus estimated probability of PGD control) with corresponding 95% confidence intervals are presented in order to estimate the influence of each biomarker in predicting PGD status. When adjusting for biomarker in the fitted models, we standardized each by their respective estimated standard deviation.
Impact of varying PGD definitions on mortality
We compared both 30-day mortality and overall survival between the different grades of PGD measured at different times. Overall survival was assessed using Kaplan-Meier methods with log rank tests. Cox proportional hazards models were constructed to compare hazard ratios between PGD grades at different time points. To estimate differences in the effect of PGD on mortality according to transplant procedure type, multiplicative interaction terms were used in logistic regression models (for 30-day mortality) and Cox models (for overall survival).
All statistical comparisons were performed using STATA version 10.1 (STATA Corp., College Station, TX), and SAS version 9.1 (SAS Institute, Cary, NC). This research protocol was approved by the Institutional Review Boards at each of the participating centers.
Results
Table 1 summarizes the characteristics of the study sample for the biomarkers analysis. The mean age was 50 (95% CI 47, 53), 45% of recipients were female, 88% of recipients were Caucasian, and the most common predisposing diagnosis was COPD. The clinical characteristics of the LTOG biomarker population according to grade 3 PGD status have been published previously.16,21
Table 1.
Clinical characteristics of the study populations.
| Biomarker population 19,24 n=128 |
Survival population n=446 |
|
|---|---|---|
| Mean Donor Age, years (95% CI) | 30 (28,33) | 32(31, 34) |
| Donor Female gender | 39% | 35% |
| Donor Race/ethnicity: | ||
| Caucasian | 68% | 76% |
| African-American | 17% | 17% |
| Hispanic | 13% | 4% |
| Other | 2% | 3% |
| Mean Recipient Age, years (95% CI) | 50 (47, 53) | 53 (52, 54) |
| Recipient Female gender | 45% | 43% |
| Recipient Race/ethnicity: | ||
| Caucasian | 88% | 89% |
| African-American | 8% | 9% |
| Hispanic | 1% | 1% |
| Other | 3% | 1% |
| Recipient Diagnosis: | ||
| Chronic obstructive pulmonary disease | 49% | 59% |
| Diffuse parenchymal lung disease | 31% | 19% |
| Cystic fibrosis | 10% | 8% |
| Pulmonary arterial hypertension | 3% | 4% |
| Others | 7% | 10% |
| Procedure type: | ||
| Single | 46% | 54% |
| Bilateral | 54% | 46% |
Differences in levels of protein C, ICAM-1, and PAI-1 were greatest for PGD grade 3 (at each time point) vs. PGD grades 0-2, supporting the higher severity of the PGD grade 3 (Figure 1). Even at the earliest time points, the levels of each biomarker were significantly different for PGD grade 3 compared to the other grades, and remained so throughout the assessed time points. Table 2 displays the corresponding risk differences of PGD (with varying definitions) conferred by plasma biomarkers at the four measurement times. Risk differences represent the difference in the risk of PGD (however defined) between patients with any value of biomarker compared to patients with a biomarker value which is 1 standardized unit higher. Across the majority of postoperative assessments, analyses of Grade 3 vs. grades 0/1/2 produced the greatest biomarker discrimination, as evidenced by the greater risk differences. In certain instances, defining PGD as grades 2-3 produced similar risk difference estimates. Although ICAM-1 and Protein C produced the best discrimination when PGD was graded at 48 or 72 hours, PAI-1 showed greater differences at the 24 hour time point.
Figure 1.



Biomarkers of acute lung injury by different PGD grades at T72: a) protein C, b) PAI-1, c) ICAM-1. The different grades appear as numbers in the lines at the different time points. Solid lines indicate grade 0; dotted lines indicate grade1; dashed lines indicate grade 2; and dash-dot lines indicate grade 3 PGD. *p<0.05; **p<0.01 for grade 3 versus the rest.
Table 2. Biomarker discrimination summaries (risk differences and 95% CIs) of patients with and without PGD, as defined by various grade thresholds, by biomarker measurement time.
| Biomarker Measurement Time (day) | PGD as defined by grade → | Grade 3 vs. 2,1,0 | Grade 3,2 vs. 1,0 | Grade 3,2,1 vs. 0 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PGD grading hour → | 24h | 48h | 72h | 24h | 48h | 72h | 24h | 48h | 72h | |
| Biomarker | ||||||||||
| 0 | Protein C | 0.01 (-0.01, 0.03) |
0.09 (0.04, 0.14) |
0.09 (0.04, 0.15) |
0.01 (-0.01, 0.02) |
0.09 (0.03, 0.14) |
0.10 (0.04, 0.16) |
0.03 (-0.00, 0.06) |
0.05 (0.01, 0.09) |
0.02 (-0.01, 0.04) |
| PAI-1 | 0.03 (0.00, 0.06) |
0.02 (-0.00, 0.05) |
0.05 (0.01, 0.09) |
0.03 (-0.00, 0.06) |
0.05 (0.01, 0.09) |
0.05 (0.01, 0.09) |
0.03 (-0.00, 0.06) |
0.01 (-0.01, 0.03) |
0.01 (-0.01, 0.03) |
|
| ICAM | 0.05 (0.00, 0.10) |
0.10 (0.03, 0.16) |
0.07 (0.01, 0.12) |
0.04 (-0.00, 0.09) |
0.06 (0.01, 0.11) |
0.08 (0.02, 0.13) |
0.00 (-0.01, 0.01) |
0.06 (0.01, 0.11) |
0.05 (0.00, 0.09) |
|
| 1 | Protein C | 0.00 (-0.00, 0.00) |
0.13 (0.07, 0.20) |
0.13 (0.06, 0.19) |
0.00 (-0.01, 0.01) |
0.08 (0.03, 0.13) |
0.13 (0.06, 0.19) |
0.02 (-0.01, 0.04) |
0.01 (-0.01, 0.03) |
0.00 (-0.00, 0.00) |
| PAI-1 | 0.17 (0.10, 0.24) |
0.14 (0.08, 0.20) |
0.09 (0.04, 0.14) |
0.18 (0.11, 0.25) |
0.14 (0.07, 0.20) |
0.10 (0.04, 0.16) |
0.09 (0.03, 0.14) |
0.05 (0.01, 0.09) |
0.04 (0.00, 0.07) |
|
| ICAM | 0.07 (0.01, 0.13) |
0.03 (-0.01, 0.08) |
0.07 (0.01, 0.12) |
0.07 (0.01, 0.12) |
0.04 (-0.01, 0.08) |
0.06 (0.01, 0.12) |
0.04 (-0.00, 0.09) |
0.09 (0.03, 0.16) |
0.12 (0.05, 0.20) |
|
| 2 | Protein C | 0.05 (0.00, 0.10) |
0.11 (0.05, 0.18) |
0.14 (0.07, 0.21) |
0.01 (-0.01, 0.03) |
0.09 (0.03, 0.15) |
0.13 (0.06, 0.20) |
0.02 (-0.01, 0.04) |
0.04 (-0.00, 0.08) |
0.00 (-0.00, 0.00) |
| PAI-1 | 0.06 (0.01, 0.12) |
0.02 (-0.01, 0.06) |
0.03 (-0.01, 0.07) |
0.04 (-0.00, 0.08) |
0.02 (-0.01, 0.04) |
0.02 (-0.01, 0.06) |
0.03 (-0.00, 0.07) |
0.02 (-0.01, 0.05) |
0.04 (0.00, 0.08) |
|
| ICAM | 0.17 (0.09, 0.26) |
0.03 (-0.01, 0.08) |
0.16 (0.07, 0.24) |
0.14 (0.06, 0.22) |
0.08 (0.02, 0.14) |
0.15 (0.07, 0.23) |
0.01 (-0.01, 0.04) |
0.10 (0.03, 0.17) |
0.12 (0.05, 0.20) |
|
| 3 | Protein C | 0.04 (-0.00, 0.07) |
0.14 (0.07, 0.21) |
0.13 (0.06, 0.20) |
0.01 (-0.01, 0.02) |
0.12 (0.05, 0.18) |
0.13 (0.06, 0.20) |
0.03 (-0.01, 0.06) |
0.04 (0.00, 0.08) |
0.02 (-0.01, 0.04) |
| PAI-1 | 0.10 (0.04, 0.15) |
0.01 (-0.01, 0.03) |
0.01 (-0.01, 0.03) |
0.05 (0.01, 0.09) |
0.01 (-0.01, 0.03) |
0.01 (-0.01, 0.03) |
0.05 (0.00, 0.09) |
0.01 (-0.01, 0.03) |
0.01 (-0.01, 0.02) |
|
| ICAM | 0.14 (0.06, 0.21) |
0.05 (-0.00, 0.10) |
0.16 (0.08, 0.24) |
0.16 (0.07, 0.24) |
0.11 (0.04, 0.17) |
0.14 (0.06, 0.22) |
0.05 (-0.00, 0.10) |
0.10 (0.03, 0.17) |
0.09 (0.02, 0.15) |
|
Table 1 displays the characteristics of the study sample in the survival analysis. The mean age was 53 (95% CI 52, 54), 43% of recipients were female and 89% Caucasian. The overall prevalence of each PGD grade according to different time points is presented in Table 3. Overall, the prevalence of higher grades of PGD significantly declined farther out from the time of transplant (test for trend p<0.001). For example, the prevalence of PGD grade 3 was 28% at T24 and was 18% at T72. On the other hand, 33% had PGD grade 0 (no PGD) at T0, whereas 47% had no PGD by T72.
Table 3.
Prevalence (with 95% confidence intervals) of different grades of PGD at different time points in the outcomes study.
| PGD grade | ||||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| T24 | 0.329 (0.284, 0.375) |
0.200 (0.163, 0.242) |
0.186 (0.151, 0.227) |
0.284 (0.242, 0.330) |
| T48 | 0.370 (0.324, 0.417) |
0.221 (0.183, 0.263) |
0.181 (0.146, 0.221) |
0.228 (0.189, 0.271) |
| T72 | 0.472 (0.423, 0.375) |
0.234 (0.195, 0.277) |
0.115 (0.086, 0.149) |
0.180 (0.145, 0.220) |
PGD grades at different time points were associated with 30-day mortality (Figure 2). Using PGD grade at T24, 30-day all-cause mortality was 24.5% (95% CI 17.2, 33.2) for Grade 3, 6.2% (95%CI 2.1, 13.9) for grade 2, 3.5% (95%CI 0.7, 9.8) for Grade 1, and 4.2% (95%CI 1.5, 9.0) for Grade 0. Using PGD grade at T72, 30 day all-cause mortality was 36.4% (95%CI 25.7, 48.1) for Grade 3, 6.1% (95%CI 1.2, 16.9) for Grade 2, 5.0% (95%CI 1.6, 11.2) for Grade 1, and 3.5% (95%CI 1.4, 7.0) for grade 0 (test for trend, p<0.001). There was no interaction between PGD grade and the type of transplant (single vs. bilateral) at any time point (all p>0.50), indicating that the impact of PGD on 30-day mortality did not vary by the type of transplant performed.
Figure 2.

Impact of PGD grades at different time points on 30-day mortality rates.
Figure 3 presents differences in overall mortality by PGD grade. The overall all-cause mortality was significantly different between the groups (p<0.001 by log rank test for each time point). While Grade 3 PGD at T24 had a significant worse outcome compared to other PGD grades, Grade 3 PGD compared to other PGD grades at T72 demonstrated more pronounced differences in survival. Likewise, at time points further from transplant, intermediate grades of lung injury (grades 1 and 2) discriminated mortality differently from grade 0 and grade 3. There was no interaction between PGD grade and type of transplant (single vs. bilateral) at any time point (p>0.50 for each time point), indicating that the impact of PGD on overall survival did not vary by the type of transplant performed.
Figure 3.
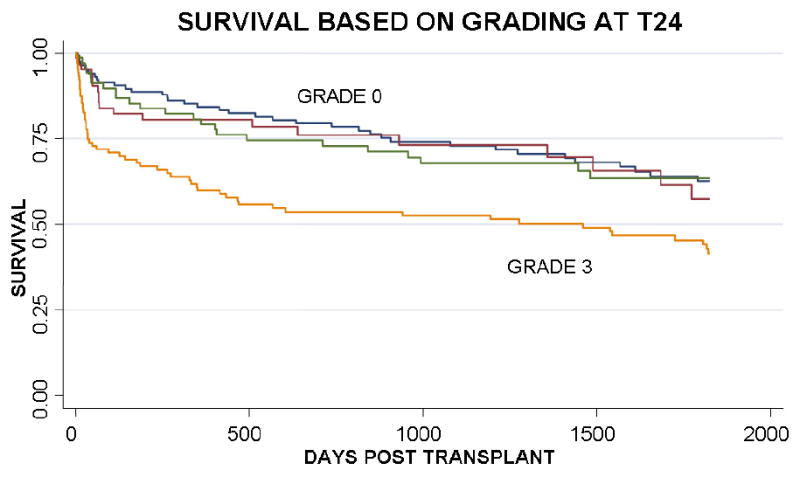


Kaplan Meier survival curves of PGD grades at different time points: a) T24, b) T48, c) T72.
Discussion
In this study, we demonstrated the discriminant validity of the ISHLT grading system for PGD for biomarker profiles and survival. PGD Grade 3 showed the most significant alterations in plasma biomarker profiles of lung injury. Grade 3 was also associated with the highest risk of death at 30 days and worse long-term survival. Grading PGD at time points farther out from transplant demonstrated sharper discrimination of both early mortality and long-term survival. These results may inform scientific definitions of PGD to be used in future studies aimed at investigating PGD risk and outcomes.
Grade 3 PGD was distinct from PGD grades 0-2 in terms of biomarker profile and survival. Therefore, studies of mechanisms, risk factors, or prevention of ischemia reperfusion injury after lung transplantation should focus on PGD Grade 3, given both the plasma biomarker differences as well as the significantly increased risk of short- and long-term mortality. These results support the use of either “any Grade 3 PGD” or “PGD Grade 3 at T72” as the primary outcome for mechanistic studies seeking a dichotomous PGD definition12,22,23 and in future clinical trials of treatment or prevention. Nonetheless, given that grade 2 PGD produced similar biomarker discrimination at some time points, investigators may choose to account for the apparent intermediate injury, either by treating PGD as an ordinal variable or by excluding grade 2 subjects.24,25
We found that as PGD grading was applied at later time points, discrimination for mortality improved. The better discrimination of PGD grade farther out from transplant for mortality may have been due to misclassification of subjects with early, mild, reversible pulmonary edema. Alternately, the better discrimination of PGD grade at later time points may represent persistent or irreversible lung injury that may have greater impact on outcome. Grading earlier after transplantation demonstrated a significantly higher prevalence of more severe PGD, reflecting either a higher risk of early acute lung injury or a distinct lung injury phenotype.
PGD grades 1-2 were associated with a higher risk of death compared to PGD grade 0 that appeared to manifest after the first 30 days. These findings are consistent with those of Daud and colleagues,22 who demonstrated a stepwise association of PGD grade with the development of bronchiolitis obliterans syndrome (BOS). That mild and moderate lung injury is associated with later chronic rejection is consistent with the injury response hypothesis26 and warrants further investigation. Likewise, the absence of clinically apparent lung injury (PGD Grade 0) at any time point was associated with the best long-term survival.
There were several limitations to our study. First, we did not perform grading at the T0 time point.15,27,28 The lack of earlier time points may have hampered our ability to demonstrate differences in PGD grade with mortality by different transplant procedure types.8 However, we did not detect a difference when testing for interaction by transplant type in our analyses of biomarkers or outcomes. These findings can be interpreted as consistent with Oto and colleagues who found the greatest differences between transplant type to be at earlier grading time points,8 whereas later grading seemed to give similar results between transplant types. In addition, the concept of earlier grading times is supported by our observation that the measured plasma biomarkers exhibited spikes at the earliest measured time point (6 hours). Finally, we only assessed short and long-term mortality. Other important end points include acute rejection and BOS, which have been linked with early allograft injury in a step-wise fashion according to PGD grade.12,22 Future studies will focus on these different outcomes in explaining mortality differences.
We conclude that the ISHLT consensus PGD definition demonstrates evidence of convergent and divergent validity using outcomes and concurrent biomarkers as constructs. PGD Grade 3 after lung transplantation appears clearly different from other PGD grades, regardless of time point. Our results suggest that use of PGD Grade 3 is a useful dichotomous definition for studies aimed at PGD mechanism or PGD prevention. In addition, our results support the specification of intermediate PGD grades for long term outcome studies. Future studies using this established definition of PGD will elucidate the mechanism and possible interventions to reduce the risk of this complication and potentially improve long term outcomes of lung transplantation.
Acknowledgments
Support: NIH HL04243, HL081619, HL087115, HL67771, HL081332, HL088263 and the Craig and Elaine Dobbin Pulmonary Research Fund
APPENDIX – Participants in the Lung Transplant Outcomes Group by site
Columbia University:
David Lederer, MD, MS (PI)
Selim Arcasoy, MD
Joshua Sonett, MD
-
Jessie Wilt, MD
Frank D'Ovidio, MD
Nilani Ravichandran, NP
Matthew Bacchetta, MD
Nadine Al-Naamani, MD
Debbie Rybak, BA
Michael Koeckert, BA
Robert Sorabella, BA
University of Pennsylvania (Coordinating site):
Jason Christie, MD, MS (PI)
Steven M. Kawut, MD, MS
Alberto Pocchetino, MD
Y. Joseph Woo, MD
Ejigayehu Demissie, MSN
Karen McGibney, RN
Robert M. Kotloff, MD
Vivek N. Ayha, MD
James Lee, MD, MS
Denis Hadjiliadis, MD, MHS
Melanie Doran, BS
Richard Aplenc, MD
Clifford Deutschman, M.D., M.S.
Benjamin Kohl, M.D.
University of Pittsburgh
Maria Crespo, MD (PI)
Joseph Pilewski, MD
Vanderbilt University:
Lorraine Ware, MD (PI)
Pali Shah, MD
Stacy Kelley-Blackburn, RN
Stanford University:
Ann Weinacker, MD (PI)
Ramona Doyle, MD
David Weill, MD
Susan Spencer Jacobs, MSN
Val Scott, MSN
University of Alabama, Birmingham:
Keith Wille, MD (PI)
Joao deAndrade, MD
Tonja Meadows, RN
Johns Hopkins University:
Jonathan Orens, MD (PI)
Ashish Shah, MD
John McDyer, MD
University of Michigan
Vibha Lama, MD, MS (PI)
Fernando Martinez, MD, MS
Emily Galopin, BS
Duke University
Scott M. Palmer, MD, MHS (PI)
David Zaas, MD, MBA
R. Duane Davis, MD
Ashley Finlen-Copeland, MSW
Footnotes
The authors have no relationships with commercial entities that have an interest in the subject matter of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arcasoy SM, Fisher A, Hachem RR, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part V: predictors and outcomes. J Heart Lung Transplant. 2005;24:1483–1488. doi: 10.1016/j.healun.2004.11.314. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Bavaria JE, Palevsky HI, et al. Primary Graft Failure following lung transplantation. Chest. 1998;114:51–60. doi: 10.1378/chest.114.1.51. [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. American Journal of Respiratory & Critical Care Medicine. 2005;171:1312–1316. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christie JD, Kotloff RM, Pochettino A, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124:1232–1241. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 5.King RC, Binns OA, Rodriguez F, et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Annals of Thoracic Surgery. 2000;69:1681–1685. doi: 10.1016/s0003-4975(00)01425-9. [DOI] [PubMed] [Google Scholar]
- 6.Christie JD, Carby M, Bag R, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–1459. doi: 10.1016/j.healun.2004.11.049. Epub 2005 Jun 1454. [DOI] [PubMed] [Google Scholar]
- 7.Christie JD, Van Raemdonck D, de Perrot M, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part I: introduction and methods. J Heart Lung Transplant. 2005;24:1451–1453. doi: 10.1016/j.healun.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Oto T, Levvey BJ, Snell GI. Potential refinements of the International Society for Heart and Lung Transplantation primary graft dysfunction grading system. Journal of Heart & Lung Transplantation. 2007;26:431–436. doi: 10.1016/j.healun.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Zaas D, Palmer SM. Respiratory failure after lung transplantation: Now that we know the extent of the problem, what are the solutions? Chest. 2003;123:14–16. doi: 10.1378/chest.123.1.14. [DOI] [PubMed] [Google Scholar]
- 10.Chatilla WM, Furukawa S, Gaughan JP, et al. Respiratory Failure After Lung Transplantation. Chest. 2003;123:165–173. doi: 10.1378/chest.123.1.165. [DOI] [PubMed] [Google Scholar]
- 11.Thabut G, Vinatier I, Stern JB, et al. Primary graft failure following lung transplantation: predicitive factors of mortality. Chest. 2002;121:1876–1882. doi: 10.1378/chest.121.6.1876. [DOI] [PubMed] [Google Scholar]
- 12.Whitson BA, Prekker ME, Herrington CS, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. Journal of Heart & Lung Transplantation. 2007;26:1004–1011. doi: 10.1016/j.healun.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Barr ML, Kawut SM, Whelan TP, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part IV: recipient-related risk factors and markers. J Heart Lung Transplant. 2005;24:1468–1482. doi: 10.1016/j.healun.2005.02.019. Epub 2005 Jul 1427. [DOI] [PubMed] [Google Scholar]
- 14.de Perrot M, Bonser RS, Dark J, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part III: donor-related risk factors and markers. J Heart Lung Transplant. 2005;24:1460–1467. doi: 10.1016/j.healun.2005.02.017. Epub 2005 Aug 1468. [DOI] [PubMed] [Google Scholar]
- 15.Oto T, Levvey BJ, Snell GI. Potential refinements of the International Society for Heart and Lung Transplantation primary graft dysfunction grading system. J Heart Lung Transplant. 2007;26:431–436. doi: 10.1016/j.healun.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Christie JD, Robinson N, Ware LB, et al. Association of protein C and type 1 plasminogen activator inhibitor with primary graft dysfunction. Am J Respir Crit Care Med. 2007;175:69–74. doi: 10.1164/rccm.200606-827OC. Epub 2006 Oct 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Covarrubias M, Ware LB, Kawut SM, et al. Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7:2573–2578. doi: 10.1111/j.1600-6143.2007.01981.x. [DOI] [PubMed] [Google Scholar]
- 18.Christie JD, Carby M, Bag R, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. Journal of Heart & Lung Transplantation. 2005;24:1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 19.Ware LB, Fang X, Matthay MA. Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;285:L514–521. doi: 10.1152/ajplung.00442.2002. [DOI] [PubMed] [Google Scholar]
- 20.Flori HR, Ware LB, Glidden D, et al. Early elevation of plasma soluble intercellular adhesion molecule-1 in pediatric acute lung injury identifies patients at increased risk of death and prolonged mechanical ventilation. Pediatr Crit Care Med. 2003;4:315–321. doi: 10.1097/01.PCC.0000074583.27727.8E. [DOI] [PubMed] [Google Scholar]
- 21.Covarrubias M, Ware LB, Kawut SM, et al. Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. American Journal of Transplantation. 2007;7:2573–2578. doi: 10.1111/j.1600-6143.2007.01981.x. [DOI] [PubMed] [Google Scholar]
- 22.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. American Journal of Respiratory & Critical Care Medicine. 2007;175:507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 23.Whitson BA, Nath DS, Johnson AC, et al. Risk factors for primary graft dysfunction after lung transplantation. Journal of Thoracic & Cardiovascular Surgery. 2006;131:73–80. doi: 10.1016/j.jtcvs.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 24.Plomin R, Haworth CMA, Davis OSP. Common disorders are quantitative traits. Nat Rev Genet. 2009;10:872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- 25.Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med. 1978;299:926–930. doi: 10.1056/NEJM197810262991705. [DOI] [PubMed] [Google Scholar]
- 26.Halloran PF, Homik J, Goes N, et al. The “injury response”: a concept linking nonspecific injury, acute rejection, and long-term transplant outcomes. Transplant Proc. 1997;29:79–81. doi: 10.1016/s0041-1345(96)00015-2. [DOI] [PubMed] [Google Scholar]
- 27.Prekker ME, Herrington CS, Hertz MI, et al. Early Trends in PaO(2)/fraction of inspired oxygen ratio predict outcome in lung transplant recipients with severe primary graft dysfunction. Chest. 2007;132:991–997. doi: 10.1378/chest.06-2752. [DOI] [PubMed] [Google Scholar]
- 28.Sekine Y, Waddell TK, Matte-Martyn A, et al. Risk quantification of early outcome after lung transplantation: donor, recipient, operative, and post-transplant parameters. J Heart Lung Transplant. 2004;23:96–104. doi: 10.1016/s1053-2498(03)00034-2. [DOI] [PubMed] [Google Scholar]


