Abstract
Nervous system development proceeds by sequential gene expression mediated by cascades of transcription factors in parallel with sequences of patterned network activity driven by receptors and ion channels. These sequences are cell type- and developmental stage-dependent and modulated by paracrine actions of substances released by neurons and glia. How and to what extent these sequences interact to enable neuronal network development is not understood. Recent evidence demonstrates that CNS development requires intermediate stages of differentiation providing functional feedback that influences gene expression. We suggest that embryonic neuronal functions constitute a series of phenotypic checkpoint signatures; neurons failing to express these functions are delayed or developmentally arrested. Such checkpoints are likely to be a general feature of neuronal development and may constitute presymptomatic signatures of neurological disorders when they go awry.
Constructing the nervous system: the scale of the problem
The complexity of the nervous system makes the developmental assembly of this structure unusually challenging. Neuronal phenotypes are specified and synaptic connections are formed with prodigious specificity. An argument can be made that the brain begins simply and that complexity is built up gradually. However, 80% of 20,000 mouse genes are expressed in the adult nervous system (1). With 1011 neurons making 1015 synapses, this number of genes is insufficient to program the development of the nervous system on a single-gene-to-single-component basis. How is such a complex program regulated during development? Cascades of transcription factors play an important role (2,3). However, there is significant potential for disruptions of neuronal development by mistakes in transcriptional machinery or perturbations of gene expression. Indeed there are a vast number of genetically or environmentally driven developmental disorders, with unfortunate societal and financial impacts.
Fortunately, developing neurons are not mute during development. They express cell and developmental stage-specific sequences of voltage-gated and transmitter receptor-linked ion channel currents that provide read-outs of their state of differentiation Often due to the expression of different channel subunits, immature currents are more “sloppy” than adult ones and their long synaptic durations account for the relatively slow kinetics that enable calcium influx at early developmental stages (4-8). Immature networks also follow a specific developmental sequence initially characterized by intrinsic, synapse-independent voltage-gated calcium currents, followed by large calcium plateaus in small neuronal populations connected by gap junctions. Subsequently, primitive spontaneous synapse-driven patterns appear, like the so-called giant depolarizing potentials (GDPs) in the hippocampus and neocortex that suppress the large calcium plateaus (9-11) (Figure 1) and retinal waves in the visual system (12-17). These simple patterns of activity then disappear as the networks become capable of generating more diversified behaviourally relevant patterns. Interestingly, these primitive patterns are essential for the correct construction of cortical ensembles but are generated at a time when sensory systems are not yet working. Thus, retinal waves are generated well before vision is functional and operate to enable adjacent neurons to fire together and make synaptic connections with adjacent targets (idem and 18). Although the timing of these patterns differs in different animal species and brain structures, the sequences appear identical in postnatal rodents and in utero primates, suggesting that they have been preserved throughout evolution. Here we suggest that these developmental sequences constitute a series of checkpoints controlling the appropriate progression of genetic programs.
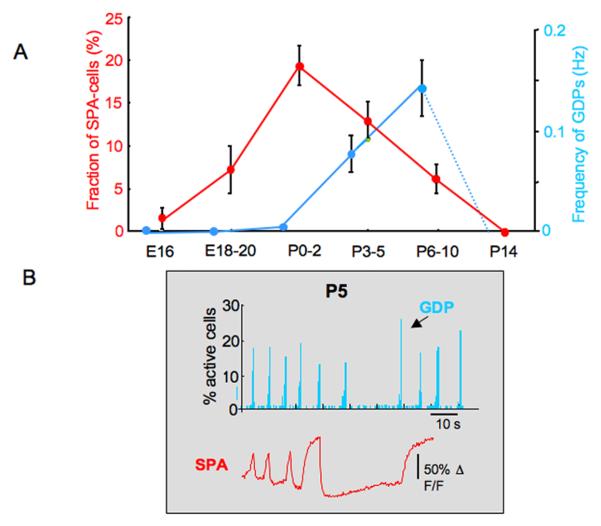
Figure 1. Network activity maturation.
(a) The first coordinated pattern of activity generated in utero in rodent cortical structures consists of large plateau elevations of intracellular calcium triggered in small populations of neurons interconnected by gap junctions (Synchronous Plateaus in cell Assemblies, SPAs). SPAs are generated by intrinsic non-synaptic, voltage-gated calcium currents. Later, synapse-driven Giant Depolarizing Potentials (GDPs) are generated synchronously by large populations of neurons until the GABA depolarizing-to-hyperpolarizing shift has occurred (around the end of the first postnatal week in rodents). (b) GDPs suppress SPAs (negative deflections measuring calcium elevation with Fura-2). When they are both present during the postnatal period, GDPs arrest SPAs that are synchronized with the end of GDPs. Moreover, blocking GDPs with receptor antagonists reinstates SPAs at an age when they have normally disappeared (not shown). Reproduced, with permission, from Ref. (9).
The general role of phenotypic checkpoints
The construction of a building requires repeated inspection and determination of the extent to which the architectural and engineering programs have been respected, before subsequent stages of construction can be initiated. Similarly, advancement in the educational system and in the workplace depends on evaluations of performance. We suggest that the expression of embryonic neuronal functions at different developmental stages satisfies a similar requirement. With this view in mind, failure to realize a given step in development –such as migration from one position to another - delays or arrests the developmental sequence of ionic currents and/or other signaling messengers at the stage at which the failure has occurred. The genetic program is impacted, as the genes and functional feedback act in series, and the affected neurons generate electrical activity corresponding to stage A rather than B. Thus checkpoints provide punctuated control of the implementation of the genetic program (Figure 2). These phenotypic feedback loops can provide developing biological systems with sufficient flexibility to accommodate perturbations to programs of gene expression and enable responses to changing environments in which the nervous system develops. They enable integration of genetic and environmental messages and provide a degree of plasticity in the construction of networks. The power of this mechanism for enabling environmental input and the ubiquity of these findings suggest that phenotypic checkpoints are a general feature of neuronal development.
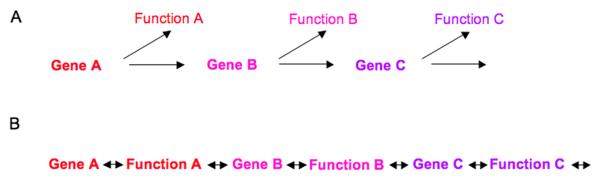
Figure 2. Phenotypic checkpoint signaling.
(a) The classical view of brain development involves serial expression of genes that lead to embryonic functions. (b) Phenotypic checkpoint signaling integrates embryonic functions in the process of gene expression by feedback and feedforward signaling.
Remarkably, major aspects of development such as proliferation, migration, and differentiation are not fully programmed genetically, but rely on phenotypic checkpoints: times and places during development at which functional validation appropriate to the stage of the cells enables the process to go forward normally, take an alternative route, or become arrested. In what follows, we marshal evidence for the role of phenotypic checkpoints during development and following disruption of normal developmental processes.
Phenotypic checkpoints in embryonic development
Proliferation checkpoint
Neural progenitors in the ventricular and subventricular zones of the developing brain undergo mitotic divisions that give rise to neuroblasts that express transmitters. Genes regulating proliferation have been identified (19-21), but electrical activity modulates this process. GABA and glutamate are secreted at early stages of development, and paracrine actions of both GABA and glutamate depolarize the progenitors, generate elevations of intracellular calcium and inhibit DNA synthesis in the ventricular zone (22-23). Release of GABA from neuroblasts also activates GABAA receptors and suppresses proliferation in the subventricular zone (24-26). On the other hand, GABA induces proliferation of postnatal rat immature cerebellar granule cells through depolarization and activation of calcium channels (27). Release of glutamate from glia ensheathing proliferating cells that express NMDA-type glutamate receptors is critical for neuroblast survival (28). Serotonin increases proliferation of neuronal progenitors (29). Expression of serotonin receptors (5-HT1A, 5-HT2), GABAA receptors and AMPA-class glutamate receptors enables functional feedback that regulates the number of neurons generated and constitutes a phenotypic checkpoint signature.
Migration checkpoint
As neurons are generated they commence migration along stereotyped pathways to their permanent locations in the brain. Although genes regulating this process have been identified (30-32), the speed and extent of both radial and tangential migration are regulated by paracrine action of neurotransmitters. The activation of glutamate receptors –produced by non vesicular release that predominates at this stage - increases migration of cerebellar granule neurons via elevations of intracellular calcium (33). Furthermore, activation of glutamate or GABAA receptors promotes migration of hippocampal and cortical neurons (34-36). In contrast, activation of GABAA receptors decreases migration of neuronal precursors from the subventricular zone to the olfactory bulb (37) and can act as the stop signal for migration (38). Both depletion and excess of serotonin reduce interneuron migration, in different systems (29, 39, 40). The expression and activation of receptor-mediated and voltage-gated currents well before synapses have been formed is required for appropriate migration and attests that the process is not an automated one independent of the influence of the external milieu. Thus, the activation of transmitter signaling provides a phenotypic checkpoint for migration.
Axon guidance checkpoint
Growth cones at the tips of axons navigate through the embryonic nervous system to reach targets with which synapses will be formed, usually interacting with a series of intermediate targets en route. Growth cones appear to express receptors appropriate for the recognition of each intermediate target, thus avoiding errors in axon guidance. For example, commissural axons grow toward the midline and then leave it again on the opposite side and normally never recross; on the contralateral side they turn anteriorly or posteriorly to reach other targets (41-43). Their growth cones are initially attracted by Netrin-1 protein secreted by cells at the midline, for which they express high levels of DCC receptor; these receptors stimulate calcium influx that drives growth cone turning (44-46). Commissural neurons are unaffected by the midline repellent protein, Slit, for which they express a low level of Robo receptor. However, after they cross the midline they become insensitive to Netrin-1 and are repelled by Slit (47) (Figure 3) as well as other repulsive molecules (48). The switch from attraction to repulsion results from insensitivity to Netrin-1 through physical interaction between DCC and activated Robo, and repulsion by Slit due to increased levels of Robo. The sequential expression of DCC and unblocking of Robo constitute a checkpoint signature, driving midline crossing. Without this critical step, later guidance steps are blocked. Growth cones exhibit local protein synthesis (49, 50) that can introduce expression of new classes of receptor (51). Thus growth cones are likely to move from one phenotypic checkpoint to the next during axonal pathfinding.
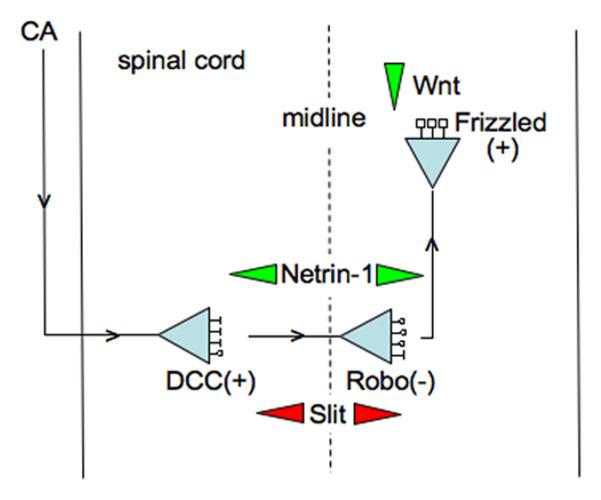
Figure 3. Axon guidance checkpoint.
Commissural axons (CA) cross the midline of the spinal cord to ascend on the opposite side. Their growth cones are initially attracted by a gradient of Netrin-1 secreted by midline cells, which binds to DCC receptors. After crossing the midline, growth cones are repelled by a gradient of Slit secreted by midline cells, which binds to Robo receptors. Insensitivity to Netrin-1 is mediated by interaction of DCC with activated Robo that constitutes a checkpoint for midline crossing. Attraction of growth cones is subsequently mediated by a gradient of Wnt binding to Frizzled receptors.
Neurotransmitter and receptor specification checkpoints
Neurons communicate by release of neurotransmitter molecules that bind to receptor proteins on other neurons and target cells. Specifying the correct transmitter in a population of neurons, from the 100 or so that have been identified, is essential for network activity. The mechanism by which appropriate transmitter specification is achieved in different classes of neurons involves a partnership between gene expression and electrical activity (52). Calcium spikes are generated in embryonic amphibian spinal neurons with cell-type specific frequencies, and increasing or decreasing spike frequencies prior to synapse formation changes transmitter specification. Suppressing calcium spiking increases the number of neurons expressing excitatory transmitters, whereas enhancing calcium spiking increases the number of neurons expressing inhibitory transmitters, typically by 30-50% (53, 54). Altering sensory input to postembryonic neurons once synapses have formed changes the specification of transmitter selectively within the activated circuit by a similar amount (55).
This phenotypic checkpoint involves the expression of developmentally transient calcium spikes, triggered by calcium-dependent action potentials resulting from large voltage-gated calcium currents largely unopposed by small voltage-gated potassium currents. Changes in transmitter receptor expression occur postsynaptically to match changes in transmitter expression (55, 56) (Figure 4), demonstrating another phenotypic checkpoint. Thus, unlike previously described checkpoints that occur prior to synapse formation, transmitter and receptor specification checkpoints at this stage can be responsive to synaptic input.
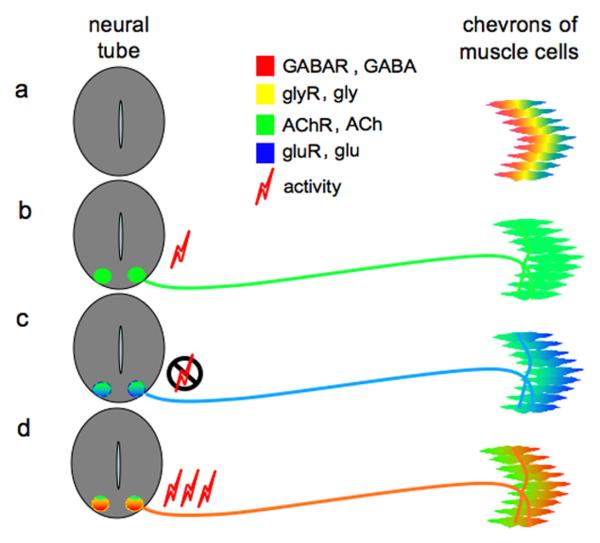
Figure 4. Neurotransmitter receptor selection checkpoint.
Activity-dependent transmitter specification acts as a checkpoint for selection of receptors at the embryonic neuromuscular junction. (a) Prior to axon outgrowth from the amphibian neural tube (Xenopus), trunk muscle cells express a range of different transmitter receptors. (b) During normal development, spontaneous neuronal calcium spike activity contributes to the expression of acetylcholine in motor neurons (localized within the neural tube), while acetylcholine receptors are stabilized on muscle cells and other classes of receptors disappear. (c) When calcium spike activity is suppressed, motor neurons express glutamate in addition to acetylcholine, and muscle cells express glutamate (NMDA and AMPA) receptors in addition to acetylcholine receptors. (d) When calcium spike activity is enhanced, motor neurons express GABA and glycine in addition to acetylcholine, and muscle cells express GABAA receptors and glycine receptors in addition to acetylcholine receptors. Reproduced, with permission, from Ref. (56).
GABA/chloride signaling checkpoint
The levels of intracellular chloride are elevated at early developmental stages in a wide range of animal species and brain structures suggesting that this has been preserved during evolution (4, 57, 58). The initially depolarizing and excitatory actions of GABA are the result of developmental expression of a chloride importer (NKCC1) prior to a chloride exporter (KCC2) (59). Activation of GABA (or glycine) receptors generates sodium and calcium currents and activates NMDA receptors by removing their voltage-dependent magnesium block, leading to a large calcium influx observed only in immature neurons (11). GABA depolarization regulates early aspects of development including proliferation, migration (see above), neurite outgrowth (60, 61) and formation of GABAergic and glutamatergic synapses (62-64). Since GABAergic neurons and synapses mature before glutamatergic ones, GABA also provides the first source of neuronal activity (65). The abrupt maternally-triggered reduction of embryonic intracellular chloride during the birthing process, via enhanced oxytocin levels, together with the corresponding hyperpolarizing action of GABA that protects embryonic neurons from anoxic insults, illustrate the important biological function of the polarity of GABA's effects (66). If this conversion in polarity of GABA signals is accelerated or prevented, subsequent stages of development are altered (61-64). Thus the switch from depolarization to hyperpolarization is an important phenotypic checkpoint during development.
Phenotypic checkpoints during development in the adult nervous system
Are phenotypic checkpoints operative in an adult environment where neurogenesis is known to take place? Or are they restricted to brain maturation? The response to this issue is complicated by the fact that neurogenesis is restricted to a small number of brain regions (67-70), hampering general conclusions as to the roles of checkpoints in the adult nervous system in general. Nevertheless, proliferation of neural progenitor cells generating dentate granule cells in the adult hippocampus is modulated by NMDA (71) and serotonin signaling (72, 73). Voluntary wheel running by rodents or seizure activity increase the proliferation of these cells, consistent with a role for physiological activity in generating new neurons (74, 75). The development of adult granule cell dendrites and synapse formation is controlled by tonic GABAergic depolarization and reducing chloride accumulation by the suppression of NKCC1 or expression of KCC2 blocks these processes (76, 77). Thus, proliferation and integration checkpoints operate in an adult environment for the correct assembly of networks, suggesting that they cannot be fabricated de novo without following the developmental sequence. However, compared to neuronal development at embryonic stages, neural development in the adult brain is significantly prolonged; acceleration of the speed of development during adult neurogenesis resulting from increased activity (e.g. seizures) or genetic defects (eg. mutations in the expression of important developmental genes, such as disrupted-in-schizophrenia-1, DISC1) can lead to deficits in neuronal development.
Checkpoint mechanisms
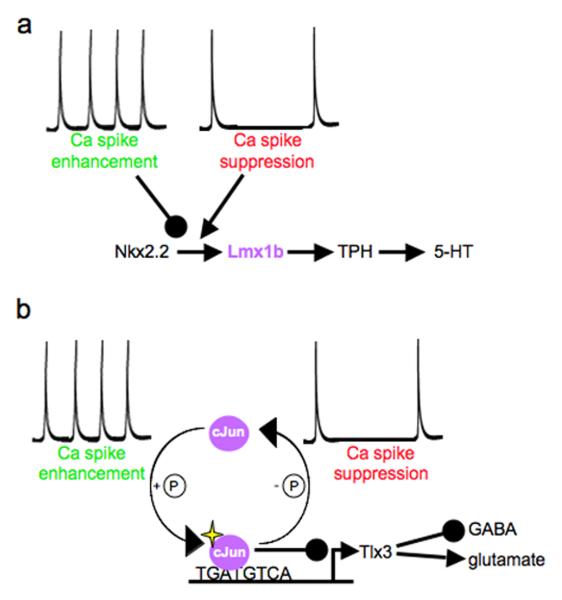
Activity-dependent regulation of transcription factors provides a mechanism for neurotransmitter specification checkpoints. Spontaneous calcium spike activity in the hindbrain of developing amphibian larvae modulates the specification of serotonergic neurons by controlling the number of neurons expressing the LIM homeobox transcription factor 1b (Lmx1b). Activity acts downstream of the Nkx2.2 homeobox transcription factor, but upstream of Lmx1b, leading to regulation of the serotonergic phenotype (54). Manipulations of activity and targeted alteration of Lmx1b expression demonstrate that these changes in the number of serotonergic neurons change larval swimming behaviour (54). Spontaneous calcium spike activity in the spinal cord of amphibian larvae regulates transcription of the GABAergic/glutamatergic selection gene tlx3 through a variant cAMP response element (CRE) in its promoter (78). Calcium signals through phosphorylation of the cJun transcription factor, which binds to this CRE site and modulates transcription, thereby integrating activity-dependent and intrinsic neurotransmitter specification. This mechanism provides a way for early activity to regulate genetic pathways at critical decision points, switching the phenotype of developing neurons (Figure 5).
Figure 5. Checkpoint mechanisms.
The intersection of calcium spike activity and gene expression determines neurotransmitter specification in the brain and spinal cord. (a) Calcium spike enhancement decreases and spike suppression increases the number of neurons expressing the LIM homeobox transcription factor 1b (Lmx1b), leading to changes in the number of neurons making tryptophan hydroxylase (TPH) and serotonin (5-HT). The number of neurons expressing the Nkx2.2 homeobox transcription factor is not affected. (b) Calcium spike enhancement and suppression modulate phosphorylation of the cJun transcription factor, which regulates transcription of the tlx3 homeobox transcription factor through the cAMP response element (TGATGTCA) in the tlx3 promoter. Tlx3 determines the glutamatergic fate over the GABAergic fate in the dorsal spinal cord. Reproduced, with permission, from Refs. (54) and (78).
Epigenetic imprinting that links environmental factors to the coding of genetic programs is highly suited to implement phenotypic checkpoints generally, since epigenetic modifications reversibly regulate gene expression preferentially during brain maturation. Molecular modifications to the structure of histone proteins and DNA (chromatin) regulate the transcription of genes without altering their nucleotide sequence. DNA methylation and histone deacetylation are two major epigenetic modifications that contribute to the stability of gene expression (79-81). Environmental stimuli such as maternal care, social interactions, as well as drugs, activate epigenetic mechanisms in post-mitotic neurons during development that result in alterations of neuronal phenotype with long-term behavioural consequences (82-85). This DNA methylation and chromatin patterning is programmed during early development and appears to be highest at early stages (86, 87) although it can also impact memory processes in adults (88). At later stages epigenetic control is less reversible, preventing potentially dangerous phenotypic checkpoint signaling. DNA methylation patterns and epigenetic factors differ in the chimpanzee and human cortex (89) and distinguish brain regions, providing a mechanism for region-specific functional specialization (90).
Epigenetic phenotype specification provides a developmental mechanism that enables rapid, efficient and quasi-permanent alterations of phenotypes during development. The Aicardi Goutière syndrome is interesting in this context since a genetic mutation and an environmental insult –a cytomegalovirus infection during gestation- converge on the same signaling cascade to generate polymicrogyria via a programmed succession of phenotypes common to both insults (91, 92). This syndrome illustrates the convergence of genes and environment in impacting brain development via signaling cascades.
Checkpoints signal branch points in genetic programs
The roles of biological checkpoints have been extensively characterized in cell division where the failure of these feedback controls leads to cell death or extensive proliferation (93-95). When neurons are misplaced or misconnected for genetic or environmental reasons they can signal this situation by arresting some of their developmental sequences. Evidence has been obtained with intrauterine short-interfering RNA (siRNA) knockdown of expression of a variety of proteins associated with major neurological disorders including Rett syndrome, lissencephaly, dyslexia, and certain forms of mental retardation (4, 96, 97). Although neurons with mutations in the genes encoding these proteins fail to migrate, they develop, arborize and form synapses with adjacent neurons, suggesting that cell death is not solely a consequence of checkpoints and that some aspects of differentiation are intrinsic to neurons. However, they generate misplaced ensembles that impinge on the construction and function of adjacent cortical units. Interestingly, following knockdown of doublecortin (DCX), neurons are “frozen” in an immature state with voltage-gated currents and oscillatory features that normally disappear when it is expressed (98, 99) (Box 1).
Box 1. When does checkpoint-control end? Re-examining the concept of critical periods.
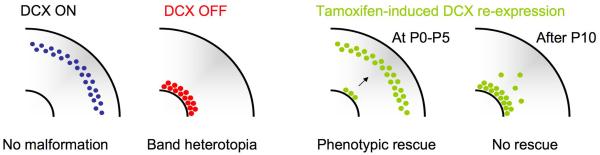
Cell proliferation, migration and differentiation are almost entirely restricted to brain maturation. Does this imply that phenotypic checkpoint signalling is terminated once the principal developmental sequences have run their course, the genetic program has been implemented, and networks are operational? Specific answers to this question are lacking, but experiments aimed at repairing the consequences of genetic mutations provide conceptual hints. In utero RNAi-mediated knockdown of doublecortin (DCX) –like many other proteins involved in neurological disorders- appears to produce an arrest in neuronal maturation at the developmental stage at which its expression has been stopped. These neurons are misplaced and are characterized by immature and aberrant electrical features (98, 99). A similar return to immature features has been observed following a variety of insults, suggesting recapitulation of an immature state (105-107). Interestingly, attempts to repair the phenotype by reintroduction of the correct gene – under tamoxifen control to enable the desired temporal expression of the gene – produces a partial rescue (108). However, this rescue only occurs when the gene is induced during early postnatal life (P0-5), and not at later points in postnatal development (> P10), suggesting that the mechanisms required for repair are no longer accessible after a certain timepoint in development. Thus, there is a window of opportunity for genetic repair during which plasticity is available. Interestingly, this window broadly corresponds to the so-called “critical period” during which wiring of networks changes in response to environmental stimuli. We suggest that the extensive wiring plasticity at this age corresponds to a window of checkpoints that closes with permanent wiring. This conclusion could have significant implications for gene therapy of neurological disorders and for our understanding of whether and how immature neuronal features can be repaired by genetic manipulations after insults such as seizures in adults.
Figure I.
In utero knockdown of DCX leads to the formation of a double cortex (band heterotopia) with neurons that fail to migrate to their assigned layer. Delayed re-expression of DCX (using conditional gene expression under the temporal control of tamoxifen) - produces a partial phenotypical rescue only when this is done during early postnatal life (i.e. before P10). Reproduced, with permission, from Ref. (108).
Early malformations can occur years or decades before the manifestation of clinical syndromes with which they are typically associated, suggesting that they provide early electrical signatures of disorders to come: the neuro-archeology concept (4). In Huntington's disease, brain malformations are revealed by brain imaging and behavioral tests well before the onset of clinical manifestations (100, 101). Other observations suggest that immature neurons are more resistant than adult neurons to insults that nevertheless produce severe long lasting sequels, indicating that mechanisms other than cell loss are involved, such as neuro-immune interactions (102-104). We suggest that early insults, whether they are genetic or environmental, are “programmatic” (i.e. mediated by alterations of developmental programs resulting from mismatches at phenotypic checkpoints). In this model, the electrical signature of neurons provides a stage-dependent readout attesting whether or not they have achieved their program adequately (4).
Conclusions
The functional feedback and feedforward provided by phenotypic checkpoints serves several purposes. Phenotypic checkpoints can play a role in specifying the “what” and “where” of development: what genes are turned on or off or what cytoskeletal components are posttranslationally modified and where these events take place. Activity-dependent transmitter specification provides an example of such regulation. Phenotypic checkpoints can specify “how much”, through positive and negative feedback loops. Neurotransmitters regulate the extent of proliferation and migration of developing neurons. Phenotypic checkpoints can specify “when”, rather like the function of a clock, turning gene expression or other processes on or off at particular times. Conversion of GABA signaling from depolarization to hyperpolarization provides a timing signal for subsequent stages of development. Thus, checkpoints provide both plasticity and precision for the assembly of the nervous system. Current evidence suggests that phenotypic checkpoint signaling terminates at the conclusion of development (see Box 1).
What is the relationship of phenotypic checkpoints to the homeostatic behavior of neurons and networks? Homeostasis is instrumental in the operation of adult networks; it maintains a balance across a wide range of crucial functions (eg. pH, temperature, etc) and prevents excessive excitation or inhibition, limiting the density of synapses on neurons and controlling a large variety of signaling cascades through feedback loops. However homeostasis does not operate on developmental processes in which genetic programs and the environment converge to generate networks of cells. Developing neurons are generally in a progressive state and not in homeostasis as they achieve their programs. Thus, blocking expression of a given phenotype in immature neurons often fails to promote appearance of a phenotype that compensates for the missing one and instead leads to sustained expression of the previous phenotype (see Figure 1). Blockade of GDPs leads to reappearance of synchronous plateaus in cell assemblies (SPAs), the earlier phenotype. Neurological disorders involving aberrant neuronal migration do not develop because homeostasis has been disrupted but because misplaced neurons create aberrant connections and generate patterns of activity that perturb normal network operation. However, as neurons begin to mature, homeostasis becomes important and is involved, for example, in neurotransmitter specification. Suppression of activity leads to an increase in the number of neurons expressing excitatory transmitters and a decrease in the number of neurons expressing inhibitory transmitters. Enhancing activity produces the opposite result. Thus, phenotypic checkpoints enable both progressive and homeostatic neuronal development.
In contrast to the construction of an inanimate machine, the brain is active at the earliest stages of its assembly and this activity is cell, network and developmental stage dependent. We suggest that this activity is an online signature of the genetic program, providing checkpoints that condition activity of previous elements and the acquisition of subsequent elements in the program. We have outlined issues that seem ready for further investigation (Box 2), which may be expected to clarify both the mechanisms and impact of phenotypic checkpoints. Such checkpoints supply critical feedback information for error correction and integrate environmental information through alterations in activity that adapt and fine-tune the realization of the program. They also appear to resolve the Nature versus Nurture debate, as both operate in series in this scheme.
Box 2. Outstanding questions.
What is the mechanism by which neuronal functions influence expression of genes at phenotypic checkpoints? Is there a common checkpoint cascade of signals for different elements or do different signals control the expression of different signaling proteins/processes?
Does sensory information during critical periods act through phenotypic checkpoints to eliminate depolarization by GABA, alter NMDA and AMPA receptor expression levels, and suppress primitive patterns of network activity?
Can checkpoints be bypassed by convergent genetic pathways for aspects of development that are so basic that functional validation is unnecessary?
Following checkpoint failure, do aberrant networks impact the operation of adjacent normal circuits? How are immature features integrated with the behaviorally relevant physiological patterns that the latter generate?
Does inappropriate activation of phenotypic checkpoints lead to neurological disorders?
What are the mechanisms involved in the delay between an early checkpoint failure and the manifestation of disease?
Does development of other organs, such as the heart or the lungs, involve phenotypic checkpoints?
Acknowledgments
We are grateful to Drs P Szepetowski, F Muscatelli, JB Manent and X Nicol for suggestions. The authors' work is supported by INSERM, FP7 (NEMO) ANR (YBA) and NINDS and NIMH (NCS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 2.Lee SK, Pfaff SL. Transcriptional networks regulating neuronal identity in the developing spinal cord. Nature Neurosci. 2001;4:1183–1191. doi: 10.1038/nn750. [DOI] [PubMed] [Google Scholar]
- 3.Goulding M, Pfaff S. Development of circuits that generate simple rhythmic behaviors in vertebrates. Curr. Opin. Neurobiol. 2005;15:14–20. doi: 10.1016/j.conb.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Ari Y. Neuro-archaeology: pre-symptomatic architecture and signature of neurological disorders. Trends Neurosci. 2008;31:626–636. doi: 10.1016/j.tins.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Moody WJ, Bosma MM. Ion channel development, spontaneous activity, and activity-dependent development in nerve and muscle cells. Physiol. Rev. 2005;85:883–941. doi: 10.1152/physrev.00017.2004. [DOI] [PubMed] [Google Scholar]
- 6.O'Dowd DK, et al. Development of voltage dependent calcium, sodium and potassium currents in Xenopus spinal neurons. J. Neurosci. 1988;8:792–805. doi: 10.1523/JNEUROSCI.08-03-00792.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris GL, et al. Changes in densities and kinetics of delayed rectifier potassium channels during neuronal differentiation. Neuron. 1988;1:739–750. doi: 10.1016/0896-6273(88)90172-9. [DOI] [PubMed] [Google Scholar]
- 8.Desarmenien MG, Spitzer NC. Role of calcium and protein kinase C in development of the delayed rectifier potassium current in Xenopus spinal neurons. Neuron. 1991;7:797–805. doi: 10.1016/0896-6273(91)90282-5. [DOI] [PubMed] [Google Scholar]
- 9.Crepel V, et al. A parturition-associated nonsynaptic coherent activity pattern in the developing hippocampus. Neuron. 2007;54:105–120. doi: 10.1016/j.neuron.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Bonifazi P, et al. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science. 2009;326:1419–1424. doi: 10.1126/science.1175509. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Ari Y, et al. GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 12.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 13.Feller MB. Retinal waves are likely to instruct the formation of eye-specific retinogeniculate projections. Neural Dev. 2009;4:24. doi: 10.1186/1749-8104-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leitch E, et al. GABA type-A activity controls its own developmental polarity switch in the maturing retina. J. Neurosci. 2005;25:4801–4805. doi: 10.1523/JNEUROSCI.0172-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerschensteiner D, Wong RO. A precisely timed asynchronous pattern of ON and OFF retinal ganglion cell activity during propagation of retinal waves. Neuron. 2008;58:851–858. doi: 10.1016/j.neuron.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron. 2002;33:357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- 17.Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat. Rev. Neurosci. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanganu IL, et al. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J. Neurosci. 2006;26:6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagoshima H, Shigesada K, Kohara Y. RUNX regulates stem cell proliferation and differentiation: insights from studies of C. elegans. J. Cell. Biochem. 2007;100:1119–1130. doi: 10.1002/jcb.21174. [DOI] [PubMed] [Google Scholar]
- 20.McClellan KA, Slack RS. Novel functions for cell cycle genes in nervous system development. Cell Cycle. 2006;5:1506–1513. doi: 10.4161/cc.5.14.2980. [DOI] [PubMed] [Google Scholar]
- 21.Zagami CJ, Zusso M, Stifani S. Runx transcription factors: lineage-specific regulators of neuronal precursor cell proliferation and post-mitotic neuron subtype development. J. Cell. Biochem. 2009;107:1063–1072. doi: 10.1002/jcb.22221. [DOI] [PubMed] [Google Scholar]
- 22.LoTurco JJ, et al. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 23.Nacher J, McEwen BS. The role of N-methyl-D-asparate receptors in neurogenesis. Hippocampus. 2006;16:267–270. doi: 10.1002/hipo.20160. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, et al. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nature Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang DD, Kriegstein AR. Defining the role of GABA in cortical development. J. Physiol. 2009;587:1873–1879. doi: 10.1113/jphysiol.2008.167635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dave KA, Bordey A. GABA increases Ca2+ in cerebellar granule cell precursors via depolarization: implications for proliferation. IUBMB Life. 2009;61:496–503. doi: 10.1002/iub.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiszman ML, Borodinsky LN, Neale JH. GABA induces proliferation of immature cerebellar granule cells grown in vitro. Brain Res. Dev. Brain Res. 1999;115:1–8. doi: 10.1016/s0165-3806(99)00035-8. [DOI] [PubMed] [Google Scholar]
- 28.Platel JC, Dave KA, Gordon V, Lacar B, Rubio ME, Bordey A. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron. 2010;65:859–872. doi: 10.1016/j.neuron.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vitalis T, Parnavelas JG. The role of serotonin in early cortical development. Dev. Neurosci. 2003;25:245–256. doi: 10.1159/000072272. [DOI] [PubMed] [Google Scholar]
- 30.Heng JI, Nguyen L, Castro DS, Zimmer C, Wildner H, Armant O, Skowronska-Krawczyk D, Bedogni F, Matter JM, Hevner R, Guillemot F. Neurogenin 2 controls cortical neuron migration through regulation of Rnd2. Nature. 2008;455:114–118. doi: 10.1038/nature07198. [DOI] [PubMed] [Google Scholar]
- 31.Takei Y, Teng J, Harada A, Hirokawa N. Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J. Cell Biol. 2000;150:989–1000. doi: 10.1083/jcb.150.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coquelle FM, Levy T, Bergmann S, Wolf SG, Bar-El D, Sapir T, Brody Y, Orr I, Barkai N, Eichele G, Reiner O. Common and divergent roles for members of the mouse DCX superfamily. Cell Cycle. 2006;5:976–983. doi: 10.4161/cc.5.9.2715. [DOI] [PubMed] [Google Scholar]
- 33.Komuro H, Rakic P. Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron. 1996;17:275–285. doi: 10.1016/s0896-6273(00)80159-2. [DOI] [PubMed] [Google Scholar]
- 34.Demarque M, et al. Paracrine intercellular communication by a Ca2+-and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002;36:1051–1061. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- 35.Manent JB, et al. A noncanonical release of GABA and glutamate modulates neuronal migration. J. Neurosci. 2005;25:4755–4765. doi: 10.1523/JNEUROSCI.0553-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uchino S, Hirasawa T, Tabata H, Gonda Y, Waga C, Ondo Y, Nakajima K, Kohsaka S. Inhibition of N-methyl-D-aspartate receptor activity resulted in aberrant neuronal migration caused by delayed morphological development in the mouse neocortex. Neuroscience. 2010;169:609–618. doi: 10.1016/j.neuroscience.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 37.Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J. Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62:53–71. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pronina T, et al. Influence of serotonin on the development and migration of gonadotropin-releasing hormone neurones in rat foetuses. J. Neuroendocrinol. 2003;15:549–558. doi: 10.1046/j.1365-2826.2003.01029.x. [DOI] [PubMed] [Google Scholar]
- 40.Riccio O, Potter G, Walzer C, Vallet P, Szabo G, Vutskits L, Kiss JZ, Dayer AG. Excess of serotonin affects embryonic interneuron migration through activation of the serotonin receptor 6. Mol. Psychiatry. 2009;14:280–290. doi: 10.1038/mp.2008.89. [DOI] [PubMed] [Google Scholar]
- 41.Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- 42.Evans TA, Bashaw GJ. Functional diversity of Robo receptor immunoglobulin domains promotes distinct axon guidance decisions. Curr. Biol. 2010;20:567–572. doi: 10.1016/j.cub.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickson BJ, Zou Y. Navigating intermediate targets: the nervous system midline. Cold Spring Harb. Perspect. Biol. 2010;2:a002055. doi: 10.1101/cshperspect.a002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403:93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- 45.Shim S, Goh EL, Ge S, Sailor K, Yuan JP, Roderick HL, Bootman MD, Worley PF, Song H, Ming GL. XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nat. Neurosci. 2005;8:730–735. doi: 10.1038/nn1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 434:898–904. doi: 10.1038/nature03478. [DOI] [PubMed] [Google Scholar]
- 47.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 48.Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell. 2000;102:363–375. doi: 10.1016/s0092-8674(00)00041-6. [DOI] [PubMed] [Google Scholar]
- 49.Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- 50.Ming GL, et al. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- 51.Brittis PA, et al. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–235. doi: 10.1016/s0092-8674(02)00813-9. [DOI] [PubMed] [Google Scholar]
- 52.Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- 53.Borodinsky LN, et al. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- 54.Demarque M, Spitzer NC. Activity-dependent expression of Lmx1b regulates specification of serotonergic neurons modulating swimming behavior. Neuron. 2010;67:321–334. doi: 10.1016/j.neuron.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dulcis D, Spitzer NC. Illumination controls dopaminergic differentiation regulating behavior. Nature. 2008;456:195–201. doi: 10.1038/nature07569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borodinsky LN, Spitzer NC. Activity-dependent neurotransmitter-receptor matching at the neuromuscular junction. Proc. Nat. Acad. Sci. USA. 2007;104:335–340. doi: 10.1073/pnas.0607450104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat. Rev. Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 58.Owens DF, Kriegstein AR. Developmental neurotransmitters? Neuron. 2002;36:989–991. doi: 10.1016/s0896-6273(02)01136-4. [DOI] [PubMed] [Google Scholar]
- 59.Rivera C, et al. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 60.Barbin G, et al. Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons. Neurosci. Lett. 1993;152:150–154. doi: 10.1016/0304-3940(93)90505-f. [DOI] [PubMed] [Google Scholar]
- 61.Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- 62.Chudotvorova I, et al. Early expression of KCC2 in rat hippocampal cultures augments expression of functional GABA synapses. J. Physiol. 2005;566:671–679.29. doi: 10.1113/jphysiol.2005.089821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akerman CJ, Cline HT. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J. Neurosci. 2006;26:5117–5130. doi: 10.1523/JNEUROSCI.0319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang DD, Kriegstein AR. GABA regulates excitatory synapse formation in the neocortex via NMDA receptor activation. J. Neurosci. 2008;28:5547–5558. doi: 10.1523/JNEUROSCI.5599-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tyzio R, et al. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J. Neurosci. 1999;19:10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tyzio R, et al. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 2006;314:1788–1792. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- 67.Barkan S, Ayali A, Nottebohm F, Barnea A. Neuronal recruitment in adult zebra finch brain during a reproductive cycle. Dev. Neurobiol. 67:687–701. doi: 10.1002/dneu.20379. [DOI] [PubMed] [Google Scholar]
- 68.Brainard MS, Doupe AJ. What songbirds teach us about learning. Nature. 2002;417:351–358. doi: 10.1038/417351a. [DOI] [PubMed] [Google Scholar]
- 69.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 70.Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu. Rev. Cell Dev. Biol. 2009;25:253–75. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 71.Cameron HA, et al. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J. Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malberg JE, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 74.van Praag H, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Nat. Acad. Sci. U.S.A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parent JM, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Markwardt SJ, et al. Input-specific GABAergic signaling to newborn neurons in adult dentate gyrus. J. Neurosci. 2009;29:15063–15072. doi: 10.1523/JNEUROSCI.2727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marek KW, Kurtz LN, Spitzer NC. cJun phosphorylation integrates calcium spike activity and tlx3 expression to regulate neurotransmitter specification. Nat. Neurosci. 2010;13:944–950. doi: 10.1038/nn.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macdonald JL, Roskams AJ. Epigenetic regulation of nervous system development by DNA methylation and histone deacetylation. Prog. Neurobiol. 2009;88:170–183. doi: 10.1016/j.pneurobio.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 80.Keverne EB, Curley JP. Epigenetics, brain evolution and behaviour. Front. Neuroendocrinol. 2008;29:398–412. doi: 10.1016/j.yfrne.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Van d.V. Genetic effects of methylation diets. Annu. Rev. Nutr. 2002;22:255–282. doi: 10.1146/annurev.nutr.22.010402.102932. [DOI] [PubMed] [Google Scholar]
- 82.Szyf M, et al. The social environment and the epigenome. Environ. Mol. Mutagen. 2008;49:46–60. doi: 10.1002/em.20357. [DOI] [PubMed] [Google Scholar]
- 83.Szyf M, et al. Maternal care, the epigenome and phenotypic differences in behavior. Reprod. Toxicol. 2007;24:9–19. doi: 10.1016/j.reprotox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 84.Romieu P, et al. Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats. J. Neurosci. 2008;28:9342–9348. doi: 10.1523/JNEUROSCI.0379-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murgatroyd C, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat. Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 86.Dolinoy DC, et al. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. U. S. A. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Francis D, et al. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 88.Miller CA, et al. Cortical DNA methylation maintains remote memory. Nat. Neurosci. 2010;13:664–666. doi: 10.1038/nn.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farcas R, et al. Differences in DNA methylation patterns and expression of the CCRK gene in human and nonhuman primate cortices. Mol. Biol. Evol. 2009;26:1379–1389. doi: 10.1093/molbev/msp046. [DOI] [PubMed] [Google Scholar]
- 90.Ladd-Acosta C, et al. DNA methylation signatures within the human brain. Am. J. Hum. Genet. 2007;81:1304–1315. doi: 10.1086/524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu. Rev. Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Crow YJ, Livingston JH. Aicardi-Goutieres syndrome: an important Mendelian mimic of congenital infection. Dev. Med. Child Neurol. 2008;50:410–416. doi: 10.1111/j.1469-8749.2008.02062.x. [DOI] [PubMed] [Google Scholar]
- 93.de Bruin RA, Wittenberg C. All eukaryotes: before turning off G1-S transcription, please check your DNA. Cell Cycle. 2009;8:214–217. doi: 10.4161/cc.8.2.7412. [DOI] [PubMed] [Google Scholar]
- 94.Putnam CD, Jaehnig EJ, Kolodner RD. Perspectives on the DNA damage and replication checkpoint responses in Saccharomyces cerevisiae. DNA Repair (Amst) 2009;8:974–982. doi: 10.1016/j.dnarep.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boye E, Skjolberg HC, Grallert B. Checkpoint regulation of DNA replication. Methods Mol. Biol. 2009;521:55–70. doi: 10.1007/978-1-60327-815-7_4. [DOI] [PubMed] [Google Scholar]
- 96.Young-Pearse TL, et al. A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J. Neurosci. 2007;27:14459–14469. doi: 10.1523/JNEUROSCI.4701-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manent JB, Jorquera I, Franco V, Ben-Ari Y, Perucca E, Represa A. Antiepileptic drugs and brain maturation: fetal exposure to lamotrigine generates cortical malformations in rats. Epilepsy Res. 2008;78:131–139. doi: 10.1016/j.eplepsyres.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 98.Ackman JB, et al. Abnormal network activity in a targeted genetic model of human double cortex. J. Neurosci. 2009;29:313–327. doi: 10.1523/JNEUROSCI.4093-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bai J, et al. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat. Neurosci. 2003;6:1277–1283. doi: 10.1038/nn1153. [DOI] [PubMed] [Google Scholar]
- 100.Nopoulos P, et al. Morphology of the cerebral cortex in preclinical Huntington's disease. Am. J. Psychiatry. 2007;164:1428–1434. doi: 10.1176/appi.ajp.2007.06081266. [DOI] [PubMed] [Google Scholar]
- 101.Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J. Neurophysiol. 2005;93:2809–2821. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- 102.Boulanger LM. MHC class I in activity-dependent structural and functional plasticity. Neuron Glia Biol. 2004;1:283–289. doi: 10.1017/S1740925X05000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc. Natl. Acad. Sci. U. S. A. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nedivi E, et al. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993;363:718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- 106.Cohen I, et al. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 107.Represa A, Ben-Ari Y. Molecular and cellular cascades in seizure-induced neosynapse formation. Adv. Neurol. 1997;72:25–34. [PubMed] [Google Scholar]
- 108.Manent JB, et al. Dcx reexpression reduces subcortical band heterotopia and seizure threshold in an animal model of neuronal migration disorder. Nat. Med. 2009;15:84–90. doi: 10.1038/nm.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]