Abstract
Control of lymphocyte homeostasis is essential to ensure efficient immune responses and to prevent autoimmunity. Splenic marginal zone B-cells are important producers of autoantibodies, and are subject to stringent tolerance mechanisms to prevent autoimmunity. In this paper, we explore the role of the Mer tyrosine kinase (Mertk) in regulating autoreactive B cells. This receptor tyrosine kinase serves to bind apoptotic cells, to mediate their phagocytosis, and to regulate subsequent cytokine production. Mice lacking Mertk suffer from impaired apoptotic cell clearance and develop a lupus-like autoimmune syndrome. Here we show that such Mertk-KO mice have expanded numbers of splenic marginal zone B cells. Mertk-KO mice bearing a DNA-specific immunoglobulin heavy-chain transgene (3H9) produced anti-DNA antibodies that appeared to be secreted largely by marginal zone B cells. Finally, Mertk-KO mice developed greater antibody responses after NP-Ficoll immunization than their B6 counterparts. Taken together, our data show that Mertk has a major effect on the development of the marginal zone B-cell compartment. Mertk is also important in establishing DNA-specific B cell tolerance in 3H9 anti-DNA transgenic mice.
Keywords: Apoptosis, Autoantibodies, Autoimmunity, Tolerance, MZ B-cell
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by autoantibodies to nuclear antigens, including double-stranded DNA (dsDNA) [1]. Though dsDNA-specific B cells are detectable in normal individuals, potent mechanisms limit their activation and subsequent antibody secretion. Even in mice expressing a dsDNA-specific immunoglobulin transgene, central and peripheral tolerance mechanisms prevent activation or maturation of anti-dsDNA secreting B cells. One such well-studied system is mice bearing the 3H9 transgene, which encodes a heavy chain that forms an anti-DNA antibody when paired with most light chains [2-4]. B cells from 3H9 transgenic mice lose their DNA specificity, mainly through receptor editing. 3H9 transgenic mice have been generated in which the 3H9 transgene is inserted within the IgH locus, thus allowing normal transcriptional control and class switching [4, 5] (herein referred to as 3H9 mice). Whether the transgene is conventionally expressed or “knocked-in”, 3H9 transgenic mice which have otherwise normal genetic backgrounds do not develop serum anti-DNA antibody titers [6].
Recently, the process of cell death has been suggested as the source of the antigenic stimulus that drives the production of anti-DNA antibodies in lupus [7-9]. Under normal conditions, the rapid and efficient clearance of apoptotic cells and their debris may prevent this form of self-immunization. The receptor tyrosine kinase Mertk is one of multiple receptors that participate in the recognition and phagocytosis of these apoptotic cells. Mertk is expressed on macrophages, dendritic cells, and to some extent, on activated B cells [10-12]. Mice deficient in Mertk expression (Mertk-KO) show an impairment of macrophage-mediated phagocytosis and clearance of apoptotic cells [13, 14]. Although these Mertk-KO mice did not display a broad spectrum of autoimmunity, these mice did develop a lupus-like serology late in their life [9]. In this report, we found a two-fold increase of marginal zone (MZ) B cells in mice lacking Mertk expression. This may contribute to the stronger T-independent antibody responses to NP-Ficoll in Mertk-KO mice. To elucidate the mechanism whereby DNA-specific B cells are inactivated, we generated 3H9 transgenic Mertk-deficient mice (3H9/Mertk-KO). Unlike Mertk sufficient 3H9 mice on a B6 background, these animals indeed produced anti-DNA antibodies, and at an earlier age than mice lacking Mertk. Consistent with previous findings, these mice developed expanded marginal zones, which appeared to contain the bulk of the anti-DNA antibody forming cells. These findings suggest that Mertk serves to prevent the expansion of MZ anti-DNA specific B cells.
2. Methods
2.1. Mice
C57BL/6 (B6) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and subsequently bred at our mouse colony. Mertk-KO mice were generated at the University of North Carolina [15] and back crossed for 10 generations to B6 in our facility. 3H9 knock-in transgenic (3H9) mice on the C57BL/6 background, originally obtained from Dr. Martin Weigert [4], University of Chicago, were crossed with Mertk-KO mice (also having been bred to the B6 background for multiple generations). 3H9 transgene positive (heterozygous) and Mertk-KO mice (3H9/Mertk-KO) were used. 6-8 week-old mice were used unless otherwise indicated. All of the experimental procedures performed on these animals were conducted according to the guidelines of our Institutional Animal Care and Use Committee.
2.2. Reagents and immunization procedure
NP-Ficoll, NP-BSA, and NP-LPS were purchased from Biosearch Technologies (Novato, CA). OVA protein was purchased from Sigma (St. Louis, MO). Antibodies labeled with various dyes used in flow cytometry were from BD Biosciences (San Diego, CA): CD80, CD19, CD86, I-Ab, CD25, CD3, CD40, CD21, and CD23. Mice were immunized i.p. with either 300μg OVA (absorbed with alum), with 50μg NP-Ficoll in PBS, or with 50μg NP-LPS in PBS. Mice sera were collected at different time points and stored at -20°C for ELISA.
2.3. Flow cytometry
Mice were sacrificed and spleens were collected in cold RPMI-1640. Single-cell suspensions were prepared and red blood cells were lysed using ACK lysing buffer. Staining was done in staining buffer (1% BSA in PBS) with various combinations of antibodies at 4°C for 45 minutes. Cells were washed once and data were collected using a FACS Canto flow cytometer and Cell Quest software (BD Immunocytometry Systems, San Jose, CA). Data analysis was performed using FlowJo software (San Carlos, CA).
2.4. ELISA
96-well plates were coated with 10ug/ml OVA or 10ug/ml NP(30)-BSA in PBS overnight at 4°C. Plates were washed once with distilled water. Plates were then blocked with 1% BSA in PBS overnight at 4°C, and incubated with various dilutions of serum for 2 hrs at 37°C. Plates were then washed with wash buffer (PBS/0.05% Tween-20) for 3 times, and either biotinylated goat anti-mouse IgM or IgG1, IgG2c, IgG2b, IgG3, IgG antibodies (Southern Biotechnology Associates, Birmingham, AL) diluted 1/5000 in 1% BSA/PBS was added for 1 h at 37°C. Plates were washed again 5 times and alkaline phosphate conjugated avidin (Southern Biotechnology, Birmingham, AL) was added for 1 h at 4°C prior to the addition of the alkaline phosphate substrate p-nitrophenyl phosphate (Sigma, St. Louis, MO). The OD was measured at 405 nm using a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA).
2.5. Anti-dsDNA ELISA
DNA was prepared from calf thymus DNA as previously described [16]. Polyvinyl microtiter plates (Dynex Technologies, Inc., Chantilly, VA) were pre-treated with poly-L-lysine and coated with 2μg/ml dsDNA in borate-buffered saline (BBS). Plates were blocked with BBT (0.5% BSA, 0.4% Tween-80 in BBS) and diluted samples (1:100 in BBT) were added and allowed to incubate overnight at 4°C. Anti-dsDNA antibodies were detected with anti-Fcγ− or anti-Fcμ-specific anti-mouse alkaline-phosphatase conjugated F(ab′)2 fragments (Jackson ImmunoResearch Laboratories, West Grove, PA) and substrate (disodium p-nitrophenyl phosphate, Sigma, St Louis, MO). For allotype-specific anti-dsDNA ELISA, monoclonal anti-allotype anti-IgG2aa or anti-IgG2ab were used as detecting antibodies. Optical density (O.D.) was read at 405nm. Data are expressed as equivalent dilution factor (EDF) based on a standard curve using serial dilutions of an MRL/lpr reference serum (EDF is the dilution of the reference serum that gives an assay O.D. equal to that of the sample serum) [17].
2.6. Enzyme-linked immunoSPOT (ELISPOT) assay
In each experiment, splenocytes from two 3H9/Mertk-KO, Mertk-KO, 3H9, or MRL/lpr mice were pooled. B cells were isolated by negative selection (Miltenyi Biotec, Auburn, CA). B cells were then incubated with anti-CD23PE, followed by anti-PE magnetic microbeads and MACS sorted. In this fashion, the CD23+ fraction was enriched for FO B cells, while the CD23- fraction was enriched for MZ B cells. Serial dilutions of cells starting at 1 × 106 were added in triplicate to microtiter wells coated with dsDNA (50 μg/mL) and incubated for 4 hours at 37°C. Anti-dsDNA B cells were revealed with standardized alkaline-phosphatase labeled anti-Igκ (HB-58, ATCC, Manassas, VA) and BCIP/NBT, and spots counted using an ELISPOT reader (Cellular Technology, Cleveland, OH).
2.7. Statistic analysis
Data are presented as the mean ± SD. Statistical significance was determined using Student's t test. Double asterisks indicate differences with p<0.01; single asterisks indicate differences with p<0.05.
3. Results
3.1. Characterization of B-cell populations in Mertk-KO mice spleens
Mice lacking all three related TAM (Tyro3/ Axl /Mertk/) receptors showed a profound expansion of T and B-lymphocytes [18]. We, therefore, characterized B-cell subpopulations in mice lacking Mertk expression. At two months, we did not observe splenomegaly in the Mertk-KO mice. The total number of splenocytes and B cells was similar between B6 and Mertk-KO mice (data not shown). The surface expression levels of MHC-II, CD86 and IgM on B1a and B2 in the peritoneal cells were comparable between Mertk-KO and WT mice (data not shown), suggesting that the B-cell populations are mostly in a resting stage as in B6 mice. Interestingly, a nearly two-fold increase of MZ B cells was consistently observed in the spleens of young (8-week) and old (40-week) Mertk-KO mice compared to age-matched WT B6 mice (Figure 1). A two-fold amplification of absolute MZ B cell numbers in Mertk-KO mice was also observed since the total numbers of B cells from Mertk-KO and B6 mice were not significantly different.
Figure 1. Increased MZ B cells in Mertk-KO mice spleen.
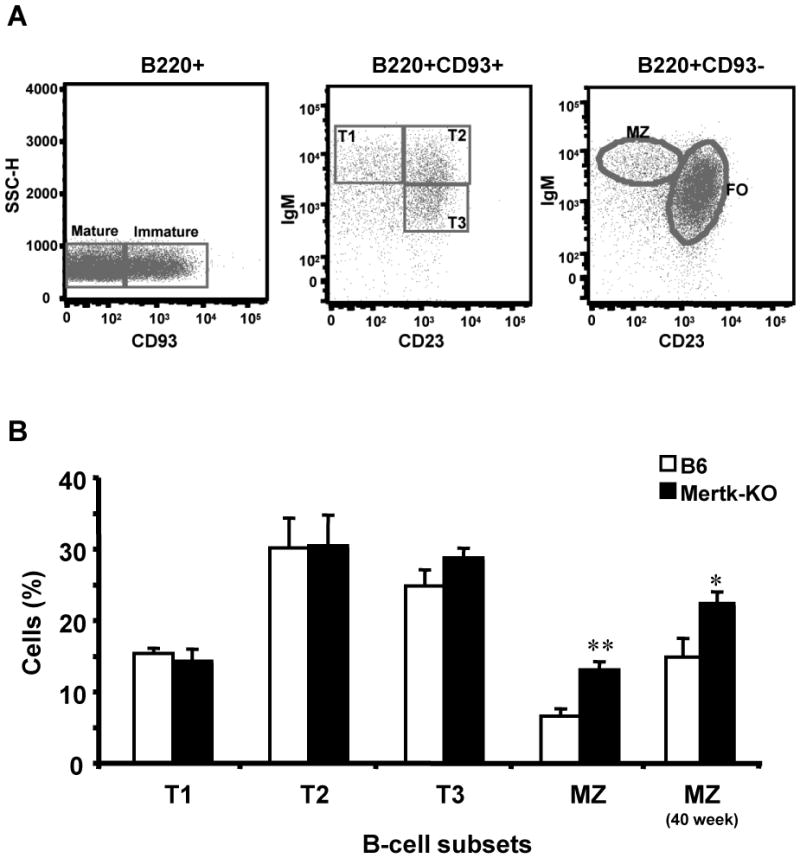
(A) Separation of splenic B cells subsets. Plots were first gated for live lymphocytes based on forward vs side scatter profiles. Using the Allman classification scheme [32], B220+CD93+ cells were subdivided based on CD23 and IgM expression. FO B cells were identified as IgM+CD23+, MZ B cells were identified as IgM+CD23-. (B) Splenic B-cell subsets in percentage.
3.2 Enhanced T-independent responses in Mertk-KO mice
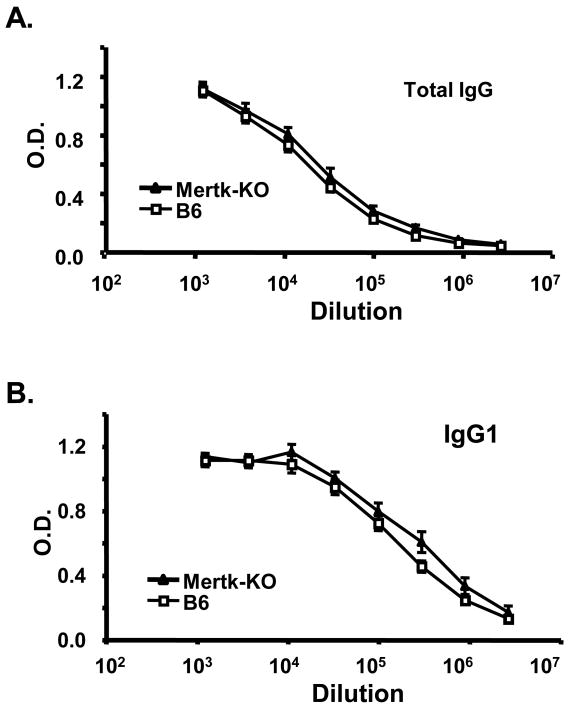
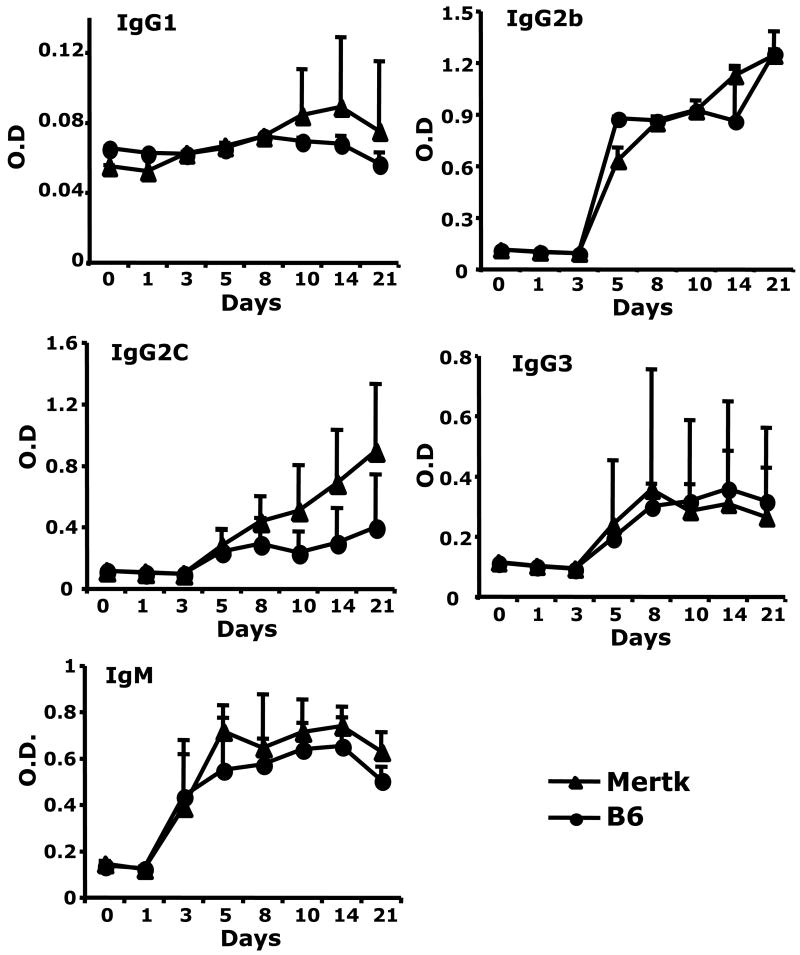
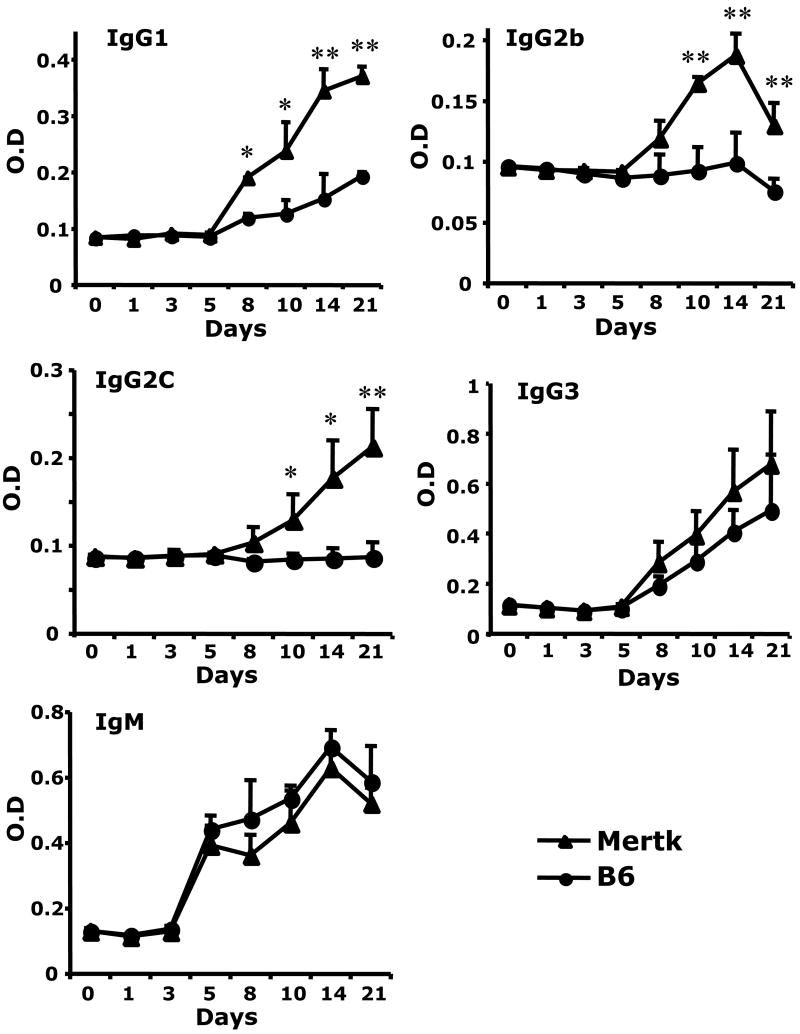
B cells localized to the splenic MZ share many functional properties with B1 B cells. These cells may serve as critical components in host defense against T-independent antigens. In contrast to FO B cells, MZ B cells are enriched for specificities common to T-independent antigens and may also present antigen to T cells efficiently. We tested whether Mertk-KO mice could develop normal antibody responses to T-dependent (TD) and T-independent (TI) antigens. As shown in figure 2, Mertk-KO mice developed similar anti-OVA antibody responses compared to B6 mice. Next, we immunized Mertk-KO mice with a type 1 TI (TI-1) antigen (NP-LPS) and a type 2 TI (TI-2) antigen (NP-Ficoll). As had been shown previously, Mertk-KO mice developed a normal TI response to NP-LPS (figure 3); yet they developed greater antibody responses to NP-Ficoll (Figure 4). The differences were most prominent in IgG1, IgG2c, and IgG2b isotypes.
Figure 2. Normal T-dependent responses in Mertk-KO mice.
Mer-KO (solid triangles) and WT (B6, open squares) mice were immunized with OVA. Serum anti-OVA Ab levels were determined by ELISA as described in the Methods. Data are representative of three replicate experiments.
Figure 3. Similar response to NP-LPS in Mertk-KO and WT mice.
Mertk-KO mice (solid triangle) and B6 mice (solid circle) were immunized with 50 μg NP-LPS in PBS. Isotypes of anti-NP antibody levels were measured by ELISA.
Figure 4. Enhanced type-2 T-independent response in Mertk-KO mice.
Mertk-KO mice (solid triangle) and B6 mice (solid circle) were immunized with 50 μg NP-Ficoll in PBS. Isotypes of anti-NP antibody levels were measured by ELISA. The single and double asterisks indicate significant differences from WT B6 mice with a p <0.05 or <0.01, respectively.
3.3. Anti-dsDNA autoantibodies in 3H9/Mertk-KO mice
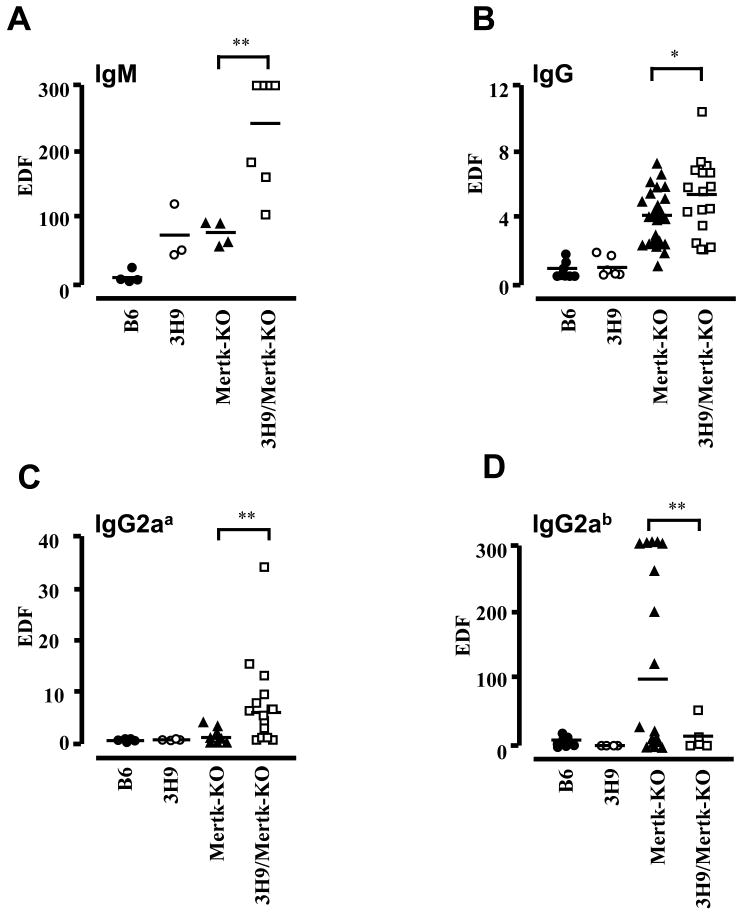
The anti-dsDNA heavy chain knock-in mice, 3H9 mice, have skewed B cell repertoire. Yet, 3H9 mice are well tolerized [3]. When 3H9 mice were bred onto Mertk-KO background, the 3H9/Mertk-KO mice developed spontaneous anti-dsDNA IgM titers as early as two months of age with significantly greater levels than either Mertk-KO or 3H9 mice (Figure 5A). At this age, no anti-dsDNA IgG is present in non-transgenic Mertk-KO mice, which develop autoimmunity over a longer timeframe. In three- to five-month old mice, anti-dsDNA IgG levels are significantly greater in 3H9/Mertk-KO than Mertk-KO mice (Figure 5B). As expected, B6 mice bearing 3H9 were well tolerized and they do not produce anti-dsDNA autoantibodies (Figure 5).
Figure 5. Anti-dsDNA antibody levels in 3H9/Mertk-KO and Mertk-KO mice.
Anti-dsDNA IgM level of two-month old mice (A) and anti-dsDNA total IgG (B), anti-dsDNA IgG2aa (C), and anti-dsDNA IgG2ab (D) levels in three- to five-month old B6 (filled circles), 3H9 (open circles), Mertk-KO (filled triangles) and 3H9/Mertk-KO (open squares) mice. Each symbol represents a different mouse. Units are in equivalent dilution factor (EDF) based on a standard curve using serial dilutions of a reference serum. Each point represents the EDF of an individual mouse. In (B) and (C), the EDF for control MRL/lpr sera was 20 and 70 respectively, while control B6.C20 sera had an EDF of 1 and 0.2 respectively. In (D), B6/lpr sera had an EDF of 300. * p<0.05, ** p<0.01.
It is possible that the anti-dsDNA antibody observed in 3H9/Mertk-KO mice was derived from endogenous heavy chains, especially since Mertk-KO mice have a propensity to spontaneously develop autoimmunity. Heavy chain editing or incomplete allelic exclusion might permit the emergence of autoreactive B cells using the endogenous heavy chain repertoire. As “a” allotype anti-dsDNA antibodies are present in 3H9/Mertk-KO mice [4, 5], it is apparent that the anti-dsDNA antibodies in 3H9/Mertk-KO mice are a product of the 3H9 transgene (Figure 5C). The production of “b” allotype autoantibody (Figure 5D) in 3H9/Mertk-KO mice corresponds well with the increased IgG level in figure 5B.
3.4. Expansion of marginal zone B cell population in 3H9/Mertk-KO mice
The splenic marginal zone has been assigned a special role in some animal models in the disposition of autoreactive B cells [19]. 3H9 mice have been shown to accumulate larger numbers of marginal zone B cells than non-transgenic controls [20], and L chain editing is thought to be especially active in this anatomic region [20-22]. 3H9/Mertk-KO mice have a similarly expanded population of marginal zone B cells (Figure 6) as 3H9 mice. Analysis of a second larger cohort of mice confirmed this expansion in 3H9/Mertk-KO mice. No difference in MZ size was observed between male and female mice (data not shown).
Figure 6. Expansion of MZ B cell populations in 3H9/Mertk-KO and 3H9 mice.
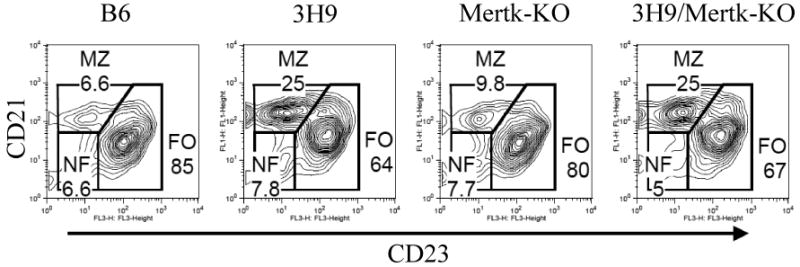
Splenocytes were harvested from four- to five-month old mice, stained and analyzed by FACS. Plots shown are gated on CD19+ lymphocytes and stained for CD21 and CD23. Data are representative of three mice per group. MZ B cells (CD21+CD23-), FO B cells (CD21+CD23+), newly formed B cells (NF, CD21-CD23-) are analyzed based on their surface markers.
3.5. Marginal zone B cells secrete anti-dsDNA autoantibody in 3H9/Mertk-KO mice
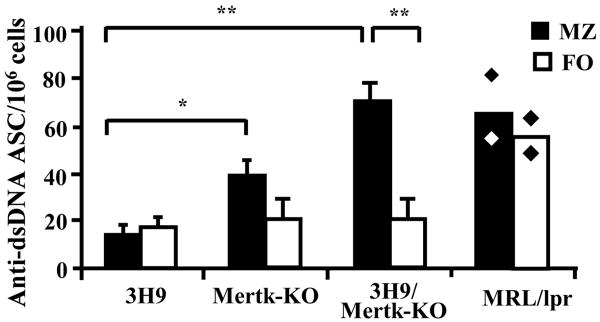
We wondered if the expanded marginal zones of the spleens in 3H9/Mertk-KO mice might contain increased numbers of anti-dsDNA producing B cells, reasoning that Mertk deficiency might impede the establishment of tolerance in this zone. To address this question, splenocytes were enriched for MZ or FO B cells and ELISPOTS performed to quantitate the relative amounts of anti-dsDNA antibody produced by B cells originating from these areas. A significantly greater number of MZ B cells from 3H9/Mertk-KO mice secreted anti-dsDNA antibodies compared to FO B cells (Figure 7) or compared to MZ B cells from control 3H9 mice (Figure 7), despite an equivalent expansion of MZ B cells in 3H9 and 3H9/Mertk-KO mice (Figure 6). Thus, the absence of Mertk in 3H9 animals appeared to provoke activation of the MZ B cells and subsequent production of anti-dsDNA antibodies.
Figure 7. MZ B cells secrete anti-dsDNA IgG in 3H9/Mertk-KO mice.
MZ (black bars) and FO (white bars) B cells were isolated from five-month old mice and anti-dsDNA antibody-secreting cells (ASC) were quantitated by ELISPOT analysis. The number of anti-dsDNA ASC out of 105 MZ or 105 FO cells is shown. Values are means ± SD of three experiments, within each experiment two mice for every strain were pooled except for MRL/lpr where values are the means of two experiments (solid rhombus).
4. Discussion
MZ B cells are localized in the marginal sinus surrounding lymphoid follicles and are thought to be the first B cells to encounter and respond to antigen, often in a T-independent manner [19, 23]. They have low activation thresholds, with elevated constitutive B7 expression levels, and an increased ability to present antigen to T lymphocytes [24, 25]. Two groups of antigens can activate B cells in a T independent manner: TI-1 antigens activate B cells without the need of second signal; TI-2 antigens need residual noncognate T help for activation of B cells. The latter antigens elicit robust and long-lasting primary antibody responses in mice [26]. Amlotet et. al showed that antibody responses to TI-2 antigens depend on stimulation of B cells in splenic environments involving antigen presentation by MZ macrophages [27]. Enhanced TI-2 responses provoked by NP-Ficoll in Mertk-KO mice correlate well with the increase MZ B cells. Macrophages lacking Mertk might be more capable of presenting TI-2 antigens, consistent with the find that Mertk can suppress DC antigen presentation [28].
Our data emphasize the important role of the splenic marginal zone in B-cell tolerance. Macrophages in this zone, via expression of Mertk [29], may serve a specialized function of eliminating apoptotic debris and thus removing the stimulus for autoimmunity. The Mertk kinase serves a key role as a receptor for apoptotic debris through its interaction with phosphatidylserine via the bridging proteins GAS6 or protein S. We have previously shown that Mertk deficiency leads to systemic autoimmunity in mice [9]. In the present studies, we have extended this work by determining the effect of Mertk on the regulation of a site-directed Ig transgene (3H9) that targets the lupus autoantigen, DNA. Normal mice bearing the 3H9 transgene secrete little to no anti-dsDNA antibodies, indicating tolerance of 3H9 autoreactive B cells [4, 30]. In contrast, 3H9 mice lacking Mertk (3H9/Mertk-KO mice) produce anti-dsDNA antibody of the transgenic allotype. Our findings suggest that Mertk, perhaps expressed on macrophages within the marginal zone, serves to prevent the expansion of anti-DNA specific B cells. This may be accomplished through reducing the burden of autoimmunogenic apoptotic debris and perhaps also through the influence of Mertk in diverting macrophages from inflammatory cytokine production. Thus, autoantigen overload may result from the defect in phagocytosis of apoptotic cells characteristic of Mertk-deficient mice, despite normal phagocytosis of other particles [9, 13]. Additionally, we and others have demonstrated that injection of apoptotic cells into Mertk-deficient mice can stimulate a transient autoantibody response, indicating the presence of a tolerant/ignorant population of autoantigen-specific B cells responsive to antigenic stimulation [9, 31]. Thus, the presence of apoptotic bodies (such as that seen in Mertk-KO mice) may induce the activation of anergic or ignorant B cells and subsequent autoantibody production, which would explain our observation of anti-dsDNA antibody levels in 3H9/Mertk-KO mice as compared to 3H9 or Mertk-KO mice. Similar studies have shown that Mertk-KO mice transgenic for an anti-Sm autoantibody develop anti-Sm titers [31]. Nevertheless, the allotype specificity from 3H9 knock-in gene allow us to further identify the MZ B cells as autoantibody secreting cells. The increased number of MZ B-cell in Mertk-KO (Figure 1) and 3H9/Mertk-KO mice (Figure 6), confirmed by gating in different ways, is probably due to the expansion of autoreactive B-cell populations.
In recent studies with NZB/W mice, a difference in anti-dsDNA antibody production between conventional 3H9 (c-3H9, randomly inserted μ H chain) transgenic mice and the 3H9 knock-in (3H9, site-directed JH knock-in) transgenic mice was noted [6]. Since the 3H9 heavy chain can be paired with any L chain in both c-3H9 and 3H9 mice and their transgenic B cells have similar surface level of IgMa, the c-3H9 and 3H9 mice are thought to have a comparable repertoire of autoreactive B cells and thus a similar susceptibility to anergy [6]. Yet, only the 3H9 NZB/W mice develop serum anti-dsDNA titers. Thus, the difference in anti-dsDNA production is thought to be due to an increased potential for activation of the 3H9 B cells rather than to an increase in susceptibility to anergy [6]. This concurs with the hypothesis that B cells in 3H9 mice are more susceptible to activation and thus primed to respond to antigenic stimulation. Additionally, anergic 3H9 B cells have been shown to break tolerance in other models, such as in the chronic graft-versus-host disease model [5]. Therefore, the accumulation of apoptotic debris in Mertk-KO mice may be responsible for the activation of DNA-specific B cells in 3H9/Mertk-KO mice.
Mertk may also play a vital role in the tolerance of autoreactive B cells by regulating cytokine secretion ([15] and unpublished data) or as a negative regulator of antigen presentation by dendritic cells [28]. The observed enrichment of the marginal zone for anti-dsDNA producing B cells is consistent with this notion. Also relevant are studies indicating that anti-Sm production by anti-Sm transgenic B cells is enhanced by exposure of B cells to apoptotic debris, and that this process is particularly concentrated among B cells of the marginal zone [31].
In conclusion, the combined studies provide support for the concept of the marginal zone as a clearinghouse for apoptotic debris, and for Mertk as an important molecule for scavenging this material and reducing its immunogenicity.
Acknowledgments
We are grateful for Dr. Ziaur S.M. Rahman for critical reading of this manuscript. This work was supported by grants from the National Institute of Dental and Craniofacial Research at the National Institute of Health (Grant number: R01DE017590) and the United States Department of Veterans Affairs.
Abbreviations
- Mertk
Mer receptor tyrosine kinase
- B6
C57BL/6
- EDF
equivalent dilution factor
- MZ
marginal zone
- FO
follicular zone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohen PL. Apoptotic cell death and lupus. Springer Semin Immunopathol. 2006;28:145–52. doi: 10.1007/s00281-006-0038-z. [DOI] [PubMed] [Google Scholar]
- 2.Radic MZ, Mascelli MA, Erikson J, Shan H, Weigert M. Ig H and L chain contributions to autoimmune specificities. J Immunol. 1991;146:176–82. [PubMed] [Google Scholar]
- 3.Ibrahim SM, Weigert M, Basu C, Erikson J, Radic MZ. Light chain contribution to specificity in anti-DNA antibodies. J Immunol. 1995;155:3223–33. [PubMed] [Google Scholar]
- 4.Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331–4. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 5.Sekiguchi DR, Jainandunsing SM, Fields ML, Maldonado MA, Madaio MP, Erikson J, Weigert M, Eisenberg RA. Chronic graft-versus-host in Ig knockin transgenic mice abrogates B cell tolerance in anti-double-stranded DNA B cells. J Immunol. 2002;168:4142–53. doi: 10.4049/jimmunol.168.8.4142. [DOI] [PubMed] [Google Scholar]
- 6.Steeves MA, Marion TN. Tolerance to DNA in (NZB × NZW)F1 mice that inherit an anti-DNA V(H) as a conventional micro H chain transgene but not as a V(H) knock-in transgene. J Immunol. 2004;172:6568–77. doi: 10.4049/jimmunol.172.11.6568. [DOI] [PubMed] [Google Scholar]
- 7.Kuan AP, Cohen PL. p53 is required for spontaneous autoantibody production in B6/lpr lupus mice. Eur J Immunol. 2005;35:1653–60. doi: 10.1002/eji.200525982. [DOI] [PubMed] [Google Scholar]
- 8.Cohen PL, Caricchio R. Genetic models for the clearance of apoptotic cells. Rheum Dis Clin North Am. 2004;30:473–86. viii. doi: 10.1016/j.rdc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–40. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao WH, Eisenberg RA, Cohen PL. The Mer receptor tyrosine kinase is required for the loss of B cell tolerance in the chronic graft-versus-host disease model of systemic lupus erythematosus. J Immunol. 2008;180:7728–35. doi: 10.4049/jimmunol.180.11.7728. [DOI] [PubMed] [Google Scholar]
- 11.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham DK, Bowman GW, Dawson TL, Stanford WL, Earp HS, Snodgrass HR. Cloning and developmental expression analysis of the murine c-mer tyrosine kinase. Oncogene. 1995;10:2349–59. [PubMed] [Google Scholar]
- 13.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–11. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 14.Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178:5635–42. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- 15.Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162:3498–503. [PubMed] [Google Scholar]
- 16.Cohen PL, Maldonado MA. Animal models for SLE. Curr Protoc Immunol. 2003;Chapter 15 doi: 10.1002/0471142735.im1520s52. Unit 15 20. [DOI] [PubMed] [Google Scholar]
- 17.Yount WJ, Cohen P, Eisenberg RA. Distribution of IgG subclasses among human autoantibodies to Sm, RNP, dsDNA, SS-B and IgG rheumatoid factor. Monogr Allergy. 1988;23:41–56. [PubMed] [Google Scholar]
- 18.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–11. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 19.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–35. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Li H, Weigert M. Autoreactive B cells in the marginal zone that express dual receptors. J Exp Med. 2002;195:181–8. doi: 10.1084/jem.20011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quong MW, Martensson A, Langerak AW, Rivera RR, Nemazee D, Murre C. Receptor editing and marginal zone B cell development are regulated by the helix-loop-helix protein, E2A. J Exp Med. 2004;199:1101–12. doi: 10.1084/jem.20031180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Li H, Ni D, Weigert M. Anti-DNA B cells in MRL/lpr mice show altered differentiation and editing pattern. J Exp Med. 2002;196:1543–52. doi: 10.1084/jem.20021560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 24.Liu YJ, Arpin C. Germinal center development. Immunol Rev. 1997;156:111–26. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 25.Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999;162:7198–207. [PubMed] [Google Scholar]
- 26.Fehr T, Skrastina D, Pumpens P, Zinkernagel RM. T cell-independent type I antibody response against B cell epitopes expressed repetitively on recombinant virus particles. Proc Natl Acad Sci U S A. 1998;95:9477–81. doi: 10.1073/pnas.95.16.9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amlot PL, Grennan D, Humphrey JH. Splenic dependence of the antibody response to thymus-independent (TI-2) antigens. Eur J Immunol. 1985;15:508–12. doi: 10.1002/eji.1830150516. [DOI] [PubMed] [Google Scholar]
- 28.Wallet MA, Sen P, Flores RR, Wang Y, Yi Z, Huang Y, Mathews CE, Earp HS, Matsushima G, Wang B, et al. MerTK is required for apoptotic cell-induced T cell tolerance. J Exp Med. 2008;205:219–32. doi: 10.1084/jem.20062293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao WH, Zhen Y, Eisenberg RA, Cohen PL. The Mer receptor tyrosine kinase is expressed on discrete macrophage subpopulations and mainly uses Gas6 as its ligand for uptake of apoptotic cells. Clin Immunol. 2009;133:138–44. doi: 10.1016/j.clim.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C, Nagy Z, Prak EL, Weigert M. Immunoglobulin heavy chain gene replacement: a mechanism of receptor editing. Immunity. 1995;3:747–55. doi: 10.1016/1074-7613(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 31.Qian Y, Wang H, Clarke SH. Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-1 B cells. J Immunol. 2004;172:625–35. doi: 10.4049/jimmunol.172.1.625. [DOI] [PubMed] [Google Scholar]
- 32.Allman D, Srivastava B, Lindsley RC. Alternative routes to maturity: branch points and pathways for generating follicular and marginal zone B cells. Immunol Rev. 2004;197:147–60. doi: 10.1111/j.0105-2896.2004.0108.x. [DOI] [PubMed] [Google Scholar]