Abstract
Muscles are significant contributors to the high joint forces developed in the knee during human walking. Not only do muscles contribute to the knee joint forces by acting to compress the joint, but they also develop joint forces indirectly through their contributions to the ground reaction forces via dynamic coupling. Thus, muscles can have significant contributions to forces at joints they do not span. However, few studies have investigated how the major lower-limb muscles contribute to the knee joint contact forces during walking. The goal of this study was to use a muscle-actuated forward dynamics simulation of walking to identify how individual muscles contribute to the axial tibio-femoral joint force. The simulation results showed that the vastii muscles are the primary contributors to the axial joint force in early stance while the gastrocnemius is the primary contributor in late stance. The tibio-femoral joint force generated by these muscles was at times greater than the muscle forces themselves. Muscles that do not cross the knee joint (e.g., the gluteus maximus and soleus) also have significant contributions to the tibio-femoral joint force through their contributions to the ground reaction forces. Further, small changes in walking kinematics (e.g., knee flexion angle) can have a significant effect on the magnitude of the knee joint forces. Thus, altering walking mechanics and muscle coordination patterns to utilize muscle groups that perform the same biomechanical function, yet contribute less to the knee joint forces may be an effective way to reduce knee joint loading during walking.
Keywords: musculoskeletal model, forward dynamic simulation, knee joint contact force, muscle contributions, walking
Introduction
The human knee joint is subjected to significant loads during walking, with peak loads well-above body weight (e.g., Anderson and Pandy, 2001; D'Lima et al., 2007; Glitsch and Baumann, 1997; Heinlein et al., 2009; Taylor et al., 2004). The high joint loading is primarily due to muscle forces (e.g., Herzog et al., 2003). Studies analyzing muscle contributions to knee joint loads during walking have focused primarily on those muscles crossing the knee joint, and found that the quadriceps and gastrocnemius are the primary contributors during early and late stance, respectively (Kim et al., 2009; Lin et al., 2010; Morrison, 1970; Schipplein and Andriacchi, 1991; Shelburne et al., 2006). Typically, joint forces are determined using the vector sum of the intersegmental joint forces calculated using inverse dynamics analysis and the compressive forces from the muscles crossing the joint. However, this method does not account for individual muscle contributions to the ground reaction forces since only the net ground reaction force is used to determine the intersegmental joint forces. Because muscles can contribute to all joint forces (even those they do not span) through their contributions to the ground reaction forces via dynamic coupling (Zajac and Gordon, 1989), it is possible for muscles spanning a joint to generate greater joint forces than the forces developed in the muscles themselves. In addition, it is possible for muscles that do not span a joint to have greater contributions to the joint force than muscles spanning the joint due to their contributions to the ground reaction forces. For example, studies have shown that the gluteus maximus and soleus have large contributions to the ground reaction forces (Anderson and Pandy, 2003; Liu et al., 2006; Neptune et al., 2004), and therefore these muscles may have significant contributions to the knee joint force even though they do not anatomically cross the joint. However, few studies have investigated how the major lower-limb muscles contribute to the knee joint contact forces during walking. Understanding how individual muscles contribute to knee joint loading has important clinical implications for developing rehabilitation strategies that focus on specific muscle groups to help reduce knee joint loads for patients with osteoarthritis and other joint disorders (e.g., Fregly et al., 2007; Mundermann et al., 2004; Mundermann et al., 2008a).
The purpose of this study was to use a muscle-driven forward dynamics simulation of normal walking to identify individual muscle contributions to the tibio-femoral joint force. Specifically, we examined the joint force component parallel to the longitudinal axis of the tibia (i.e., axial force), which is the dominant force component (D'Lima et al., 2007). We hypothesized that 1) muscles spanning the knee joint can generate greater tibio-femoral joint forces than the forces developed in the muscles themselves, and 2) muscles that do not span the knee joint can have significant contributions to the tibio-femoral joint forces through their contributions to the ground reaction forces.
Methods
Musculoskeletal model
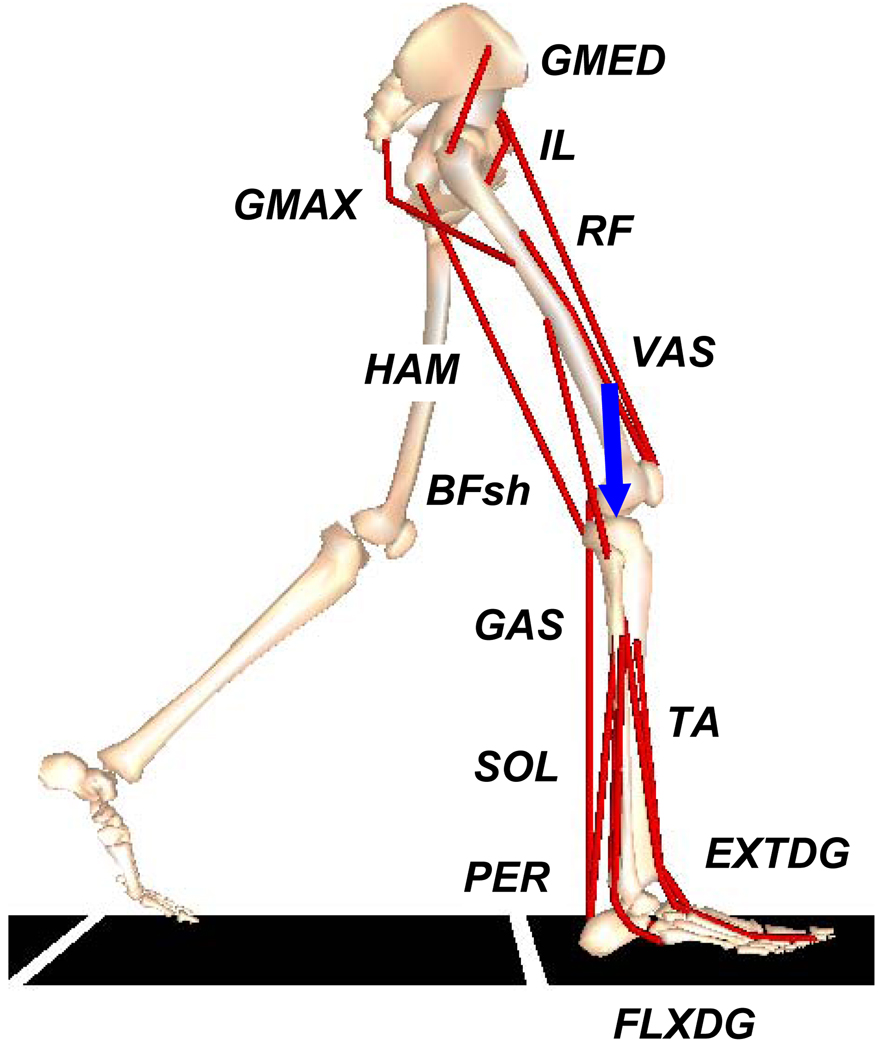
A musculoskeletal model (Fig. 1) was generated using SIMM (MusculoGraphics Inc., Santa Rosa, CA), which consisted of a trunk segment (head, torso and arms) and two legs (femur, tibia, patella, calcaneus, mid-foot and toe for each leg). The model had a total of thirteen degrees of freedom in the sagittal-plane (translations and rotation of the trunk, flexion-extension at the hip, knee, ankle, mid-foot and toe joints for both legs). The motion of the patella and the tibia relative to the femur were prescribed as functions of knee flexion (Yamaguchi and Zajac, 1989). The model was driven by 25 Hill-type muscle actuators per leg, with activation-deactivation dynamics governed by a first-order differential equation (Raasch et al., 1997). The muscles were grouped into thirteen functional groups, with muscles within each group receiving the same excitation pattern (Fig. 1). Muscle electromyography (EMG) data (see Experimental data below) were used to define the muscle excitation patterns. Block patterns were used for muscles where EMG data were not available. Passive torques representing the forces applied by ligaments, passive tissue and joint structures were applied at the hip, knee and ankle joints (Davy and Audu, 1987). The passive torques for the mid-foot and toe joints were defined using the following equation:
where joint angle was defined as the angular displacement from the neutral anatomical position expressed in radians, and constants k, b were (750, 0.05) for the mid-foot joint, and (25, 0.03) for the toe joint expressed in N·m and N·m·s, respectively. Thirty-one visco-elastic elements were attached to each foot segment to model the foot-ground contact (Neptune et al., 2000).
Fig. 1.
The musculoskeletal model consisted of a trunk segment (head, torso and arms) and two legs (femur, tibia, patella, calcaneus, mid-foot and toe for each leg). The muscles included in the model were the GMAX (gluteus maximus, adductor magnus), GMED (anterior and posterior portions of gluteus medius), IL (psoas, iliacus), VAS (three vastii muscles), RF (rectus femoris), HAM (medial hamstrings, biceps femoris long head), BFsh (biceps femoris short head), SOL (soleus, tibialis posterior), GAS (medial and lateral gastrocnemius), TA (tibialis anterior, peroneus tertius), PER (peroneus longus, peroneus brevis), EXTDG (extensor digitorum longus, extensor hallucis longus), FLXDG (flexor digitorum longus, flexor hallucis longus). The axial knee joint force (arrow) is the force component parallel to the longitudinal axis of the tibia.
Forward dynamics simulation of walking
A forward dynamics simulation of walking was generated using Dynamics Pipeline (MusculoGraphics, Inc., Santa Rosa, CA) and SD/FAST (PTC, Needham, MA). Muscle excitation patterns were fine-tuned using dynamic optimization (e.g. Neptune and Hull, 1998), where the differences in kinematics and ground reaction forces between experimental and simulation data were minimized over a full gait cycle (from right heel-strike to the subsequent right-heel strike). In the optimization, a simulated annealing algorithm was used to minimize the following cost function:
where wi,m, is the weighting factor for variable m, Yi,m, is the experimental measurement of variable m, Ŷi,m, is the simulation data corresponding to Yi,m, and SDi,m, is the standard deviation of experimental variable m at time step i. The excitation timing for each muscle was constrained based on the EMG data to ensure that the muscles generated force at the appropriate time in the gait cycle.
Experimental data
Previously collected experimental kinematic, ground reaction force and EMG data (Neptune and Sasaki, 2005) were used. Briefly, ten able-bodied subjects (5 males and 5 females; age 29.6 ± 6.1 years old, height 169.7 ± 10.9 cm, body mass 65.6 ± 10.7 kg) walked on a split-belt instrumented treadmill (TecMachine, France) at 1.2 m/s while data were collected for 15 seconds. Kinematic data collected at 120 Hz (Motion Analysis Corp, Santa Rosa, CA) using a modified Helen Hays marker set were digitally low-pass filtered at 6 Hz. Ground reaction force data were collected at 480 Hz and low-pass filtered at 20 Hz. Surface bi-polar EMG data (Noraxon, Scottsdale, AZ) were collected at 1200 Hz from the soleus, tibialis anterior, medial gastrocnemius, vastus medialis, rectus femoris, biceps femoris long head and gluteus maximus. The EMG signals were band-pass filtered (20–400 Hz), fully rectified and then low-pass filtered at 10 Hz to generate linear envelope signals. All digital filters were fourth-order zero-lag Butterworth filters. All data were normalized to the gait cycle, averaged across steps and then across subjects to obtain group-averaged data.
Muscle contributions to the tibio-femoral joint force
The axial tibio-femoral joint force was computed as the component of joint contact force parallel to the longitudinal axis of the tibia, which includes all forces acting on the joint (i.e., intersegmental joint forces and muscle compressive forces). Individual muscle contributions to the axial joint force were obtained at each time step in the simulation by 1) performing a ground reaction force decomposition to determine individual muscle contributions to the ground reaction forces (Neptune et al., 2001), 2) applying only the muscle force of interest and corresponding ground reaction forces to the system and 3) solving the equations of motion to determine the axial tibio-femoral joint contact force. This process was repeated for each muscle at each time step over the entire gait cycle.
Results
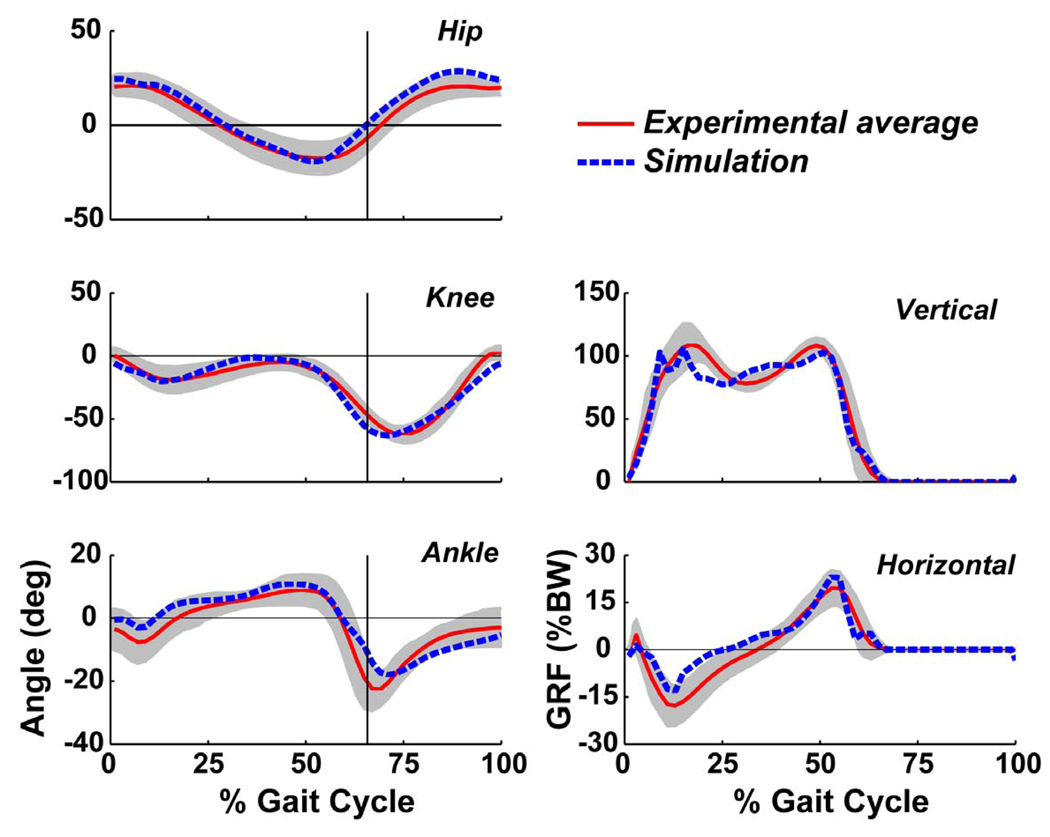
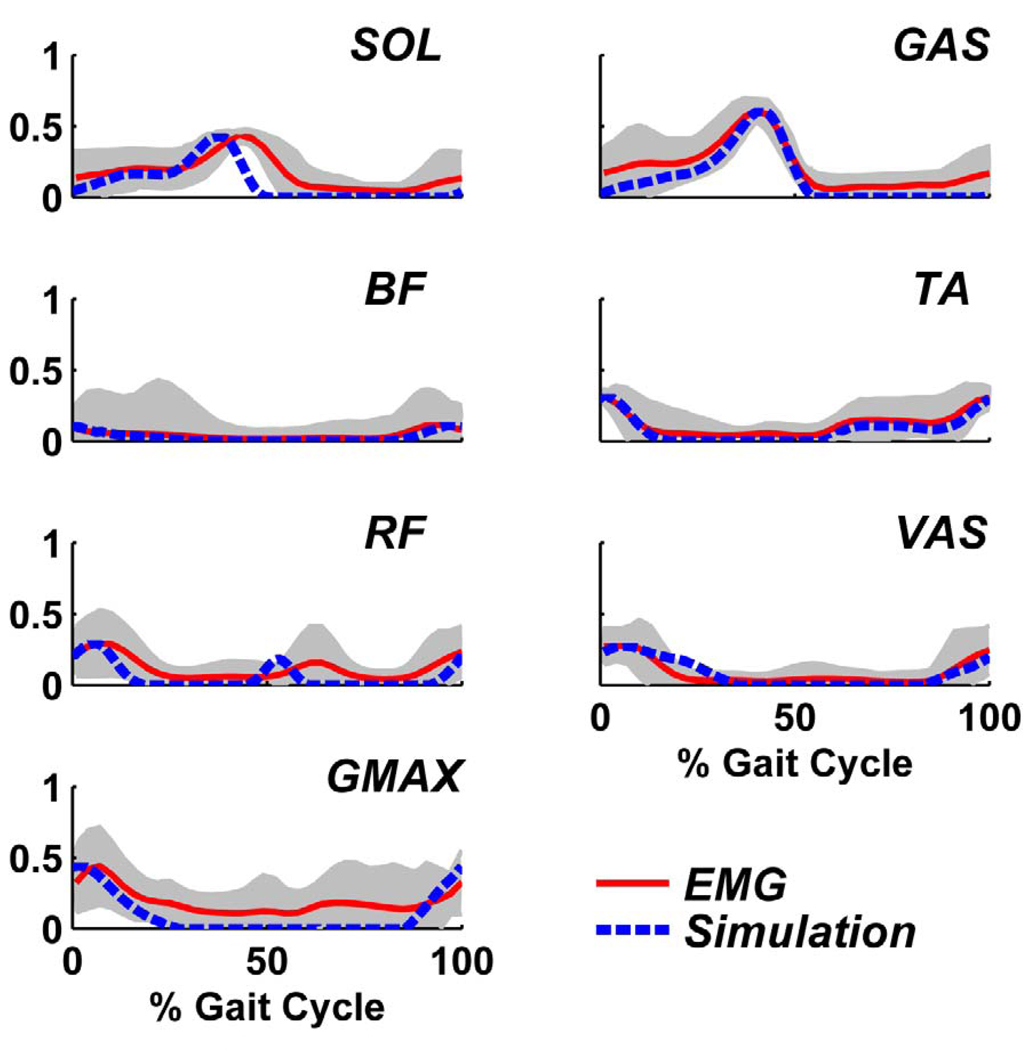
The simulation emulated well the experimentally measured sagittal-plane walking kinematics and ground reaction forces (Fig. 2). The mean absolute errors over the gait cycle for the hip, knee and ankle angles, and vertical and horizontal ground reaction forces were 3.9, 4.7 and 3.2 degrees, and 5.6 % and 2.7 % body weight (BW), respectively. In addition, the resulting muscle excitation patterns matched closely with the experimentally measured EMG patterns (Fig. 3).
Fig. 2.
Comparison between the group average experimental (solid line) and simulation (dashed line) hip, knee and ankle joint angles, and vertical (vGRF) and horizontal (hGRF) ground reaction forces (units: normalized to body weight) over the gait cycle. The shaded regions indicate ± two standard deviations. The vertical lines indicate toe-off.
Fig. 3.
Comparison between the group average EMG (solid line) and simulation muscle excitation (dashed line) patterns. The shaded regions indicate ± one standard deviation. The EMG data were normalized to the maximum value of the corresponding simulation data for comparison.
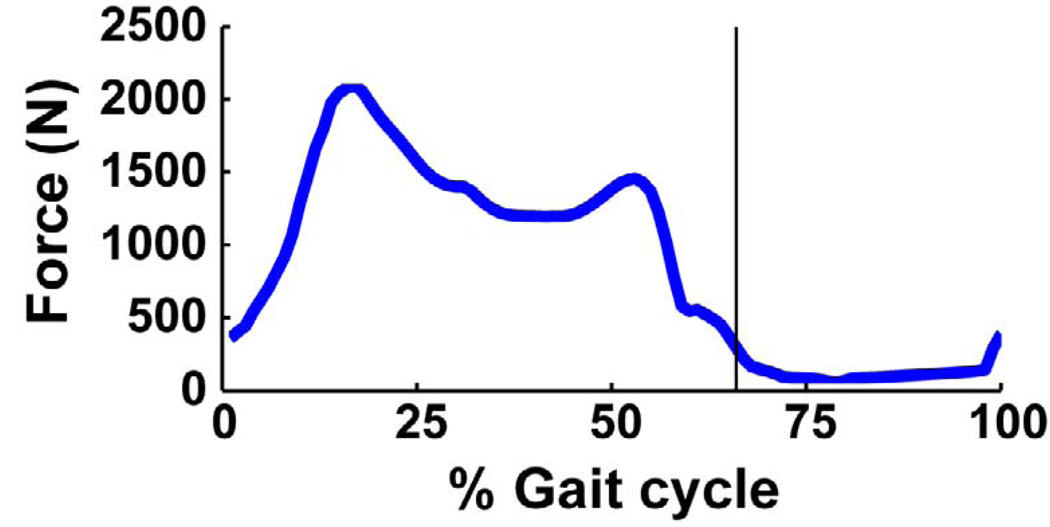
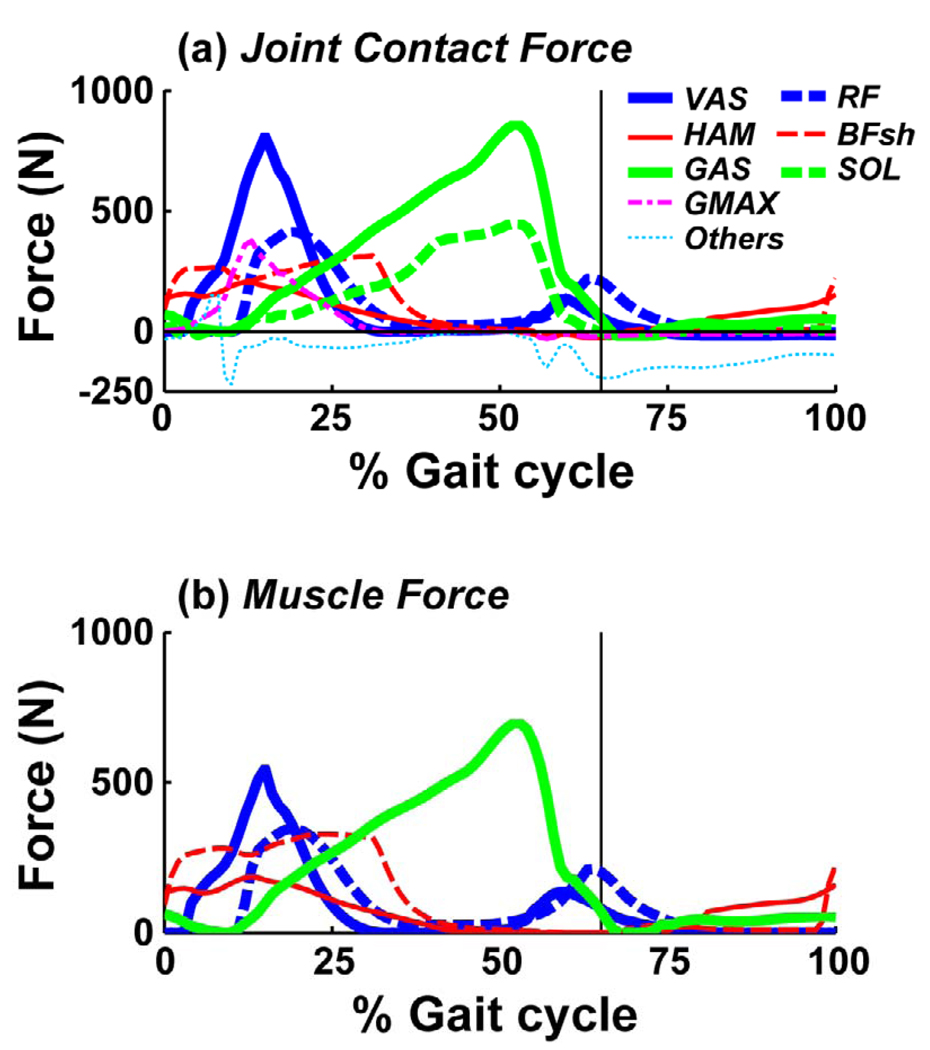
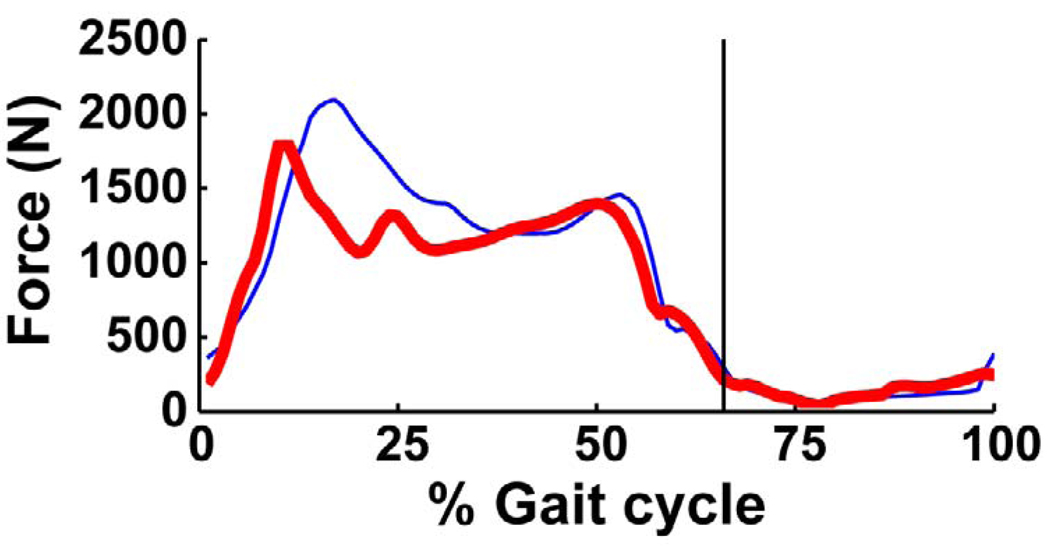
The axial tibio-femoral joint force had two major peaks during the stance phase (Fig. 4). The first peak occurred in early stance (Fig. 4: ~15% gait cycle), reaching a magnitude of ~2100 N (2.8 BW). The vastii muscles were the primary contributors to the joint force, with a peak magnitude of 810 N (Fig. 5a). The rectus femoris, hamstrings, biceps femoris short head and gluteus maximus also contributed to the first peak force (Fig. 5a: RF, HAM, BFsh and GMAX, 0–25% gait cycle). The second peak in late stance was lower than the first peak (Fig. 4: ~50% gait cycle), with a magnitude of ~1450 N (2.0 BW). This peak was primarily due to the gastrocnemius, which had a peak magnitude of 860N (Fig. 5a: GAS), with the soleus also having a significant contribution (Fig. 5a: SOL). The knee joint force during the swing phase was negligible compared to its value in stance. Both the vastii and gastrocnemius muscles generated greater axial tibio-femoral joint forces than the forces developed in the muscles themselves (Fig. 5b: VAS and GAS: the peak muscle forces were 530 N and 700 N, respectively).
Fig. 4.
Axial knee joint contact force over the gait cycle. The model’s body weight was 737 N. The vertical line indicates toe-off.
Fig. 5.
(a) Individual muscle contributions to the axial knee joint contact force over the gait cycle. (b) Muscle force component parallel to the longitudinal axis of the tibia developed in those muscles crossing the knee joint. The vertical lines indicate toe-off.
Some muscles contributed negatively to the axial joint force (Fig. 5a: Others). During the stance phase, this occurred as some muscles (e.g., the tibialis anterior) contributed negatively to the vertical ground reaction force (i.e., they acted to offload the leg), resulting in a negative axial force. During the swing phase, muscles accelerated the shank or thigh in a way that acted to reduce the axial force.
Discussion
The overall objective of this study was to identify individual muscle contributions to the axial tibio-femoral joint force during normal steady-state walking. The knee joint loading pattern during stance had two major peak forces, with the first peak occurring in early stance and the second occurring in late stance (Fig. 4). This pattern was consistent with previous studies (Anderson and Pandy, 2001; Collins, 1995; D'Lima et al., 2006; Glitsch and Baumann, 1997; Heinlein et al., 2009; Shelburne et al., 2006; Taylor et al., 2004; Zhao et al., 2007), although other studies have shown an additional sharp peak occurring immediately after heel strike (D'Lima et al., 2005; Morrison, 1970; Schipplein and Andriacchi, 1991). The vastii and gastrocnemius muscles were the primary contributors to the first and second peak forces, respectively (Fig. 5a: VAS: ~15% gait cycle, GAS: ~50% gait cycle). These results were consistent with previous modeling studies investigating muscle and knee joint forces (Kim et al., 2009; Lin et al., 2010; Schipplein and Andriacchi, 1991; Shelburne et al., 2006), which focused on those muscles crossing the knee joint. However, these studies may have underestimated the contributions of these muscles by not accounting for individual muscle contributions to the ground reaction forces, which indirectly contribute to the knee joint contact force. Our results show that the vastii and gastrocnemius contributions to the axial tibio-femoral joint force (Fig. 5a: VAS, GAS) can be greater than the muscle forces themselves (Fig. 5b: VAS, GAS).
The results also highlighted how muscles that do not cross the knee joint (e.g., the gluteus maximus and soleus) can have significant contributions to the knee joint force (Fig. 5a: GMAX, SOL). This occurs through dynamic coupling (Zajac and Gordon, 1989) that allows their contributions to the ground reaction forces (Anderson and Pandy, 2003; Liu et al., 2006; Neptune et al., 2004) to influence the joint loads. In fact, the soleus had a higher peak value than the rectus femoris and hamstring (HAM and BFsh) muscles, which all cross the knee joint (Fig. 5a). Thus, when seeking to understand how individual muscles contribute to joint loading, it is important to consider their individual contributions to the ground reaction forces.
The peak axial force occurring in early stance reached ~2.8 body weight (BW), which was slightly higher than previous in vivo studies using an instrumented knee implant (~2.2–2.5 BW, D'Lima et al., 2007; D'Lima et al., 2006; Heinlein et al., 2009; Mundermann et al., 2008b; Zhao et al., 2007). One possible reason for our higher value could be due to differences between studies in muscle coordination and walking mechanics in early stance. For example, the study by D’Lima et al. (2007) showed representative data of in vivo tibio-femoral joint forces, ground reaction forces and knee flexion angles over the gait cycle. During early stance, the joint force was less than two times body weight, with the vertical ground reaction force being less than bodyweight and knee flexion being approximately 10 degrees (Fig. 3 in D'Lima et al., 2007). This suggests that the subject in their study walked with minimal knee flexion, which would require lower vastii forces (i.e., quadriceps avoidance gait) and result in a lower tibio-femoral joint force. To test this hypothesis, we generated a post-hoc simulation that emulated a quadriceps avoidance gait pattern with a reduced knee flexion angle during weight acceptance. To generate the simulation, the experimental knee joint angles used in the cost function (Yi,m in Equation 1) were decreased by 10 degrees during 10–25% gait cycle when knee flexion reaches its maximum value (Fig. 2: Knee), and the simulation was re-optimized. When the peak knee flexion was decreased by 10 degrees, the corresponding vertical ground reaction force also decreased. These reductions were due to a decrease in the vastii force, which decreased by ~400 N. In addition to the vastii force decrease, the force in the biceps femoris short head also decreased, resulting in a reduction of the peak axial joint force to 2.4 BW (Fig. 6). The contributions from other muscles (e.g., the gluteus maximus) were insensitive to the change in knee flexion. Thus, reducing knee flexion during weight acceptance (i.e., quadriceps avoidance gait) appears to be an effective mechanism to reduce the tibio-femoral joint force through decreased vastii muscle forces. This strategy appears to be used by knee osteoarthritis (OA) patients, as previous studies have shown that knee OA patients walk with reduced knee flexion angles and knee extensor torques during weight acceptance (Astephen et al., 2008; Childs et al., 2004; Deluzio and Astephen, 2007; Zeni and Higginson, 2009).
Fig. 6.
Axial knee joint contact forces over the gait cycle with reduced knee flexion during weight acceptance (thick line). The force developed during normal walking (thin line) is shown for comparison. The vertical line indicates toe-off.
In addition to modifying knee joint kinematics, altering muscle coordination patterns to utilize functionally redundant muscles may provide an effective way to reduce joint loading. For example, both the gluteus maximus and vastii muscles provide body support in early stance (Anderson and Pandy, 2003; Liu et al., 2006; Neptune et al., 2004). However, the vastii muscles contribute twice as much to the tibio-femoral joint force as the gluteus maximus (Fig. 5a: VAS, GMAX, 15% gait cycle). Thus, an effective mechanism to reduce the tibio-femoral joint force in early stance would be to rely less on the vastii muscles and more on the gluteus maximus to provide body support. Similarly, the gastrocnemius is the primary contributor to the tibio-femoral joint force in late stance and provides body support and leg swing initiation (Neptune et al., 2001). Body support and leg swing initiation are also provided by the soleus and uniarticular hip flexors, respectively (Neptune et al., 2004). Thus, an effective mechanism to reduce knee joint loading in late stance may be to alter one’s muscle coordination pattern to utilize more soleus and hip flexor activity and less gastrocnemius activity.
There are some potential limitations with this study. One limitation is that the simulations were restricted to the sagittal plane. A previous simulation analysis of walking showed that different excitation patterns can occur for muscles crossing the hip joint (e.g., the gluteus medius) between 2D and 3D simulations (Xiao and Higginson, 2008). Similarly, a previous inverse dynamics analysis showed that 2D models can underestimate joint forces compared to 3D models (Glitsch and Baumann, 1997). Thus, we may be underestimating the contribution of some muscles to the tibio-femoral joint force. Future 3D simulation analyses are needed to assess this possibility. In addition, the anterior-posterior displacement of the tibia relative to the femur, which was defined as a function of the knee flexion angle in our simulations (Yamaguchi and Zajac, 1989), may influence the magnitude of knee joint forces because the moment-arms of muscles spanning the knee would be altered. To test this possibility, we performed a sensitivity analysis where the relative tibia-femur displacement was altered by ± 5 mm. When the muscle excitation patterns were re-optimized, the net knee joint moment was nearly identical with minor modifications of the excitation in the biceps femoris muscles, and the peak joint force in early stance changed little (max difference ~50 N). Also of note is that the analysis is based upon a generic model. Subject-specific muscle coordination and anatomy will influence specific muscle contributions to the joint forces. However, we believe the mechanisms identified in this study will be similar in subject-specific models. Future work integrating similar analyses with subject-specific models is needed to confirm this belief.
Another limitation is that our analysis does not attempt to distribute the tibio-femoral joint forces between medial and lateral compartments of the tibia. Studies have shown that the loading on the medial compartment is greater than the loading on the lateral compartment (Mundermann et al., 2008b; Schipplein and Andriacchi, 1991; Shelburne et al., 2006; Zhao et al., 2007). Thus, it is possible that muscles contribute to the medial and lateral knee joint forces differently. For example, the study by Shelburne et al. (2006) showed large quadriceps contributions to the knee adduction torque during stance (Shelburne et al., 2006), which suggests the quadriceps contribute significantly to the medial knee joint force. Future work is needed with a more detailed knee joint model to assess individual muscle contributions to the medial and lateral knee joint forces. Finally, some muscles and connective tissues crossing the knee joint were not included in the model (e.g., knee ligaments or the tensor fasciae latae, gracilis and sartorius muscles), which may contribute to the knee joint force. However, previous studies have shown that their influence is much lower than the quadriceps (Lin et al., 2010; Shelburne et al., 2006), although some studies have shown that knee ligaments can influence knee joint loading depending on the movement task (Collins, 1995; Kim et al., 2009; Shelburne et al., 2004). Thus, future work is needed to assess the relative contributions of muscles and ligaments to the total tibio-femoral joint force and how these contributions change with different walking mechanics and muscle coordination patterns.
In summary, the present study examined individual muscle contributions to the axial tibio-femoral joint forces during walking and found 1) the vastii and gastrocnemius muscles are the primary contributors to the joint force in early and late stance, respectively, with the peak force being greater than the muscle forces themselves, and 2) muscles that do not anatomically cross the knee joint (e.g., gluteus maximus and soleus) can have significant contributions to the joint force. Finally, small changes in walking kinematics (e.g., knee flexion angle) can have a significant effect on the magnitude of the knee joint forces. Thus, altering walking mechanics and muscle coordination patterns to utilize muscle groups that perform the similar biomechanical functions yet contribute less to the knee joint forces may be an effective way to reduce knee joint loading during walking.
Acknowledgements
This works was funded in part by NIH grant R01 NS55380.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors confirm that the authors were fully involved in the study and preparation of the manuscript and that the material within has not been and will not be submitted for publication elsewhere.
Conflict of interest statement
None.
References
- Anderson FC, Pandy MG. Static and dynamic optimization solutions for gait are practically equivalent. Journal of Biomechanics. 2001;34:153–161. doi: 10.1016/s0021-9290(00)00155-x. [DOI] [PubMed] [Google Scholar]
- Anderson FC, Pandy MG. Individual muscle contributions to support in normal walking. Gait & Posture. 2003;17:159–169. doi: 10.1016/s0966-6362(02)00073-5. [DOI] [PubMed] [Google Scholar]
- Astephen JL, Deluzio KJ, Caldwell GE, Dunbar MJ, Hubley-Kozey CL. Gait and neuromuscular pattern changes are associated with differences in knee osteoarthritis severity levels. Journal of Biomechanics. 2008;41:868–876. doi: 10.1016/j.jbiomech.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Childs JD, Sparto PJ, Fitzgerald GK, Bizzini M, Irrgang JJ. Alterations in lower extremity movement and muscle activation patterns in individuals with knee osteoarthritis. Clinical Biomechanics. 2004;19:44–49. doi: 10.1016/j.clinbiomech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Collins JJ. The redundant nature of locomotor optimization laws. Journal of Biomechanics. 1995;28:251–267. doi: 10.1016/0021-9290(94)00072-c. [DOI] [PubMed] [Google Scholar]
- D'Lima DD, Patil S, Steklov N, Chien S, Colwell CW., Jr In vivo knee moments and shear after total knee arthroplasty. Journal of Biomechanics. 2007;40 Suppl 1:S11–S17. doi: 10.1016/j.jbiomech.2007.03.004. [DOI] [PubMed] [Google Scholar]
- D'Lima DD, Patil S, Steklov N, Slamin JE, Colwell CW., Jr In vivo knee forces after total knee arthroplasty. Clinical Orthopaedics and Related Research. 2005;440:45–49. doi: 10.1097/01.blo.0000186559.62942.8c. [DOI] [PubMed] [Google Scholar]
- D'Lima DD, Patil S, Steklov N, Slamin JE, Colwell CW., Jr Tibial forces measured in vivo after total knee arthroplasty. Journal of Arthroplasty. 2006;21:255–262. doi: 10.1016/j.arth.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Davy DT, Audu ML. A dynamic optimization technique for predicting muscle forces in the swing phase of gait. Journal of Biomechanics. 1987;20:187–201. doi: 10.1016/0021-9290(87)90310-1. [DOI] [PubMed] [Google Scholar]
- Deluzio KJ, Astephen JL. Biomechanical features of gait waveform data associated with knee osteoarthritis: an application of principal component analysis. Gait & Posture. 2007;25:86–93. doi: 10.1016/j.gaitpost.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Fregly BJ, Reinbolt JA, Rooney KL, Mitchell KH, Chmielewski TL. Design of patient-specific gait modifications for knee osteoarthritis rehabilitation. IEEE Transactions On Biomedical Engineering. 2007;54:1687–1695. doi: 10.1109/TBME.2007.891934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch U, Baumann W. The three-dimensional determination of internal loads in the lower extremity. Journal of Biomechanics. 1997;30:1123–1131. doi: 10.1016/s0021-9290(97)00089-4. [DOI] [PubMed] [Google Scholar]
- Heinlein B, Kutzner I, Graichen F, Bender A, Rohlmann A, Halder AM, Beier A, Bergmann G. ESB Clinical Biomechanics Award 2008: Complete data of total knee replacement loading for level walking and stair climbing measured in vivo with a follow-up of 6–10 months. Clinical Biomechanics. 2009;24:315–326. doi: 10.1016/j.clinbiomech.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Herzog W, Longino D, Clark A. The role of muscles in joint adaptation and degeneration. Langenbecks Archives of Surgery. 2003;388:305–315. doi: 10.1007/s00423-003-0402-6. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Fernandez JW, Akbarshahi M, Walter JP, Fregly BJ, Pandy MG. Evaluation of predicted knee-joint muscle forces during gait using an instrumented knee implant. Journal of Orthopaedic Research. 2009;27:1326–1331. doi: 10.1002/jor.20876. [DOI] [PubMed] [Google Scholar]
- Lin Y-C, Walter JP, Banks SA, Pandy MG, Fregly BJ. Simultaneous prediction of muscle and contact forces in the knee during gait. Journal of Biomechanics. 2010;43:945–952. doi: 10.1016/j.jbiomech.2009.10.048. [DOI] [PubMed] [Google Scholar]
- Liu MQ, Anderson FC, Pandy MG, Delp SL. Muscles that support the body also modulate forward progression during walking. Journal of Biomechanics. 2006;39:2623–2630. doi: 10.1016/j.jbiomech.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Morrison JB. The mechanics of the knee joint in relation to normal walking. Journal of Biomechanics. 1970;3:51–61. doi: 10.1016/0021-9290(70)90050-3. [DOI] [PubMed] [Google Scholar]
- Mundermann A, Asay JL, Mundermann L, Andriacchi TP. Implications of increased medio-lateral trunk sway for ambulatory mechanics. Journal of Biomechanics. 2008a;41:165–170. doi: 10.1016/j.jbiomech.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Mundermann A, Dyrby CO, D'Lima DD, Colwell CW, Jr, Andriacchi TP. In vivo knee loading characteristics during activities of daily living as measured by an instrumented total knee replacement. Journal of Orthopaedic Research. 2008b;26:1167–1172. doi: 10.1002/jor.20655. [DOI] [PubMed] [Google Scholar]
- Mundermann A, Dyrby CO, Hurwitz DE, Sharma L, Andriacchi TP. Potential strategies to reduce medial compartment loading in patients with knee osteoarthritis of varying severity: reduced walking speed. Arthritis & Rheumatism. 2004;50:1172–1178. doi: 10.1002/art.20132. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Hull ML. Evaluation of performance criteria for simulation of submaximal steady-state cycling using a forward dynamic model. Journal of Biomechanical Engineering. 1998;120:334–341. doi: 10.1115/1.2797999. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. Journal of Biomechanics. 2001;34:1387–1398. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Sasaki K. Ankle plantar flexor force production is an important determinant of the preferred walk-to-run transition speed. Journal of Experimental Biology. 2005;208:799–808. doi: 10.1242/jeb.01435. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Wright IC, van den Bogert AJ. A method for numerical simulation of single limb ground contact events: application to heel-toe running. Computer Methods in Biomechanics and Biomedical Engineering. 2000;3:321–334. doi: 10.1080/10255840008915275. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Zajac FE, Kautz SA. Muscle force redistributes segmental power for body progression during walking. Gait & Posture. 2004;19:194–205. doi: 10.1016/S0966-6362(03)00062-6. [DOI] [PubMed] [Google Scholar]
- Raasch CC, Zajac FE, Ma B, Levine WS. Muscle coordination of maximum-speed pedaling. Journal of Biomechanics. 1997;30:595–602. doi: 10.1016/s0021-9290(96)00188-1. [DOI] [PubMed] [Google Scholar]
- Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. Journal of Orthopaedic Research. 1991;9:113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- Shelburne KB, Pandy MG, Anderson FC, Torry MR. Pattern of anterior cruciate ligament force in normal walking. Journal of Biomechanics. 2004;37:797–805. doi: 10.1016/j.jbiomech.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Shelburne KB, Torry MR, Pandy MG. Contributions of muscles, ligaments, and the ground-reaction force to tibiofemoral joint loading during normal gait. Journal of Orthopaedic Research. 2006;24:1983–1990. doi: 10.1002/jor.20255. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Heller MO, Bergmann G, Duda GN. Tibio-femoral loading during human gait and stair climbing. Journal of Orthopaedic Research. 2004;22:625–632. doi: 10.1016/j.orthres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Xiao M, Higginson JS. Muscle function may depend on model selection in forward simulation of normal walking. Journal of Biomechanics. 2008;41:3236–3242. doi: 10.1016/j.jbiomech.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi GT, Zajac FE. A planar model of the knee joint to characterize the knee extensor mechanism. Journal of Biomechanics. 1989;22:1–10. doi: 10.1016/0021-9290(89)90179-6. [DOI] [PubMed] [Google Scholar]
- Zajac FE, Gordon ME. Determining muscle's force and action in multi-articular movement. Exercise and Sport Sciences Reviews. 1989;17:187–230. [PubMed] [Google Scholar]
- Zeni JA, Jr, Higginson JS. Differences in gait parameters between healthy subjects and persons with moderate and severe knee osteoarthritis: a result of altered walking speed? Clinical Biomechanics. 2009;24:372–378. doi: 10.1016/j.clinbiomech.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Banks SA, D'Lima DD, Colwell CW, Jr, Fregly BJ. In vivo medial and lateral tibial loads during dynamic and high flexion activities. Journal of Orthopaedic Research. 2007;25:593–602. doi: 10.1002/jor.20362. [DOI] [PubMed] [Google Scholar]