Abstract
Using the X-ray structure of human group X secreted phospholipase A2 (hGX), we carried out structure-based design of indole-based inhibitors and prepared the compounds using a new synthetic route. The most potent compound inhibited hGX and the mouse orthologue with an IC50 of 75 nM. This compound is the most potent hGX inhibitor reported to date and was also found to inhibit a subset of the other mouse and human sPLA2s.
Secreted phospholipases A2 (sPLA2s) are a group of enzymes with conserved active sites and calcium-binding loops that catalyze the hydrolysis of the sn-2 ester of glycero-phospholipids to release free fatty acids and 2-lysophospholipids.1–4 The sPLA2s are low molecular weight (~14–20 kDa) enzymes with 5–8 disulfide bonds and require Ca2+ as a catalytic cofactor.1–4 To date, there are 10 distinct mammalian sPLA2s (groups IB, IIA, IIC, IID, IIE, IIF, III, V, X, XIIA), with group IIC present as a pseudogene in humans.1,2 Biomedical interest in this class of enzymes stems from the observation that one or more plays a role in the liberation of arachidonic acid from cellular phospholipids for the biosynthesis of eicosanoids. Mammalian cells also contain a cytosolic phospholipase (cPLA2-α).5 Recent evidence shows that in macrophages and other cells, sPLA2s and cPLA2-α work together to liberate arachidonic acid in agonist-stimulated mammalian cells.6–9 The molecular basis for the cross-talk between these two enzymes is not known. Potent and selective inhibitors of the sPLA2 enzymes would help resolve some of the questions surrounding the mechanism of arachidonate release as well as probe other physiological functions of sPLA2s.
For the past decade, the group IIA sPLA2 has received the most attention among the sPLA2s because it was the first nonpancreatic sPLA2 to be discovered, and it is found in high levels in patients suffering from inflammatory diseases including rheumatoid arthritis10 and acute pancreatitis.11 The group IIA sPLA2 has been the focus of medicinal chemistry efforts at several pharmaceutical companies.12–16 Workers at Lilly Labs and Shionogi & Co. Ltd. have reported on substituted indoles, exemplified by compound I, as potent inhibitors of group IIA sPLA2 (Figure 1).12–15 Among the various reported inhibitors of sPLA2,17 these compounds appear to be the most potent and also appear to have the most drug-like properties. With the discovery of additional sPLA2 enzymes, we have been interested in exploring these indole analogues as inhibitors of all of the members of the sPLA2 enzyme class.18 We are particularly interested in the group X sPLA2 because it appears to have the highest specific activity in promoting arachidonic acid release from mammalian cells.7,19,20 To date, very few inhibitors of the group X sPLA2 have been reported, with the general sPLA2 inhibitor YM-26734 being the most potent at 0.20 μM against the group X enzyme.21 To this end, we recently determined the X-ray structure of the indole-based inhibitor Me-Indoxam bound to human group X sPLA2 (hGX).18 We now report the development of a potent indole-based inhibitor of hGX that was designed based on this structural information.
Figure 1.

Representative structure of a substituted indole inhibitor of group IIA sPLA2. Indole positions 1–7 are shown.
In our first attempt to develop potent indole-based inhibitors of sPLA2s other than the group IIA enzyme, we made a library of analogues in which the substituent attached to N1 of the indole ring was varied.18 The range of IC50 values for this library was not very broad, and potent inhibitors of hGX were not obtained. Subsequent X-ray structural studies showed that this substituent largely points out of the active site of the sPLA2, toward what would be the membrane plane when the enzyme is bound to the phospholipid bilayer.18 Thus, we turned to chemical modifications of other areas of the indole ring.
Inspection of the X-ray structure of Me-Indoxam bound to hGX sPLA218 reveals a large hydrophobic pocket near the 2-substituent that makes little interaction with the inhibitor. We hypothesized that a larger 2-alkyl substituent would bind to the hydrophobic pocket and increase the binding affinity of the inhibitor. Also, the 6-position of the indole points out of the enzyme active site and does not contribute to binding affinity. However, modification of the 6-position may be useful for modifying the physiochemical properties of the indole for subsequent use in whole animal studies to affect pharmacokinetics. Docking studies were performed on the hGX enzyme in which the indole inhibitor was modified to include a 2-ethyl and 6-methyl, and the N1 substituent replaced with a benzyl (Figure 2). As suspected, the larger 2-ethyl group contacts the inner wall of the enzyme much better than a 2-methyl group, and a 6-methyl group sticks out of the enzyme active site and should not affect binding. As the reported synthesis12 of substituted 2-ethyl indoles was unsuccessful in our laboratory and the starting material for introduction of the 6-methyl substituent is not commercially available, a novel synthesis for 2-ethyl-6-methyl indoles was developed (Scheme 1). As there are few known literature reactions to functionalize the 6-position of an indole, the indole core had to be built up from pyrrole. Michael addition of nitromethane to tert-butyl crotonate followed by deprotection of this ester and subsequent treatment with thionyl chloride produced the acyl chloride 2. This was then added to benzenesulfonyl protected pyrrole in the presence of aluminum trichloride to give ketone 3. Treatment of 3 with NaOH in MeOH at low temperature followed by concentrated H2SO4 yielded dimethyl acetal 4. Ring closure to form the 4-oxyethanol indole 5 was accomplished by addition of a catalytic amount of acid with refluxing in toluene/ethylene glycol solvent. Conversion to the chloride followed by addition of excess n-butyllithium and benzyl protection yielded indole 6. Addition of n-butyllithium and acetic anhydride produced the desired 2-acetyl compound 7 due to the ortho-lithiating director used to protect the N1-position of the indole. Removal of the benzenesulfonyl protecting group and reduction of the ketone was accomplished in one step by refluxing in excess lithium aluminum hydride. Deoxygenation at the 2-position was accomplished using NaBH4 and trifluoroacetic acid to produce the 2-ethyl indole 9. N1-benzylation and 4-hydroxy deprotection followed by addition of tert-butyl bromoacetate yielded the tert-butyl oxyethanoate 11. Treatment of compound 11 with dilute oxalyl chloride followed by ammonia gas and deprotection of the tert-butyl ester with trifluoroacetic acid yielded the desired substituted 2-ethyl-6-methyl indole (compound A). Compounds B–D (Figure 3) were synthesized similarly (Supporting Information).
Figure 2.
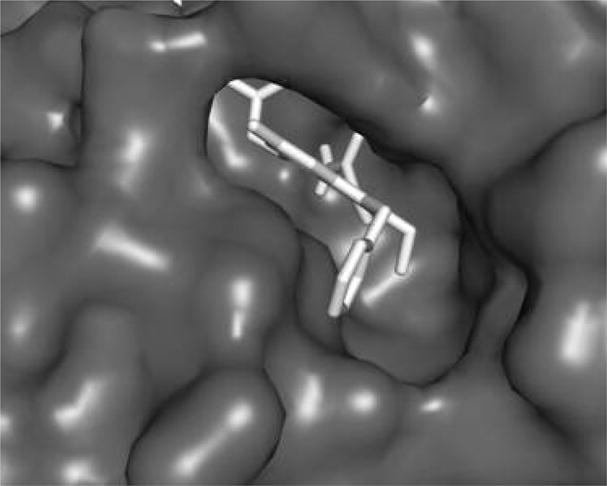
Compound A docked into the active site of hGX sPLA2. As can be seen, the 2-ethyl substituent makes contact with the active site wall and the 6-methyl group sticks out of the active site.
Scheme 1.
Synthesis of SPLA2 Inhibitor Aa
a Reagents: (a) CH3NO2, KF, 18-Crown-6, ACN, reflux; (b) (i) H2SO4, CHCl3, reflux; (ii) SOCl2, reflux; (c) AlCl3, ClCH2CH2Cl; (d) (i) NaOH, −20 °C, MeOH; (ii) H2SO4, −20 °C, MeOH; (e) p-TsOH, toluene, HOCH2CH2OH, reflux; (f) CCl4, PPh3; (g) 12 equiv of n-BuLi, THF; (h) NaH, BnBr, DMF; (i) n-BuLi, THF, −78 °C, Ac2O; (j) LAH, THF, reflux; (k) NaBH4, TFA, THF; (l) NaH, BnBr, DMF; (m) H2, Pd/C, MeOH; (n) NaH, BrCH2CO2t-Bu, DMF; (o) (i) (ClCO)2, CH2Cl2; (ii) NH3; (p) TFA, CH2Cl2.
Figure 3.
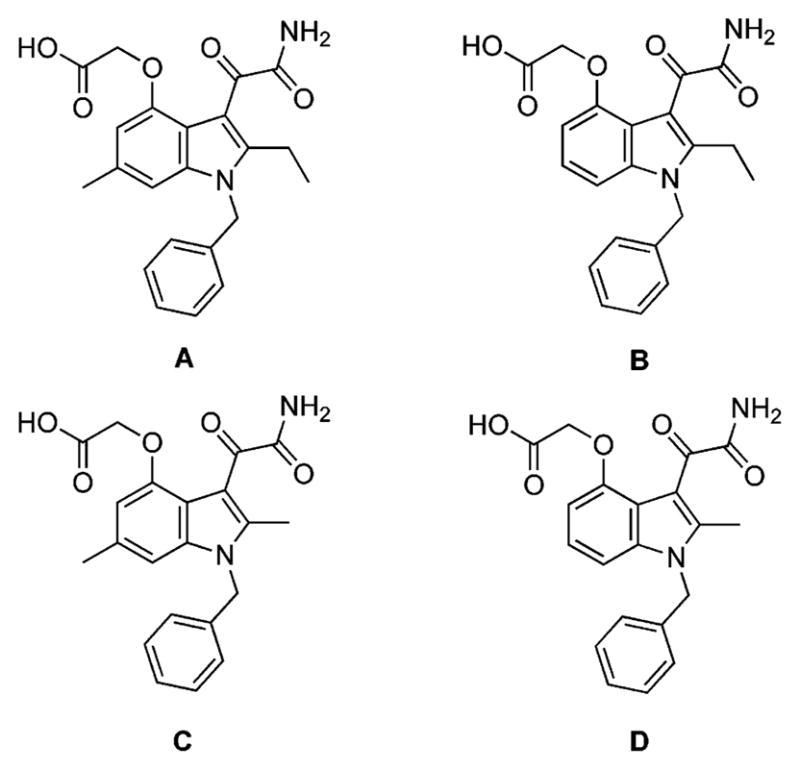
Structures of sPLA2 inhibitors with varying alkyl groups at the 2- and 6-positions.
To test the indole analogues as sPLA2 inhibitors, we used a fluorometric assay consisting of unilamellar vesicles of 1-hexa-decanoyl-2-(10-pyrenedecanoyl)-sn-glycero-3-phosphoglycerol.22 The sPLA2-catalyzed liberation of 10-pyrenedecanoic acid allows the fluorophore to dislodge from the vesicles and bind to albumin in the buffer phase where it now undergoes monomer fluorescent emission rather than excimer emission. The assay results (Table 1) show the 2-ethyl substituent to have a dramatic affect on binding to the hGX, with IC50 values of 75 nM for compounds A and B. The 2-ethyl compounds (A and B) are 26-fold more potent than the analogous 2-methyl compounds (C and D) against hGX, which have IC50 values of 2 μM. The 6-methyl substituent has no effect on hGX binding; compounds A and B have identical IC50 values. The inhibitors were then screened against a panel of recombinant human and mouse sPLA2s (hGIB, mGIB, hGIIA, mGIIA, hGIIE, mGIIE, hGV, mGV, hGX, and mGX). In all cases the 2-ethyl compounds are more potent than the 2-methyl derivatives, and the 6-methyl group is tolerated (Table 1). Compounds A and B should be useful in distinguishing the groups X and V sPLA2s based on the ~10- fold increased potency for the former. This is significant because current evidence favors a role of these two sPLA2s in arachidonate liberation in mammalian cells. Although these compounds are also potent inhibitors of the group IIA sPLA2s, the original lead compound Me-Indoxam is 50-fold more potent on hGIIA and mGIIA versus hGX and mGX.18 Thus, by carrying out studies with a combination of inhibitors, it should be possible to probe for the role of specific sPLA2s in cellular processes.
Table 1.
Inhibition Data against Mammalian sPLA2s for Compounds A–Da
| sPLA2 | compound IC50 (μM) |
|||
|---|---|---|---|---|
| A | B | C | D | |
| hGIB | 0.80 ± 0.10 | 0.75 ± 0.15 | 2.00 ± 0.20 | 2.50 ± 0.25 |
| mGIB | 0.20 ± 0.05 | 0.14 ± 0.075 | 2.00 ± 0.10 | 2.20 ± 0.15 |
| hGIIA | 0.125 ± 0.03 | 0.125 ± 0.02 | 0.30 ± 0.05 | 0.275 ± 0.05 |
| mGIIA | 0.05 ± 0.01 | 0.07 ± 0.02 | 0.125 ± 0.02 | 0.125 ± 0.02 |
| hGIIE | 0.05 ± 0.01 | 0.05 ± 0.02 | 0.125 ± 0.03 | 0.075 ± 0.01 |
| mGIIE | 0.075 ± 0.02 | 0.075 ± 0.02 | 0.40 ± 0.05 | 0.40 ± 0.04 |
| hGV | 0.50 ± 0.1 | 0.50 ± 0.05 | 0.80 ± 0.05 | 0.80 ± 0.05 |
| mGV | 0.75 ± 0.15 | 0.75 ± 0.10 | 0.85 ± 0.05 | 1.00 ± 0.075 |
| hGX | 0.075 ± 0.01 | 0.075 ± 0.01 | 2.20 ± 0.10 | 2.00 ± 0.15 |
| mGX | 0.075 ± 0.01 | 0.075 ± 0.01 | 2.50 ± 0.15 | 2.50 ± 0.20 |
IC50s are based on duplicate or triplicate analyses.
In conclusion, the first potent inhibitor against hGX and mGX sPLA2s has been discovered. A new chemical route to these indole-based sPLA2 inhibitors has been developed.
Supplementary Material
Footnotes
Supporting Information Available: Experimental details including the synthesis of all compounds and assay procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Valentin E, Lambeau G. Increasing molecular diversity of secreted phospholipases A(2) and their receptors and binding proteins. Biochim Biophys Acta. 2000;1488:59–70. doi: 10.1016/s1388-1981(00)00110-4. [DOI] [PubMed] [Google Scholar]
- 2.Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 3.Berg OG, Gelb MH, Tsai MD, Jain MK. Interfacial enzymology: the secreted phospholipase A(2)-paradigm. Chem Rev. 2001;101:2613–2654. doi: 10.1021/cr990139w. [DOI] [PubMed] [Google Scholar]
- 4.Murakami M, Kudo I. Phospholipase A2. J Biochem (Tokyo) 2002;131:285–292. doi: 10.1093/oxfordjournals.jbchem.a003101. [DOI] [PubMed] [Google Scholar]
- 5.Leslie CC. Properties and regulation of cytosolic phospholipase A2. J Biol Chem. 1997;272:16709–16712. doi: 10.1074/jbc.272.27.16709. [DOI] [PubMed] [Google Scholar]
- 6.Satake Y, Diaz BL, Balestrieri B, Lam BK, Kanaoka Y, Grusby MJ, Arm JP. Role of group V phospholipase A2 in zymosan-induced eicosanoid generation and vascular permeability revealed by targeted gene disruption. J Biol Chem. 2004;279:16488–16494. doi: 10.1074/jbc.M313748200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mounier CM, Ghomashchi F, Lindsay MR, James S, Singer AG, Parton RG, Gelb MH. Arachidonic acid release from mammalian cells transfected with human groups IIA and X secreted phospholipase A(2) occurs predominantly during the secretory process and with the involvement of cytosolic phospholipase A(2)-alpha. J Biol Chem. 2004;279:25024–25038. doi: 10.1074/jbc.M313019200. [DOI] [PubMed] [Google Scholar]
- 8.Balsinde J, Balboa MA, Yedgar S, Dennis EA. Group V phospholipase A(2)-mediated oleic acid mobilization in lipopolysaccharide-stimulated P388D(1) macrophages. J Biol Chem. 2000;275:4783–4786. doi: 10.1074/jbc.275.7.4783. [DOI] [PubMed] [Google Scholar]
- 9.Kuwata H, Nonaka T, Murakami M, Kudo I. Search of factors that intermediate cytokine-induced group IIA phospholipase A2 expression through the cytosolic phospholipase A2- and 12/15-lipoxygenase-dependent pathway. J Biol Chem. 2005;280:25830–25839. doi: 10.1074/jbc.M500168200. [DOI] [PubMed] [Google Scholar]
- 10.Hara S, Kudo I, Chang HW, Matsuta K, Miyamoto T, Inoue K. Purification and characterization of extracellular phospholipase A2 from human synovial fluid in rheumatoid arthritis. J Biochem (Tokyo) 1989;105:395–399. doi: 10.1093/oxfordjournals.jbchem.a122675. [DOI] [PubMed] [Google Scholar]
- 11.Gronroos JM, Nevalainen TJ. Increased concentrations of synovial-type phospholipase A2 in serum and pulmonary and renal complications in acute pancreatitis. Digestion. 1992;52:232–236. doi: 10.1159/000200958. [DOI] [PubMed] [Google Scholar]
- 12.Dillard RD, Bach NJ, Draheim SE, Berry DR, Carlson DG, Chirgadze NY, Clawson DK, Hartley LW, Johnson LM, Jones ND, McKinney ER, Mihelich ED, Olkowski JL, Schevitz RW, Smith AC, Snyder DW, Sommers CD, Wery JP. Indole inhibitors of human nonpancreatic secretory phospholipase A2. 1. Indole-3-acetamides. J Med Chem. 1996;39:5119–5136. doi: 10.1021/jm960485v. [DOI] [PubMed] [Google Scholar]
- 13.Dillard RD, Bach NJ, Draheim SE, Berry DR, Carlson DG, Chirgadze NY, Clawson DK, Hartley LW, Johnson LM, Jones ND, McKinney ER, Mihelich ED, Olkowski JL, Schevitz RW, Smith AC, Snyder DW, Sommers CD, Wery JP. Indole inhibitors of human nonpancreatic secretory phospholipase A2. 2. Indole-3-acetamides with additional functionality. J Med Chem. 1996;39:5137–5158. doi: 10.1021/jm960486n. [DOI] [PubMed] [Google Scholar]
- 14.Draheim SE, Bach NJ, Dillard RD, Berry DR, Carlson DG, Chirgadze NY, Clawson DK, Hartley LW, Johnson LM, Jones ND, McKinney ER, Mihelich ED, Olkowski JL, Schevitz RW, Smith AC, Snyder DW, Sommers CD, Wery JP. Indole inhibitors of human nonpancreatic secretory phospholipase A2. 3. Indole-3-glyoxamides. J Med Chem. 1996;39:5159–5175. doi: 10.1021/jm960487f. [DOI] [PubMed] [Google Scholar]
- 15.Schevitz RW, Bach NJ, Carlson DG, Chirgadze NY, Clawson DK, Dillard RD, Draheim SE, Hartley LW, Jones ND, Mihelich ED, Olkowski JL, Snyder DW, Sommers C, Wery JP. Structure-based design of the first potent and selective inhibitor of human nonpancreatic secretory phospholipase A2. Nat Struct Biol. 1995;2:458–465. doi: 10.1038/nsb0695-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaton HG, Bennion C, Connolly S, Cook AR, Gensmantel NP, Hallam C, Hardy K, Hitchin B, Jackson CG, Robinson DH. Discovery of new nonphospholipid inhibitors of the secretory phospholipases A2. J Med Chem. 1994;37:557–559. doi: 10.1021/jm00031a001. [DOI] [PubMed] [Google Scholar]
- 17.Reid RC. Inhibitors of secretory phospholipase A2 group IIA. Curr Med Chem. 2005;12:3011–3026. doi: 10.2174/092986705774462860. [DOI] [PubMed] [Google Scholar]
- 18.Smart BP, Pan YH, Weeks AK, Bollinger JG, Bahnson BJ, Gelb MH. Inhibition of the complete set of mammalian secreted phospholipases A(2) by indole analogues: a structure-guided study. Bioorg Med Chem. 2004;12:1737–1749. doi: 10.1016/j.bmc.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Bezzine S, Koduri RS, Valentin E, Murakami M, Kudo I, Ghomashchi F, Sadilek M, Lambeau G, Gelb MH. Exogenously added human group X secreted phospholipase A(2) but not the group IB, IIA, and V enzymes efficiently release arachidonic acid from adherent mammalian cells. J Biol Chem. 2000;275:3179–3191. doi: 10.1074/jbc.275.5.3179. [DOI] [PubMed] [Google Scholar]
- 20.Hanasaki K, Ono T, Saiga A, Morioka Y, Ikeda M, Kawamoto K, Higashino K, Nakano K, Yamada K, Ishizaki J, Arita H. Purified group X secretory phospholipase A(2) induced prominent release of arachidonic acid from human myeloid leukemia cells. J Biol Chem. 1999;274:34203–34211. doi: 10.1074/jbc.274.48.34203. [DOI] [PubMed] [Google Scholar]
- 21.Hamaguchi K, Kuwata H, Yoshihara K, Masuda S, Shimbara S, Oh-ishi S, Murakami M, Kudo I. Induction of distinct sets of secretory phospholipase A(2) in rodents during inflammation. Biochim Biophys Acta. 2003;1635:37–47. doi: 10.1016/j.bbalip.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Dudler T, Chen WQ, Wang S, Schneider T, Annand RR, Dempcy RO, Crameri R, Gmachl M, Suter M, Gelb MH. High-level expression in Escherichia coli and rapid purification of enzymatically active honey bee venom phospholipase A2. Biochim Biophys Acta. 1992;1165:201–210. doi: 10.1016/0005-2760(92)90188-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.