Abstract
Gene therapy promises to become an alternative choice for the treatment of hepatic cancer. In many cancers, the delivery of chimeric proteins by adenovirus vector has been reported to induce apoptosis. This study was performed to evaluate whether the recombinant adenovirus interleukin (IL)-24-Bax can induce apoptosis in hepatocellular carcinoma cells in vitro and in vivo. Several recombinant adenoviruses were constructed, and the expression of their encoded proteins was measured. The effects of the recombinant adenovirus on hepatocellular carcinoma cells and the normal hepatocyte cell line were investigated through cell viability and apoptosis assays after the cells were treated with Ad.Luc, Ad.IL-24, Ad.Bax or Ad.IL-24-Bax. The mechanism involved was also explored. A tumor-bearing mouse model was used to evaluate the effects of the adenovirus on tumor volume and cell apoptosis in vivo. Ad.IL-24-Bax selectively suppressed growth of hepatocellular carcinoma cells and induced apoptosis, but it had little influence on the normal hepatocytes. The mechanism of this response may include the effect of the 10HRE/VEGF385 promoter and the synergistic effect of IL-24 and Bax. Ad.IL-24-Bax also suppressed tumor growth in nude mice and induced apoptosis. Ad.IL-24-Bax may be a useful tool for gene therapy of hepatic cancer.
Keywords: IL-24, Bax, IL-24-Bax, recombinant adenovirus, hepatic cancer, apoptosis
Introduction
Current therapies for malignancies do not have a satisfactory patient survival rate, so novel approaches to treat these diseases have been extensively studied. Gene therapy represents an alternative form of treatment for cancer and may be used to treat malignancies. One strategy is to combine molecular targeting with a toxic molecule.1 Some chimeric toxins have already undergone clinical trials. However, several disadvantages limit the clinical application of chimeric toxins.2, 3, 4 One disadvantage of targeted chimeric toxins is that the bacterial toxins are recognized as foreign by the immune system of the recipients; another is that the nonspecific toxicity of the chimeric toxins limits its dosage and persistent application. To overcome these problems, a new generation of chimeric proteins have been developed using human apoptosis-inducing factors. Interleukin (IL)-2-Bax is the first prototype of this class. IL-2-Bax specifically targets IL-2R-expressing cells and induces cell specific, dose-dependent apoptosis.5, 6
Bax is a proapoptotic member of the Bcl-2 protein family, and has been reported to induce apoptosis in cancer cells after transfection; it can also inhibit growth of many tumor types. Bax can increase the permeability of the mitochondrial outer membrane, leading to the release of apoptosis molecules such as cytochrome C.7, 8, 9 p53 has been shown to initiate the mitochondrial apoptosis by modulating the expression of Bcl-2 family members, including Bax.10 Mutations in p53 frequently occur in tumors, so a chimeric toxin harboring Bax may be able to activate the mitochondrial apoptosis pathway regardless of p53 status. In other words, Bax could successfully replace bacterial or phytotoxins. For this study, Bax fused with IL-2 or IL-3 was confirmed to accumulate to the target cell and induce cell death.
IL-24, previously named melanoma differentiation associated gene-7 (mda-7), was originally identified by subtraction hybridization as a novel molecule of unknown function, and its expression is elevated in terminally differentiated human melanoma cells.11 IL-24 was initially shown to possess cellular growth arresting properties.12, 13 Several independent studies have shown that a majority of human cancer derived cell lines, including melanoma, prostate, breast, cervical, lung, fibrosarcoma, pancreatic, colorectal and glioblastoma underwent apoptosis when exposed to mda-7/IL-24.14, 15, 16 Most tumor cell surfaces express IL-24 receptor, and purified mda-7/IL-24 can specifically induce apoptosis of tumor cell in a receptor-dependent way.17, 18, 19 Thus, IL-24 not only can specifically induce tumor cell apoptosis, but also can act as a targeting molecule like IL-2 or IL-3, specifically targeting other molecules into a tumor cell.
Reports have shown that p53 mutations and decreased Bax activation are common in hepatocellular carcinoma cells.20, 21 mda-7/IL-24 has been reported to induce apoptosis in hepatocellular carcinoma cells.22, 23 In this study, IL-24 and Bax were fused and then introduced into adenovirus (Ad.IL-24-Bax). The activity of the chimeric protein IL-24-Bax was examined both in vitro and in vivo, especially its effect on apoptosis in hepatocellular carcinoma cells and in tumor-bearing nude mice model. Furthermore, the possible mechanism was also investigated and discussed.
Materials and methods
Cell lines and culture conditions
Human embryonic kidney cells (HEK-293), human hepatocellular carcinoma cell lines (HepG2, Hep3B, PLC/PRF/5) and human hepatocyte line L02 were originally obtained from the American Type Tissue Culture Collection (Rockville, MD). HepG2 and L02 cells were incubated in Dulbecco's Modified Eagle Media supplemented with 10% fetal bovine serum, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin at 37 °C in an atmosphere of 5% CO2 and 100% humidity. Hep3B and PLC/PRF/5 cells were incubated in RPMI 1640 (Invitrogen, Karlsruhe, Germany) supplemented with 10% fetal bovine serum (PAA Laboratories GmbH, Pasching, Austria), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin at 37 °C in an atmosphere of 5% CO2 and 100% humidity. The cells were passaged twice per week, and 0.25% trypsin with 1 mM EDTA was used to isolate the cells.
Construction of recombinant adenovirus
The recombinant adenovirus was commercially constructed by Vector Gene Technology Company Limited (Beinjing, China). For recombinant adenovirus IL-24-Bax (Ad.IL-24-Bax), 515 bp 10 × HRE/VEGF385 (HRE: 5′-GTGACTACGTGCTGCCTAG-3′) was introduced into the pGL3-SV40 promoter vector using SacI/XhoI restriction sites. A segment from human IgG1 (gag gct tct ggt ggt ggt cct gaa) was used to link the cDNA of Baxα and the cDNA of IL-24. A 6 × His (ATGATGATGATGATGATG) tag was introduced before the stop codon. Then, the recombinant segment of IL-24-Bax was introduced into the plasmid with the 10 × HRE/VEGF385 promoter using the Xbal/HindIII restriction sites. After restriction digestion with KpnI/SalI, the 2.3 kb recombinant segment was introduced into the AdEasy Adenoviral Vector System (Quantum Biotech, Montreal, Canada). Subsequently, the vector was enclosed, amplified and purified in HEK-293 cells. Other recombinant adenoviruses (Ad.IL-24, Ad.Bax and Ad.Luc) were similarly constructed.
To assess the optimal multiplicity of infection (MOI) for maximal transgene expression, Hep3B cells were infected with Ad.IL-24-Bax at various MOIs and examined by fluorescence microscopy. Twenty-four hours before transfection with recombinant adenovirus vector at different MOIs, cells were transferred to a six-well plate at a density of 2 × 105 cells per well. The medium was changed 2 h after the transfection was completed, then the cells were cultured for another 72 h before observation by fluorescent microscopy.
Western blotting analysis
After transfection, the expression of recombinant IL-24-Bax in transfected cells was detected using western blotting analysis. The analysis was performed 48 h after the transfection. Cellular proteins were collected and the concentration was determined using the Bio-Rad method (Bio-Rad, San Francisco, CA). The protein was analyzed by SDS-PAGE electrophoresis and electrotransferred to a nitrocellulose membrane. Then the filters were incubated in 5% nonfat milk solution at room temperature for 2 h to block nonspecific antibody binding. Subsequently, the filters were incubated in primary antibody solution for 2 h. The respective primary antibodies were as follows: rabbit anti-human IL-24 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-human p53 (Santa Cruz Biotechnology), rabbit anti-human BiP/GRP78 (Biovision Research Products, Mountain View, CA), rabbit anti-human Caspase-12 (Biovision Research Products), rabbit anti-human Caspase-3 (Cell Signaling, Beverly, MA), rabbit anti-human β-actin (Cell Signaling) and mouse anti-human Bax (Santa Cruz Biotechnology). The filters were then incubated in peroxidase-labeled goat anti-rabbit IgG or goat anti-mouse IgG (Santa Cruz Biotechnology). The filters were developed by ECL (Pierce, Rockford, IL), and images were generated after exposure to X-ray film. The images were analyzed by Quantity One. The relative gray scale value was the ratio of the gray scale of the test protein band vs the gray scale of the β-actin band. The relative gray scale value represented the relative protein expression.
Western blotting analysis was also performed to investigate the recombinant protein expression under normal condition and anoxia condition. L02, Hep3B, HepG2 and PLC/PRF/5 cells were cultured in six-well plates at density of 2 × 105 per well. After the cells adhered, the recombinant adenovirus (MOI=10) was added to the cells and incubated for 48 h. Then cells were randomly divided into two groups. One group was cultured in an atmosphere of 95% air and 5% CO2, whereas another group was cultured in an atmosphere of 95% N2 and 5% CO2. Before protein collection, cells were cultured in serum-free medium for 4, 8 and 16 h, respectively. The expression of IL-24-Bax was detected by western blotting analysis as described earlier.
Cell viability assay
The effects of recombinant adenovirus on cell growth were determined using a methyl thiazolyl tetrazolium cell viability assay as described earlier. Cells in logarithmic growth phase were cultured for 24 h in a 96-well plate at a density of 105 cells × 100 μl per well. Then the recombinant adenovirus at different MOI (0.01–100) diluted in serum-free medium was added to the wells. Cells were cultured for another 3 days. Then, the culture medium was replaced with 100 μl serum-free medium with 20 μl methyl thiazolyl tetrazolium (5 mg ml−1) at 37 °C for 5 h. Finally, the medium was discarded and 150 μl dimethyl sulfoxide solution was added to each well to lyse cells. Absorbance was measured at A570 nm. Each experiment was repeated three times. Cell viability was calculated as the ratio of the absorbance of the test group vs the absorbance of the control group. A cell growth curve was constructed based on the cell viability data.
Apoptosis assay by flow cytometry
Before induction of cell apoptosis, the cultured cells were treated for 30 min with the endoplasmic reticulum (ER) inhibitor calpastatin I (ALLN, N-Ac---norleucinal, 25 μ, Calbiochem, Darmstadat, Germany). Then the cultured cells were incubated with adenovirus (MOI=10) for 48 h. Later, cells were isolated using trypsin and collected by centrifugation. A total of 2 × 106 cells were analyzed by flow cytometry. Before analysis, cells were fixed in 75% ethanol overnight and stained with Annexin V-fluorescein isothiocyanate/proliferation index for 30 min. Cell cycle analysis was performed using a FACSaria flow cytometer (Becton Dickinson, Franklin Lakes, NJ). All data were analyzed using Modifit3.0 software.
Western blots were performed to investigate the mechanism involved in the induction of apoptosis. The expression of mitochondrial pathway related proteins (caspase-3, Bax) and ER-stress pathway related proteins (Bip/GRP78, caspase-12) were measured after Hep3B cells were treated with Ad.Luc, Ad.Bax, Ad.IL-24 or Ad.IL-24-Bax (MOI=10) for 48 h. On the other hand, after the ER-stress pathway was blocked by ALLN, the expression of related proteins was also measured after Hep3B cells were similarly treated by recombinant adenovirus.
In vivo study using tumor-bearing nude mouse models
All procedures were performed in accordance with the guidelines and approval of the local Institutional Animal Experimentation Ethics Committee. BALB/c-nu/nu mice were purchased from Shanghai Laboratory Animal Research Center of the Chinese Academy of Sciences and were bred in the specific pathogen-free animal laboratory in the Second Military Medical University. All mice were maintained in specific pathogen-free facilities and fed with irradiated food. BALB/c-nu/nu mice were age matched (4–5 weeks of age) at the beginning of each experiment. Hep3B cells were injected subcutaneously into the back of athymic mice (5 × 106 cells/each). Mice were observed and weighed everyday.
When the tumor diameter reached 4–5 mm, 40 mice bearing tumors were randomly divided into four groups: Ad.IL-24-Bax, Ad.IL-24, Ad.Bax and Ad.Luc. The adenovirus was intratumorally injected at a single site at the dose of 2 × 108 PFU per 100 μl once every 4 days for three times. Three weeks after the first injection, mice were killed by dislocation of cervical vertebra. Tumors were weighed and tumor volume was measured using a vernier caliper. The tumor volume was calculated using the following formula: tumor volume =0.5 × a × b2, where a is the longer diameter and b is the shorter diameter. Tumors were fixed in 10% paraformaldehyde solution before further analysis.
Immunochemistry and apoptosis assay
Tumor tissue was first sliced, fixed and dehydrated and then it underwent immunochemistry using the EliVision plus assay kit (Zymed Biotechnology, Zymed, CA) following the manufacture's instructions. Rabbit anti-human Ki-67 monoclonal antibody (Santa Cruz Biotechnology) was applied in the assay. The known positive slide was used as the positive control, and phosphate buffered saline was used as the negative control. Five areas ( × 400) were randomly observed. For each area, 200 cells were counted and the positive cell number was recorded. The count was repeated three times and the percentage of positive cells was calculated.
Apoptosis assays were performed using the transferase-mediated dUTP nick-end-labeling method. The tumor cells were labeled using the Fluorescein-FragEL DNA Fragmentation Detection Kit following the manufacturer's protocol (Oncogene Research Products, Boston, MA) and then observed under a fluorescent microscope. Areas ( × 400) where positive cells were evenly distributed were observed. Three hundred cells were counted and the number of positive cells was recorded. The count was repeated three times and the percentage of positive cells was calculated.
Statistical analysis
All statistical analysis was performed using the SPSS10.0 software. The statistical significance was determined by a t-test of independent samples and analysis of variance. P-values for significance were set at 0.05.
Results
Construction of recombinant adenovirus
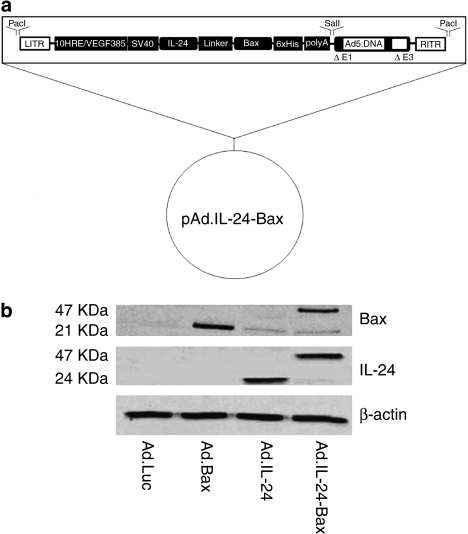
As Figure 1a shows, the recombinant adenovirus IL-24-Bax was constructed as well as Ad.IL-24, Ad.Bax and Ad.Luc. When Hep3B cells were transfected with Ad.IL-24-Bax, the transfection efficiency was related to the MOI of the recombinant adenovirus. As the MOI increased, the efficiency of transfection also increased. When MOI was higher than 25, the efficiency of transfection was as high as 90%. Considering the cytotoxicity of the virus, an MOI of 10 for the virus was used in transfection experiments in this study.
Figure 1.
Construction and expression of Ad.IL-24-Bax. (a) Construction of recombinant adenovirus vector that encodes the IL-24-Bax. (b) Western blot analysis of the expression of the IL-24-Bax fusion protein. Hep3B cells were treated with Ad.IL-24-Bax, Ad.IL-24, Ad.Bax or Ad.Luc. Forty-eight hours after treatment, proteins were extracted from cells for western blot analysis. β-Actin was used as the internal standard.
The results of the western blotting analysis confirmed the expression of the chimeric protein (Figure 1b). Antibodies to IL-24 and Bax, respectively, detected the protein segment of IL-24 and Bax.
Anoxia induces IL-24-Bax expression in hepatocellular carcinoma cells but not in normal hepatocytes
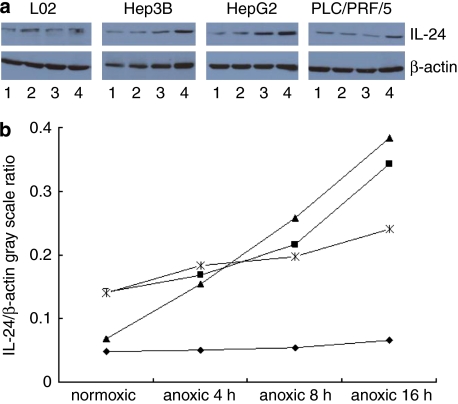
Figure 2 shows the expression of IL-24-Bax in Hep3B, HepG2, L02 and PLC/PRF/5 cells after cells were cultured in normal or anoxic conditions. Generally, the expression was higher when cells were cultured in anoxic conditions than in cells cultured under normal conditions. The expression increased over time in anoxic conditions. After the cells were cultured under anoxic conditions for 16 h, the expression of IL-24-Bax in Hep3B, HepG2 and PLC/PRF cells was 1.71, 4.16 and 1.5 times that of cells cultured in normal conditions. However, the expression of IL-24-Bax in L02 cells remained the same regardless of the culture conditions. The results indicated that insertion of the HRE promoter repeats enhanced the specific expression of Ad.IL-24-Bax under anoxic conditions in hepatocytes.
Figure 2.
Anoxia conditions induced the expression of IL-24 after cells were transfected with Ad.IL-24-Bax. (a) L02, Hep3B, HepG2 and PLC/PRF/5 cells were treated with Ad.IL-24-Bax. Forty-eight hours after treatment, the transfected cells were divided into two groups. One group was cultured under normal conditions (95% air, 5% CO2), and the other under anoxic conditions (95% N2 and 5% CO2) in serum-free medium for 4, 8 or 16 h (1: normoxic; 2: anoxic 4 h; 3: anoxic 8 h; 4: anoxic 16 h). The expression of IL-24 increased progressively at 4, 8 and 16 h in Ad.IL-24-Bax transfected L02, Hep3B, HepG2 and PLC/PRF/5 cells. β-Actin was used as the internal standard. (b) IL-24/β-actin gray scale ratio. L02 (⧫), Hep3B (▪), HepG2 (▴) and PLC/PRF/5 cells ( ).
).
Ad.IL-24-Bax inhibited hepatocellular carcinoma cell growth and prompted apoptosis in vitro
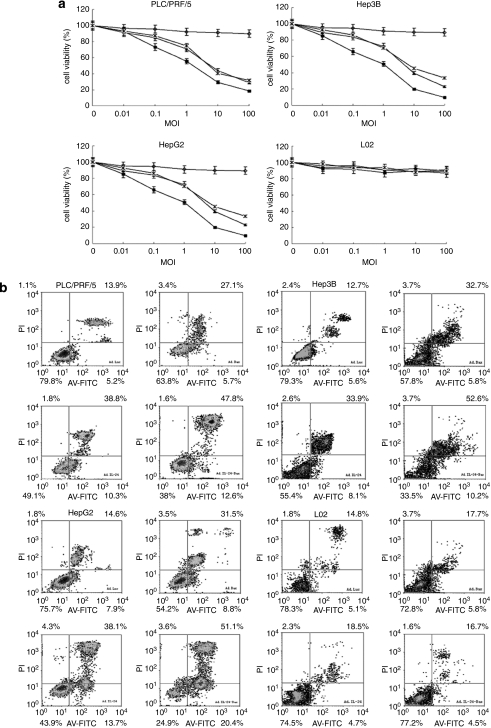
Figure 3a illustrated the cell viability of Hep3B, PLC/PRF/5, HepG2 and L02 cells after transfection with the recombinant adenovirus at different MOIs. When transfected and cultured with Ad.IL-24 and Ad.IL-24-Bax at high MOI, the growth of hepatocellular carcinoma cells was significantly inhibited. When the MOI was >10, the survival rate of hepatocellular carcinoma cells was significantly lower when transfected and cultured with Ad.IL-24-Bax than with Ad.IL-24 (P<0.05). Meanwhile, the growth curve of normal hepatocyte L02 was not significantly changed (P>0.05) when transfected and cultured with Ad.Bax, Ad.IL-24 or Ad.IL-24-Bax. When transfected and cultured with Ad.Luc, the growth curves of normal hepatocytes and hepatocellular carcinoma cells were similar.
Figure 3.
Ad.IL-24-Bax suppressed the proliferation of hepatocellular carcinoma cells and induced apoptosis in hepatocellular carcinoma in vitro. (a) Cell viability after Ad.IL-24-Bax treatment by methyl thiazolyl tetrazolium assays. Normal hepatocyte (L02) and hepatocellular carcinoma cells (HepG2, Hep3B, PLC/PRF/5) were treated with Ad.Luc (◊), Ad.Bax ( ), Ad.IL-24 (▴) or Ad.IL-24-Bax (▪) at different multiplicities of infection (MOI). Three days after infection, the cell survival rate was checked. Ad.IL-24-Bax inhibited hepatocellular carcinoma cell growth, while having little influence on the growth of normal liver cells. (b) Percentage of apoptotic cells after Ad.IL-24-Bax treatment using the Annexin V-fluorescein isothiocyanate/proliferation index staining method. Normal hepatocytes (L02) and hepatocellular carcinoma cells (HepG2, Hep3B, PLC/PRF/5) were treated with Ad.Luc, Ad.Bax, Ad.IL-24 or Ad.IL-24-Bax (MOI=10) for 72 h. Cells were harvested for Annexin V-fluorescein isothiocyanate/proliferation index staining analysis.
), Ad.IL-24 (▴) or Ad.IL-24-Bax (▪) at different multiplicities of infection (MOI). Three days after infection, the cell survival rate was checked. Ad.IL-24-Bax inhibited hepatocellular carcinoma cell growth, while having little influence on the growth of normal liver cells. (b) Percentage of apoptotic cells after Ad.IL-24-Bax treatment using the Annexin V-fluorescein isothiocyanate/proliferation index staining method. Normal hepatocytes (L02) and hepatocellular carcinoma cells (HepG2, Hep3B, PLC/PRF/5) were treated with Ad.Luc, Ad.Bax, Ad.IL-24 or Ad.IL-24-Bax (MOI=10) for 72 h. Cells were harvested for Annexin V-fluorescein isothiocyanate/proliferation index staining analysis.
The results of the flow cytometry assay (Figure 3b) indicated that Ad.IL-24-Bax promoted apoptosis of hepatocellular carcinoma cells but had no obvious cytotoxicity to normal hepatocytes. The apoptosis rate of three hepatocellular carcinoma cell lines was significantly higher when transfected with Ad.IL-24-Bax than those transfected with Ad.IL-24 or Ad.Bax (P<0.05). The apoptosis rate of the normal hepatocyte line L02 was not significantly different when transfected with Ad.Luc, Ad.IL-24, Ad.Bax or Ad.IL-24-Bax (P>0.05). The results indicated that Ad.IL-24-Bax robustly inhibited hepatocellular carcinoma cell growth and induced apoptosis while having no obvious toxic side effects.
The possible mechanism involved in the cell apoptosis
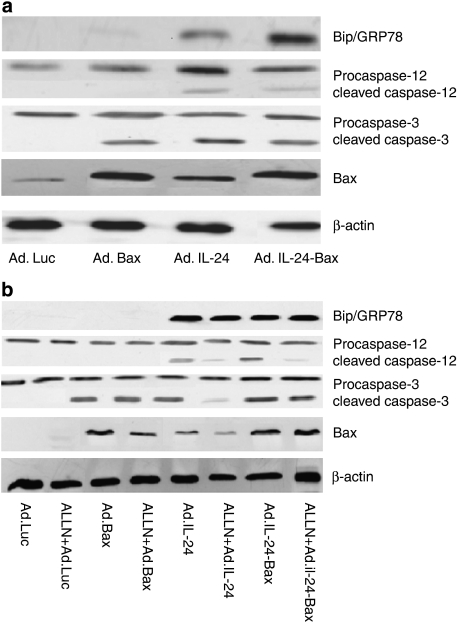
To determine whether apoptosis was induced by the ER-stress pathway, the expression of related proteins in p53-absent Hep3B cells was examined. Figure 4a showed that after transfection with Ad.IL-24-Bax, BiP/GRP78 was highly expressed, caspase-12 was activated and endogenous Bax was highly expressed in Hep3B cells. Furthermore, after transfection with Ad.IL-24-Bax and treatment with the ALLN, an inhibitor of the ER-stress pathway, the activation of caspase-12 was significantly inhibited, whereas the expression of Bax and the activation of caspase-3 were not significantly influenced (Figure 4b).
Figure 4.
The potential mechanism involved in the cell apoptosis. (a) Expression of mitochondrial apoptosis-related and ER-stress-related proteins by western blot analysis. Hep3B cells were treated with Ad.Luc, Ad.Bax, Ad.IL-24 or Ad.IL-24-Bax (MOI=10) for 48 h. BiP/GRP78, caspase-12, caspase-3 and Bax proteins were detected by western blot analysis. β-Actin was used as the internal standard. (b) Expression of the mitochondrial apoptosis-related proteins after blocking the ER-stress pathway by western blot analysis. Hep3B cells were treated with ALLN (25 μ) for 30 min, then infected with Ad.Luc, Ad.Bax, Ad.IL-24 or Ad.IL-24-Bax (MOI=10) for 48 h. BiP/GRP78, caspase-12, caspase-3 and Bax proteins were detected by western blot analysis. β-Actin was used as the internal standard.
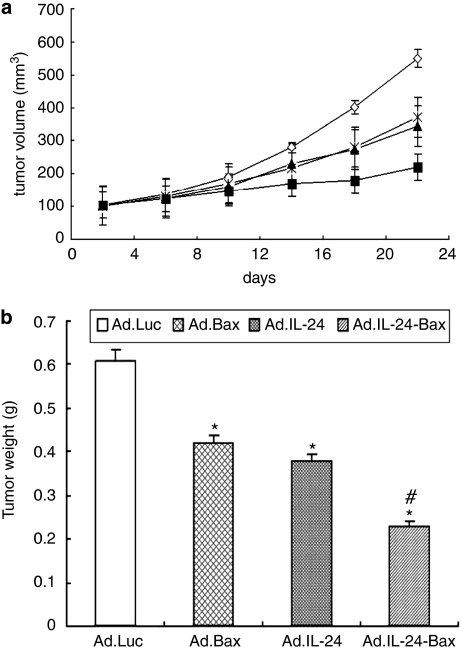
Ad.IL-24-Bax suppressed tumor growth and induced cell apoptosis in tumor-bearing nude mice
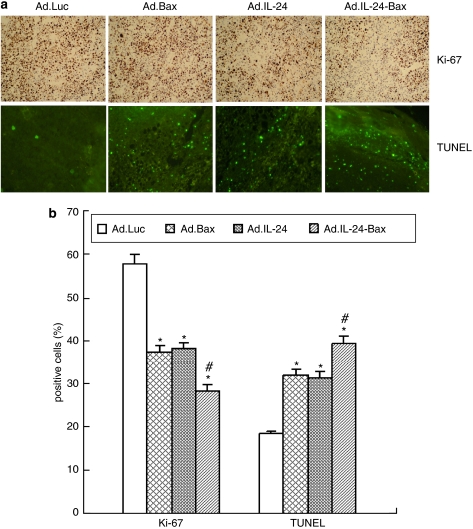
Figure 5 shows that the tumor volume in the Ad.IL-24-Bax group was significantly smaller compared with that of the Ad.Luc, Ad.Bax or Ad.IL-24 group (P<0.05). More importantly, compared with the other groups, the expression of Ki-67 was significantly lower in the Ad.IL-24-Bax group, as evidenced by immunohistochemistry and assessment of the proliferation index (Figure 6).
Figure 5.
Ad.IL-24-Bax suppressed liver tumor growth in tumor-bearing nude mice model. (a) The tumor-bearing BALB/c mice (n=10) were subcutaneously injected with Ad.Luc (⋄), Ad.Bax ( ), Ad.IL-24 (▴) or Ad.IL-24-Bax (▪) (2 × 108 PFU per 100 μl). The treatment was performed every 3 days for a total of three times for each mouse and killing the mice after 3 weeks. The tumor volume of mice treated with Ad.IL-24-Bax were significantly (P<0.05) lower than those of the other groups. (b) Ad.IL-24-Bax decreased tumor weight on the tumor-bearing mice. *P<0.05, vs the Ad.Luc group. #P<0.05 vs the Ad.IL-24 group.
), Ad.IL-24 (▴) or Ad.IL-24-Bax (▪) (2 × 108 PFU per 100 μl). The treatment was performed every 3 days for a total of three times for each mouse and killing the mice after 3 weeks. The tumor volume of mice treated with Ad.IL-24-Bax were significantly (P<0.05) lower than those of the other groups. (b) Ad.IL-24-Bax decreased tumor weight on the tumor-bearing mice. *P<0.05, vs the Ad.Luc group. #P<0.05 vs the Ad.IL-24 group.
Figure 6.
Ad.IL-24-Bax suppressed the proliferation of hepatocellular carcinoma cells and induced apoptosis in hepatocellular carcinoma in vivo. (a) The treatment was performed every 3 days for a total of three times for each mouse (n=5) and killed the mice after 3 weeks. Expression of ki-67 in Hep3B tumors was analyzed by immunohistochemistry 3 weeks after the initial injection of transgenic virus, and apoptotic Hep3B cells were detected by transferase-mediated dUTP nick-end-labeling. (b) The number of positive cells was counted as described in Materials and methods. *P<0.05, vs the Ad.Luc group. #P<0.05 vs the Ad.IL-24 group. The results were expressed as means±s.d. of three randomly selected mice.
As measured by transferase-mediated dUTP nick-end-labeling assay (Figure 6), the number of apoptotic Hep3B cells was significantly more in the Ad.IL-24-Bax group than in other groups. These results indicate that Ad.IL-24-Bax inhibited the growth and proliferation of Hep3B tumors and induced tumor apoptosis in vivo.
Discussion
Hepatocellular carcinoma is a major malignant solid tumor worldwide, and its incidence keeps increasing in the United States as well as in other parts of the world. The current therapies include traditional surgical procedures and newly emerged minimally invasive therapy. However, the post-operation recurrence rate and the metastasis rate remain high. Hepatocellular carcinoma is insensitive to chemotherapy and radiotherapy and can easily develop tolerance. Generally, most patients with hepatocellular carcinoma do not respond well to therapy. Thus, new therapies, such as gene therapy and molecular targeted therapy should be studied for the treatment of hepatocellular carcinoma.24
Recent studies including our study have shown that mda-7/IL-24 could selectively induce apoptosis of hepatocellular carcinoma cells,22, 23 and its effect was related to the ER-stress pathway.23 In this study, IL-24 and Bax, with different apoptosis promoting mechanisms, were combined to enhance their respective proapoptosis effects on hepatocellular carcinoma cells. Briefly, the complete cDNA of IL-24 and the cDNA of Bax α chain were linked by a hinge zone, EASGGPE, coding for a region of human IgG1 gene. A 6 × His tag was introduced before the stop codon for the convenience of identification and purification of the expressed recombinant protein. VEGF385 with a 10 × HRE sequence was designed in the promoter zone to modulate the expression of IL-24-Bax, as most solid tumors are anoxic. We found Hep3B could express adenovirus-mediated recombinant protein IL-24-Bax with a molecular weight of 47 kDa. Under anoxic conditions, the 10HRE/VEGF385 promoter could increase the expression of recombinant IL-24-Bax in hepatocellular carcinoma cells, and the expression level was increased over time under anoxic conditions.
Studies25, 26 have also shown that the growth inhibition of tumor cells induced by IL-24 is independent of tumor suppressor genes and apoptosis-related genes such as p53, p16, ras, Bax and caspase-3. Bax could induce permeation of mitochondrial outer membrane, thus directly causing the release of apoptosis molecules such as cytochrome C. One of p53's anti-tumor effects is to activate mitochondrial-mediated apoptosis by Bax and other Bcl-2 family members.10 Three human hepatocellular carcinoma cell lines with differing p53 situations were selected as test cells in this study: Hep3B is p53-null, PLC/PRF/5 has mutant p53, and HepG2 has wild-type p53. The results showed that regardless of p53 status, Ad.IL-24-Bax could inhibit hepatocellular carcinoma cell growth and induce apoptosis, and the effects were increased with the MOI. In addition, when compared with Ad.IL-24 or Ad.Bax alone, the effects of Ad.IL-24-Bax were more significant and had no obvious cytotoxic effects on the normal hepatocyte cell line, L02. The results in the nude mouse hepatoma model were similar: Ad.IL-24-Bax postponed the growth of the hepatoma and the effect was more significant than Ad.IL-24 or Ad.Bax alone. In the hepatoma tissue slice, the expression of the hepatocellular carcinoma cell proliferation related antigen ki-67 decreased, and many hepatocellular carcinoma cells showed signs of apoptosis. It can be inferred that as the environment of most solid tumors is anoxic, IL-24-Bax, whose expression was modulated by the HRE promoter, could enhance the sensitivity and specificity of the test cells to the anoxic conditions of hepatocellular carcinoma cells. Moreover, IL-24 or Bax-mediated gene therapy could inhibit the growth of many kinds of tumor cells. IL-24 and Bax retained their own independent effects on tumor cell apoptosis or might act synergistically, thus Ad.IL-24-Bax's effect of inhibiting hepatocellular carcinoma cell growth and promoting apoptosis were more significant than those of Ad.Bax or Ad.IL-24. On the other hand, normal human hepatocytes were not obviously affected after infection with Ad.Luc, Ad.Bax, Ad.IL-24 or Ad.IL-24-Bax. This was due to the selectivity of IL-24-induced tumor cell apoptosis and the limited expression of Bax in normal hepatocytes, as the promoter was regulated by anoxia.
Both IL-24 and Bax can promote apoptosis, only by different mechanisms. An obvious difference is that IL-24 can activate the ER-stress pathway and induce cell apoptosis.18, 23, 27, 28, 29, 30, 31 We addressed this issue by asking whether the different mechanisms of the recombinant protein IL-24-Bax can result in an enhanced anti-hepatoma effect. Did IL-24 and Bax function respectively or synergistically? Did many apoptosis pathways function at the same time? To answer these questions, p53-absent Hep3B cells were selected for further study. To determine whether the ER-stress pathway was involved or not, we performed western blots to assess the activation of the ER-stress pathway related protein Bip/GPR78 and caspase-12, as well as the expression of the mitochondrial pathway related protein Bax and activated caspase-3. We inferred from the results that apoptosis induced by Ad.IL-24-Bax may involve both the ER-stress pathway and the mitochondrial pathway. Then, we inhibited the activation of caspase-12 in the ER-stress pathway with the calpain inhibitor I (ALLN) to investigate the relationship between the ER-stress pathway and the mitochondrial pathway. The results showed that after ALLN treatment, the activation of caspase-12 in Ad.IL-24-Bax infected Hep3B cells was significantly inhibited, whereas the expression of Bax and caspase-3 were not changed, indicating that the mechanism by which Ad.IL-24-Bax activates the ER-stress pathway was different from that of Ad.IL-24. Because of Bax, Ad.IL-24-Bax may induce hepatocellular carcinoma cell apoptosis by changing the permeabilization of the mitochondrial membrane or in other unknown ways.
To conclude, the constructed Ad.IL-24-Bax has an enhanced effect of selectively inducing hepatocellular carcinoma cell apoptosis, whereas IL-24 and Bax retain their respective apoptosis-inducing effect. The mechanism of this enhanced proapoptosis activity involves the dual effect of the ER-stress pathway and the mitochondrial pathway. IL-24, a member of the IL-10 family of cytokines, is produced by monocytes and Th2 cells. Interestingly, immune cells do not seem to express specific IL-24 receptor chains; therefore it is unlikely that IL-24 has classical immune-modulating properties.32 And the initial interest in IL-24 did not arise from its physiological signaling properties through its cognate receptors, but rather from its tentative ability to selectively kill different cancer cells.33 Whether the selectivity was related to the specificity of the receptor or by the bystander effect of secreted IL-24 protein still remains unknown. We will analyze whether IL-24 can act as targeting molecule to introduce recombinant molecules to hepatocellular carcinoma cells and enhance biological activities. It should be noted that we assessed the anti-tumor activity of chimeric protein IL-24-Bax using nude mice; but further studies are still needed to determine its anti-tumor or immunomodulatory activities in euthymic mice, for the result will determine whether chimeric protein IL-24-Bax have the potential to develop into highly specific therapeutic agent against cancer.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30500477, 30873020) and China National Key Projects for Infectious Disease (No. 2008ZX10002-021).
The authors declare no conflict of interest.
References
- Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin treatment of cancer. Annu Rev Med. 2007;58:221–237. doi: 10.1146/annurev.med.58.070605.115320. [DOI] [PubMed] [Google Scholar]
- Jarboe JS, Johnson KR, Choi Y, Lonser RR, Park JK. Expression of interleukin-13 receptor alpha2 in glioblastoma multiforme: implications for targeted therapies. Cancer Res. 2007;67:7983–7986. doi: 10.1158/0008-5472.CAN-07-1493. [DOI] [PubMed] [Google Scholar]
- Litzinger MT, Fernando R, Curiel TJ, Grosenbach DW, Schlom J, Palena C. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 2007;110:3192–3201. doi: 10.1182/blood-2007-06-094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A, Liu JS, Rizzieri D, Hogge D. Phase I clinical study of diphtheria toxin-interleukin 3 fusion protein in patients with acute myeloid leukemia and myelodysplasia. Leuk Lymphoma. 2008;49:543–553. doi: 10.1080/10428190701799035. [DOI] [PubMed] [Google Scholar]
- Aqeilan R, Yarkoni S, Lorberboum-Galski H. Interleukin 2-Bax: a novel prototype of human chimeric proteins for targeted therapy. FEBS Lett. 1999;457:271–276. doi: 10.1016/s0014-5793(99)01050-9. [DOI] [PubMed] [Google Scholar]
- Ben-Yehudah A, Aqeilan R, Belostotsky R, Azar Y, Lorberboum-Galski H. Utilizing chimeric proteins for exploring the cellular fate of endogenous proteins. Biochem Biophys Res Commun. 2002;290:332–338. doi: 10.1006/bbrc.2001.6163. [DOI] [PubMed] [Google Scholar]
- Dansen TB, Whitfield J, Rostker F, Brown-Swigart L, Evan GI. Specific requirement for Bax, not Bak, in Myc-induced apoptosis and tumor suppression in vivo. J Biol Chem. 2006;281:10890–10895. doi: 10.1074/jbc.M513655200. [DOI] [PubMed] [Google Scholar]
- Toyota H, Kondo S, Kyo S, Mizuguchi J. Enforced expression of a truncated form of Bax-alpha (tBax) driven by human telomerase reverse transcriptase (hTERT) promoter sensitizes tumor cells to chemotherapeutic agents or tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) Anticancer Res. 2006;26:99–105. [PubMed] [Google Scholar]
- Krop EJ, Matsui EC, Sharrow SD, Stone MJ, Gerber P, van der Zee JS, et al. Recombinant major urinary proteins of the mouse in specific IgE and IgG testing. Int Arch Allergy Immunol. 2007;144:296–304. doi: 10.1159/000106318. [DOI] [PubMed] [Google Scholar]
- Tobiume K. Involvement of Bcl-2 family proteins in p53-induced apoptosis. J Nippon Med Sch. 2005;72:192–193. doi: 10.1272/jnms.72.192. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–2486. [PubMed] [Google Scholar]
- Schweinfest CW, Nelson PS, Graber MW, Demopoulos RI, Papas TS. Subtraction hybridization cDNA libraries. Methods Mol Biol. 1995;37:13–30. doi: 10.1385/0-89603-288-4:13. [DOI] [PubMed] [Google Scholar]
- Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Lebedeva IV, Sarkar D, Gopalkrishnan RV, Sauane M, Sigmon C, et al. Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene. 2003;22:1164–1180. doi: 10.1038/sj.onc.1206062. [DOI] [PubMed] [Google Scholar]
- Ellerhorst JA, Prieto VG, Ekmekcioglu S, Broemeling L, Yekell S, Chada S, et al. Loss of MDA-7 expression with progression of melanoma. J Clin Oncol. 2002;20:1069–1074. doi: 10.1200/JCO.2002.20.4.1069. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, et al. mda-7 (IL-24) mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci USA. 2002;99:10054–10059. doi: 10.1073/pnas.152327199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauane M, Su ZZ, Gupta P, Lebedeva IV, Dent P, Sarkar D, et al. Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proc Natl Acad Sci USA. 2008;105:9763–9768. doi: 10.1073/pnas.0804089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauane M, Gopalkrishnan RV, Lebedeva I, Mei MX, Sarkar D, Su ZZ, et al. Mda-7/IL-24 induces apoptosis of diverse cancer cell lines through JAK/STAT-independent pathways. J Cell Physiol. 2003;196:334–345. doi: 10.1002/jcp.10309. [DOI] [PubMed] [Google Scholar]
- Chada S, Mhashilkar AM, Ramesh R, Mumm JB, Sutton RB, Bocangel D, et al. Bystander activity of Ad-mda7: human MDA-7 protein kills melanoma cells via an IL-20 receptor-dependent but STAT3-independent mechanism. Mol Ther. 2004;10:1085–1095. doi: 10.1016/j.ymthe.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Friedman SL, Shaulian E, Littlewood T, Resnitzky D, Oren M. Resistance to p53-mediated growth arrest and apoptosis in Hep 3B hepatoma cells. Oncogene. 1997;15:63–70. doi: 10.1038/sj.onc.1201149. [DOI] [PubMed] [Google Scholar]
- Zhao J, Wang H, Wei L, Habib NA, Lu X, Wu M, et al. The cytotoxic effect of E1B 55-kDa mutant adenovirus on human hepatocellular carcinoma cell lines. Cancer Gene Ther. 2001;8:333–341. doi: 10.1038/sj.cgt.7700316. [DOI] [PubMed] [Google Scholar]
- Chen WY, Cheng YT, Lei HY, Chang CP, Wang CW, Chang MS. IL-24 inhibits the growth of hepatoma cells in vivo. Genes Immun. 2005;6:493–499. doi: 10.1038/sj.gene.6364233. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kang X, Shi L, Li J, Xu W, Qian H, et al. mda-7/IL-24 induces apoptosis in human HepG2 hepatoma cells by endoplasmic reticulum stress. Oncol Rep. 2008;20:437–442. [PubMed] [Google Scholar]
- El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Sarkar D, Su ZZ, Kitada S, Dent P, Stein CA, et al. Bcl-2 and Bcl-x(L) differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda-7/IL-24. Oncogene. 2003;22:8758–8773. doi: 10.1038/sj.onc.1206891. [DOI] [PubMed] [Google Scholar]
- Yacoub A, Mitchell C, Hong Y, Gopalkrishnan RV, Su ZZ, Gupta P, et al. MDA-7 regulates cell growth and radiosensitivity in vitro of primary (non-established) human glioma cells. Cancer Biol Ther. 2004;3:739–751. doi: 10.4161/cbt.3.8.968. [DOI] [PubMed] [Google Scholar]
- Sieger KA, Mhashilkar AM, Stewart A, Sutton RB, Strube RW, Chen SY, et al. The tumor suppressor activity of MDA-7/IL-24 is mediated by intracellular protein expression in NSCLC cells. Mol Ther. 2004;9:355–367. doi: 10.1016/j.ymthe.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Sauane M, Gupta P, Lebedeva IV, Su ZZ, Sarkar D, Randolph A, et al. N-glycosylation of MDA-7/IL-24 is dispensable for tumor cell-specific apoptosis and ‘bystander' antitumor activity. Cancer Res. 2006;66:11869–11877. doi: 10.1158/0008-5472.CAN-06-1887. [DOI] [PubMed] [Google Scholar]
- Park MA, Yacoub A, Sarkar D, Emdad L, Rahmani M, Spiegel S, et al. PERK-dependent regulation of MDA-7/IL-24-induced autophagy in primary human glioma cells. Autophagy. 2008;4:513–515. doi: 10.4161/auto.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W, Liu J, Tong Y, Yan S, Yang C, Yang M, et al. Enhanced antitumor activity by a selective conditionally replicating adenovirus combining with MDA-7/interleukin-24 for B-lymphoblastic leukemia via induction of apoptosis. Leukemia. 2008;22:361–369. doi: 10.1038/sj.leu.2405034. [DOI] [PubMed] [Google Scholar]
- Yacoub A, Park MA, Gupta P, Rahmani M, Zhang G, Hamed H, et al. Caspase-, cathepsin-, and PERK-dependent regulation of MDA-7/IL-24-induced cell killing in primary human glioma cells. Mol Cancer Ther. 2008;7:297–313. doi: 10.1158/1535-7163.MCT-07-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada S, Sutton RB, Ekmekcioglu S, Ellerhorst J, Mumm JB, Leitner WW, et al. MDA-7/IL-24 is a unique cytokine--tumor suppressor in the IL-10 family. Int Immunopharmacol. 2004;4:649–667. doi: 10.1016/j.intimp.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Kreis S, Philippidou D, Margue C, Behrmann I. IL-24: a classic cytokine and/or a potential cure for cancer. J Cell Mol Med. 2008;12:2505–2510. doi: 10.1111/j.1582-4934.2008.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]