Abstract
Vitamin D deficiency is a global public-health concern, even in tropical regions where the risk of deficiency was previously assumed to be low due to cutaneous vitamin D synthesis stimulated by exposure to sun. Poor vitamin D status, indicated by low serum concentrations of 25-hydroxyvitamin D [25(OH)D], has been observed in South Asian populations. However, limited information is available on the vitamin D status of young infants in this region. Therefore, to gain preliminary insights into the vitamin D status of infants in rural Bangladesh, 25(OH)D was assessed in a group of community-sampled control participants in a pneumonia case-control study in rural Sylhet, Bangladesh (25°N) during the winter dry season (January-February). Among 29 infants aged 1-6 months, the mean 25(OH)D was 36.7 nmol/L [95% confidence interval (CI) 30.2-43.2]. The proportion of infants with vitamin D deficiency defined by 25(OH)D <25 nmol/L was 28% (95% CI 10-45), 59% (95% CI 40-78) had 25(OH)D<40 nmol/L, and all were below 80 nmol/L. From one to six months, there was a positive correlation between age and 25(OH)D (Spearman=0.65; p=0.0001). Within a larger group of 74 infants and toddlers aged 1-17 months (cases and controls recruited for the pneumonia study), young age was the only significant risk factor for vitamin D deficiency [25(OH)D <25 nmol/L]. Since conservative maternal clothing practices (i.e. veiling) and low frequency of intake of foods from animal source (other than fish) were common among the mothers of the participants, determinants of low maternal-infant 25(OH)D in Bangladesh deserve more detailed consideration in future studies. In conclusion, the vitamin D status in young infants in rural Sylhet, Bangladesh, was poorer than might be expected based on geographic considerations. The causes and consequences of low 25(OH)D in infancy and early childhood in this setting remain to be established.
Key words: Risk factors, 25-hydroxyvitamin D, Vitamin D, Vitamin D deficiency, Bangladesh
INTRODUCTION
Throughout most of the previous century, vitamin D deficiency and rickets were predominantly perceived as problems of industrialized countries at northern latitudes, where insufficient exposure to sun and intake of vitamin D were linked to inadequate intestinal absorption of calcium and impaired skeletal mineralization (1). However, since vitamin D status has become readily estimable based on serum/plasma 25-hydroxyvitamin D concentration [25(OH)D] (2), epidemiologic research aimed at identifying previously-uncharacterized at-risk populations and characterizing novel disease associations with vitamin D status has been greatly facilitated. Thus, vitamin D deficiency has re-emerged as a global public-health concern and is now presumptively linked to a range of infectious, inflammatory and neoplastic diseases throughout the life course and around the world (1).
Low 25(OH)D is surprisingly common in South Asia, where systemic vitamin D deficits would be expected to be prevented by cutaneous vitamin D synthesis stimulated by exposure to sun at relatively low latitudes (3). Few studies on vitamin D status in infancy have been conducted in South Asia (Table 1). In Bangladesh, two published reports on childhood 25(OH)D are available but neither reported 25(OH)D in early infancy (Table 1).
Table 1.
Summary of studies of vitamin D status of infants and children aged 1 month to <5 years in Bangladesh, Pakistan, and India
| Study | Participants | Location | Latitude | Season | No. | Mean 25(OH)D (nmol/L) | Categorical descriptions of vitamin D status |
|---|---|---|---|---|---|---|---|
| Combs et al., 2008 (9) | Aged 1-5 years; no clinical signs of rickets | Chakaria subdistrict, Cox's Bazar, Bangladesh | 21°N | Unknown | 158 | 68.3 |
|
| Fischer et al., 1999 (10) | Cases in case-control study of rickets; aged 10-120 months | Chakaria subdistrict, Bangladesh | 21°N | Unknown | 14 | 50 |
|
| Controls | Chakaria subdistrict, Bangladesh | 21°N | Unknown | 13 | 62.5 |
|
|
| Atiq et al., 1998 (11) | Aged 6 weeks-11 months; immunization clinic | Karachi, Pakistan | 25°N | November-March | 23 | 24.5 |
|
| April-October | 48 | 40.7 | |||||
| Agarwal et al., 2002 (12) | Aged 9-24 months; high-pollution area | Morigate, Delhi, India | 28°N | March-April | 26 | 31 |
|
| Aged 9-24 months; low-pollution area | Gurgaon, Delhi, India | 28°N | March-April | 31 | 68 |
|
|
| Bhalala et al., 2007 (13) | Healthy breastfed infants aged 3 months | Mumbai, India | 18°N | Throughout the year | 35 | 45.5 |
|
| Tiwari and Puliyel, 2004 (14) | Aged 9-30 months living in impoverished neighbourhoods | Sundernagari, Delhi, India | 28°N | January | 47 | 96.3 |
|
| Rajiv Colony, Delhi, India | 28°N | February | 49 | 23.8 |
|
||
| Rajiv Colony, Delhi, India | 28°N | August | 48 | 17.8 |
|
||
| Gurgaon, Delhi, India | 28°N | August | 52 | 19.2 |
|
||
| Wayse et al., 2004 (15) | Healthy controls in case-control study; aged <5 years | Indapur, India | 17°N | May-June | 70 | 38.4 |
|
Knowledge of the vitamin D status of young children and infants is needed to design studies targeting the aetiologic mechanisms and potential health implications of deficiency. A case-control study on the association between acute lower respiratory tract infection (ALRI) and vitamin D status in infants and young children conducted in Zakiganj subdistrict of Sylhet district in Bangladesh, during January-February 2008, provided an opportunity to gain preliminary insights into the vitamin D status of infants in northeastern rural Bangladesh (4). Here, we aimed to describe the vitamin D status of the source population and briefly review the potential determinants of low infant 25(OH)D in this setting.
MATERIALS AND METHODS
Setting
Zakiganj subdistrict (upazila) is in Sylhet district of northeastern Bangladesh (25°N), on the border with India. This region has low average household income and maternal literacy and has limited access to healthcare compared to neighbouring subdistricts in Sylhet (5). The study was facilitated by strong existing partnerships with local community organizations, the subdistrict health complex, and the Ministry of Health and Family Welfare, based on an ongoing collaborative neonatal health intervention trial infrastructure (Projahnmo) (6).
Participants
ALRI cases who met a clinical definition of ALRI were recruited from among infants and young children, aged one month to two years, admitted to the Zakijang subdistrict hospital. Control participants were selected by sampling from among children who lived in the same villages as the cases, were matched to a case on age (±2 months) and gender, and had no signs of ALRI at recruitment or reported past history of ALRI/pneumonia. To identify controls, a rapid household census was conducted in the village of residence of each case participant to generate a list of eligible controls aged 1-23 months. In an order based on closeness in age to the index case, caregivers of the listed children were approached until a control participant was recruited, consent was obtained, and a blood specimen was collected. If an eligible control was not enrolled, the census and eligible control identification process was repeated in the nearest neighbouring village. Some recruited children not considered eligible for the primary case-control study were included in the present analysis. The major reasons for this difference were that children enrolled during a one-week pilot phase were not included in the case-control study but were included here, and the strict requirement that gross haemolysis be absent on visual inspection of serum specimens included in the case-control study was relaxed for the present analysis. This latter decision was made based on post-hoc findings that the mean 25(OH)D of grossly haemolyzed specimens was only slightly and non-significantly lower than that of non-haemolyzed serum specimens (difference of means=3.6 nmol/L, p=0.335, after adjustment for case-control status).
Collection of data
Caregivers (mothers) of participants were administered a questionnaire that addressed selected infant, maternal and household characteristics potentially associated with vitamin D status. Maternal intake of foods from animal sources, which included potential sources of vitamin D and rich sources of calcium, was assessed based on the reported frequency of consumption of food items/categories over the seven days preceding enrollment. Participants were further categorized as to whether the mother had consumed each food item/category at least once in the preceding seven days. Weight of infant was the average of two measurements recorded to the nearest 0.1 kg (Seca 354 infant scale), and length was the average of two measurements, to the nearest 0.5 cm (Seca 210 measuring mat). Gender-specific weight-for-age (WA), length-for-age (LA), and body mass index (BMI) z-scores were calculated according to the growth standards of the World Health Organization (7). According to convention, participants with z-score values of less than -2 for each of the anthropometric indices were considered to have stunting (LA), underweight (WA), and low body mass index (BMI). Since reliable information on gestational age at birth was unavailable, anthropometric measures were interpreted under the assumption of term gestation.
A venous blood specimen was collected by standard methods, separated into serum aliquots, and stored at -20 °C or less. At the completion of the study, sera were shipped to the laboratory of Dr. Bruce Hollis, Medical University of South Carolina, Charleston, USA, for measurement of the total serum 25(OH)D concentration by radio-immunoassay (8).
Outcomes
The primary outcome of the study was the estimated mean 25(OH)D among infants and children aged one month to two years, in the referral area of Zakiganj subdistrict hospital, based only on the healthy control participants sampled from the community. Since most controls (29/35) from whom blood samples were obtained were aged less than six months, the analysis was focused on this age subgroup. Estimates of the prevalence of vitamin D deficiency were based on the proportion of participants with 25(OH)D lower than pre-specified cut-offs (<25 nmol/L, <40 nmol/L, and <80 nmol/L).
As a secondary exploratory analysis, observations relating to infant, maternal or household factors that potentially influenced vitamin D status were drawn from the complete sample of 74 participants (cases and controls) in whom 25(OH)D was measured. Comparisons were made between groups of participants categorized by vitamin D status using a 25-nmol/L cut-off. ALRI cases were included in this analysis to maximize the size of the available sample of infants in whom risk factors could be explored. However, in the primary case-control study, the mean 25(OH)D was significantly lower among ALRI cases compared to controls (4).
Statistical analysis
The distribution of 25(OH)D among control participants aged less than six months (n=29) was described by its mean and 95% confidence interval (CI), standard deviation (SD), median and interquartile range (IQR), and the proportions (and 95% CIs) of participants with 25(OH)D less than each of the cut-off values. Other than age and 25(OH)D, which were compared across groups by one-way analysis of variance, the statistical significance of bivariate associations between the vitamin D status and the maternal, infant or household characteristics in the entire sample (n=74) was assessed by non-parametric tests, including chi-square tests, Mann-U Whitney tests, and Spearman's rank correlation coefficient. Given that the determinants of vitamin D status may differ across age-groups, associational analyses were repeated among participants aged less than six months. Analyses were performed using the Stata software (version 10.1) (Stata Corporation, College Station, TX, USA). By convention, the p values of less than 0.05 were considered significant.
Ethics
Caregivers provided signed permission before enrollment. The Institutional Review Board of the Johns Hopkins Bloomberg School of Public Health and the ethics committee of the Bangladesh Institute for Child Health at the Dhaka Shishu Hospital, Bangladesh, approved the study.
RESULTS
Characteristics of study participants
Serum 25(OH)D was measured in the 74 participants (39 ALRI cases and 35 controls) during the study. Of 58 potential controls identified in village censuses and approached for participation, 14 were not enrolled due to parental refusal, six due to inability to bring the child to the hospital for study procedures, and three due to history of ALRI. The subgroup of 29 community control participants, aged 1-6 months, consisted mainly of boys (a result of the male predominance among the ALRI cases to whom the controls were sex-matched), with maternal and household characteristics typical of rural Sylhet (Table 2).
Table 2.
Characteristics of a sample of infants aged 1-6 months in rural Sylhet district, Bangladesh
| Characteristics | Mean±SD or no. (%) |
|---|---|
| No. | 29 |
| Age (days) | 71±32 |
| Boys | 25 (86) |
| Age (years) of mothers | 23.8±4.2 |
| Mother attended any school | 19 (66) |
| Father attended any school | 18 (62) |
| Family owns their own home | 19 (66) |
| Lives in a house with components made of manufactured materials (i.e. cement, brick, tin) | |
| Floor | 1 (3) |
| Walls | 9 (31) |
| Roof | 27 (93) |
| Occupation of primary income-earner of household | |
| Day labourer | 15 (52) |
| Farmer on leased land | 2 (7) |
| Land owner | 2 (7) |
| Non-agricultural business-owner | 5 (17) |
| Salaried non-agricultural job | 5 (17) |
| Muslim religion | 27 (93) |
| Mother wears a burka when in public | 27 (93) |
| Exclusively breastfeeding | 23 (79) |
| Anthropometric measures | |
| Weight-for-age z-score | -1.58±1.29 |
| Length-for-age z-score | -1.08±1.57 |
| Body mass index z-score | -1.40±1.67 |
SD=Standard deviation
Vitamin D status of infants aged 1-6 months
Serum 25(OH)D ranged from 9.5 to 73.9 nmol/L among the community-sampled infants aged 1-6 months (Fig. 1). Their mean 25(OH)D was 36.7 nmol/L (95% CI 30.2-43.2; SD=17.1 nmol/L), and the median was 38.2 nmol/L (IQR 25.5). The proportion of infants with 25(OH)D below each cut-off level was 28% (95% CI 10-45) <25 nmol/L, and 59% (95% CI 40-78) <40 nmol/L; all were below 80 nmol/L. From one to six months, there was a positive correlation between age and 25(OH)D (Spearman ρ=0.65; p=0.0001) (Fig. 1). Among the youngest 22 infants aged one to less than three months, 36% (95% CI 15-58) had 25(OH)D <25 nmol/L, and 73% (95% CI 53-93) had 25(OH)D <40 nmol/L.
Fig. 1.
Association between serum 25-hydroxyvitamin D concentration and age in 29 community-sampled infants aged 1-6 months in Sylhet district, Bangladesh
Boys are represented by filled circles and girls by hollow squares; the solid line is a linear leastsquares fit line for boys and girls combined
Characteristics associated with low 25(OH)D
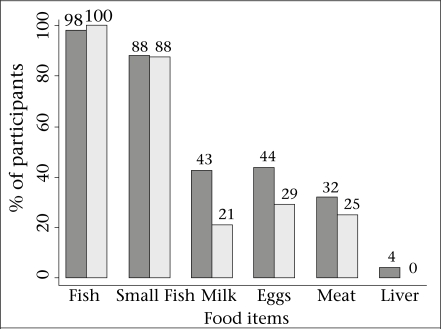
Of the 74 participants (cases and controls) aged 1-17 months, the mean 25(OH)D was 32.6 nmol/L (95% CI 29.1-36.2), the highest 25(OH)D in any participant was 73.9 nmol/L, 24 (32%) had 25(OH)D <25 nmol/L, and 52 (70%) had 25(OH)D <40 nmol/L. In this group, age was the only factor that was significantly associated with vitamin D deficiency defined by 25(OH)D <25 nmol/L (Table 3). However, deficient infants/toddlers tended to be of lower socioeconomic status based on household ownership and housing materials and were somewhat more likely to be stunted and at low BMI (Table 3). Although there were no significant associations between maternal dietary intake and infant/toddlers’ 25(OH)D (data not shown) or vitamin D deficiency (Table 3), a higher proportion of the mothers of infants with 25(OH)D ≥25 nmol/L consumed milk, meat, and eggs on at least one occasion during the week preceding the interview (Fig. 2). Results of analyses of associations of vitamin D status with potential risk factors were similar when repeated in a group restricted to participants aged less than six months (data not shown).
Table 3.
Comparison of selected characteristics of infants and toddlers categorized by vitamin D status in Sylhet, Bangladesh
| Characteristics | 25(OH)D <25 nmol/L | 25(OH)D ≥25 nmol/L | p value |
|---|---|---|---|
| No. | 24 | 50 | |
| Mean 25(OH)D±SD | 16.3±5.8 | 40.5±11.8 | <0.001 |
| Age (mean days±SD) | 71.3±43.4 | 136.6±126.7 | <0.001 |
| <6 months | 23 (96) | 39 (78) | 0.051 |
| Boys | 21 (88) | 40 (80) | 0.427 |
| Birth-order (median±IQR) | 2±4.5 | 2±5 | 0.455 |
| Anthropometric indicators | |||
| Underweight | 11 (46) | 25 (48) | 0.861 |
| Stunted | 10 (42) | 16 (32) | 0.415 |
| Low body mass index | 14 (58) | 20 (40) | 0.138 |
| Age of mothers (mean years±SD) | 23.7±5.6 | 24.8±5.3 | 0.410 |
| Years of schooling of mother (median±IQR) | 1.5±6.5 | 5±6 | 0.613 |
| Years of schooling of father (median±IQR) | 0±5 | 4±5 | 0.177 |
| Family owns their own homestead | 14 (58) | 32 (64) | 0.638 |
| Manufactured housing materials (i.e. cement, brick, or tin) | |||
| Floor | 0 | 3 (6) | 0.221 |
| Walls | 6 (25) | 16 (32) | 0.537 |
| Roof | 20 (83) | 45 (90) | 0.411 |
| Child is/was usually swaddled during the first 6 months of life | 24 (100) | 46 (92) | 0.154 |
| Child was deliberately exposed to sunlight before learning to crawl/walk | 18 (75) | 36 (72) | 0.786 |
| Mother's clothing practices when outdoors | |||
| Head usually covered | 23 (96) | 50 (100) | 0.146 |
| Face usually covered | 0 | 0 | - |
| Arms usually covered | 24 (100) | 48 (96) | 0.321 |
| Usually wears burka in public | 21 (91) | 47 (94) | 0.672 |
| Number of meals at which mother reportedly consumed food items from animal source during the past week (median±IQR) | |||
| Any type of fish | 13.0±5 | 14.5±12 | 0.158 |
| Small fish with bones | 6.0±7 | 6±8 | 0.561 |
| Milk | 0±0 | 0±7 | 0.119 |
| Meat | 0±0.5 | 0±2 | 0.415 |
| Eggs | 0±1.5 | 0±2 | 0.247 |
| Liver | 0±0 | 0±0 | 0.324 |
Figures in parentheses indicate percentages;
IQR=Interquartile range;
SD=Standard deviation
Fig. 2.
Percentage of the study participants whose mothers consumed the specified food item/category at least once during the preceding 7 days, among those participants with 25(OH)D ≥25 nmol/L (dark bars; n=50), and those with 25(OH)D <25 nmol/L (light gray bars; n=24)
Fish referred to any fish whereas small fish referred to those typically consumed with bones (e.g. mola); meat referred to flesh (i.e. poultry, beef, or mutton)
DISCUSSION
This preliminary study in Sylhet during the winter dry season revealed that the vitamin D status of young infants in rural Bangladesh might be poor enough to put many at risk of rickets and other potential vitamin D-related health consequences. Applying a very conservative definition of vitamin D deficiency [25(OH)D <25 nmol/L], we estimated that about one-third of infants aged 1-6 months may be vitamin D-deficient. To our knowledge, this is the first report of vitamin D status in young infants in Bangladesh. However, the causes and consequences of low 25(OH)D in this setting remain to be determined.
To put these findings in a global context, it is first useful to draw a comparison with what is perhaps the only young infant ‘reference’ group studied at the equator where vitamin D status would be expected to be optimal throughout the year in the absence of dietary supplementation or fortification. In a sample of infants in Oyem, Gabon (1°N), the mean 25(OH)D was 110 nmol/L (SD 43) at birth, 149 nmol/L (SD 54) at three months of age, and 151 nmol/L (SD 64) at six months of age (16). These values suggest a very wide variation but that most infants in that setting were well above the 25(OH)D threshold currently considered optimal (>80 nmol/L) (17). The present findings from Bangladesh were somewhat intermediary between the results of two other studies in South Asia. In Karachi, Pakistan (25°N), 38 breastfed infants aged less than six months had a mean 25(OH)D of 25 nmol/L (18 SD), and 71% of infants (12/17) aged less than three months, had 25(OH)D <40 nmol/L (11). Further south, in Mumbai, India (18°N), 35 breastfed infants at three months of age had a mean 25(OH)D of 49 nmol/L (SD 24), and 51% had values of <37.5 nmol/L (13). In the United Arab Emirates (UAE), at latitude 24°N (about the same as Bangladesh), 78 breastfed term infants aged 1-4 months, born to women with low milk intake and a habitual practice of covering the skin entirely when outdoors, had a median 25(OH)D of only 11.5 nmol/L, and 82% had 25(OH)D <25 nmol/L (18).
In Iowa, USA (41°N), in a longitudinal study of predominantly white infants who were all exclusively breastfed and not receiving supplements, the mean 25(OH)D at about three and half months of age was 33 nmol/L for those assessed in the summer (50% at <27.5 nmol/L) and 17 nmol/L in the winter (79% at <27.5 nmol/L); at approximately six months of age, the mean 25(OH)D increased to 45 nmol/L in the summer (32% at <27.5 nmol/L) but remained at 17 nmol/L for those measured in the winter (82% <27.5 nmol/L) (19). An exogenous vitamin D source is recommended for all infants in North America. So, it is worthwhile noting that, among formula-fed or vitamin D-supplemented infants (mean vitamin D intake of ∼370 IU per day) aged 1-6 months (n=37) enrolled in a hospital-based study during the winter in Alberta, Canada (53°N) (20), the mean 25(OH)D was 78 nmol/L (Roth D et al. unpublished observations). Although there was a wide variation (17-152 nmol/L), only one infant aged 1.3 months had 25(OH)D <40 nmol/L, demonstrating the real-world effect of supplementation/fortification policies.
Therefore, these data from rural Bangladesh, in combination with earlier findings from infants in urban Pakistan, India, and UAE, have demonstrated that the vitamin D status of young infants in South Asia and the Middle East may be no better than that of unsupplemented infants at much higher northern latitudes in North America, where guidelines support the provision of routine vitamin D supplementation to all breastfed infants (21,22). This implies that a tropical climate does not necessarily protect against low 25(OH)D in early infancy. Although cutaneous pre-vitamin D3 synthesis is expected to occur year-round in South Asia on the basis of latitude (23), there is a substantial seasonal variation in ultraviolet B irradiance (24). In fact, a seasonal differential in vitamin D status was observed among Pakistani infants (11). Therefore, the representativeness of our data is limited because they reflect vitamin D status during the winter, when ultraviolet radiation exposure is at its nadir, and when cutaneous vitamin D synthesis would be expected to be relatively minimal (24). Moreover, in the Bengal region, the attenuation of actual summer-time ultraviolet radiation exposure due to monsoon cloud-cover (24) may prevent sufficient endowment of vitamin D stores during the summer and, thus, further increase the risk of deficiency during the winter. Our cross-sectional observations suggest that 25(OH)D in Bangladeshi infants may rise within the first few months of life. However, these age-dependent differences may have been confounded by seasonal timing of gestation—a younger age implied that the third trimester coincided with the expected seasonal nadir of vitamin D synthesis, when maternal vitamin D stores might be relatively depleted and, thus, when transfer of vitamin D metabolites to the foetus may have been minimized.
To further explain the apparent ‘vitamin D paradox’ in South Asia (3), a range of hypothetical mechanisms can be proposed (Table 3). Young infants depend almost entirely on the transplacental transfer of vitamin D and 25(OH)D, which explains the consistent association between maternal and cord-blood 25(OH)D (25) and the observation that maternal antenatal vitamin D supplementation augments both maternal and cord-blood 25(OH)D (26). Therefore, the major reason that Bangladeshi infants start life with poor vitamin D stores is low maternal antenatal 25(OH)D, which has been documented in urban and rural Bangladeshi women of reproductive age (27). Islam et al. recently studied female workers in a garment factory in Dhaka and speculated that their long day-time hours in indoors, brief exposure to low-intensity sunlight in the early morning, outdoor air pollution, and widespread sunscreen use, in combination with darkly-pigmented skin, may contribute to their poor vitamin D status (mean 25(OH)D of 37 nmol/L, and 15% of the participants had 25(OH)D <25 nmol/L) (28). Conservative dress, including almost complete skin coverage by traditional veils or cloaks, has been emphasized as a contributor to vitamin D deficits in Muslim women in South Asia and the Middle East because it limits cutaneous vitamin D synthesis regardless of the intensity of ambient ultraviolet B (29).
Inferences regarding the determinants of infant/toddlers’ vitamin D status in this study were limited, largely because of the small sample-size and substantial uniformity with respect to selected maternal clothing and dietary practices. We also acknowledge that pooling of ALRI cases and controls to maximize our available sample-size may have led to selection biases. However, the observation that mothers of infants with relatively low 25(OH)D seemed less likely to consume foods from animal sources (other than fish) deserves consideration in future studies. The amount of vitamin D in the local diet is unknown but low calcium intake (or reduced absorption of calcium due to high phytate intake), typical of low-income diets in Bangladesh (30,31), may accelerate 25(OH)D use, leading to relatively-increased vitamin D demands (32). Since the concentration of vitamin D metabolites in breastmilk is determined by maternal vitamin D status, maternal vitamin D deficiency during lactation may cause ongoing deficits in postnatal infants’ vitamin D intake (42); yet, maternal factors cannot entirely account for the persistence of low 25(OH)D among toddlers (Table 1) who experience direct exposure to sun and should be unaffected by the conservative clothing practices of their mothers. Therefore, much remains to be learnt about the determinants of vitamin D status throughout infancy and childhood, particularly where 25(OH)D appears discrepant from that which would be expected based on latitude.
Table 4.
Hypothetical mechanisms that may contribute to risk of maternal-infant vitamin D deficiency in South Asia
| General mechanism | Examples of specific hypothesized mechanisms |
|---|---|
| Endogenous cutaneous vitamin D production | |
| Dietary vitamin D intake |
|
| Intestinal vitamin D absorption and storage | |
| Hepatic conversion of vitamin D to 25-hydroyxyvitamin D |
|
| Destruction or use of 25-hydroxyvitamin D or 1,25-dihydroxyvitamin D |
|
Despite the current enthusiasm for vitamin D supplementation in the USA (22), the effects of vitamin D deficits during early infancy are still not fully understood, and meaningful inflection points in 25(OH)D-outcome relationships have not been established. The most widely-accepted manifestation of severe vitamin D deficiency in infancy is rickets, the classical childhood metabolic bone disease associated with skeletal hypomineralization and deformities, muscle weakness, and growth impairment (1). Young infants with severe congenital vitamin D deficiency may present with hypocalcaemic tetany or seizures, with absent or subtle skeletal pathology (43–45). Greer noted that there is no clear or consistent association between 25(OH)D and the risk of rickets or other functional outcomes (46). Although 25(OH)D <25 nmol/L is typical of clinically-apparent rickets, early stages of the disease may occur at higher 25(OH)D (∼40 nmol/L) (47), with declines in 25(OH)D occurring as the disease progresses and vitamin D stores are depleted. The frequent occurrence of 25(OH)D >25 nmol/L among toddlers and older children with rickets has been presumptively attributed to dietary calcium deficits (48); however, rickets among breastfed infants with 25(OH)D >25 nmol/L (49) suggests that factors other than calcium intake may be implicated.
In Bangladesh, rickets may be more common than previously thought, based on surveys of lower limb deformities in ambulating children (50,51). Recent data from a nationwide survey suggest that about 0.6% of Bangladeshi children, aged 1-15 years, may have radiologic evidence of rickets, with the highest prevalence in Chittagong and Sylhet divisions (51). The incidence of symptomatic hypocalcaemia secondary to vitamin D deficiency in early infancy is unknown. Some investigators have played down the role of vitamin D in rickets in Bangladesh (10, 52), instead blaming dietary calcium deficits or other mineral deficiencies or excesses, e.g. aluminum (53). However, in case-control studies, dietary calcium intake between rickets-affected and unaffected households did not differ (54) whereas the mean 25(OH)D in cases was significantly lower than in controls (10). A plausible hypothesis is that vitamin D deficiency acts synergistically with other causes of inadequate bone mineralization and that an individual's 25(OH)D concentration below which clinical signs emerge depends on the severity and multiplicity of other genetic and environmental factors.
Beyond rickets, speculation abounds regarding the potential extra-skeletal consequences of suboptimal vitamin D status during foetal development and infancy. The vitamin D receptor has been found within virtually every organ-system (55), and the active metabolite of vitamin D is well-described as a potent mediator of cell proliferation and differentiation, particularly noted for its range of effects on immune function in laboratory models (56). The case-control study for which the data for the present analysis were primarily collected revealed an inverse association between 25(OH)D and the odds of hospitalization for ALRI (4), corroborating the findings in neonates in Turkey (57) and children in India (15). If vitamin D deficiency is confirmed as a risk factor for pneumonia, interventions to improve maternal-infant vitamin D status could reduce the global burden of ALRI, the single most important cause of early childhood death in the world (58). Other postulated consequences of antenatal or infant vitamin D deficiency include growth faltering (59,60), type 1 diabetes (61), and asthma (62). However, rigorous studies of the broad health benefits of interventions to improve the antenatal or postnatal vitamin D status in South Asian mothers and infants have yet to be reported.
Limitations
This study was limited by its small sample-size, restricted geographic scope, and cross-sectional design. We aimed to select control participants for the main case-control study in a manner that would enable inferences about the source population. Selection of control was necessarily non-random from the perspective of age and gender and, thus, unfortunately led to an over-representation of boys; however, there is unlikely to be a gender differential in vitamin D status during early infancy (63). Also, the requirement for an absence of reported history of ALRI probably produced negligible bias since only three otherwise eligible children were excluded for this reason. Aside from these caveats, the community-based sampling was likely random with respect to most determinants of vitamin D status. The group of infants aged 1-6 months was small but adequate to estimate the mean 25(OH)D within a 13-nmol/L range with 95% confidence. However, data were insufficient to yield precise estimates of the associations between infant and maternal characteristics and 25(OH)D. Another limitation was the lack of ancillary biochemical or radiographic data that may have revealed evidence of adverse consequences of low 25(OH)D. We did not report the findings of physical examinations because scoring systems for rickets are not very useful in early infancy, and a protocol for a standardized musculoskeletal clinical examination was not satisfactorily implemented. However, none of the toddlers had any clinical evidence of rickets according to the physician's examination (data not shown).
Conclusions
This study provides initial observations on the vitamin D status of young infants in northeastern rural Bangladesh. However, it remains to be determined whether the relatively left-shifted distribution of 25(OH)D in this study sample is representative of the broader population and causally associated with an excess burden of rickets, symptomatic hypocalcaemia, growth faltering, or extra-skeletal health outcomes. Therefore, recommendations for universal antenatal and/or infant vitamin D supplementation in Bangladesh based on biochemical data alone would be premature. The causes and consequences of low 25(OH)D in young infants in South Asia must be further investigated.
ACKNOWLEDGEMENTS
The authors thank the participants and caregivers for their kind cooperation and also thank the study field staff, the Projahnmo Sylhet team, and personnel at the Zakiganj subdistrict hospital. They particularly thank Kazi Moksedur Rahman (Deputy Executive Director, SHIMANTIK), Dr. Arun Kumar Roy (Project Research Physician, Projahnmo), Dr. Daniel Hossain (Project Research Manager, Projahnmo), Dr. Sirajul Islam (Upazila Health and Family Planning Officer), and Dr. Jonme Joy Dutta Shankar (Medical Officer, Zakiganj subdistrict hospital). The authors are grateful to Dr. Bruce Hollis and Dr. Carole Wagner (Medical University of South Carolina, USA), Dr. Samir K. Saha (Bangladesh Institute of Child Health, Dhaka Shishu Hospital, Dhaka, Bangladesh), and the Department of International Health at the Johns Hopkins Bloomberg School of Public Health. D. Roth was supported by training grants from the Canadian Institutes for Health Research and the Alberta Heritage Foundation for Medical Research.
REFERENCES
- 1.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–72. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87:1087S–91S. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- 3.Joshi SR. Vitamin D paradox in plenty sunshine in rural India—an emerging threat. J Assoc Physicians India. 2008;56:749–52. [PubMed] [Google Scholar]
- 4.Roth DE, Shah R, Black RE, Baqui AH. Vitamin Dstatus and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr. 2010;99:389–93. doi: 10.1111/j.1651-2227.2009.01594.x. [DOI] [PubMed] [Google Scholar]
- 5.Baqui AH, Arifeen SE, Darmstadt GL, Ahmed S, Seraji HR, Winch PJ, et al. Bangladesh Projahnmo Study Group. Differentials in neonatal mortality in two adjacent rural areas of Bangladesh: lessons for neonatal health interventions. Global Public Health. 2008;3:366–82. [Google Scholar]
- 6.Baqui AH, Arifeen SE, Darmstadt GL, Ahmed S, Williams EK, Seraji HR, et al. Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: a cluster-randomised controlled trial. Lancet. 2008;371:1936–44. doi: 10.1016/S0140-6736(08)60835-1. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO child growth standards: length/height-for-age, weight-for-age, weigh-t-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: World Health Organization; 2006. p. 312. [Google Scholar]
- 8.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with a 125I-labeled tracer. Clin Chem. 1993;39:529–33. [PubMed] [Google Scholar]
- 9.Combs GF, Jr, Hassan N, Dellagana N, Staab D, Fischer P, Hunt C, et al. Apparent efficacy of food-based calcium supplementation in preventing rickets in Bangladesh. Biol Trace Elem Res. 2008;121:193–204. doi: 10.1007/s12011-007-8053-z. [DOI] [PubMed] [Google Scholar]
- 10.Fischer PR, Rahman A, Cimma JP, Kyaw-Myint TO, Kabir AR, Talukder K, et al. Nutritional rickets without vitamin D deficiency in Bangladesh. J Trop Pediatr. 1999;45:291–3. doi: 10.1093/tropej/45.5.291. [DOI] [PubMed] [Google Scholar]
- 11.Atiq M, Suria A, Nizami SQ, Ahmed I. Maternal vitamin-D deficiency in Pakistan. Acta Obstet Gynecol Scand. 1998;77:970–3. [PubMed] [Google Scholar]
- 12.Agarwal KS, Mughal MZ, Upadhyay P, Berry JL, Mawer EB, Puliyel JM. The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Arch Dis Child. 2002;87:111–3. doi: 10.1136/adc.87.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhalala U, Desai M, Parekh P, Mokal R, Chheda B. Subclinical hypovitaminosis D among exclusively breastfed young infants. Indian Pediatr. 2007;44:897–901. [PubMed] [Google Scholar]
- 14.Tiwari L, Puliyel JM. Vitamin D level in slum children of Delhi. Indian Pediatr. 2004;41:1076–7. [PubMed] [Google Scholar]
- 15.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–7. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 16.Nguema-Asseko B, Ganga-Zandzou PS, Ovono F, Lendoye E, Lemamy GJ, Akendengue B, et al. [Vitamin D status in Gabonese children] Arch Pediatr. 2005;12:1587–90. doi: 10.1016/j.arcped.2005.06.018. [French]. [DOI] [PubMed] [Google Scholar]
- 17.Heaney RP. Vitamin D: criteria for safety and efficacy. Nutr Rev. 2008;66(10 Suppl 2):S178–81. doi: 10.1111/j.1753-4887.2008.00102.x. [DOI] [PubMed] [Google Scholar]
- 18.Dawodu A, Agarwal M, Hossain M, Kochiyil J, Zayed R. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in summer: a justification for vitamin D supplementation of breast-feeding infants. J Pediatr. 2003;142:169–73. doi: 10.1067/mpd.2003.63. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler EE, Hollis BW, Nelson SE, Jeter JM. Vitamin D deficiency in breastfed infants in Iowa. Pediatrics. 2006;118:603–10. doi: 10.1542/peds.2006-0108. [DOI] [PubMed] [Google Scholar]
- 20.Roth DE, Jones AB, Prosser C, Robinson JL, Vohra S. Vitamin D status is not associated with the risk of hospitalization for acute bronchiolitis in early childhood. Eur J Clin Nutr. 2009;63:297–9. doi: 10.1038/sj.ejcn.1602946. [DOI] [PubMed] [Google Scholar]
- 21.Canadian Paediatric Society. Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health. 2007;12:583–98. [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner CL, Greer FR, American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–52. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr. 1995;61(3 Suppl):638S–645S. doi: 10.1093/ajcn/61.3.638S. [DOI] [PubMed] [Google Scholar]
- 24.Bachelet D, Barnes PW, Brown D, Brown M. Latitudinal and seasonal variation in calculated ultraviolet-B irradiance for rice-growing regions of Asia. Photochem Photobiol. 1991;54:411–22. [Google Scholar]
- 25.Hollis BW, Pittard WB., 3rd Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J Clin Endocrinol Metab. 1984;59:652–7. doi: 10.1210/jcem-59-4-652. [DOI] [PubMed] [Google Scholar]
- 26.Specker B. Vitamin D requirements during pregnancy. Am J Clin Nutr. 2004;80(6 Suppl):1740S–7S. doi: 10.1093/ajcn/80.6.1740S. [DOI] [PubMed] [Google Scholar]
- 27.Islam MZ, Lamberg-Allardt C, Kärkkäinen M, Outila T, Salamatullah Q, Shamim AA. Vitamin D deficiency: a concern in premenopausal Bangladeshi women of two socio-economic groups in rural and urban region. Eur J Clin Nutr. 2002;56:51–6. doi: 10.1038/sj.ejcn.1601284. [DOI] [PubMed] [Google Scholar]
- 28.Islam MZ, Shamim AA, Kemi V, Nevanlinna A, Akhtaruzzaman M, Laaksonen M, et al. Vitamin D deficiency and low bone status in adult female garment factory workers in Bangladesh. Br J Nutr. 2008;99:1322–9. doi: 10.1017/S0007114508894445. [DOI] [PubMed] [Google Scholar]
- 29.Dawodu A, Absood G, Patel M, Agarwal M, Ezimokhai M, Abdulrazzaq Y, et al. Biosocial factors affecting vitamin D status of women of childbearing age in the United Arab Emirates. J Biosoc Sci. 1998;30:431–7. doi: 10.1017/s0021932098004313. [DOI] [PubMed] [Google Scholar]
- 30.Islam MZ, Lamberg-Allardt C, Kärkkäinen M, Ali SM. Dietary calcium intake in premenopausal Bangladeshi women: do socio-economic or physiological factors play a role? Eur J Clin Nutr. 2003;57:674–80. doi: 10.1038/sj.ejcn.1601597. [DOI] [PubMed] [Google Scholar]
- 31.Hels O, Hassan N, Tetens I, Haraksingh Thilsted S. Food consumption, energy and nutrient intake and nutritional status in rural Bangladesh: changes from 1981–1982 to 1995–96. Eur J Clin Nutr. 2003;57:586–94. doi: 10.1038/sj.ejcn.1601567. [DOI] [PubMed] [Google Scholar]
- 32.Clements MR, Johnson L, Fraser DR. A new mechanism for induced vitamin D deficiency in calcium deprivation. Nature. 1987;325:62–5. doi: 10.1038/325062a0. [DOI] [PubMed] [Google Scholar]
- 33.Matsuoka LY, Wortsman J, Dannenberg MJ, Hollis BW, Lu Z, Holick MF. Clothing prevents ultraviolet-B radiation-dependent photosynthesis of vitamin D3. J Clin Endocrinol Metab. 1992;75:1099–103. doi: 10.1210/jcem.75.4.1328275. [DOI] [PubMed] [Google Scholar]
- 34.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–6. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 35.Lu Z, Chen TC, Zhang A, Persons KS, Kohn N, Berkowitz R, et al. An evaluation of the vitamin D3 content in fish: is the vitamin D content adequate to satisfy the dietary requirement for vitamin D? J Steroid Biochem Mol Biol. 2007;103:642–4. doi: 10.1016/j.jsbmb.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamboni G, Piemonte G, Bolner A, Antoniazzi F, Dall'Agnola A, Messner H, et al. Influence of dietary taurine on vitamin D absorption. Acta Paediatr. 1993;82:811–5. doi: 10.1111/j.1651-2227.1993.tb17616.x. [DOI] [PubMed] [Google Scholar]
- 37.Leichtmann GA, Bengoa JM, Bolt MJ, Sitrin MD. Intestinal absorption of cholecalciferol and 25-hydroxycholecalciferol in patients with both Crohn's disease and intestinal resection. Am J Clin Nutr. 1991;54:548–52. doi: 10.1093/ajcn/54.3.548. [DOI] [PubMed] [Google Scholar]
- 38.Glahn RP, Beers KW, Bottje WG, Wideman RF, Jr, Huff WE, Thomas W. Aflatoxicosis alters avian renal function, calcium, and vitamin D metabolism. J Toxicol Environ Health. 1991;34:309–21. doi: 10.1080/15287399109531570. [DOI] [PubMed] [Google Scholar]
- 39.Ogunkolade WB, Boucher BJ, Bustin SA, Burrin JM, Noonan K, Mannan N, et al. Vitamin D metabolism in peripheral blood mononuclear cells is influenced by chewing “betel nut” (Areca catechu) and vitamin D status. J Clin Endocrinol Metab. 2006;91:2612–7. doi: 10.1210/jc.2005-2750. [DOI] [PubMed] [Google Scholar]
- 40.Fraser DR. Vitamin D-deficiency in Asia. J Steroid Biochem Mol Biol. 2004;89–90:491–5. doi: 10.1016/j.jsbmb.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 41.Allegretto EA, Shevde N, Zou A, Howell SR, Boehm MF, Hollis BW, et al. Retinoid X receptor acts as a hormone receptor in vivo to induce a key metabolic enzyme for 1,25-dihydroxyvitamin D3. J Biol Chem. 1995;270:23906–9. doi: 10.1074/jbc.270.41.23906. [DOI] [PubMed] [Google Scholar]
- 42.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79:717–26. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed I, Atiq M, Iqbal J, Khurshid M, Whittaker P. Vitamin D deficiency rickets in breast-fed infants presenting with hypocalcaemic seizures. Acta Paediatr. 1995;84:941–2. doi: 10.1111/j.1651-2227.1995.tb13798.x. [DOI] [PubMed] [Google Scholar]
- 44.Hatun S, Ozkan B, Orbak Z, Doneray H, Cizmecioglu F, Toprak D, et al. Vitamin D deficiency in early infancy. J Nutr. 2005;135:279–82. doi: 10.1093/jn/135.2.279. [DOI] [PubMed] [Google Scholar]
- 45.Balasubramanian S, Shivbalan S, Kumar PS. Hypocalcemia due to vitamin D deficiency in exclusively breastfed infants. Indian Pediatr. 2006;43:247–51. [PubMed] [Google Scholar]
- 46.Greer FR. 25-Hydroxyvitamin D: functional outcomes in infants and young children. Am J Clin Nutr. 2008;88:529S–33S. doi: 10.1093/ajcn/88.2.529S. [DOI] [PubMed] [Google Scholar]
- 47.Arnaud SB, Stickler GB, Haworth JC. Serum 25-hydroxyvitamin D in infantile rickets. Pediatrics. 1976;57:221–5. [PubMed] [Google Scholar]
- 48.DeLucia MC, Mitnick ME, Carpenter TO. Nutritional rickets with normal circulating 25-hydroxyvitamin D: a call for reexamining the role of dietary calcium intake in North American infants. J Clin Endocrinol Metab. 2003;88:3539–45. doi: 10.1210/jc.2002-021935. [DOI] [PubMed] [Google Scholar]
- 49.Kreiter SR, Schwartz RP, Kirkman HN, Jr, Charlton PA, Calikoglu AS. Davenport ML. Nutritional rickets in African American breast-fed infants. J Pediatr. 2000;137:153–7. doi: 10.1067/mpd.2000.109009. [DOI] [PubMed] [Google Scholar]
- 50.Hellen Keller International. Rickets in Bangladeshi children: a small focus or a widespread problem? Dhaka: Hellen Keller International; 2001. p. 4. (Nutrition surveillance bulletin no. 4). [Google Scholar]
- 51.International Centre for Diarrhoeal Disease Research, Bangladesh. National rickets survey in Bangladesh, 2008. Health Sci Bull. 2009;7:7–11. [Google Scholar]
- 52.Craviari T, Pettifor JM, Thacher TD, Meisner C, Arnaud J, Fischer PR, Rickets Convergence Group Rickets: an overview and future directions, with special reference to Bangladesh. A summary of the Rickets Convergence Group meeting, Dhaka, 26–27 January 2006. J Health Popul Nutr. 2008;26:112–21. [PMC free article] [PubMed] [Google Scholar]
- 53.Cimma JP, Arnaud J, Labarere J, Guillard O, Nugues F, Marrauld A, et al. Effect of consumption of food cooked in aluminium or stainless-steel pots on Bangladeshi children with calcium-deficient rickets: an eight month trial. J Trace Elem Med Biol. 2004;17:249–53. doi: 10.1016/S0946-672X(04)80026-9. [DOI] [PubMed] [Google Scholar]
- 54.Combs GF, Hassan N. The Chakaria food system study: household-level, case-control study to identify risk factor for rickets in Bangladesh. Eur J Clin Nutr. 2005;59:1291–301. doi: 10.1038/sj.ejcn.1602242. [DOI] [PubMed] [Google Scholar]
- 55.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491S–9S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 56.van Etten E, Stoffels K, Gysemans C, Mathieu C, Overbergh L. Regulation of vitamin D homeostasis: implications for the immune system. Nutr Rev. 2008;66(10 Suppl 2):S125–34. doi: 10.1111/j.1753-4887.2008.00096.x. [DOI] [PubMed] [Google Scholar]
- 57.Karatekin G, Kaya A, Salihoğlu O, Balci H, Nuhoğlu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr. 2009;63:473–7. doi: 10.1038/sj.ejcn.1602960. [DOI] [PubMed] [Google Scholar]
- 58.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 59.Brooke OG, Butters F, Wood C. Intrauterine vitamin D nutrition and postnatal growth in Asian infants. Br Med J. 1981;283:1024. doi: 10.1136/bmj.283.6298.1024. (Clin Res Ed); [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajah J, Jubeh JA, Haq A, Shalash A, Parsons H. Nutritional rickets and z scores for height in the United Arab Emirates: to D or not to D? Pediatr Int. 2008;50:424–8. doi: 10.1111/j.1442-200X.2008.02700.x. [DOI] [PubMed] [Google Scholar]
- 61.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93:512–7. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 62.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–95. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gordon CM, Feldman HA, Sinclair L, Williams AL, Kleinman PK, Perez-Rossello J, et al. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Pediatr Adolesc Med. 2008;162:505–12. doi: 10.1001/archpedi.162.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]