Abstract
Active surveillance is becoming a more widely accepted management strategy in men with low-risk localized prostate cancer. This is in recognition of the knowledge that most men with such cancer are likely to die from other causes. The obvious benefits of active surveillance are reduced morbidity by delaying or avoiding radical gland therapy. These advantages should be balanced against appropriate selection criteria and triggers for moving to radical therapy while on active surveillance. The optimal method by which to identify the small number of men who will progress by use of clinical, biopsy, and imaging data is yet to be defined. Nevertheless, active surveillance is an appealing management option in selected men with prostate cancer and represents a solution to the significant problem of the overdiagnosis of clinically insignificant disease that accompanies prostate-specific antigen (PSA) screening.
Keywords: Needle biopsy, Outcome assessment, Prostate neoplasms, Prostate-specific antigen, Review
INTRODUCTION
Active surveillance (AS), since first being described in 2002, is now an accepted treatment strategy for men with low-risk prostate cancer (PCa) who previously received radical whole-gland treatment (surgery, external-beam radiation, or brachytherapy) [1]. The concept of AS evolved from watchful waiting, which meant no treatment until progression to metastatic or locally advanced disease, followed by androgen ablation therapy [2]. The concept is to cure clinically significant PCa rather than to wait for the development of metastatic disease. For low-risk PCa, AS and radical treatment both have merits and disadvantages. AS has minimal morbidity with the inherent risk of progression associated with expectant management; radical therapies have an impact on erectile function and continence but provide definitive treatment [3,4]. Somewhere between AS and radical treatment lies focal therapy. Focal therapy has been limited to small cohorts with no extensive follow-up and cannot be recommended outside study protocols [5]. The impact of focal therapy on the natural history of favorable-risk disease remains uncertain. Most men with favorable-risk PCa and their physicians will choose between AS and radical therapy.
In this review, we will focus on the rationale, patient selection, method of follow-up, triggers for intervention, and recent results of this approach.
RATIONALE AND ADVANTAGES OF ACTIVE SURVEILLANCE
AS for favorable-risk PCa has emerged as a credible management strategy within populations in which PCa screening is widespread. This is due to the observation that PCa screening by use of digital rectal exam (DRE), prostate-specific antigen (PSA), and biopsy results in the detection of disease that is not clinically significant in many patients (i.e., untreated, the cancer would not pose a threat to health or cause death). Furthermore, by treating men with favorable-risk PCa, we risk morbidity and even mortality when the disease, due to its long natural history, may never have been destined to have any clinical manifestations during the patient's lifetime. Hence, AS is a solution to the widely acknowledged problems of overdiagnosis and overtreatment of clinically insignificant disease that accompany the early detection of PCa [6].
AS is flexible in that it allows for initial assessment of disease prior to deciding on it as a course of management. This is possible by incorporating a period of initial observation into patient management with the belief that if appropriate triggers for intervention are followed, then the patient will still have a favorable outcome when undergoing radical therapy. This approach helps to manage the subset of patients with initially apparent favorable-risk PCa who actually are at risk, due to either higher risk disease undetected at diagnosis or progression to a more aggressive phenotype of PCa over the period of surveillance. AS relies on defined triggers to detect and predict higher risk disease while on such a program. Tools used in the published surveillance series include serial PSAs, DREs, and repeat prostate biopsies. Magnetic resonance imaging (MRI) and biomarkers have an emerging role. These will be discussed later in greater detail.
The approach of AS has been summarized as that which (1) identifies patients who have a low likelihood of disease progression during their lifetime on the basis of clinical and pathologic features of the disease and patient age and co-morbidity, (2) includes adherence to close monitoring over time, (3) includes reasonable criteria or triggers for intervention that will both identify more aggressive disease in a timely fashion and also not result in excessive treatment, and (4) improves communication to reduce the psychological burden of living with an untreated cancer [6].
POTENTIAL DISADVANTAGES OF ACTIVE SURVEILLANCE
The obvious disadvantage of AS where selective delayed therapy is relied upon is that the "window of opportunity" for cure may be missed. Individual risk of disease progression is difficult to assign, so of concern is a small but real possibility of progression to death in the AS population because of the loss of opportunity for cure during the surveillance period. PCa has an exceptionally long natural history, characterized typically by initiation in the 30s, clinical diagnosis in the 50-60s, and death from disease in the 80s. This represents a 50-year time course. Thus, most believe that a treatment delay of 1 or 2 years in patients who are reclassified as higher risk and then treated is unlikely to significantly alter cancer mortality. Further, although AS may appear to have little morbidity, several studies have shown a deterioration of quality of life (QoL) and sexual function [7-11]. Alternatively, QoL is likely to deteriorate if all men with low-risk disease are offered radical treatments with the known impacts on sexual function and continence, the very reasons AS was established as a strategy.
Finally, a small attrition rate can be expected because of men who are unable or unwilling to tolerate surveillance. This must be accepted from the outset for any individual [12].
UPTAKE OF ACTIVE SURVEILLANCE
Although AS has gained popularity, it is still infrequently utilized in some regions. Patients and/or their physicians appear to want to treat the PCa once diagnosed. For example, in the United States, only approximately 10% of eligible men are put on AS protocols [13], and even in countries where AS is largely accepted as a treatment strategy, only 30% of eligible men are on AS [14]. It must be noted that the discussion and acceptance of AS in guidelines and recommendations by learned bodies has been far greater [15,16].
SELECTION CRITERIA FOR ACTIVE SURVEILLANCE
A genuine concern is that patients thought to have clinically insignificant PCa might actually harbor cancer with unfavorable pathological features [17]. However, in any individual patient, it may be difficult to perfectly differentiate between clinically insignificant and life-threatening PCa [16]. This requires the selection for AS patients to be stringent. Prospective studies outlining the ideal selection married with adequate follow-up and intervention data are lacking. No randomized trials comparing selection criteria exist, and data are likely to be obtained from larger prospective cohorts. At present, an array of selection criteria to define favorable cases or even low-risk or clinically insignificant cancer confronts a urologist [14,18-21] (Table 1). Most of the data for selecting patients work backward from the concept of an insignificant tumor found at radical prostatectomy, which in itself may be a flawed concept. This is because given the different theories regarding the relevance of PCa as multifocal disease and the idea of a dominant or index lesion being responsible for progression or metastases, prospective data are likely to be more enlightening, but are not yet available.
TABLE 1.
Selection criteria for active surveillance based on different protocols currently used worldwide
PSA: prostate-specific antigen, a: and <50% of cancer in any core, b: for patients over age 70, these criteria were relaxed to include Gleason ≥7 (3+4) and/or ≤PSA 15 ng/ml, c: age 50-80 years
Outcomes in men outside the more accepted selection criteria (e.g., Gleason score of 6) for AS are lacking. In selected patients with screening-detected Gleason score of 3+4=7 PCa, AS might be an option, especially in those with comorbidity or a short life expectancy [22]. Interestingly, the original Toronto cohort of AS included men with Gleason 7 disease who were over 70, but the protocol was amended to only accept men with Gleason 6 [18].
OUTCOMES OF ACTIVE SURVEILLANCE
A pooled analysis by Chodak et al of original data from 828 patients treated by the more traditional watchful waiting with intervention with metastatic disease as described earlier was the catalyst for current AS strategies [23]. The pooled data were based on patients from six nonrandomized studies in which cancer-specific survival and metastasis-free survival were reported with up to 10 years of follow-up. Low- grade tumors did better than high-grade tumors, with the prospect of metastasis development at 10 years being approximately twice as great in the low-grade groups as in the high-grade groups. This also translated into poorer survival for the higher risk tumors.
The largest prospective series of modern AS from Toronto pioneered by Klotz et al has recently reported the outcome of AS with selective delayed intervention by using clinical PSA or histologic progression as treatment indications for clinically localized PCa [18]. With a median follow-up of around 8 years (range, 1-15 years), the total of 453 men represents the largest AS cohort in a prospective, single-arm, cohort study. Definitive intervention was offered to those patients with a PSA doubling time of less than 3 years, Gleason score progression (≥4+3), or unequivocal clinical progression. Overall survival was 79%. The 10-year PCa actuarial survival was 97%. Among the 30% of patients (n=117) who were reclassified as higher risk and who were treated, PSA failure was relatively common at 50% (13% of the total cohort). Interestingly, data from the Swedish section of the European Randomized Study of Screening for Prostate Cancer did not find differences in intermediate outcomes between immediate radical prostatectomy (RP) and delayed RP [24]. There were limited patient numbers available for analysis and of course the delayed RP group may have been subject to a selection bias. Overall, more prospective datas are required to resolve this issue.
Notably, in the Klotz series, a PSA doubling time of 3 years or less was associated with an 8.5 times higher risk of biochemical failure after definitive treatment compared with a doubling time of >3 years [18]. However, this must be balanced against an observed low rate of PCa mortality because other-cause mortality accounted for almost all of the deaths. Certainly, the conclusion that additional studies are warranted to improve the identification of patients who harbor more aggressive disease despite favorable clinical parameters at diagnosis remains valid. Other robust-sized AS cohorts led by Carter et al [20] at Johns Hopkins and Dall'Era [25] at the University of California at San Francisco, both in the United States, are not mature enough yet but in the future may provide the supportive data necessary to help to refine selection and interventional criteria.
TRIGGERS OR CRITERIA FOR INTERVENTION IN MEN UNDERGOING ACTIVE SURVEILLANCE
Again, as with the selection criteria for AS, the triggers for leaving surveillance and having radical therapy are not well defined. Klotz focused on PSA kinetics [18], whereby a doubling time of less than 3 years is of concern, although this is only likely to happen in around a quarter of men in a large series. Others have elected to follow more regular biopsies, whereas others still believe that the criteria that patients were entered upon should be deemed exit points even after multiple biopsies [20,25]. What most agree on at this stage is that a combination of regular DRE, PSA, and biopsies at least between one and three yearly should all be factored into the decision to progress to radical therapy in the hope of obtaining a cure in men who were initially believed to have low-risk disease. Increasingly, dynamic contrast-enhanced and diffusion-weighted MRI is being incorporated into the algorithm to enhance the identification of men with large volume, usually anterior disease. This is particularly useful in patients with minimal Gleason 6 disease on biopsy and adverse PSA kinetics.
IMAGING AND BIOMARKERS IN ACTIVE SURVEILLANCE
Useful adjuncts to current selection criteria are imaging and biomarkers. MRI is emerging as a useful tool [26]. The key features of MRI are that it is noninvasive, may differentiate low-grade and high-grade PCa, and has much more sensitivity for large volume, clinically significant cancers [27]. Many candidates for AS will have tumor volumes well below 0.5 cc. A 'negative' MRI in a patient whose biopsy shows minimal disease therefore increases the likelihood that the patient does not harbor a significant volume of disease. MRI will have an increasing a role in selecting patients for AS and as a trigger for re-biopsy or intervention during surveillance. A particular group that may benefit are those who exhibit adverse PSA kinetics in the absence of benign prostatic hyperplasia (BPH) or inflammation, with minimal disease on repeat biopsy. Recent datas indicate that current biopsy schemes often miss large-volume anterior tumors. This has been termed the prostatic-evasive anterior tumor syndrome, or PEATS [28]. MRI may help to uncover such patients and direct further biopsies. A current challenge is to better understand MRI images in men considering or being managed with AS. This requires the pooling of data from individual patients' tumors with the combination of different sequences, use of diffusion-weighted MRI (DW-MRI), magnetic resonance spectroscopy, and other contrast manipulations combined with biopsy data [29]. The idea that men on AS could have cancer volume progression and tumor grade documented with MRI is enticing [26].
Many disease biomarkers are currently being evaluated. These include multiparametric tissue-based assays using a systems pathology approach (i.e., the Aureon test), mitochondrial deletion assays (Mitomics), somatic cell SNP analysis, and the PCa antigen 3 (PCA3) urine-based assay. The PCA3 test may allow prebiopsy risk stratification. PCA3-based nomograms have been applied and validated in a large, external, European cohort of men at risk of PCa [30], which adds data to the data already published in the United States [31]. A biomarker assay that accurately predicts tumor aggressivity (or benignity) would enhance patient selection, PCA3 follow-up, timing, and need for biopsies in AS protocols. Although the conventional PSA and biopsy based approach to surveillance is associated with an extremely low PCa mortality rate, approximately one third of patients are eventually subjected to radical therapy. The benefit of the imaging and biomarker enhanced approach would be to lower the proportion of patients on surveillance requiring definitive intervention and to generate an earlier signal for intervention in the remaining minority who are reclassified as higher risk. Ultimately, biomarkers and imaging may be combined together to create individualized and tailored 'biological signatures.'
MORTALITY FROM ACTIVE SURVEILLANCE
Currently, there are few datas regarding mortality from AS. The reported data for patients dying while on active surveillance is best addressed by the University of Toronto cohort. Krakowsky et al reported that out of a series of around 450 patients on AS, 5 died of PCa [32]. All of them had a PSA doubling time of 1.6 years or less, thus triggering a recommendation for radical therapy. Radical intervention was performed in three of the five patients. Two received radiation and one underwent radical prostatectomy. Of the 2 patients who did not receive definitive treatment, one was lost to follow-up and was treated conservatively by his family doctor, whereas the other elected androgen-deprivation therapy rather than radical treatment. Overall, there was a low cancer-specific mortality in this, thus providing support for an AS approach to favorable-risk PCa. This is notable because only 1 of the 453 patients presented with favorable disease had a time course of disease progression that left open the possibility that he might have suffered a preventable death. This analysis reinforces the importance of close monitoring and of definitive treatment for those in whom disease is reclassified as higher risk over time.
ACTIVE SURVEILLANCE IN ASIAN MEN
This was a retrospective study of 131 Korean men who underwent RP for clinically insignificant PCa as defined by contemporary Epstein criteria as unfavorable PCa (pathological Gleason sum >7 and/or extraprostatic extension) [17]. Their results showed that a significant proportion of contemporary Korean patients who meet all the conditions of the contemporary Epstein criteria for prediction of clinically insignificant PCa might actually harbor cancer with unfavorable pathological features. The large Japanese multicenter study by Kakehi et al demonstrated a pathological progression rate at 1-year re-biopsy of 33% [21]. However, almost half of the patients remained on AS for a maximal observation period of 54 months, thus giving credence to AS in Asian men. Given the low mortality from PCa in Asian men compared with Western men, AS is even more appealing in Asians, not withstanding the results of this small trial.
THE FUTURE OF ACTIVE SURVEILLANCE
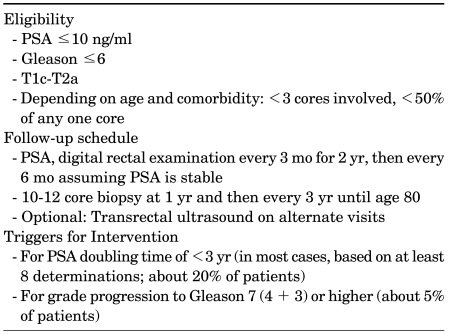
AS is increasingly becoming popular as a strategy for selected men in Europe, Canada, and Australia, with the United States tentatively following. Suggested eligibility criteria, follow-up schedule, and triggers for intervention are summarized on the basis of current knowledge (Table 2). However, the future of AS and uptake as a modality to manage low-risk PCa will depend on better patient selection and improved identification of when the disease process has altered such that radical intervention is required prior to local advancement or metastases. On the first point, the two most likely candidates for achieving this are imaging, particularly with MRI and biomarkers.
TABLE 2.
Active surveillance: suggested algorithm for eligibility and follow-upa
PSA: prostate-specific antigen, a: adapted from Klotz and Nam [2]. These are guidelines and should be modified according to patient age and comorbidity.
Regarding the ability to better identify those at risk of progression while on AS, the tools available are PSA and PSA velocity, DRE, repeat biopsy, and serial imaging. Most of these tools are currently used in AS protocols. Imaging has been used where discordance between PSA and other biopsy findings exists with anteriorly placed tumors using MRI [28]. Biomarkers for this indication are in development.
Another major development may be data supporting the use of 5-alpha reductase inhibitors (5ARIs) in the AS setting. Two large trials, PCPT [33] and REDUCE [34], have reported that the rate of PCa diagnosis is decreased by 30% with 5ARIs. Many men in these studies harbored undiagnosed PCa at entry. Thus, it is a reasonable inference that these drugs act to stabilize or reduce the volume of existing PCa; indeed, that may be their main mode of action as prevention agents. One study testing this hypothesis in surveillance patients, the REDEEM study [35], has been completed but has not yet reported. It is possible that for many men with favorable-risk PCa, a 5ARI represents a low-cost, minimal intervention that is sufficient to further reduce their risk of progression to exceedingly low levels. At this point, however, there is no direct evidence to support this hypothesis. While placing men on surveillance on 5ARIs is appealing, particularly if they have other indications for the drug (i.e., BPH symptoms), it should not be considered a definitive therapy. Such patients still require close monitoring and periodic biopsies. The PSA kinetics in men on 5ARI are simply recalibrated from the new baseline nadir.
A large randomized controlled trial comparing standard treatment with surgery or radiation against active surveillance, or the Surveillance Therapy Against Radical Treatment (START) trial, is currently recruiting and will answer the question of which management strategy is best for patients with low-risk PCa [36]. The PROTECT study of AS, radical prostatectomy, or radiation therapy for localized PCa from the United Kingdom [37] and the European-based large, prospective, observational AS registry known as the Prostate Cancer Research International: Active Surveillance (PRIAS) study will both add much needed data to help urologists and patients decide on the best management strategy for low-risk PCa [38].
AS is an appealing option for men with favorable-risk PCa, particularly those whose extent of disease appears minimal. It has been demonstrated to be safe in the intermediate time frame. The quality of life benefits are indisputable. The controversy in this field is now related to optimal patient selection and the ideal triggers for intervention.
Footnotes
The authors have nothing to disclose.
References
- 1.Choo R, Klotz L, Danjoux C, Morton GC, DeBoer G, Szumacher E, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol. 2002;167:1664–1669. [PubMed] [Google Scholar]
- 2.Klotz L, Nam R. Active surveillance with selective delayed intervention for favourable risk prostate cancer: clinical experience and a "number needed to treat" analysis. Eur Urol Suppl. 2006;5:479–486. [PubMed] [Google Scholar]
- 3.Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 4.Klotz L. Active surveillance for favorable risk prostate cancer: what are the results, and how safe is it? Semin Radiat Oncol. 2008;18:2–6. doi: 10.1016/j.semradonc.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Lindner U, Weersink RA, Haider MA, Gertner MR, Davidson SR, Atri M, et al. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J Urol. 2009;182:1371–1377. doi: 10.1016/j.juro.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 6.Klotz L. Active surveillance for favorable-risk prostate cancer: who, how and why? Nat Clin Pract Oncol. 2007;4:692–698. doi: 10.1038/ncponc0966. [DOI] [PubMed] [Google Scholar]
- 7.Bacon CG, Giovannucci E, Testa M, Kawachi I. The impact of cancer treatment on quality of life outcomes for patients with localized prostate cancer. J Urol. 2001;166:1804–1810. [PubMed] [Google Scholar]
- 8.Galbraith ME, Ramirez JM, Pedro LW. Quality of life, health outcomes, and identity for patients with prostate cancer in five different treatment groups. Oncol Nurs Forum. 2001;28:551–560. [PubMed] [Google Scholar]
- 9.Litwin MS, Lubeck DP, Spitalny GM, Henning JM, Carroll PR. Mental health in men treated for early stage prostate carcinoma: a posttreatment, longitudinal quality of life analysis from the Cancer of the Prostate Strategic Urologic Research Endeavor. Cancer. 2002;95:54–60. doi: 10.1002/cncr.10651. [DOI] [PubMed] [Google Scholar]
- 10.Arredondo SA, Downs TM, Lubeck DP, Pasta DJ, Silva SJ, Wallace KL, et al. Watchful waiting and health related quality of life for patients with localized prostate cancer: data from CaPSURE. J Urol. 2004;172:1830–1834. doi: 10.1097/01.ju.0000140758.04424.77. [DOI] [PubMed] [Google Scholar]
- 11.Steineck G, Helgesen F, Adolfsson J, Dickman PW, Johansson JE, Norlén BJ, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347:790–796. doi: 10.1056/NEJMoa021483. [DOI] [PubMed] [Google Scholar]
- 12.Dall'Era MA, Cooperberg MR, Chan JM, Davies BJ, Albertsen PC, Klotz LH, et al. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer. 2008;112:1650–1659. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
- 13.Barocas DA, Cowan JE, Smith JA, Jr, Carroll PR. What percentage of patients with newly diagnosed carcinoma of the prostate are candidates for surveillance? An analysis of the CaPSURE database. J Urol. 2008;180:1330–1334. doi: 10.1016/j.juro.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 14.van den Bergh RC, Roemeling S, Roobol MJ, Aus G, Hugosson J, Rannikko AS, et al. Outcomes of men with screen-detected prostate cancer eligible for active surveillance who were managed expectantly. Eur Urol. 2009;55:1–8. doi: 10.1016/j.eururo.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer V.3.2010. 2010. [DOI] [PubMed] [Google Scholar]
- 16.Heidenreich A, Aus G, Abbou C. Guidelines on Prostate Cancer. European Association of Urology; 2007. [Google Scholar]
- 17.Lee SE, Kim DS, Lee WK, Park HZ, Lee CJ, Doo SH, et al. Application of the Epstein criteria for prediction of clinically insignificant prostate cancer in Korean men. BJU Int. 2010;105:1526–1530. doi: 10.1111/j.1464-410X.2009.09070.x. [DOI] [PubMed] [Google Scholar]
- 18.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2009;28:126–131. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 19.Carter HB, Walsh PC, Landis P, Epstein JI. Expectant management of nonpalpable prostate cancer with curative intent: preliminary results. J Urol. 2002;167:1231–1234. [PubMed] [Google Scholar]
- 20.Carter HB, Kettermann A, Warlick C, Metter EJ, Landis P, Walsh PC, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007;178:2359–2364. doi: 10.1016/j.juro.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kakehi Y, Kamoto T, Shiraishi T, Ogawa O, Suzukamo Y, Fukuhara S, et al. Prospective evaluation of selection criteria for active surveillance in Japanese patients with stage T1cN0M0 prostate cancer. Jpn J Clin Oncol. 2008;38:122–128. doi: 10.1093/jjco/hym161. [DOI] [PubMed] [Google Scholar]
- 22.van den Bergh RC, Roemeling S, Roobol MJ, Aus G, Hugosson J, Rannikko AS, et al. Gleason score 7 screen-detected prostate cancers initially managed expectantly: outcomes in 50 men. BJU Int. 2009;103:1472–1477. doi: 10.1111/j.1464-410X.2008.08281.x. [DOI] [PubMed] [Google Scholar]
- 23.Chodak GW, Thisted RA, Gerber GS, Johansson JE, Adolfsson J, Jones GW, et al. Results of conservative management of clinically localized prostate cancer. N Engl J Med. 1994;330:242–248. doi: 10.1056/NEJM199401273300403. [DOI] [PubMed] [Google Scholar]
- 24.van den Bergh RC, Steyerberg EW, Khatami A, Aus G, Pihl CG, Wolters T, et al. Is delayed radical prostatectomy in men with low-risk screen-detected prostate cancer associated with a higher risk of unfavorable outcomes? Cancer. 2010;116:1281–1290. doi: 10.1002/cncr.24882. [DOI] [PubMed] [Google Scholar]
- 25.Dall'Era MA, Konety BR, Cowan JE, Shinohara K, Stauf F, Cooperberg MR, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112:2664–2670. doi: 10.1002/cncr.23502. [DOI] [PubMed] [Google Scholar]
- 26.Raz O, Haider M, Trachtenberg J, Leibovici D, Lawrentschuk N. MRI for men undergoing active- surveillance or with rising PSA and negative biopsies. Nat Rev Urol. 2010;7:543–551. doi: 10.1038/nrurol.2010.143. [DOI] [PubMed] [Google Scholar]
- 27.Franiel T, Lüdemann L, Taupitz M, Rost J, Asbach P, Beyersdorff D. Pharmacokinetic MRI of the prostate: parameters for differentiating low-grade and high-grade prostate cancer. Rofo. 2009;181:536–542. doi: 10.1055/s-0028-1109168. [DOI] [PubMed] [Google Scholar]
- 28.Lawrentschuk N, Haider MA, Daljeet N, Evans A, Toi A, Finelli A, et al. 'Prostatic evasive anterior tumours': the role of magnetic resonance imaging. BJU Int. 2010;105:1231–1236. doi: 10.1111/j.1464-410X.2009.08938.x. [DOI] [PubMed] [Google Scholar]
- 29.Shukla-Dave A, Hricak H, Kattan MW, Pucar D, Kuroiwa K, Chen HN, et al. The utility of magnetic resonance imaging and spectroscopy for predicting insignificant prostate cancer: an initial analysis. BJU Int. 2007;99:786–793. doi: 10.1111/j.1464-410X.2007.06689.x. [DOI] [PubMed] [Google Scholar]
- 30.Auprich M, Haese A, Walz J, Pummer K, de la Taille A, Graefen M, et al. External validation of urinary PCA3-based nomograms to individually predict prostate biopsy outcome. Eur Urol. 2010 doi: 10.1016/j.eururo.2010.06.038. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Sokoll LJ, Ellis W, Lange P, Noteboom J, Elliott DJ, Deras IL, et al. A multicenter evaluation of the PCA3 molecular urine test: pre-analytical effects, analytical performance, and diagnostic accuracy. Clin Chim Acta. 2008;389:1–6. doi: 10.1016/j.cca.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol. 2010;184:131–135. doi: 10.1016/j.juro.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 33.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 34.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 35.Fleshner N, Gomella LG, Cookson MS, Finelli A, Evans A, Taneja SS, et al. Delay in the progression of low-risk prostate cancer: rationale and design of the Reduction by Dutasteride of Clinical Progression Events in Expectant Management (REDEEM) trial. Contemp Clin Trials. 2007;28:763–769. doi: 10.1016/j.cct.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Klotz L, Kibel A, Sanda M. Observation or Radical Treatment in Patients With Prostate Cancer. US National Institutes of Health, ClinicalTrials.gov; 2007. NCT00499174. [Google Scholar]
- 37.Hamdy F. Active surveillance, radical prostatectomy, or radiation therapy in treating patients with loocalized prostate cancer. US national institutes of health, clinical trials.gov; 2008. NCT00632983. [Google Scholar]
- 38.van den Bergh RC, Roemeling S, Roobol MJ, Roobol W, Schröder FH, Bangma CH. Prospective validation of active surveillance in prostate cancer: the PRIAS study. Eur Urol. 2007;52:1560–1563. doi: 10.1016/j.eururo.2007.05.011. [DOI] [PubMed] [Google Scholar]