Abstract
Purpose
The Gleason score (GS) is an important factor that is considered when making decisions about prostate cancer and its prognosis. However, upgrading of the GS can occur between transrectal ultrasonography (TRUS) biopsy and radical prostatectomy. This study analyzed the clinical factors predictive of upgrading of the GS after radical prostatectomy compared with that at the time of TRUS biopsy.
Materials and Methods
We analyzed the medical records of 107 patients who had undergone radical prostatectomy. Patients were divided into two groups. Group 1 consisted of patients in whom the GS was not upgraded, and group 2 consisted of patients in whom the GS was upgraded. Associations between preoperative clinical factors and upgrading of the GS were analyzed. Preoperative clinical factors included age, prostate-specific antigen (PSA), prostate volume, PSA density, GS of TRUS biopsy, maximum core percentage of cancer, percentage of positive cores, number of biopsies, location of positive core with maximum GS, high-grade prostatic intraepithelial neplasia (HGPIN), inflammation on biopsy, and clinical stage.
Results
Among 85 patients, 42 (49%) patients had an upgraded GS after operation. TRUS biopsy core number of 12 or fewer (p=0.029) and prostate volume of 36.5 ml or less (p<0.001) were associated with upgrading of the GS. Preoperative clinical factors associated with nonupgrading of the GS were the detection of positive cores with a maximum GS at the apex (p=0.002) or in a hypoechoic lesion (p=0.002) in TRUS.
Conclusions
If the positive cores with maximum GS are located at the apex or in a hypoechoic lesion in TRUS, we can expect that the GS will not be upgraded. In patients with the clinical predictive factors of a prostate volume of 36.5 ml or less and TRUS biopsy core number of less than 12, we can expect upgrading of the GS after radical prostatectomy, and more aggressive treatment may be needed.
Keywords: Biopsy, Prostatectomy, Prostatic neoplasms
INTRODUCTION
After the introduction of prostate-specific antigen (PSA) as a screening test for prostate cancer, the number of patients diagnosed as having prostate cancer and the proportion of low-risk prostate cancer have increased [1]. A diagnosis of prostate cancer is confirmed by transrectal ultrasonography (TRUS) biopsy. The biopsy result includes the Gleason score, which indicates the tumor grade. The Gleason score, which is a combination of PSA, prostate size, and clinical stage, represents the aggressiveness of the prostate cancer and is an important clinical factor that is considered when making decisions about treating prostate cancer and its prognosis [2].
However, according to recent studies of TRUS biopsy, pathological upgrading of the Gleason score can occur between the pathologic reports of the TRUS biopsy specimen and the radical prostatectomy specimen. The discordance rate of the Gleason score from TRUS biopsy and radical prostatectomy is known to range from 24% to 50% [3-7]. Various preoperative clinical factors reported to be associated with upgrading of the Gleason score are PSA, prostate volume, number of biopsy cores taken, number of positive cores, and inter- and intra-observer variation [8,9].
Clinically insignificant cancer is defined as cancer with a tumor volume <0.2 cm3, a Gleason score ≤6 from TRUS biopsy, and pathologic stage of T2c or less. Predictive factors for this cancer are a clinical stage of T1c, absence of Gleason grade 4 or 5 on TRUS biopsy, PSA density less than 0.15 ng/ml/cm3, cancer detected at less than 3 cores, and core density less than 50%.
Watchful waiting or active surveillance can be considered as one of the treatment modalities in patients matched to these criteria [10]. However, in cases of prostate cancer with a Gleason grade ≥4 or score (or sum) ≥7, watchful waiting or active surveillance is not appropriate because of the poor prognosis of this cancer [11] and the higher rate of capsular invasion or biochemical recurrence [12]. Thus, the Gleason score is associated with the aggressiveness and prognosis of prostate cancer [13]. Therefore, accurate grading is crucial in deciding treatment modalities for prostate cancer such as radical prostatectomy, radiation therapy, or active surveillance [11,14].
According to D'Amico et al, patients with stage T1c, T2a, PSA level ≤10 ng/ml and Gleason score ≤6 are low-risk prostate cancer and these patients has low risk for death [12]. Therefore it is alleged that radical prostatectomy to low risk patients is overtreatment. However, the discordance rate in the Gleason score from TRUS biopsy and that from radical prostatectomy is known to be up to 50%. Therefore, even though a patient is diagnosed as having low-risk prostate cancer after TRUS biopsy, preoperative clinical factors predictive of Gleason score upgrading should be considered when treating prostate cancer.
The aim of this study was to identify the factors predictive of upgrading of the Gleason score, which can change the treatment modality combined with PSA, prostate volume, and clinical stage, and to identify a means of more accurately diagnosing the Gleason score by TRUS biopsy.
MATERIALS AND METHODS
We retrospectively analyzed the medical records of 107 patients who had undergone radical prostatectomy under a diagnosis of prostate cancer from January 2000 to February 2010. Every patient was diagnosed with prostate cancer by TRUS biopsy. TRUS biopsies were performed in patients with elevated serum PSA, abnormal digital rectal examination findings, and the presence of a hypoechoic lesion on TRUS. TRUS biopsy was performed with the ultrasound machine SA-8000 Ex Prime (Medison, Seoul, Korea). The number of biopsy cores taken ranged from 6 to 12 and an additional target biopsy was performed if needed. Biopsy slides were deciphered by a pathologist at our institute applying the 2005 International Society of Urological Pathology (ISUP) consensus. Biopsy slides of other institutes were deciphered by the pathologist of our institute again to confirm the diagnosis of prostate cancer and Gleason score. Biopsy results included the number of cores taken, length of biopsy core, Gleason score of positive cores, location of positive cores, and tumor length in positive cores. The 2002 TNM staging system of the American Joint Committee on Cancer (AJCC) was used for clinical staging. Serum PSA was measured before TRUS biopsy. Patients who received androgen deprivation therapy or radiation therapy, those diagnosed by transurethral resection of the prostate before radical prostatectomy, and those who did not have a sufficient TRUS biopsy result were excluded.
Open retropubic radical prostatectomy or robot-assisted radical prostatectomy was performed in all patients with localized and locally advanced prostate cancer. The radical prostatectomy specimen was sliced into 3 mm serial sections and deciphered by applying the same method of biopsy and by 2 pathologists when the result was conflicting.
The maximum Gleason scores in the TRUS biopsy and radical prostatectomy specimens were compared. Upgrading of Gleason score was defined as an elevation of the Gleason score after radical prostatectomy compared with TRUS biopsy. Patients were divided into two groups. Group 1 comprised patients in whom the Gleason score was not upgraded, and group 2 comprised patients in whom the Gleason score was upgraded after radical prostatectomy. We analyzed the data for any associative factors between upgrading of the Gleason score and preoperative parameters. Preoperative parameters included age, PSA, prostate volume, PSA density, number of biopsy cores taken, maximum core percentage, Gleason score of TRUS biopsy, location of the positive core with the maximum Gleason score, percentage of positive cores, high-grade prostatic intraepithelial neplasia (HGPIN), inflammation on biopsy, and clinical stage.
Statistical analysis was performed by use of Student's t-test for continuous variables and the Pearson chi-square test and Fisher exact test for categorical variables. The software used for statistical analysis was SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA), and a p-value of less than 0.05 was considered to be statistically significant.
RESULTS
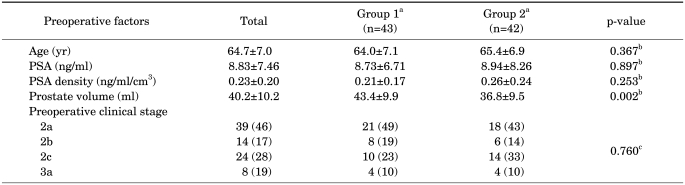
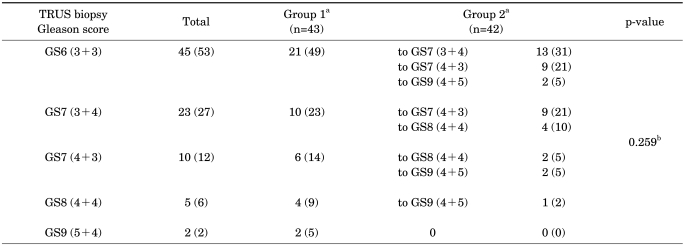
The medical records of a total of 107 patients were reviewed; 22 patients were excluded because of neoadjuvant hormonal therapy, diagnosis by TURP, or not enough TRUS biopsy information. A total of 50 patients underwent open retropubic prostatectomy and 35 patients underwent robot-assisted radical prostatectomy. The mean age of the patients who underwent radical prostatectomy was 64.7±7.0 years, their mean PSA was 8.83±7.46 ng/ml, their mean TRUS volume was 40.2±10.2 ml, and their mean PSA density was 0.23±0.20 ng/ml/cm3 (Table 1). Among 85 patients, 45 (53%) had a Gleason score of 6 (3+3), 23 (27%) had a Gleason score of 7 (3+4), 10 (12%) had a Gleason score of 7 (4+3), 5 (6%) had a Gleason score of 8 (4+4), and 2 (2%) had a Gleason score of 9 (5+4) (Table 2). The distribution of clinical T stage was 39 (46%) at cT2a, 14 (17%) at cT2b, 24 (28%) at cT2c, and 8 (19%) at cT3 (Table 1).
TABLE 1.
Comparison of preoperative factors between group 1 and group 2
PSA: prostate-specific antigen, a: Group 1 - Gleason score not upgraded after radical prostatectomy; Group 2 - Gleason score upgraded after radical prostatectomy, b: Student's t-test; Mean±SD, c: chi-square test; n (%)
TABLE 2.
Comparison of Gleason score between TRUS biopsy and radical prostatectomy
TRUS: transrectal ultrasonography, GS: Gleason score, a: Group 1 - Gleason score not upgraded after radical prostatectomy; Group 2 - Gleason score upgraded after radical prostatectomy, b: chi-square test; n (%)
The Gleason score was upgraded in 42 (50%) patients after radical prostatectomy. Among the patients with an upgraded Gleason score, 13 patients had their Gleason score upgraded from a Gleason score of 6 (3+3) to a Gleason score of 7 (3+4), 9 patients from a 6 to a Gleason score of 7 (4+3), and 2 patients from a 6 to a Gleason score of 9 (4+5). Nine patients had their Gleason score upgraded from a Gleason score of 7 (3+4) to a Gleason score of 7 (4+3), and 4 patients from a 7 to a Gleason score of 8 (4+4). Two patients had their score upgraded from a Gleason score of 7 (4+3) to a Gleason score of 8 (4+4), and 2 patients from a 7 to a Gleason score of 9 (4+5). Lastly, the score in 1 patient was upgraded from a Gleason score of 8 (4+4) to a Gleason score of 9 (4+5) (Table 2).
There were no statistically significant differences in age, PSA, or PSA density between the two groups. However, prostate volume was significantly smaller in group 2 (43.4 ml vs. 36.8 ml, p=0.002) (Table 1).
The cutoff value for prostate volume that was a predictive parameter for upgrading of Gleason score by Student's t-test was calculated by receiver operating curve (ROC). A prostate volume of less than 36.5 ml was shown to be a predictive parameter for upgrading of the Gleason score. Sensitivity was 0.744 and specificity was 0.690 (area under curve (AUC): 00.720, p<0.001, 95% confidence interval: 0.610-0.829).
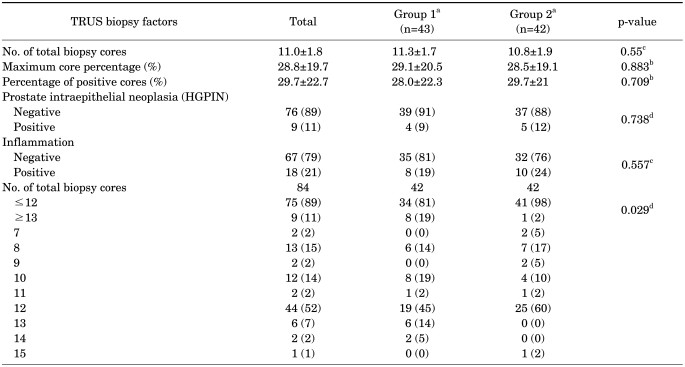
Among 85 patients, 1 patient did not have information about the number of biopsy cores. In the remaining 84 patients, biopsy cores numbering 13 or more showed statistical significance for nonupgrading (p=0.029). Gleason score, maximum core percentage, percentage of positive cores, clinical stage, HGPIN, and inflammation on biopsy were not statistically significant for upgrading (Tables 2, 3).
TABLE 3.
Comparison of TRUS biopsy factors between group 1 and group 2
TRUS: transrectal ultrasonography, In Group 1: one patient did not have information about the number of biopsy cores, a: Group 1 - Gleason score not upgraded after radical prostatectomy; Group 2 - Gleason score upgraded after radical prostatectomy, b: Student's t-test; Mean±SD, c: chi-square test; n (%), d: Fisher exact test; n (%)
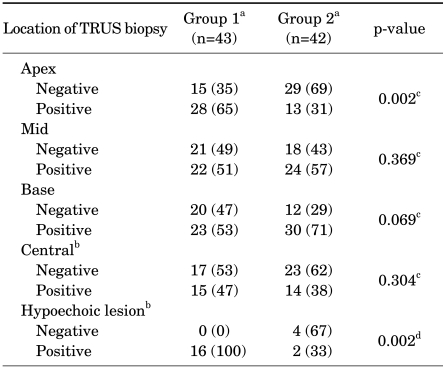
TRUS biopsy was performed in all patients at the apex, mid, and base, but not at the other cores. Our results show that the location of positive cores with a maximal Gleason score at the apex (p=0.002) or obtained through target biopsy on a hypoechoic lesion (p=0.002) were statistically significant for nonupgrading (Table 4).
TABLE 4.
Comparison of location of positive cores with maximum Gleason score between group 1 and group 2
TRUS: transrectal ultrasonography, a: Group 1 - Gleason score not upgraded after radical prostatectomy; Group 2 - Gleason score upgraded after radical prostatectomy, b: not all patients had TRUS biopsy samples taken at these cores; data were collected in patients who had biopsy results for these cores, c: chi-square test; n (%), d: Fisher exact test; n (%)
DISCUSSION
Gleason score, PSA, clinical stage, and prostate volume are crucial parameters to be considered when treating prostate cancer. The Gleason score is the most reliable factor in determining the biological characteristics of prostate cancer, tumor stage, and its prognosis. Therefore, accurate diagnosing of the Gleason score is very important for choosing proper treatment modalities in prostate cancer [14].
Recent studies have focused on the discordance in Gleason score between TRUS biopsy and radical prostatectomy specimens; discordance is reported to range from 24% to 50% [4-7]. Several factors including PSA level, prostate volume, size of the tumor mass, preoperative clinical stage, and inter- and intra-observer variation are known to be associated with upgrading of the Gleason score between TRUS biopsy and radical prostatectomy [1,15].
Nayyar et al reported that upgrading of the Gleason score is not associated with a high PSA level [16]. Unlike the report from Nayyar et al, Dong et al reported that 55% of patients were upgraded from a Gleason score of 6 to a Gleason score of 7 or higher and that a PSA level greater than 5.0 ng/ml is associated with upgrading of the Gleason score [7]. Freedland et al and Tilki et al also reported that a high PSA level is associated with upgrading of the Gleason score [9,17]. In our study, however, we did not determine the PSA level to be a predictive parameter of upgrading.
Moussa et al reported that smaller prostate volume is associated with upgrading of the Gleason score [14]. Furthermore, Turley et al reported that patients with a TRUS volume of 20 cm3 or less had more than five times the risk of upgrading compared with patients with a TRUS volume of more than 60 cm3 [18]. In our study, patients with a TRUS volume of 36.5 ml or less had a relatively higher risk of upgrading of the Gleason score after radical prostatectomy. Associations between smaller prostate volume and upgrading of the Gleason score can be explained by several theories. Kassouf et al reported that PSA elevation induced by hyperplasia in large prostates leads to earlier TRUS biopsy and this enables the detection of early prostate cancer with a well-differentiated component [19]. Turley et al reported that prostate cancer arising in small prostates is more biologically aggressive and is associated with a higher pathologic grade of tumor [18].
In our study, 53% of patients diagnosed as having a Gleason score of 6 by TRUS biopsy had an upgraded Gleason score after radical prostatectomy. This result implies that nearly half of the patients diagnosed as having a Gleason score of 6 by TRUS biopsy had more aggressive cancer than they seemed to have. If the actual Gleason score is 7 in patients who are undergoing radiation therapy under the diagnosis of prostate cancer with a Gleason score of 6, this difference could tremendously influence the treatment strategy. Therefore, even if patients are diagnosed with low-risk prostate cancer after TRUS biopsy, physicians should always consider parameters that can be predictive of upgrading to high-risk prostate cancer.
Kahl et al reported that an extended biopsy scheme with cores numbering more than 12 can help to acquire more accurate Gleason scores than a biopsy scheme with cores numbering less than 12 [20]. This report shows similar outcomes with previous reports stating that an extended biopsy scheme can improve the concordance of the Gleason score [21,22]. In this study, cores numbering from 7 to 11 were composed of 6 routine biopsy cores and additional target biopsies on hypoechoic lesions. Cores numbering from 13 to 15 were composed of 12 routine biopsy cores and additional target biopsies on hypoechoic lesions. In the 6-core biopsy scheme, there was a high rate of upgrading despite target biopsy. In the 12-core biopsy scheme, however, there was a remarkable decrease in the rate of upgrading of the Gleason score with target biopsy. Therefore, biopsy cores numbering more than 13 composed of the 12 routine biopsy cores and additional target biopsy should be taken for accurate grading of the Gleason score. This conclusion was supported with statistical significance (p=0.029).
Most prostate cancer arises from the peripheral zone. Tumor frequency is reported to be 85.5% at midgland and 82.3% at the apex. Also, prostate cancers are significantly denser in the apex to midgland, particularly in the anterior half of the gland [23]. However, the correlation between upgrading or downgrading of the Gleason score and the locations of cores taken during TRUS biopsy has not yet been clearly defined. In this study, there was statistical significance in nonupgrading between the maximum Gleason score of cores taken at the apex and obtained through target biopsy on hypoechoic lesions and that of radical prostatectomy (p=0.002 and p=0.002). Giving consideration to the facts of a higher tumor density at the apex, denser tumor cells at hypoechoic lesions on TRUS, and the results of our study, physicians can acquire more accurate information on Gleason score preoperatively through target biopsy of hypoechoic lesions and biopsy at the apex.
The results of the present study suggest that at least 13 cores should be taken during TRUS biopsy and more aggressive biopsy should be performed at the apex and of hypoechoic lesions for more precise Gleason score preoperatively. In cases of a prostate volume of 36.5 mg or less, upgrading of the Gleason score can be predicted and more aggressive treatment may be needed.
There are several limitations to the present study. First, this study was conducted retrospectively at a single institute and the study population was relatively small. Second, this study did not identify the relation between upgrading and factors such as the PSA doubling time, free PSA, and Gleason score <6 after TRUS biopsy. Third, because the biopsy strategy was not consistent, the biopsy specimens were not taken at exactly the same location in every patient. However, our study excluded patients taking neoadjuvant hormonal therapy and radiation therapy and provides comprehensive information on both localized and locally advanced prostate cancer, as well as the correlations between the locations of cores taken during TRUS biopsy and upgrading of the Gleason score. Further large-scale, prospective, multi-center studies are needed to confirm the correlations between preoperative parameters and upgrading of the Gleason score.
CONCLUSIONS
Among patients who had undergone radical prostatectomy after a diagnosis of prostate cancer, the Gleason score was upgraded in 50% of patients after radical prostatectomy. Non-upgrading could be predicted in patients with more than 13 TRUS biopsy cores and when the maximum Gleason score was detected at the apex or in a hypoechoic lesion. In cases of a prostate volume of 36.5 mg or less or a Gleason score of 6, upgrading of the Gleason score can be predicted and more aggressive treatment may be needed.
Footnotes
The authors have nothing to disclose.
References
- 1.Kim SC, Hong JH, Song K, Jeong IG, Song C, Kim CS, et al. Predictive factors for upgrading or upstaging in biopsy Gleason score 6 prostate cancer. Korean J Urol. 2009;50:836–842. [Google Scholar]
- 2.Rubin MA, Bismar TA, Curtis S, Montie JE. Prostate needle biopsy reporting: how are the surgical members of the Society of Urologic Oncology using pathology reports to guide treatment of prostate cancer patients? Am J Surg Pathol. 2004;28:946–952. doi: 10.1097/00000478-200407000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Patel AR, Jones JS. Optimal biopsy strategies for the diagnosis and staging of prostate cancer. Curr Opin Urol. 2009;19:232–237. doi: 10.1097/mou.0b013e328329a33e. [DOI] [PubMed] [Google Scholar]
- 4.Lim T, Park SC, Jeong YB, Kim HJ, Rim JS. Predictors of Gleason score upgrading after radical prostatectomy in low-risk prostate cancer. Korean J Urol. 2009;50:1182–1187. [Google Scholar]
- 5.Miyake H, Kurahashi T, Takenaka A, Hara I, Fujisawa M. Improved accuracy for predicting the Gleason score of prostate cancer by increasing the number of transrectal biopsy cores. Urol Int. 2007;79:302–306. doi: 10.1159/000109713. [DOI] [PubMed] [Google Scholar]
- 6.Hong SK, Han BK, Lee ST, Kim SS, Min KE, Jeong SJ, et al. Prediction of Gleason score upgrading in low-risk prostate cancers diagnosed via mult (> or =12)-core prostate biopsy. World J Urol. 2009;27:271–276. doi: 10.1007/s00345-008-0343-3. [DOI] [PubMed] [Google Scholar]
- 7.Dong F, Jones JS, Stephenson AJ, Magi-Galluzzi C, Reuther AM, Klein EA. Prostate cancer volume at biopsy predicts clinically significant upgrading. J Urol. 2008;179:896–900. doi: 10.1016/j.juro.2007.10.060. [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni GS, Lockwood G, Evans A, Toi A, Trachtenberg J, Jewett MA, et al. Clinical predictors of Gleason score upgrading: implications for patients considering watchful waiting, active surveillance, or brachytherapy. Cancer. 2007;109:2432–2438. doi: 10.1002/cncr.22712. [DOI] [PubMed] [Google Scholar]
- 9.Freedland SJ, Kane CJ, Amling CL, Aronson WJ, Terris MK, Prest JC., Jr Upgrading and downgrading of prostate needle biopsy specimens: risk factors and clinical implications. Urology. 2007;69:495–499. doi: 10.1016/j.urology.2006.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 11.Carter HB, Allaf ME, Partin AW. Diagnosis and staging of prostate cancer. In: Wein AJ, Kavoussi LR, Norvick AC, Partin AW, Peters CA, editors. Campbell-Walsh urology. 9th ed. Philadelphia: Saunders; 2007. pp. 2912–2931. [Google Scholar]
- 12.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 13.Epstein JI, Partin AW, Sauvageot J, Walsh PC. Prediction of progression following radical prostatectomy. A multivariate analysis of 721 men with long-term follow-up. Am J Surg Pathol. 1996;20:286–292. doi: 10.1097/00000478-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Moussa AS, Li J, Soriano M, Klein EA, Dong F, Jones JS. Prostate biopsy clinical and pathological variables that predict significant grading changes in patients with intermediate and high grade prostate cancer. BJU Int. 2009;103:43–48. doi: 10.1111/j.1464-410X.2008.08059.x. [DOI] [PubMed] [Google Scholar]
- 15.Montironi R, Mazzucchelli R, Scarpelli M, Lopez-Beltran A, Mikuz G, Algaba F, et al. Prostate carcinoma II: prognostic factors in prostate needle biopsies. BJU Int. 2006;97:492–497. doi: 10.1111/j.1464-410X.2006.05973.x. [DOI] [PubMed] [Google Scholar]
- 16.Nayyar R, Singh P, Gupta NP, Hemal AK, Dogra PN, Seth A, et al. Upgrading of Gleason socre on radical prostatectomy specimen compared to the pre-operative needle core biopsy: an Indian experience. Indian J Urol. 2010;26:56–59. doi: 10.4103/0970-1591.60445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilki D, Schlenker B, John M, Buchner A, Stanislaus P, Gratzke C, et al. Clinical and pathological predictors of Gleason Sum upgrading in patients after radical prostatectomy: results from a single institution series. Urol Oncol. 2009 doi: 10.1016/j.urolonc.2009.07.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Turley RS, Hamilton RJ, Terris MK, Kane CJ, Aronson WJ, Presti JC, Jr, et al. Small transrectal ultrasound volume predicts clinically significant Gleason score upgrading after radical prostatectomy: results from the SEARCH database. J Urol. 2008;179:523–527. doi: 10.1016/j.juro.2007.09.078. [DOI] [PubMed] [Google Scholar]
- 19.Kassouf W, Nakanishi H, Ochiai A, Babaian KN, Troncoso P, Babaian RJ. Effect of prostate volume on tumor grade in patients undergoing radical prostatectomy in the era of extended prostatic biopsies. J Urol. 2007;178:111–114. doi: 10.1016/j.juro.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Kahl P, Wolf S, Adam A, Heukamp LC, Ellinger J, Vorreuther R, et al. Saturation biopsy improves preoperative Gleason scoring of prostate cancer. Pathol Res Pract. 2009;205:259–264. doi: 10.1016/j.prp.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 21.King CR, McNeal JE, Gill H, Presti JC., Jr Extended prostate biopsy scheme improves reliability of Gleason grading: implications for radiotherapy patients. Int J Radiat Oncol Biol Phys. 2004;59:386–391. doi: 10.1016/j.ijrobp.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 22.San Francisco IF, DeWolf WC, Rosen S, Upton M, Olumi AF. Extended prostate needle biopsy improves concordance of Gleason grading between prostate needle biopsy and radical prostatectomy. J Urol. 2003;169:136–140. doi: 10.1016/S0022-5347(05)64053-0. [DOI] [PubMed] [Google Scholar]
- 23.Takashima R, Egawa S, Kuwao S, Baba S. Anterior distribution of Stage T1c nonpalpable tumors in radical prostatectomy specimens. Urology. 2002;59:692–697. doi: 10.1016/s0090-4295(02)01525-x. [DOI] [PubMed] [Google Scholar]