Abstract
Purpose
To experimentally test whether chronic sleep restriction, which is common among adolescents, is causally related to poor learning, inattentive behaviors, and diminished arousal in a classroom-like situation.
Methods
Sixteen healthy adolescents underwent a sleep manipulation that included, in counterbalanced order, 5 consecutive nights of sleep deprivation (6½ hours in bed) versus 5 nights of healthy sleep duration (10 hours in bed). At the end of each condition, participants viewed educational films and took related quizzes in a simulated classroom. Eight participants also underwent video and electroencephalography (EEG) monitoring to assess levels of inattentive behaviors and arousal, respectively.
Results
Compared to the healthy sleep condition, while sleep-deprived the participants had lower quiz scores, p=.05, more inattentive behaviors, p<.05, and lower arousal, p=.08.
Conclusions
These pilot data complement previous correlational reports by showing that chronic sleep restriction during adolescence can cause inattention, diminished learning, and lowered arousal in a simulated classroom.
Keywords: attention, arousal, learning, adolescence, pediatrics, sleep deprivation, experiment
Nearly half of US adolescents sleep less than 7 hours each school night, far below the recommended 9 hours [1, 2]. Chronic sleep restriction has been correlated with neuropsychological and scholastic dysfunction, but correlations can have hidden confounds [2]. Experimental studies, such as those published on adults [3], could provide complementary evidence of causality. However, experimental studies of adolescents have largely focused on sleepiness. We recently reported that experimental sleep restriction can affect parent and self-reported attention and metacognitive skills in adolescents [4]. That paper focused on real-life outcomes, but the reporters were not blind to the experimental manipulation. Here we report objective findings from these adolescents who were asked to learn within a simulated classroom setting, some of whom underwent monitoring of their behaviors and level of tonic arousal (general level of cerebral activation [5]). We hypothesized that, after five nights of restricted sleep, participants would learn less, be less attentive, and have lower arousal than after five nights of more optimal sleep duration.
Methods
The experimental sleep protocol and sample are detailed elsewhere [4] and were approved by the local Institutional Review Board. Twenty 13.9–16.9 year-old participants were recruited via mass e-mail and screened via parent-report for sleep disorders (in part using the Child Sleep Habits Questionnaire [6]), a history of neurological illness or injury, and current illness, injury or medication known to impact sleep or daytime functioning. Throughout the 3-week experiment, prescribed wake time was held constant at the time each participant reported that they would need to arise to attend an 8:30 am meeting. During the baseline week, participants self-selected their bedtimes. During the next two weeks, bedtimes were modified to create two conditions (order counterbalanced): sleep deprivation (SD; Monday-Friday nights limited to 6.5 hours in bed) and healthy duration (HD; 10 hours in bed Monday-Friday nights). Saturday and Sunday nights were “washout” periods, during which bedtime was participant-selected. Participants slept at home, monitored via actigraphy and sleep diary. Participants also used a daily diary to record caffeine intake and napping, though both were quite limited [4]. Participants were reimbursed $50 weekly.
Between 09:00 and 11:30 on the Saturday mornings at the end of each experimental week, participants were assessed in a simulated classroom analogous to that used in younger children to assess attention [7]. Participants were seated behind a table and viewed 30-minute educational films presented on a 19-inch television ~6 feet away. The two films, presented one per week in counterbalanced order, were selected from The Western Tradition series (Annenberg Foundation, Washington, DC) based on their developmentally-appropriate content. Afterwards, participants completed related 12-item multiple-choice quizzes. Twelve participants experienced the simulated classroom in groups of 2–3, while eight did so individually while undergoing video and electroencephalography (EEG) monitoring. A limited montage of EEG leads was secured to the scalps of these eight prior to the films. During the films, video of each participant was recorded, and linked EEG data was amplified and digitized at a rate of 256 data points per second (Hz).
There were three primary outcomes. Quiz performance was computed as the number of items correct. The two quizzes correlated moderately, r = .53, and had equivalent means and variance within each condition. Inattentive behavior, operationalized as occurrences in which the participant looked away from the television for ≥ 3 consecutive seconds, was coded by a condition-blind rater in 30-second epochs. Secondarily, the rater coded sleepy behaviors, including yawning, eye rubbing, and eyes closed or head on the table for ≥3 consecutive seconds. Tonic arousal was operationalized by spectral power in the relatively slow theta range (4–7 Hz) in the C3/A2 EEG channel commonly used while coding sleep/wake data. Spectral power was computed via Fourier transformation after manual screening for artifacts, with higher theta power reflecting lower arousal [5], and log-transformed to approximate the Gaussian distribution.
Results and Discussion
Complete data were available for all eight participants who underwent video/EEG monitoring. Of the other 12, one dropped out and quiz data for three were lost due to examiner error, leaving n=16 for quiz scores. As shown in Table 1, these subsets were similar to each other and showed excellent adherence to the sleep protocol.
Table 1.
Demographics and Sleep Data on 16 Participants Who Completed the Quizzes and the Subset of 8 who were Monitored by Video/EEG
| Baseline | Sleep Deprivation (SD) | Healthy Duration (HD) | ||||
|---|---|---|---|---|---|---|
| Quiz | Video/EEG | Quiz | Video/EEG | Quiz | Video/EEG | |
| % Caucasian | 88% | 88% | ||||
| % Male | 56% | 38% | ||||
| Age (yrs) | 15.2 ± 0.8 | 15.3 ± 0.8 | ||||
| Actigraphy Sleep Data | ||||||
| Sleep Onset Time | 0:38 ± 1:15 | 0:43 ±+ 1:30 | 1:10 ± 0:49 | 1:29 ± 0:58 | 22:34±0:33** | 23:08 ± 0:38* |
| Sleep Offset Time | 8:18 ± 1:37 | 8:43 ± 2:05 | 7:16 ± 0:35 | 7:30 ± 0:35 | 7:16 ± 0:49 | 7:40 ± 0:40 |
| Sleep Duration (hr) | 7.7 ± 1.1 | 8.0 ± 1.0 | 6.1 ± 0.6 | 6.0 ± 0.8 | 8.7 ± 0.6** | 8.5 ± 0.7** |
| # of wake episodes/night | 14.9 ± 7.9 | 13.6 ± 7.4 | 9.8 ± 4.2 | 9.1 ± 5.4 | 17.2 ± 7.4** | 18.2 ± 9.5* |
| Wake episode duration (min) | 4.4 ± 3.4 | 3.1 ± 1.5 | 3.3 ± 2.0 | 2.9 ± 2.0 | 5.0 ± 3.0 | 5.8 ± 4.2 |
| # of wakings >5 min duration | 4.1 ± 4.4 | 3.0 ± 3.0 | 1.8 ± 1.4 | 1.7 ± 1.6 | 4.4 ± 3.0** | 4.8 ± 3.5 |
| Sleep Duration (hr) | 7.7 ± 1.1 | 8.0 ± 1.0 | 6.1 ± 0.6 | 6.0 ± 0.8 | 8.7 ±0.6** | 8.5 ± 0.7** |
| Sleep Diary Data | ||||||
| Sleep Onset Time | 0:49 ± 1:11 | 1:01 ± 0:53 | 1:52 ± 0:32 | 1:03 ± 0:49 | 22:12±0:59** | 22:21 ± 1:04* |
| Sleep Offset Time | 8:02 ± 1:37 | 8:15 ± 1:58 | 7:11 ± 0:38 | 7:19 ± 0:43 | 7:23 ± 0:49 | 7:41 ± 0:43 |
| Sleep Duration (hr) | 7.2 ± 1.6 | 7.2 ± 1.4 | 6.3 ± 0.3 | 6.1 ± 0.6 | 9.2 ± 0.5** | 9.3 ± 0.7** |
p<.005,
p<.001 in planned contrasts (paired-sample t-tests) comparing the sleep deprivation and healthy sleep duration conditions. See [4] for a detailed description of the collection of sleep data via actigraphy and sleep diary. Participants averaged about 2½ hours more sleep per night (range 1.6–3.2) in the HD condition than the SD condition.
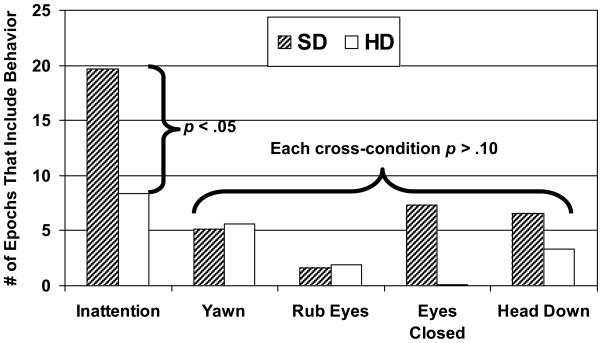
A repeated-measures general linear model examined the impact of the sleep manipulation on quiz scores, using intelligence as a covariate. Intelligence was estimated from pre-manipulation scores on the Vocabulary and Block Design subtests from the Wechsler Intelligence Scale for Children [8]. Intelligence was associated with quiz scores, F(2,13)=3.7, p=.05, but not inattentive behaviors or theta power, F(2,5)<3.0, p>.10, so analyses for the latter were simplified to paired-sample t-tests. Compared to the HD condition, while in the SD condition participants performed worse on the quizzes, F(2,15)=4.6, p=.05, ηp2=.25, tended to have higher theta power, t(7)=1.5, p=.08, d=.53, and displayed more inattentive behaviors, t(7)=2.6, p<.05, d=.98. Effect sizes were medium to large. Although not statistically significant, the medium-sized effects for “Eyes Closed” and “Head down” (d=.49–.50; Figure 1) may have reached significance in a larger sample.
Figure 1. Effect of the sleep manipulation on behaviors in the simulated classroom.
SD = Sleep Deprivation Condition, HD = Healthy Sleep Duration Condition. Adolescents’ behaviors, recorded while participants watched educational videos, were rated as present or absent within 30-second epochs. Inattentive events were coded when the participant looked away from the video for at least 3 consecutive seconds. Detailed coding criteria for inattention and the other coded behaviors are available upon request.
Findings support the assertion that chronic sleep restriction during adolescence causes inattentive behaviors, poorer learning, and diminished arousal in the classroom. Coupled with previous questionnaire findings [4], these results suggest that the adverse effects of adolescent sleep restriction extend beyond basic sleepiness to include attention regulation and learning.
Current results are intriguing, but preliminary. The small sample constrained statistical power and, although the simulated classroom yielded objective outcomes, our technique has not been previously validated and warrants psychometric attention. Even so, present findings may have implications for public policy. Studies of public policy changes such as later school start times have been quasi-experimental, had subjective outcome measures, and have not shown a clear effect on student learning [9]. Much of the public still views adolescent sleep restriction as “normal”, and lay publications often reflect acceptance or even praise for adolescents who hold evening jobs or stay up late studying (e.g, [10]). Our experimental findings add to the mounting evidence that, while chronic sleep restriction may be statistically typical for adolescents, it is neither healthy nor benign.
Acknowledgments
Funding: NIH grants K23HL075369 and M01RR08084. Drs. Beebe, Rose, and Amin each declare that they do not have a conflicting personal, financial or professional interest with respect to this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]
- 2.Wolfson AR, Carskadon MA. Understanding adolescents’ sleep patterns and school performance: A critical appraisal. Sleep Med Rev. 2003;7:491–506. doi: 10.1016/s1087-0792(03)90003-7. [DOI] [PubMed] [Google Scholar]
- 3.Van Dongen HP, Maislin G, Mullington JM, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 4.Beebe DW, Fallone G, Godiwala N, et al. Feasibility and behavioral effects of an at-home multi-night sleep restriction protocol for adolescents. Jn Child Psychol Psychiatry. 2008;49:915–923. doi: 10.1111/j.1469-7610.2008.01885.x. [DOI] [PubMed] [Google Scholar]
- 5.Oken BS, Salinsky MS, Elsas SM. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clin Neurophysiol. 2006;117:1885–1901. doi: 10.1016/j.clinph.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens J, Spirito A, McGuinn M. The children’s sleep habits questionnaire: Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. [PubMed] [Google Scholar]
- 7.Barkley RA. Attention-Deficit Hyperactivity Disorder: A handbook for diagnosis and treatment. 2. New York: Guilford; 1998. [Google Scholar]
- 8.Wechsler D. WISC-IV Manual. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 9.Wahistrom KL. Changing times: Findings from the first longitudinal study of later high school start times. National Association of Secondary School Principals Bulletin. 2002;96(633):3–21. [Google Scholar]
- 10.Medley M. Teen’s busy life includes hunting for cancer cure; Winnipeg student gets by on two or three hours of sleep a night. Ontario National Post. 2007 May 29;:A2. [Google Scholar]