Abstract
Along standing hypothesis in the field of cancer biology is that aneuploidy causes cancer by promoting loss of chromosomes that contain tumor suppressor genes. By crossing aneuploidyprone Bub1 hypomorphic mice onto a heterozygous null background for p53, we provided conclusive evidence for this idea.1 Surprisingly, the tumors that developed in this model had not just lost the chromosome 11 copy harboring wild-type p53, but had also gained an extra copy of chromosome 11 bearing the p53null allele. Here we report that a similar chromosome-reshuffling blueprint drives colonic tumorigenesis in Bub1 hypomorphic mice that are heterozygous for ApcMin, but now involving chromosome 18. These extended studies highlight that in order for whole chromosome instability to drive tumorigenesis, it needs to establish tumor suppressor gene loss of heterozygosity while retaining two copies of the other genes on the chromosome. Additional restrictions seem to apply to whole chromosome instability as a cancer causing mechanism, which will be discussed in this paper.
Keywords: Bub1, ApcMin, mitotic checkpoint, loss of heterozygosity, colon cancer, aneuploidy
Introduction
The majority of all human cancers consist of cells that exhibit an abnormal complement of chromosomes, referred to as aneuploidy.2-4 However, it has been difficult to obtain insight into the role of aneuploidy in tumorigenesis.2 Experimental evidence suggests two possible, although quite contradictory, scenarios. Chromosome number imbalances have been shown to exert detrimental effects on cellular growth and organismal fitness,5,6 suggesting that early aneuploid cells are selected against during the origin of tumors. Because later stage tumors have abnormal numbers of chromosomes,4 it seems that these tumors were successful in bypassing this selective pressure, perhaps by acquiring alterations in certain cancer-critical genes. On the other hand, a 100-year-old theory of the German biologist Theodor Boveri proposes that aneuploidy is causally implicated in tumor development.7 Although it has been difficult to prove this theory, several studies provide evidence to support it.2,8-11 Perhaps the greatest validation of Boveri's aneuploid theory comes from the observation that some of the engineered mouse models for aneuploidy are prone to tumorigenesis.12-17 However, studies of mice with chromosomal instability gene mutations have also raised several important questions.2 For instance, why is it that genes with a universal role in proper chromosome segregation, only promote tumor development in a few tissues when mutated, and why do tumor prone tissues vary between the different mouse models? Furthermore, why are some of the models not prone to spontaneous tumors despite the presence of substantial numbers of aneuploid cells,18-22 and why do some chromosomal instability gene defects exert tumor suppressive ability?16,23-26 One way to provide more definitive insight into the contribution of aneuploidy in cancer would be to decipher the underlying mechanisms. One of the classical mechanistic hypotheses in the field is that aneuploidy may promote tumorigenesis by increasing loss of tumor suppressor genes.27 The basic idea is that in cases where one copy of a given tumor suppressor gene is mutated an aberrant mitosis causes inactivation of the remaining intact gene copy through loss of the entire chromosome harboring it. To test this hypothesis, we bred Bub1 mutant mice that inaccurately segregate their chromosomes onto p53+/-, ApcMin/+, Rb+/- or Pten+/- backgrounds and monitored the rate of tumorigenesis.1,13 For simplicity, whole chromosome instability specifically due to deficiencies of Bub1 will be abbreviated (W-CIN) from this point forward.
W-CIN Drives Lymphomagenesis Through LOH of p53
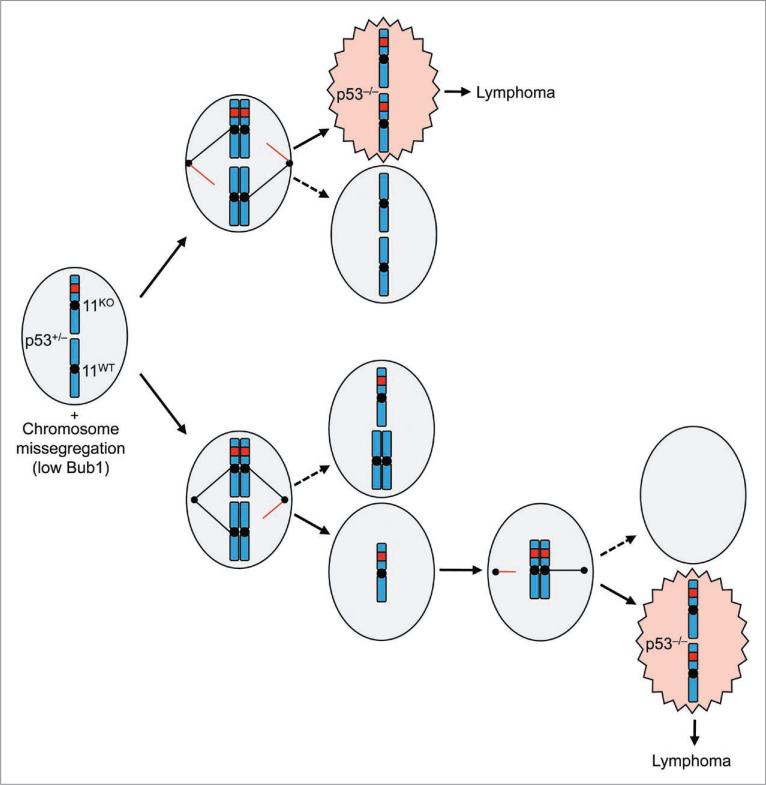
Intriguingly, at low Bub1 levels p53+/- mice became highly susceptible to T cell lymphoma,1 a tumor type that is prevalent in p53-/- mice.28 Genotyping of Bub1-/H/p53+/- thymic lymphomas revealed that they indeed had a loss of heterozygosity (LOH) of the remaining wild-type allele of p53.1 Unexpectedly, all dividing lymphoma cells had two copies of chromosome 11. Simple sequence length polymorphism (SSLP) analysis of lymphoma DNA demonstrated that both chromosome 11 copies were identical, indicating that duplication of the p53- allele resulted from whole chromosome missegregation. This may have involved two missegregation events occurring in separate cell divisions (Fig. 1). For instance, cells might obtain an extra copy of chromosome 11 containing the p53- allele in a first erroneous division, and then lose chromosome 11 containing wild-type p53 allele in a subsequent division. Alternatively, only one erroneous division may have been implicated. Due to syntelic microtubule-kinetochore attachments both chromosome 11 copies with inactive p53 may move toward one pole and those harboring the p53+ allele toward the opposite one. Syntelic attachments are quite common in cells with low amounts of Bub1, indicating that the latter scenario is certainly feasible.13,29,30
Figure 1.
Proposed mechanism for LOH of p53 in thymocytes of Bub1 deficient animals. when a p53+/- thymocyte enters into mitosis, it has two copies each of the p53 null and wild-type allele. Under normal situations, each daughter cell will receive one mutant allele and one wild-type allele, thereby maintaining the heterozygous p53 condition (not shown). However, due to insufficiencies of Bub1 and a weakened mitotic checkpoint, nondisjunction or aberrant segregation of sister chromatids occurs, leading to daughter cells that obtain two copies of the p53 null allele in potentially one (top) or two (bottom) steps.
The consistent gain of an extra copy of chromosome 11 harboring the knockout allele suggests a strong selective pressure for p53 LOH without losing the other genes on the chromosome. What could be the underlying cause of this phenomenon? Studies in yeast and mouse embryonic fibroblasts show that an extra copy of a single chromosome leads to cellular stress and reduced proliferation, perhaps because of overexpression of genes located on the chromosome that was gained.5,6,31 Along the same lines, loss of an entire chromosome might impair cellular fitness because of cellular stress associated with underexpression of genes on the affected chromosome.
W-CIN Induces Colon Cancer Through Apc LOH
ApcMin/+ mice are highly susceptible to small intestinal tumors and occasionally develop colonic tumors. However, at low Bub1 levels, these mice become highly prone to colonic tumors.1 Importantly, all colon tumors from Bub1-/H/ApcMin/+ mice that we examined lacked the Apc+ allele, indicating that Apc LOH is a requirement for colon tumor formation. Again, instead of only losing the chromosome containing the Apc+ allele, such loss seemed to be accompanied by the gain of a copy of chromosome 18 bearing the ApcMin allele, because most cancer cells showed two FISH signals for chromosome 18. Unfortunately, we were unable to provide conclusive evidence for this model because the pure C57BL/6 congenic background of the Bub1-/H/ApcMin/+ mice used in this study precluded the use of SSLP analysis.
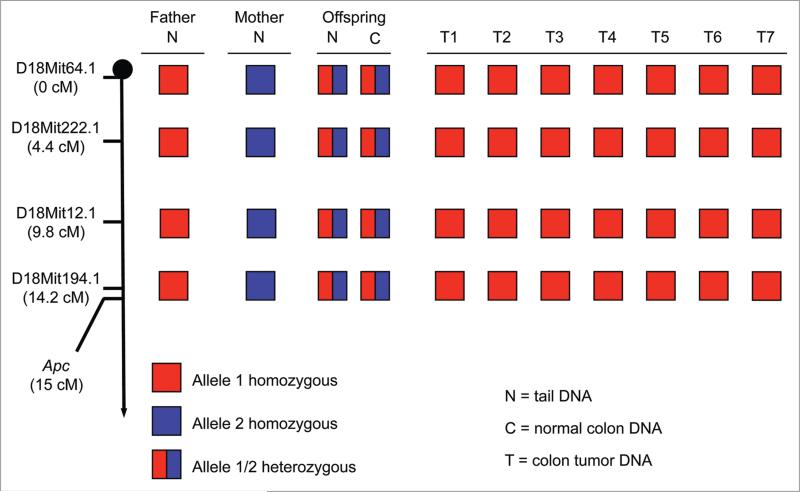
To resolve this problem, we established a cohort of Bub1-/H/ApcMin/+ mice on a 129 × C57BL/6 mixed genetic background. We verified that paternal and maternal chromosome 18 copies of mice in the cohort were distinguishable by SSLP markers. Bub1-/H/ApcMin/+ mice on the 129 × C57BL/6 mixed genetic background formed colonic tumors at very high rates, just like their counterparts on the pure C57BL/6 genetic background (data not shown). Seven of the tumors we collected from these mice were subjected to SSLP analysis using four markers distal to the Apc gene on chromosome 18 (Fig. 2). Importantly, all seven tumors were found to be homozygous for each of the four markers. This, combined with the finding that the tumor cells lack the APC+ allele and have two chromosome 18 FISH signals, demonstrates that the tumor cells had acquired two identical copies of chromosome 18 containing the ApcMin allele.
Figure 2.
Colon cancer of Bub1-/H/ApcMin/+ mice is driven by loss of chromosome 18 harboring the Apc+ locus and gain of an extra chromosome 18 harboring the ApcMin allele. Haplotypes of tail tissue (N) from the father (a Bub1-/H/ApcMin/+ mouse) and mothers (Bub1H/H/Apc+/+) generating Bub1-/H/ApcMin/+ offspring heterozygous at 4 markers in tail (N) and normal colon (C) DNa. Colon tumors (t) of these animals have reverted to being homozygous for chromosome 18 containing the ApcMin allele, which was inherited from their father.
Thus, for two independent tumor suppressor genes, p53 and Apc, whole chromosome missegregation has now been shown to drive neoplastic growth through LOH, thereby firmly establishing that aneuploidy is causally implicated in neoplastic growth. Furthermore, the consistent gain of an extra copy of the chromosome harboring the inactive tumor suppressor allele in two independent tumor models suggests that whole chromosome haploinsufficiency is selected against during the evolution of certain tumors.
W-CIN's Ability to Drive Tumor Formation Through LOH has Restrictions
Bub1 insufficiency failed to accelerate tumorigenesis in Rb+/- and Pten+/- mice.1 In fact, Bub1 insufficiency greatly reduced the number of early stage neoplastic lesions in prostate glands of Pten+/- mice. These findings are very important in that they seem to suggest that W-CIN's ability to drive tumorigenesis through tumor suppressor gene LOH is restricted to certain tumor suppressor genes and tissue/cell types. We can currently only speculate about the basis of these restrictions. One explanation would be that certain cell types are very efficient at inducing tumor suppressor gene LOH via mechanisms other than chromosome missegregation. Rb LOH in Rb+/- pituitary glands and Pten LOH in Pten+/- prostate glands are both early events that initiate neoplastic growth.32-34 This, together with the knowledge that both these tissues develop numerous independent lesions, suggests they indeed contain mechanisms for efficient LOH of relevant tumor suppressor genes. We suspect that W-CIN from Bub1 insufficiency contributes little to the overall rate of LOH of these tissues.
A second possibility is that cellular responses to aneuploidy might vary from cell type to cell type. For instance, erroneous chromosome segregation might impair proliferation or trigger apoptosis in certain cell types, while having little or no impact on growth and survival in others. It has been difficult to comprehensively test this idea because most primary cells are not amenable to combined analysis of cell fate and chromosome missegregation by live cell imaging.13 Importantly, in instances where aneuploidy were to reduce cell growth and survival, mutations neutralizing these adverse effects would have to be acquired to allow for tumor progression.5 We suspect that this scenario might explain our observations in Bub1-/H/Pten+/- prostate glands. These glands have significantly fewer low-grade PIN lesions than Pten+/- mice and the lesions that do develop show evidence of reduced cell proliferation and increased cell death, indicating that W-CIN acts to inhibit early neoplastic development in Pten+/- prostate glands.1 Later stage PIN lesions in Bub1-/H/Pten+/- prostate glands, on the other hand, show high rates of cell growth and survival, suggesting that aneuploidy creates a selective pressure for mutations that allow cells to neutralize the initial adverse effects of chromosome imbalances.
The third possibility would be that the aneuploidy rates in Bub1-/H/Rb+/- and Bub1-/H/Pten+/- mice were suboptimal. While a gradient of Bub1 levels was used in LOH studies involving p53 and Apc, only a single level of Bub1 reduction (the lowest possible one) was used in Rb and Pten studies. It would be interesting to evaluate development of PIN lesions in Pten+/- mice at various levels of Bub1 reduction to see if milder aneuploidies have the ability to accelerate cell transformation by promoting Pten LOH without adversely impacting cell growth and survival. While the Bub1 level of reduction in Bub1-/H/Pten+/- prostates may have been too severe, in Bub1-/H/Rb+/- pituitaries it may not have been extreme enough to cause substantial chromosome missegregation. Unfortunately, technical limitations precluded accurate assessment of aneuploidy rates in pituitary glands as well as in other primary tissues and cells. Until these limitations are resolved, it will be difficult to determine the incidence and severity of aneuploidy in mouse tissues and cell types other than hematopoietic cells.
Early versus Late W-CIN in Tumorigenesis
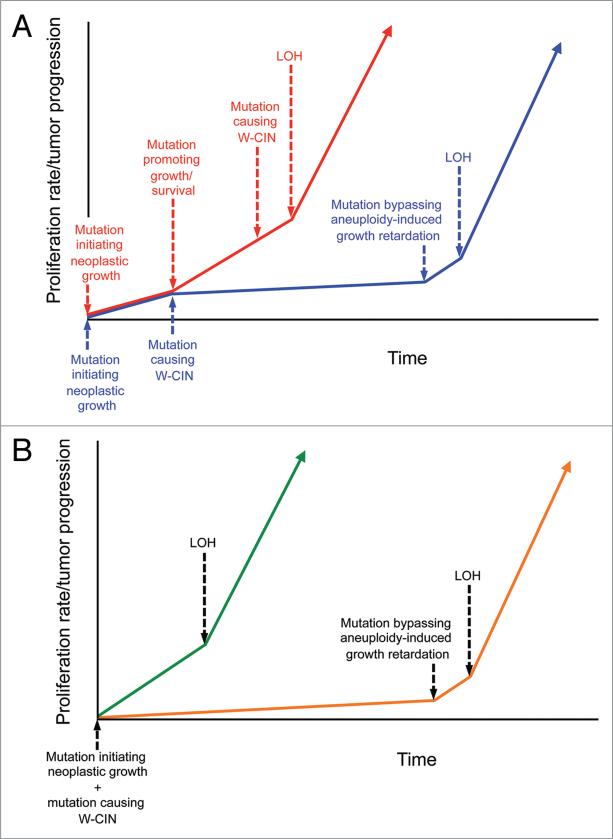
Cancers arise via a multi-step process driven by a series of DNA alterations that affect genes that control cellular processes such as proliferation, migration, polarity and apoptosis.35 Since most human cancers are aneuploid, it is reasonable to assume that developing tumor cells obtain mutations in chromosomal instability genes somewhere during tumor evolution. The timing of these mutations may in fact be critical to the impact they have on tumor progression (Fig. 3A). For instance, tumor cells that have reached an advanced malignant growth state may already contain mutations that counteract the growth inhibiting effects of chromosome imbalances, so that when these cells obtain mutations in W-CIN mutations, they only experience tumor-promoting effects of chromosome reshuffling. On the other hand, more benign tumors might enter a prolonged period of slow growth after encountering a W-CIN gene mutation (Fig. 3A). Ultimately, mutations that bypass the aneuploidy-induced growth retardation may be acquired, allowing chromosome reshuffling to accelerate tumor progression by promoting tumor suppressor gene LOH.
Figure 3.
Hypothetical models for how timing of w-CiN gene mutations may influence tumorigenesis. (a) Models in which neoplastic growth is initiated by mutation of a cancer-critical gene (other than a w-CiN gene). in scenario 1 (red line), tumor progression is driven by mutations that promote cell growth and survival. these mutations counteract the inhibiting effects of chromosome imbalances. So once w-CiN occurs, it may rapidly promote LOH and tumor advancement because only the tumor-promoting effects of aneuploidy are experienced in this tissue. in scenario 2 (blue line), w-CiN mutations may occur prior to those mutations that promote growth and survival, thereby leading to tumor cell growth inhibition (or reduced survival). the acquisition of additional mutations needed to bypass this inhibition may delay tumor development for prolonged periods of time, but after these changes occur, the underlying w-CiN mutation may rapidly promote LOH and tumorigenesis. So the timing of w-CiN gene defects may be critical to its impact on tumor progression. (B) Models prone to develop aneuploidy due to germline mutations in the mitotic checkpoint have different tissue-specific tumor susceptibilities in combination with particular tumor suppressor gene haplo-insufficiencies. in certain tissues (green line), such as thymocytes or colon epithelial cells of Bub1 deficient animals, w-CiN promotes the early loss of the chromosome containing the wild-type allele of tumor suppressor genes especially important for preventing neoplasia in that tissue, thereby accelerating the rapid onset of tumors. in other tissues (orange line), such as prostate epithelial cells of Bub1-/H mice, aneuploidy initially has detrimental effects on cell growth and survival. a selective pressure exists to bypass these adverse effects by acquiring new mutations. Once cells have acquired these changes, aneuploidy due to w-CiN may promote LOH of tumor suppressor genes.
It is important to keep in mind that W-CIN was an early event in our LOH studies because the mice had germline mutations in Bub1 and contained aneuploid cells at birth (Fig. 3B).13 In p53+/- thymocytes and ApcMin/+ colon epithelial cells, the early W-CIN seemed to have no negative impact on cell growth and survival because tumors developed so quickly in these tissues. Perhaps, the same holds true for Rb+/- pituitary glands, with the exception that Bub1 insufficiency may have been unable to accelerate Rb LOH in this particular tissue. In Pten+/- prostate epithelia, however, early W-CIN appeared to cause growth retardation and reduced survival. Perhaps if W-CIN had been introduced at a more advanced stage, it might have accelerated prostate carcinogenesis progression through LOH (Fig. 3B). It will be interesting to further test this concept by crossing Pten+/- mice with mouse models with doxycycline inducible W-CIN.17,36
Future Challenges and Perspectives
To further advance our understanding of the role of aneuploidy in tumorigenesis, it will be important to develop methods for accurate assessment of chromosome missegregation and aneuploidy rates in primary cells and tissues. A surprising finding was that many cells of Bub1-/H/p53+/- thymic lymphomas had very mild numerical abnormalities.1 These data were obtained by SKY and chromosome counts. Both these techniques require metaphase spreads from actively dividing cells, indicating that the proliferating cells within the tumor had near-diploid aneuploidy. However, it is quite possible that the non-proliferating cells had a dramatically different degree of aneuploidy, which is then masked by these current techniques. Utilization of FISH on paraffin tissue is limited to visualizing only a small subset of the total chromosomal complement and is prone to errors in detecting minor numerical changes. Using FISH on potentially non-proliferating end stage tumor cells may reveal higher levels of aneuploidy and may easily be detected using FISH probes, especially if the cell had undergone DNA endoreduplication, but how do these cells relate to the initial stages of the tumor? Although CIN is often a marker for advanced stage cancer,4,37,38 perhaps these tumor cells have already become irreversibly tumorigenic due to minor numerical changes and compensatory means much earlier in the tumorigenic process. Now that we have a technique for FISH on primary colon cancer cells that do not divide in culture,1 it would be interesting to test whether colon adenocarcinomas of Bub1-/H/ApcMin/+ mice become more severely aneuploid if they are given the opportunity to progress beyond 90 days.
Now that W-CIN has been shown to drive tumorigenesis through tumor suppressor gene LOH, it is important to determine whether it can also do so through duplication of oncogenes.39 Perhaps using a similar approach of crossing Bub1 deficient animals to various oncogenic models may elucidate whether a similar mechanism of gaining a second copy of the chromosome harboring the oncogene exists. In these scenarios, are the tumor cells now triploid for this chromosome or do selective pressures exist to maintain diploidy, and does this then indicate a selection for homozygosity? Finally, the larger question remains, do human cancers fit the same selective pressures and what interventions can be derived to target cells with minor chromosomal imbalances for elimination? Clearly the link of aneuploidy and tumor initiation/progression remains highly complex that is likely to remain subject of intense investigation for years to come.
Acknowledgements
We thank Robin Ricke for feedback on the manuscript and the Mayo Clinic Genotyping shared resource for SSLP analysis. This work was supported by the National Institutes of Health (grants CA126828 and CA91956) and the Robert and Arlene Kogod Center on Aging.
References
- 1.Baker DJ, Jin F, Jeganathan KB, van Deursen JM. Whole chromosome instability caused by Bub1 insufficiency drives tumorigenesis through tumor suppressor gene loss of heterozygosity. Cancer Cell. 2009;16:475–86. doi: 10.1016/j.ccr.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricke RM, van Ree JH, van Deursen JM. Whole chromosome instability and cancer: a complex relationship. Trends Genet. 2008;24:457–66. doi: 10.1016/j.tig.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holland AJ, Cleveland DW. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat Rev Mol Cell Biol. 2009;10:478–87. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schvartzman JM, Sotillo R, Benezra R. Mitotic chromosomal instability and cancer: mouse modelling of the human disease. Nat Rev Cancer. 2010;10:102–15. doi: 10.1038/nrc2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres EM, Williams BR, Amon A. Aneuploidy: cells losing their balance. Genetics. 2008;179:737–46. doi: 10.1534/genetics.108.090878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torres EM, Sokolsky T, Tucker CM, Chan LY, Boselli M, Dunham MJ, et al. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science. 2007;317:916–24. doi: 10.1126/science.1142210. [DOI] [PubMed] [Google Scholar]
- 7.Boveri T. Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. J Cell Sci. 2008;121:1–84. doi: 10.1242/jcs.025742. [DOI] [PubMed] [Google Scholar]
- 8.Duesberg P, Rasnick D, Li R, Winters L, Rausch C, Hehlmann R. How aneuploidy may cause cancer and genetic instability. Anticancer Res. 1999;19:4887–906. [PubMed] [Google Scholar]
- 9.Duesberg P. Chromosomal chaos and cancer. Sci Am. 2007;296:52–9. doi: 10.1038/scientificamerican0507-52. [DOI] [PubMed] [Google Scholar]
- 10.Duesberg P, Li R. Multistep carcinogenesis: a chain reaction of aneuploidizations. Cell Cycle. 2003;2:202–10. [PubMed] [Google Scholar]
- 11.Baker DJ, Chen J, van Deursen JM. The mitotic checkpoint in cancer and aging: what have mice taught us? Curr Opin Cell Biol. 2005;17:583–9. doi: 10.1016/j.ceb.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Iwanaga Y, Chi YH, Miyazato A, Sheleg S, Haller K, Peloponese JM, Jr, et al. Heterozygous deletion of mitotic arrest-deficient protein 1 (MAD1) increases the incidence of tumors in mice. Cancer Res. 2007;67:160–6. doi: 10.1158/0008-5472.CAN-06-3326. [DOI] [PubMed] [Google Scholar]
- 13.Jeganathan K, Malureanu L, Baker DJ, Abraham SC, van Deursen JM. Bub1 mediates cell death in response to chromosome missegregation and acts to suppress spontaneous tumorigenesis. J Cell Biol. 2007;179:255–67. doi: 10.1083/jcb.200706015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michel LS, Liberal V, Chatterjee A, Kirchwegger R, Pasche B, Gerald W, et al. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature. 2001;409:355–9. doi: 10.1038/35053094. [DOI] [PubMed] [Google Scholar]
- 15.Sotillo R, Hernando E, Diaz-Rodriguez E, Teruya-Feldstein J, Cordon-Cardo C, Lowe SW, et al. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weaver BA, Silk AD, Montagna C, Verdier-Pinard P, Cleveland DW. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer cell. 2007;11:25–36. doi: 10.1016/j.ccr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Diaz-Rodriguez E, Sotillo R, Schvartzman JM, Benezra R. Hec1 overexpression hyperactivates the mitotic checkpoint and induces tumor formation in vivo. Proc Natl Acad Sci USA. 2008;105:16719–24. doi: 10.1073/pnas.0803504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babu JR, Jeganathan KB, Baker DJ, Wu X, Kang-Decker N, van Deursen JM. Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation. J Cell Biol. 2003;160:341–53. doi: 10.1083/jcb.200211048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36:744–9. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 20.Baker DJ, Jeganathan KB, Malureanu L, Perez-Terzic C, Terzic A, van Deursen JM. Early aging-associated phenotypes in Bub3/Rae1 haploinsufficient mice. J Cell Biol. 2006;172:529–40. doi: 10.1083/jcb.200507081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeganathan KB, Baker DJ, van Deursen JM. Securin associates with APCCdh1 in prometaphase but its destruction is delayed by Rae1 and Nup98 until the metaphase/anaphase transition. Cell Cycle. 2006;5:366–70. doi: 10.4161/cc.5.4.2483. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Yu R, Melmed S. Mice lacking pituitary tumor transforming gene show testicular and splenic hypoplasia, thymic hyperplasia, thrombocytopenia, aberrant cell cycle progression, and premature centromere division. Mol Endocrinol. 2001;15:1870–9. doi: 10.1210/mend.15.11.0729. [DOI] [PubMed] [Google Scholar]
- 23.Weaver BA, Cleveland DW. The role of aneuploidy in promoting and suppressing tumors. J Cell Biol. 2009;185:935–7. doi: 10.1083/jcb.200905098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao CV, Yang YM, Swamy MV, Liu T, Fang Y, Mahmood R, et al. Colonic tumorigenesis in BubR1+/- ApcMin/+ compound mutant mice is linked to premature separation of sister chromatids and enhanced genomic instability. Proc Natl Acad Sci USA. 2005;102:4365–70. doi: 10.1073/pnas.0407822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeganathan KB, Baker DJ, van Deursen JM. Securin Associates with APC(Cdh1) in Prometaphase but its Destruction is Delayed by Rae1 and Nup98 until the Metaphase/Anaphase Transition. Cell Cycle. 2006;5:366–70. doi: 10.4161/cc.5.4.2483. [DOI] [PubMed] [Google Scholar]
- 26.Weaver BA, Cleveland DW. Aneuploidy: instigator and inhibitor of tumorigenesis. Cancer Res. 2007;67:10103–5. doi: 10.1158/0008-5472.CAN-07-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michor F, Iwasa Y, Vogelstein B, Lengauer C, Nowak MA. Can chromosomal instability initiate tumori-genesis? Semin Cancer Biol. 2005;15:43–9. doi: 10.1016/j.semcancer.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 29.Perera D, Tilston V, Hopwood JA, Barchi M, Boot-Handford RP, Taylor SS. Bub1 maintains centromeric cohesion by activation of the spindle checkpoint. Dev Cell. 2007;13:566–79. doi: 10.1016/j.devcel.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Meraldi P, Sorger PK. A dual role for Bub1 in the spindle checkpoint and chromosome congression. EMBO J. 2005;24:1621–33. doi: 10.1038/sj.emboj.7600641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams BR, Prabhu VR, Hunter KE, Glazier CM, Whittaker CA, Housman DE, et al. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science. 2008;322:703–9. doi: 10.1126/science.1160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 33.Hu N, Gutsmann A, Herbert DC, Bradley A, Lee WH, Lee EY. Heterozygous Rb-1 delta 20/+ mice are predis-posed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene. 1994;9:1021–7. [PubMed] [Google Scholar]
- 34.Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, et al. Mutation of Pten/ Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA. 1999;96:1563–8. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberg R. The biology of cancer. Garland Science. 2007 [Google Scholar]
- 36.Shonn MA, Murray AL, Murray AW. Spindle checkpoint component Mad2 contributes to biorientation of homologous chromosomes. Curr Biol. 2003;13:1979–84. doi: 10.1016/j.cub.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 37.Glinsky GV. Death-from-cancer signatures and stem cell contribution to metastatic cancer. Cell Cycle. 2005;4:1171–5. doi: 10.4161/cc.4.9.2001. [DOI] [PubMed] [Google Scholar]
- 38.Carter SL, Eklund AC, Kohane IS, Harris LN, Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet. 2006;38:1043–8. doi: 10.1038/ng1861. [DOI] [PubMed] [Google Scholar]
- 39.Sotillo R, Schvartzman JM, Benezra R. Very CIN-ful: whole chromosome instability promotes tumor suppressor loss of heterozygosity. Cancer Cell. 2009;16:451–2. doi: 10.1016/j.ccr.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]