Abstract
Objectives
Physical activity (PA) has potential to improve outcomes in both arthritis and diabetes, but these conditions are rarely examined together. Our objective was to explore whether persons with arthritis alone or those with both arthritis and diabetes could improve amounts of PA with a home-based counseling intervention.
Methods
As part of the Veterans LIFE Study, veterans ages 70–92 were randomized to usual care or a twelve month PA counseling program. Arthritis and diabetes were assessed via self-report. Mixed models were used to compare trajectories for minutes of endurance and strength training PA for persons with no arthritis (n=85), arthritis (n=178), and arthritis plus diabetes (n=84).
Results
Recipients of PA counseling increased minutes of PA per week independent of disease status (treatment arm by time interaction P<0.05 for both; endurance training time P=0.0006 and strength training time P<0.0001). Although PA was lower at each wave among persons with arthritis, and even more so among persons with arthritis plus diabetes, the presence of these conditions did not significantly influence response to the intervention (Arthritis/Diabetes group X time interactions P>0.05 for both outcomes) as each group experienced a nearly two-fold or more increase in PA.
Conclusions
A home-based PA intervention was effective in increasing minutes of weekly moderate intensity endurance and strength training PA in older veterans, even among those with arthritis or arthritis plus diabetes. This program may serve as a useful model to improve outcomes in older persons with these pervasive diseases.
Key Indexing Terms: Physical Activity, Arthritis, Diabetes, Counseling
As the world’s population grows older and more obese, arthritis is becoming increasingly pervasive. Global estimates for osteoarthritis indicate that more than 10% of persons over age 60 are limited by arthritis, and that radiographic evidence of osteoarthritis is present in almost all individuals over age 70[1]. These numbers highlight that arthritis and related conditions cause significant disability and are one of the most prevalent causes of morbidity in aging persons[2].
Similarly, with an aging and overweight population, type 2 diabetes rates are often described as epidemic[3]. As such, diabetes is emerging as an important co-morbidity in older persons with arthritis[3]. In individuals 65 years and older with arthritis, the presence of diabetes contributes to more than a one and a half fold decline in function over two years[4].
Physical activity (PA) has potential to improve outcomes in both diseases. Exercise training can improve pain, function, quality of life, muscle strength and even mental health in persons with arthritis[5, 6]. Similarly, even in the absence of weight loss, exercise training contributes to improvements in risk for diabetes[7, 8], and regular exercise is an essential component of the comprehensive management to improve glycemic control, mediate weight control and reduce cardiovascular risk in those with type 2 diabetes[9].
The co-existence of these two conditions complicates prescriptions for PA, especially in older individuals. In addition to barriers to exercise found in the non-arthritic population, persons with arthritis exhibit unique barriers to exercise. Barriers identified in persons with arthritis include disease severity[10], pain, fatigue, lack of mobility, lack of support from physicians[11], and an inability to find exercise classes of the appropriate challenge level[12]. Further, in persons with arthritis, illnesses[12] and co-morbid conditions[11] have been recognized as factors influencing poor compliance with an exercise program. For example, one recent report suggested that pain associated with arthritis might serve as a barrier to PA in persons with diabetes[13].
Additionally, while community facility-based[14] and internet-based[15] PA interventions have been proven useful in persons with arthritis, there are limited data regarding home-based interventions using telephone counseling as a means of increasing PA in persons with arthritis. Similarly, it is not clear if home-based interventions would be equally effective for increasing PA in older persons with both arthritis and diabetes. Here, our objective was to explore whether persons with arthritis alone or those with both arthritis and diabetes could improve amounts of PA with a home-based PA counseling intervention. We hypothesized that persons with arthritis and those with both arthritis and diabetes would exhibit unique, less desirable, trajectories with a home-based counseling intervention as compared to those without arthritis and/or diabetes.
Methods
Design and Subjects
Participants were enrolled in the Veterans LIFE (Learning to Improve Fitness and Function in Elders) Study[16]. This randomized controlled trial was conducted at the Durham Veterans Affairs Medical Center where US veterans ages 70–92 were randomized to usual care or a 12-month PA counseling program. All procedures were approved by the institutional review board and were in accordance with the Helsinki Declaration of 1975, as revised in 1983. Inclusion criteria included age 70 years or greater, ability to walk 30 feet without human assistance, and engaging in less than 150 minutes per week of PA. Participants were excluded from enrollment if there was a medical chart diagnosis of chronic pain that prevented exercise, a terminal diagnosis, unstable angina, history of ventricular tachycardia, chronic obstructive lung disease requiring two hospitalizations in last 12 months, uncontrolled hypertension, stroke with moderate to severe aphasia, diagnosis of mental or behavioral disorder, active substance abuse, dementia, or severe hearing or visual loss. Please see Figure 1 for flow of participants through this trial. This report compared participants with no self-report of arthritis or diabetes (n=85), those self-reporting arthritis (n=178), and those reporting both arthritis plus diabetes (n=84). Persons reporting diabetes only (n=53) were not included in this analysis. The outcomes were baseline to12 month changes in minutes per week of moderate or higher intensity endurance and strength training PA.
Figure 1.
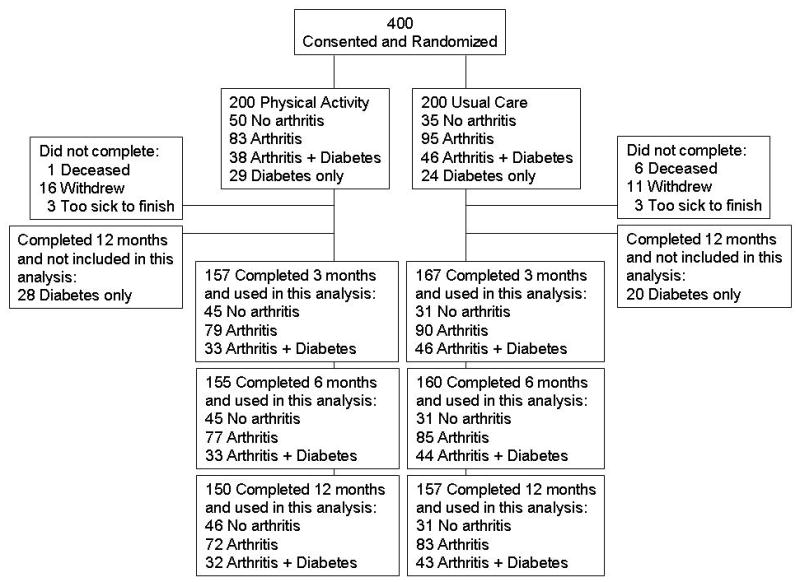
Flow of VA LIFE participants.
Intervention
This program was based on the social cognitive theoretical models used in the Activity Counseling Trial[17] and targeted goals of moderate intensity endurance training 30 minutes per day, at least five days per week, and leg strength training 15 minutes per day, at least three days per week. Walking and lower extremity strength training were emphasized since these have been associated with higher levels of physical function[18]. Details of the multicomponent intervention and primary outcomes have been reported previously[16]. To summarize, each person randomized to intervention received a baseline in-person counseling session during which personally relevant PA benefits were identified, short term PA goals were established, barriers to PA were discussed, and a source of social support for PA was identified. Individuals received a workbook with targeted reading materials including “Exercise: A guide from the NIA,” elastic resistance bands, an exercise poster depicting lower extremity resistance training exercises, and a pedometer. Following the baseline counseling session the same health counselor delivered three biweekly telephone calls within the first six weeks and then monthly telephone calls for the remainder of the trial to reinforce PA behavior, modify PA goals and problem solve as needed. Additional components of the counseling arm included an endorsement of PA by the primary care provider during one clinic visit, a monthly automated telephone PA endorsement by the primary care provider, and quarterly mailed progress reports tailored to describe each participant’s progress towards endurance and strength training goals. All participants in the intervention arm completed the baseline counseling session, clinical endorsements by the providers were documented for more than 90% of study participants, and 98% of the automated telephone messages were successfully delivered indicating successful delivery of the intervention.
Measures
Assessments were performed at baseline, three, six, and 12 months. At each wave, amounts of reported PA were determined using the Community Health Activities Model Program for seniors (CHAMPS)[19]. CHAMPS is a self-report questionnaire which assesses weekly frequency and duration of social and physical activities typically performed by older adults and has established construct validity, reliability, and sensitivity to change[20]. For this analysis, we computed the total duration (ie. minutes reported) for all moderate or higher intensity endurance (walk briskly, jog or run, ride a bicycle or stationary cycle, do aerobic machines) and strength (moderate to heavy strength training, light strength training, general conditioning such as light calisthenics) activities. Subjects reported their diabetes and arthritis status by endorsing the appropriate medical conditions on the Older Americans Resources and Services (OARS) survey[21]. A co-morbidity score was calculated as the sum of the remainder of the 33 OARS self-identified conditions.
Data Analysis
Cross sectional baseline analyses were conducted using analysis of variance for continuous measures and logistic regression for categorical measures, as appropriate. For both types of analyses, the group reporting no arthritis was used as the reference group. For these baseline analyses, the general analytic strategy involved two phases: 1) conducting an omnibus 2 degree of freedom test for distributional differences between the three groups (no arthritis, arthritis only, and arthritis plus diabetes); and 2) if the omnibus test was significant, conducting explicit pairwise comparisons between disease groups. Completion rates were computed as number of participants who completed the 12 month assessment divided by number of participants randomized x 100.
For longitudinal analyses, mixed models were used to compare trajectories for minutes of PA (endurance and strength) for persons with no self-report of arthritis, arthritis only, and arthritis plus diabetes. To assess effectiveness of the intervention, two- way interactions of treatment arm (intervention/control) by time were performed for each of the PA outcomes. Stratified analyses were performed separately for each intervention arm (PA counseling and usual care) to explore responses for those with arthritis, arthritis plus diabetes, or free of disease. Arthritis/Diabetes group X time interactions were investigated for the trajectory analyses for each outcome. All analyses were adjusted for baseline PA, body mass index (BMI), race, age, and education. Statistics were performed using SAS Version 9.1 (SAS Institute, Cary, NC).
Results
Baseline differences between the three groups are shown in Table 1. When compared to those with no arthritis, persons reporting arthritis and diabetes had a greater BMI (Table 1; P=0.002) and were less likely to have any college education (P=0.03). Persons with arthritis alone and those with both arthritis and diabetes, reported significantly more co-morbid conditions at baseline (P<0.05 for both). Baseline minutes per week of endurance PA between groups was not significantly different (P=0.87). Compared to persons with no arthritis, those with both arthritis and diabetes reported fewer minutes per week of strength PA (omnibus P=0.08; post-hoc between groups P=0.03). This subset (n=347) of participants did not significantly differ from the remainder of the original LIFE participants (n=53) in age, sex, race, BMI, education, or baseline exercise (P>0.05 for all) but did report more co-morbidities (5.4 ± 2.5) than those not included (3.6 ± 2.1; P=0.02). In general, completion rates for the intervention arm were very good: 92% percent of those with no arthritis, 87% of those with arthritis only, and 84% of those with both arthritis and diabetes completed the 12 month assessment. In the usual care arm rates of completion were as follows: no arthritis 89%, arthritis only 87%, and arthritis and diabetes 93%.
Table 1.
Baseline Demographics and Characteristics
| No Arthritis n =85 | Arthritis n =178 | Arthritis + Diabetes n =84 | |
|---|---|---|---|
| Age - years ± SD | 77.7 ± 5.5 | 77.7 ± 5.1 | 77.3 ± 4.1 |
| Sex - Men (%) | 100 | 99.4* | 100* |
| Race - Caucasian (%) | 76.5 | 80.3 | 72.6 |
| BMI (weight/height2) - mean ± SD | 28.2 ± 4.6 | 28.6 ± 4.4 | 30.4 ± 4.9† |
| Education - No college (%) | 42.4 | 52.8† | 69.1† |
| Modified Co-Morbidity Score - mean ± SD (range**) | 3.7 ± 2.1 (0–10) | 4.6 ± 2.4† (0–15) | 4.9 ± 2.7† (0–15) |
| Moderate endurance training PA (min/w) - mean ± SD | 41.7 ± 78.8 | 35.4 ± 93.0 | 32.3 ± 76.7 |
| Moderate strength training PA (min/w) - mean ± SD | 19.7 ± 44.0 | 28.4 ± 62.3 | 12.3 ± 30.1† |
P-value not estimable
P value < 0.05 for comparison with arthritis-/diabetes- group. Statistical comparisons for minutes of PA were adjusted for age, race, and BMI.
The possible range for the Modified Co-Morbidity Score was from 0 to 33.
Independent of arthritis or diabetes group, the home-based PA intervention was effective in increasing minutes per week of both endurance (treatment arm by time interaction P= 0.04; time P=0.0006; Figure 2A and Table2) and strength PA (treatment arm by time interaction P<0.0001; time P<0.0001; Figure 2B and Table 2). Although minutes of PA was attenuated relative to the no arthritis group at each wave, the proportion of change was clinically relevant; individuals with arthritis only increased their endurance and strength PA by 146 and 164% respectively and individuals with both arthritis and diabetes increased their endurance and strength PA by 53 and 241% respectively. No arthritis/diabetes group by time interactions were observed indicating successful uptake of the intervention (P>0.05 for all). There were no overall changes over time noted for three groups assigned in the usual care arm (endurance training, P<0.17; strength training, P<0.51; Figure 2 and Table 2).
Figure 2.
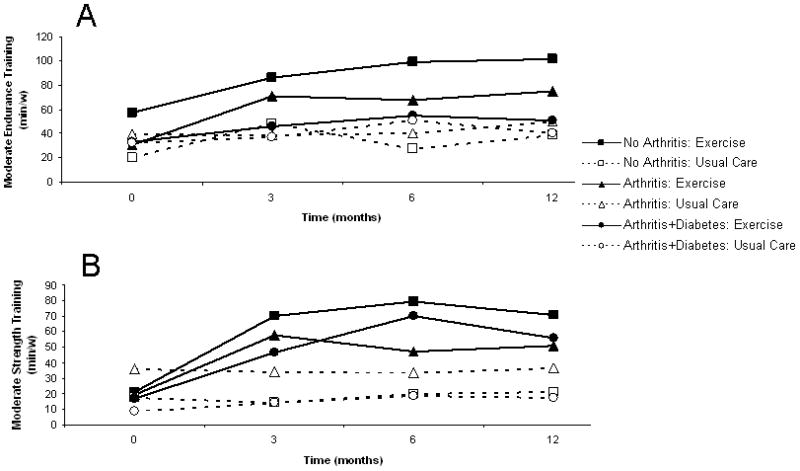
Trajectories of reported PA per week. Complete lines and closed figures are used to depict trajectories for those assigned to the counseling intervention, and dashed lines and open figures represent those who received usual care alone. Squares indicate those with no arthritis. Triangles are used to represent persons with arthritis with no diabetes. Circles indicate those with both arthritis and diabetes.
A. Minutes of reported endurance training PA per week. Time effects for PA counseling P=0.0006 and for usual care P=0.17.
B. Minutes of reported strength training PA per week.
Time effects for PA counseling P<0.0001 and for usual care P=0.51.
Table 2.
Minutes of Reported PA over Time with Counseling Intervention and Usual Care
| Baseline | 3 months | 6 months | 12 months | Time Main Effect P Value | Arthritis/Diabetes Main Effect P Value | Time X Arthritis/Diabetes Group Interaction P Value¶ | |
|---|---|---|---|---|---|---|---|
| Home-Based PA Intervention | |||||||
| Endurance Training PA (min/w) | |||||||
| No Arthritis | 57.2 ± 89.3 | 86.2 ± 99.7 | 98.7 ± 96.8 | 101.6 ± 120.5 | 0.0006 | 0.071 | 0.52 |
| Arthritis | 30.4 ± 80.4 | 70.6 ± 101.2 | 67.4 ± 88.8 | 74.8 ± 137.1 | |||
| Arthritis + Diabetes | 33.0 ± 95.9 | 46.2 ± 67.6 | 54.6 ± 72.6 | 50.6 ± 70.3 | |||
| Strength Training PA (min/w) | |||||||
| No Arthritis | 21.4 ± 47.3 | 70.4 ± 56.3 | 79.5 ± 81.5 | 70.5 ± 54.1 | <0.0001 | 0.0263 | 0.32 |
| Arthritis | 19.3 ± 46.1 | 57.7 ± 54.2 | 47.3 ± 47.3 | 51.1 ± 57.1 | |||
| Arthritis + Diabetes | 16.5 ± 38.0 | 46.5 ± 37.9 | 70.4 ± 66.9 | 56.2 ± 55.6 | |||
| Usual Care | |||||||
| Endurance Training PA (min/w) | |||||||
| No Arthritis | 19.9 ± 55.5 | 48.6 ± 84.5 | 27.5 ± 43.8 | 38.6 ± 85.6 | 0.17 | 0.82 | 0.98 |
| Arthritis | 39.8 ± 102.8 | 38.3 ± 74.5 | 40.6 ± 82.4 | 49.6 ± 116.3 | |||
| Arthritis + Diabetes | 31.9 ± 57.9 | 36.7 ± 64.9 | 51.1 ± 91.5 | 40.3 ± 69.7 | |||
| Strength Training PA (min/w) | |||||||
| No Arthritis | 17.4 ± 39.6 | 14.4 ± 36.1 | 20.2 ± 40.7 | 21.1 ± 41.8 | 0.51 | 0.31 | 0.82 |
| Arthritis | 36.3 ± 72.8 | 34.3 ± 67.8 | 33.4 ± 77.3 | 36.5 ± 75.4 | |||
| Arthritis + Diabetes | 9.0 ± 21.6 | 14.0 ± 29.1 | 18.5 ± 34.6 | 17.2 ± 33.9 | |||
Data are means ± standard deviation. Analyses were performed using mixed models adjusted for baseline PA, BMI, race, age, and education.
Full models were performed, and interaction terms from full models are displayed above. Interaction terms were removed and main effects models were rerun; time and group effects displayed above represent those from main effects models.
Discussion
While regular PA is essential to successful aging, older persons often have multiple morbidities that complicate exercise prescriptions. Recognition of the world’s expanding aging and obese population delivers an imperative to develop interventions that can effectively increase amounts of PA for older persons with co-morbidities such as arthritis and diabetes. Here, in contrast to our hypothesis, we found that persons with arthritis alone or arthritis and diabetes substantially improved minutes of reported PA with a home-based PA counseling program.
While the World Health Organization (WHO) is in the process of developing global guidelines for PA for health, current WHO recommendations follow US guidelines for PA[22]. For individuals over 65 years of age, current US recommendations for PA include aerobic, muscle strengthening, flexibility and balance exercise guidelines[23, 24] For aerobic activity, recommendations include a minimum 150 minutes per week of moderate intensity endurance activity or 75 minutes per week of vigorous intensity endurance activity[23, 24]. Combinations of vigorous and moderate intensity activities can be used to meet endurance activity goals[23, 24]. Additionally, older individuals should perform strength training PA targeting major muscle groups on at least 2 or more nonconsecutive days per week[23, 24]. For older individuals and those with co-morbidities affecting function, flexibility exercises are recommended at least twice weekly for 10 minutes, and regular balance exercises are recommended to reduce fall risk[23, 24]. PA guidelines are similar for individuals with arthritis and diabetes[25]. Of particular relevance to this study, the most recently released PA guidelines specifically address co-morbidities including osteoarthritis and type 2 diabetes with the recommendation that persons with chronic conditions should be as “physically active as their conditions allow”[23].
While neither of the arthritis and diabetes groups on average reached the minimum recommended 150 minutes per week of moderate intensity endurance training PA, we observed an increase in the number of participants meeting recommended endurance training guidelines. Percentages of participants meeting recommended endurance training (150 minutes per week or more) goals increased from 6% at baseline to 17% at 12 months for those with arthritis alone and from 9% to 19% for those with both arthritis and diabetes. In addition to percentages meeting guidelines, the relative increases achieved with this intervention are remarkable and important. For those with both arthritis and diabetes, the mean observed increase of 17.6 minutes per week corresponds to a 53% increase in minutes per week of activity. Those with arthritis alone demonstrated even greater, 146%, improvements. Such relative increases in minutes per week of activity are likely to confer significant health benefits [26, 27]. In fact, the foundation for existing PA guidelines is based on extensive evidence of a curvilinear dose-response relationship between PA and health outcomes. Small increases the low end of the PA range produce more robust health benefits which is particularly relevant to older adults living with multiple chronic conditions[27]. Also, there is considerable evidence that any deviation from sedentary behavior towards regular PA improves morbidity and mortality.
In addition to increases in endurance training, we observed significant gains in minutes per week of reported strength training that were independent of the presence of arthritis or diabetes. Less than 15% of older persons perform resistance training twice weekly as recommended[28]. After 12 months of the counseling intervention, participants with arthritis alone and with arthritis and diabetes were averaging more than 50 minutes per week of resistance training activity such that many of these participants were exceeding recommendations[24]. Specifically, percentages of participants meeting recommendations for strength training increased from 17% at baseline to 53% at 12 months for those with arthritis alone and from 13% to 56% for those with both arthritis and diabetes. In older individuals with arthritis and diabetes where disability rates are very high, increasing amounts of resistance training activity will likely confer significant functional and health benefits[24, 29].
It is important to recognize that this investigation has several limitations. First, this intervention was not designed to detect differences between persons with arthritis and diabetes and those without, and, as such, we were underpowered to detect small response differences between the arthritis/diabetes groups. Additionally, our observed response differences between groups were relatively small. Given this, we can not exclude the possibility that a statistically significant poorer response in the co-morbid groups would be detected in a much larger investigation. However, our findings are consistent with other investigations that have shown that persons with both knee arthritis and cardiovascular disease were able to improve aerobic fitness with a supervised exercise program[30]. Also, despite the increasing prevalence of arthritis and diabetes in the aging population, we believe we are the first to assessing the impact of these two specific diseases on response to a training intervention. With the critical need to improve PA in these individuals, we believe that it is important to highlight an intervention which has proven effective in increasing minutes of PA in persons with arthritis alone and both arthritis and diabetes.
It is also worth noting that the focus of this investigation was on older persons with arthritis such that we sought to understand if responses to this intervention would be effective for persons with arthritis as well as for those with the combination of arthritis and diabetes. As such, persons with diabetes alone were not presented but showed similar improvements in minutes of PA (data not shown). This arthritis-centered focus has particular relevance to rheumatology where there is an increasing recognition of cardiovascular disease and diabetes associated with rheumatologic conditions.
An additional limitation is that this investigation was performed in an almost entirely male US veteran population. It is well recognized that older men are more likely than women to engage in PA[31, 32], and we can not exclude the possibility that women might have not responded to the intervention as well. Nonetheless, as might be expected in a veteran population, minorities and those with less than a college education were well represented and strengthen the applicability of our findings to the general population.
Finally, much of our findings are based on self-reports of PA and co-morbidities. By measuring self-reported PA, we have most likely over-estimated true PA in these individuals. However, the construct validity of CHAMPS is well established, and this measure had been correlated with other self-report PA measures[20], pedometer step counts[33], and accelerometer measured PA[34]. Further, current recommendations for PA are based on recognition of over-reported amounts of PA, and we have no evidence that persons with arthritis or diabetes will report PA less or more accurately than those without these conditions.
Despite the clarity and seeming simplicity of these recommendations, the reality of introducing PA as a lifestyle intervention for older individuals with co-morbidities may seem daunting to health care providers faced with competing time constraints in the management of complex chronic diseases. Here, we have shown that independent of arthritis and diabetes, successful improvements in minutes per week of moderate intensity activity can be achieved with a relatively easy to implement home-based counseling intervention. This intervention relies on a baseline counseling session, best delivered by an individual trained in health promotion and PA. With instruction, similar exercise counseling can be delivered by nursing staff, other allied health care professionals, or certified exercise counselors[35]. Similarly, the additional components of the counseling intervention, including in person endorsements from primary care providers, periodic telephone contacts, and mailings could be implemented in most practice settings at relatively low cost.
Thus, we report that a home-based PA counseling intervention can significantly improve minutes of moderate intensity activity in older persons with co-morbidities. Independent of self-report of arthritis alone or arthritis and diabetes, significant improvements in moderate intensity endurance activity were achieved, and many participants achieved recommended goals for resistance training. Given these findings, this program may serve as a useful model to improve both functional and health outcomes in older persons with arthritis and diabetes.
Acknowledgments
We would like to recognize the contribution and participation of the primary care providers and veterans at the Durham VA Medical Center. We would also like to recognize the contributions of the remainder of the VA LIFE research team including Dee Carbuccia, Jennifer Chapman, Patricia Cowper, Gail Crowley, Heather MacDonald, and Eleanor McConnell. We appreciate support for this project from the Duke Pepper Center, NIH/NIAP30 AGO28716-01 and VA Rehabilitation and Research Development Service grant E3386R. Dr. Huffman was supported by the ACR-REF/ASP Junior Career Development Award in Geriatric Medicine funded via Atlantic Philanthropies, ACR-REF, John A. Hartford Foundation and ASP as well as NIH/NIAMS K23AR054904.
References
- 1.The burden of musculoskeletal conditions at the start of the new millennium. World Health Organ Tech Rep Ser. 2003;919:i–x. 1–218. back cover. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Prevalence and most common causes of disability among adults--United States, 2005. MMWR Morb Mortal Wkly Rep. 2009 May 1;58(16):421–6. [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004 May;27(5):1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Dunlop DD, Semanik P, Song J, Manheim LM, Shih V, Chang RW. Risk factors for functional decline in older adults with arthritis. Arthritis Rheum. 2005 Apr;52(4):1274–82. doi: 10.1002/art.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ottawa Panel. Ottawa panel evidence-based clinical practice guidelines for therapeutic exercises and manual therapy in the management of osteoarthritis. Phys Ther. 2005 Sep;85(9):907–71. [PubMed] [Google Scholar]
- 6.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: US Department of Health and Human Services; 2008. [Google Scholar]
- 7.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol. 2004;96(1):101–6. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- 8.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006 Sep;29(9):2102–7. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006 Jun;29(6):1433–8. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 10.Gecht MR, Connell KJ, Sinacore JM, Prohaska TR. A survey of exercise beliefs and exercise habits among people with arthritis. Arthritis Care Res. 1996 Apr;9(2):82–8. doi: 10.1002/1529-0131(199604)9:2<82::aid-anr1790090203>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox S, Der Ananian C, Abbott J, Vrazel J, Ramsey C, Sharpe PA, et al. Perceived exercise barriers, enablers, and benefits among exercising and nonexercising adults with arthritis: results from a qualitative study. Arthritis Rheum. 2006 Aug 15;55(4):616–27. doi: 10.1002/art.22098. [DOI] [PubMed] [Google Scholar]
- 12.Schoster B, Callahan LF, Meier A, Mielenz T, DiMartino L. The People with Arthritis Can Exercise (PACE) program: a qualitative evaluation of participant satisfaction. Prev Chronic Dis. 2005 Jul;2(3):A11. [PMC free article] [PubMed] [Google Scholar]
- 13.Center for Disease Control and Prevention (CDC) Arthritis as a potential barrier to physical activity among adults with diabetes--United States, 2005 and 2007. MMWR Morb Mortal Wkly Rep. 2008 May 9;57(18):486–9. [PubMed] [Google Scholar]
- 14.Callahan LF, Mielenz T, Freburger J, Shreffler J, Hootman J, Brady T, et al. A randomized controlled trial of the people with arthritis can exercise program: symptoms, function, physical activity, and psychosocial outcomes. Arthritis Rheum. 2008 Jan 15;59(1):92–101. doi: 10.1002/art.23239. [DOI] [PubMed] [Google Scholar]
- 15.van den Berg MH, Ronday HK, Peeters AJ, le Cessie S, van der Giesen FJ, Breedveld FC, et al. Using internet technology to deliver a home-based physical activity intervention for patients with rheumatoid arthritis: A randomized controlled trial. Arthritis Rheum. 2006 Dec 15;55(6):935–45. doi: 10.1002/art.22339. [DOI] [PubMed] [Google Scholar]
- 16.Morey MC, Peterson MJ, Pieper CF, Sloane R, Crowley GM, Cowper PA, et al. The Veterans Learning to Improve Fitness and Function in Elders Study: a randomized trial of primary care-based physical activity counseling for older men. J Am Geriatr Soc. 2009 Jul;57(7):1166–74. doi: 10.1111/j.1532-5415.2009.02301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King AC, Sallis JF, Dunn AL, Simons-Morton DG, Albright CA, Cohen S, et al. Overview of the Activity Counseling Trial (ACT) intervention for promoting physical activity in primary health care settings. Activity Counseling Trial Research Group. Med Sci Sports Exerc. 1998 Jul;30(7):1086–96. doi: 10.1097/00005768-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Morey MC, Pieper CF, Cornoni-Huntley J. Physical fitness and functional limitations in community-dwelling older adults. Med Sci Sports Exerc. 1998 May;30(5):715–23. doi: 10.1097/00005768-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Stewart AL, Mills KM, Sepsis PG, King AC, McLellan BY, Roitz K, et al. Evaluation of CHAMPS, a physical activity promotion program for older adults. Ann Behav Med. 1997 Fall;19(4):353–61. doi: 10.1007/BF02895154. [DOI] [PubMed] [Google Scholar]
- 20.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001 Jul;33(7):1126–41. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Fillenbaum G. Multidimensional Functional Assessment of Older Adults. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 22.World Health Organization (WHO) Global Strategy on Diet, Physical Activity and Health: Recommendations for Physical Activity. 2009 [updated 2009; cited]; Available from: http://www.who.int/dietphysicalactivity/factsheet_recommendations/en/index.html.
- 23.US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2008 www.health.gov/PAGuidelines.
- 24.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007 Aug 28;116(9):1094–105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 25.Minor MA, Stenstrom CH, Klepper SE, Hurley M, Ettinger WH. Work group recommendations: 2002 Exercise and Physical Activity Conference, St. Louis, Missouri. Session V: evidence of benefit of exercise and physical activity in arthritis. Arthritis Rheum. 2003 Jun 15;49(3):453–4. doi: 10.1002/art.11125. [DOI] [PubMed] [Google Scholar]
- 26.Wolinsky FD, Stump TE, Clark DO. Antecedents and consequences of physical activity and exercise among older adults. Gerontologist. 1995 Aug;35(4):451–62. doi: 10.1093/geront/35.4.451. [DOI] [PubMed] [Google Scholar]
- 27.Kesaniemi YK, Danforth E, Jr, Jensen MD, Kopelman PG, Lefebvre P, Reeder BA. Dose-response issues concerning physical activity and health: an evidence-based symposium. Med Sci Sports Exerc. 2001 Jun;33(6 Suppl):S351–8. doi: 10.1097/00005768-200106001-00003. [DOI] [PubMed] [Google Scholar]
- 28.Center for Disease Control and Prevention (CDC) Trends in strength training--United States, 1998–2004. MMWR Morb Mortal Wkly Rep. 2006 Jul 21;55(28):769–72. [PubMed] [Google Scholar]
- 29.Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil. 2004 Jul;85(7 Suppl 3):S31–42. doi: 10.1016/j.apmr.2004.03.010. quiz S3–4. [DOI] [PubMed] [Google Scholar]
- 30.Woodard C, Berry M, Rejeski W, Ribisl P, Miller H. Exercise Training in Patients With Cardiovascular Disease and Coexistent Knee Arthritis. Journal of Cardiopulmonary Rehabilitation. 1994;14(4):255–61. [Google Scholar]
- 31.Center for Disease Control and Prevention (CDC) Trends in leisure-time physical inactivity by age, sex, and race/ethnicity--United States, 1994–2004. MMWR Morb Mortal Wkly Rep. 2005 Oct 7;54(39):991–4. [PubMed] [Google Scholar]
- 32.Center for Disease Control and Prevention (CDC) Prevalence of self-reported physically active adults--United States, 2007. MMWR Morb Mortal Wkly Rep. 2008 Dec 5;57(48):1297–300. [PubMed] [Google Scholar]
- 33.Giles K, Marshall AL. Repeatability and accuracy of CHAMPS as a measure of physical activity in a community sample of older Australian adults. J Phys Act Health. 2009 Mar;6(2):221–9. doi: 10.1123/jpah.6.2.221. [DOI] [PubMed] [Google Scholar]
- 34.Pruitt LA, Glynn NW, King AC, Guralnik JM, Aiken EK, Miller G, et al. Use of accelerometry to measure physical activity in older adults at risk for mobility disability. J Aging Phys Act. 2008 Oct;16(4):416–34. doi: 10.1123/japa.16.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubbert PM, Morey MC, Kirchner KA, Meydrech EF, Grothe K. Counseling for home-based walking and strength exercise in older primary care patients. Arch Intern Med. 2008 May 12;168(9):979–86. doi: 10.1001/archinte.168.9.979. [DOI] [PubMed] [Google Scholar]


