Abstract
Purpose
Asthma morbidity in children is associated with family psychosocial functioning. While the family plays a pivotal role in maintaining optimal asthma care the mechanism of how family support influences asthma outcomes is not well understood. The purpose of this study was to examine the role of barriers to adherence in mediating the impact of family support on asthma outcomes in adolescents.
Methods
The sample included 126 adolescents with asthma aged 13 to 20 years, living in the Northeast United States. The sample consisted of 49% Whites and 51% minorities including primarily Blacks (38%) followed by Hispanic (11%). Adolescents provided self-reported data. Structural equation modeling was performed to examine the direct and indirect relationships between family support and asthma outcomes.
Results
Family support was positively associated with asthma control and quality of life. These significant associations were mediated by barriers to adherence. Particularly, family support was found to reduce barriers concerning adolescents’ negative attitudes toward medication and healthcare providers, which in turn improved asthma control and quality of life symptoms, emotional functioning and activity domains. Adolescents’ cognitive difficulty also tended to mediate the relationship between family support and emotional functioning.
Conclusion
This study highlights the beneficial effects of family support in improving asthma outcomes in adolescents. Family support exerts the positive effect by ameliorating barriers to treatment adherence in adolescents, particularly the barriers associated with negative attitudes and cognitive challenges. The findings underscore the importance of incorporating family assessment and intervention in caring for adolescents with asthma.
Keywords: Asthma, Adolescents, Family support, Asthma control, Quality of life, Barriers
Family functioning and family contextual factors have received increased attention in research examining health outcomes in children with chronic illness such as asthma. An extensive literature has substantiated that asthma outcomes in children are affected by a variety of factors germane to the family [1]. The family plays a pivotal role in maintaining optimal asthma care. Adherence to asthma treatment is also reinforced and ensured through the effective structuring of family routine and providing nurturing climate that fosters effective problem-solving [2]. Studies have indicated that well functioning families are more effective in managing or coping with asthma in their children, thus improving adherence in children with asthma [2-4] and resulting in favorable asthma outcomes [1]. Adherence is defined as patient’s range of behaviors such as taking medications, monitoring disease conditions, making lifestyle changes, that align with medical and health advice to achieve optimum health outcomes [5]. In this study, we used the concept of barriers to adherence as a proxy measure of adherence given the strong correlation between barriers and actual adherence (self-reported or provider-reported) in adolescents with asthma [5].
Literature collectively identifies four types of barriers to adherence in adolescents: (1) adolescents’ negative perceptions about treatment and providers [6]; (2) cognitive difficulty in following medical advice due to forgetfulness or limited intellectual capacity [7, 8]; (3) Social barriers pertaining to adolescents reluctance to take their medication in the presence of their peers [6, 8, 9]; and (4) adolescents’ tendency for denial characterized by underestimation of asthma symptoms and downplaying the consequence of not taking medication [5, 9]. It is speculated that the family could effectively address or reduce the barriers by providing adequate informational, emotional, and structural support to its adolescents.
Asthma control and asthma-related quality of life are often assessed in evaluating asthma outcomes. Expert Panel Review 3 (EPR3) [10] recommends the assessment of four key symptom expressions (daytime symptoms, nocturnal symptoms, frequency of Short Acting Beta Agonist [SABA] use and activity impairment) to gauge the levels of asthma control. Quality of life reflects individuals’ subjective perception or experiences with asthma symptoms and functional capabilities such as activity limitations and emotional experiences related to asthma [11]. Because quality of life is a unique indicator of asthma status independent of reported asthma symptoms [12], EPR3 recommends that quality of life be measured in addition to asthma control. Thus, this study assessed asthma control and quality of life as separate asthma outcomes.
Taken together, it is assumed that family support could influence asthma outcomes including asthma control and quality of life by modifying and ameliorating various types of barriers that compromise adolescents’ adherence. However, the indirect mechanisms between the family support and asthma outcomes via the barriers are not well understood. Existing evidence supporting the influence of the family on asthma have been based mostly on young children or a broad age range encompassing childhood and adolescence. Therefore, there is a need for a study that exclusively targets adolescents and examines how the family could affect adolescents’ asthma outcomes. Because adolescence is the period of developing independence that propels an individual’s development of identity separate from the parents, adolescents’ perception of their family and its function can be different from their parents. Thus, it is particularly important to assess implications of adolescents’ own perception of family support for asthma outcomes, instead of relying on parents’ reports as in other studies [3, 4, 13]. Moreover, there is a need for a better understanding about the mechanisms involving barriers to adherence in explaining the links between family support and asthma outcomes. Having recognized the gaps, we sought to examine the extent to which family support as perceived by adolescents was associated with asthma outcomes and to explore the mediating role of barriers using path analysis. We hypothesized that the greater levels of family support perceived by adolescents be associated with higher levels of asthma control and quality of life, and that the relationship is mediated by barriers to adherence.
Methods
Study Design and Sample
This cross-sectional study design examined the direct and indirect pathways among study variables including family support, asthma outcomes and barriers to adherence using structural equation modeling. The data reported were part of a larger study designed to determine the efficacy of implementing an asthma self-management program for adolescents. For that larger study, a priori power analysis performed as part of the design yielded an estimated required total sample size of 104 as a function of power (.80) with a large effect size (μ1–μ2/σ=.75) and α=0.05 for comparing the intervention and control groups. This secondary analysis used baseline data from the study sample of 126 adolescents (13-20 years) with an asthma diagnosis.
Participants either were on controller medication or had asthma defined as “persistent” according to the severity classification of the EPR3 guidelines (i.e., >2 days/week of daytime symptoms, >3-4 times of nighttime awakening, >2 days/week of SABA use, or any interference with normal activities due to asthma) [10]. Adolescents with emotional disorders or chronic illness other than asthma were excluded. Participants included 59.5% females, The sample consisted of 49% Whites, 38% Blacks, 11% Hispanic and 2% Asians. The mean age of participants was 15.5 years (SD=1.7). Fifty-eight percent were from families with annual household income <$50,000 which was below the US median household income [14]. About 79% were on controller medication. The average number of years with asthma diagnosis was 10 years (SD=4.7).
Measurement
Perceived Family Support (PFS) [15] is a 20 item scale measuring the extent to which individuals perceive that their needs for support, information and feedback are fulfilled by their family. The scale consisted of declarative statements such as “My family give me the moral support I need” or “My family is sensitive to my personal needs” to which participants responded “Yes (1),” “No (0)” or “Don’t know (not scored).” The total score ranges from 0 to 20; the higher the score, the greater the perceptions of family support. Criterion validity was established by demonstrating positive correlations between PFS and a social competency scale and a social network questionnaire including support provided by family members [15]. Reliability in this sample of adolescents was .85.
Barriers to Adherence consisted of 27-items measured on a 5-point scale assessing the barriers that increase the risk for poor self-management in adolescents with chronic illness [5]. Logan et al.[5] provided evidence of validity by demonstrating positive relationships between the scale scores and the perceptions of drawbacks to medication, and negative relationships between the scale scores and adherence reported by both self and providers. In a previous study, we conducted exploratory factor analysis which retained 20 items and resulted in four subscales including negativity, cognitive difficulty, social barriers, and denial [16]. The negativity subscale (9 items, α=.80) described barriers that indicated poor relationships with the providers (e.g., “I don’t always trust the doctors and nurses”) and negative perceptions about medication (e.g., “My medication has side effects that I really don’t like”). The cognitive difficulty subscale (5 items, α=.76) pertained cognitive challenges in adhering treatment (e.g., “When there are changes to my regimen I sometimes get confused”). The social barrier subscale (4 items, α=.64) explained barriers involving peers (e.g., “I don’t want my friends to know about my illness”). The denial subscale (2 items, α=.52) captured a tendency for downplaying and denial (e.g., “Nothing bad would happen to me if I didn’t follow my regimen”). Alpha for the total 20-item scale in the current sample was .84. Each subscale was summed, with higher scores indicating greater barriers to adherence.
Asthma Control Questionnaire was constructed to assess the levels of asthma control based on four types of asthma-related impairment as recommended by EPR3 [10] including daytime symptoms, night-time symptoms, and SABA use, and levels of activity limitation. The four items were measured on a 4-point scale. A total summated score (range 4-16) was computed to represent the level of asthma control, with higher values indicating higher levels of asthma control. Cronbach’s alpha was .71.
Pediatric Asthma Quality of Life Questionnaire (PAQLQ) (27 items) measures asthma-specific quality of life (QOL) [17] by assessing the extent to which children perceive difficulties as they live with asthma. The questionnaire consisted of three domains including symptoms (10 items) emotional function (8 items) and activity limitation (5 items). Items were rated on a 7-point scale; with 1 indicating maximum impairment, and 7 no impairment. The total score was computed for each domain. Higher scores indicated better levels of functioning. Validity of the measure was established [17]. Cronbach’s alphas of QOL-activity, QOL-emotion, and QOL-symptom in the current sample were .84, .93 and .95, respectively.
Demographic Information including age, race, gender, family income, and parental education was collected from the parents. Race was dichotomized into whites and non-whites due to the small number of minority participants other than blacks. Annual family income was assessed on a 7-point scale ranging from $9,999 or less (1) to $100,000 or more (7).
Procedures
Participants were recruited through flyers and referrals from clinicians and school nurses. Three primary practices and a pediatric pulmonary practice were used for clinician referrals. Of those, three practices (two primary clinics and a pulmonary specialty clinic) were affiliated with a major university medical center, serving predominantly minority children and adolescents of low income inner city families. School nurses in five local schools also made referrals. Two of those schools, located in the inner city, had the high enrollment of minority students (65% Black, 20% Hispanic) and those who were eligible for free lunch (69%). In total, 61% were recruited from the clinics and 39% from school systems. The study nurse screened for eligibility via phone when contacted by interested adolescents and parents. Of 173 screened adolescents, 30 were found ineligible for a range of reasons, and 17 declined to participate (for time constraints or lack of interest) or were lost after screening. Eligible adolescents and parents met with the study nurse at the university facility affiliated with the principal investigator (PI) for informed consent and data collection. Adolescents completed study measures independent of parents. Demographic variables were collected from the parents through interviews in a separate room. Study procedures were approved by the Institutional Review Board (IRB) in a university affiliated with the PI. No additional IRB approval was required by participating recruitment sites.
Statistical Analysis
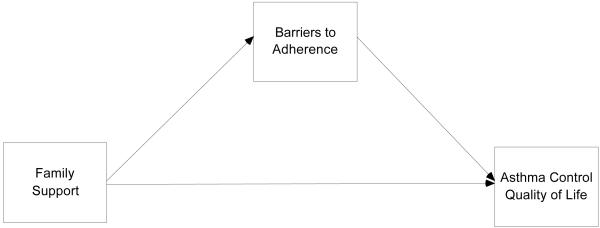
T-tests and Pearson correlations were performed to describe relationships among the variables included in the study. Direct models between family support and asthma outcomes were examined using regression analysis. Structural equation modeling was used to test proposed linkages between variables (see Figure 1). In the model, perceived family support was modeled as a predictor while qualify of life and asthma control were entered as outcome variables, and barrier perception was included in the model as a mediator. We first examined the direct effects of family support on asthma outcomes. We then investigated the indirect effects of family support on asthma outcomes via barrier perception (mediator). When there was a reduction in the size of direct path coefficients from the first steps or a disappearance of statistical significance in the second steps, we suspected the possibility of mediation. Lastly we calculated a Sobel z score [18] for each mediator. The Sobel -test indicates whether the coefficient associated with the mediation is greater than zero. If the coefficient is significantly greater than zero, mediation is indicated [19]. Criteria used to test the structural model were the chi-square, comparative fix index (CFI), Tucker-Lewis index (TLI), and RMSEA. An adequate fit of the data to the model is indicated by RMSEA value > .05 and < .08, CFI and TLI > .90 [20]. MPLUS was used to estimate the model parameters.
Figure 1.
Theoretical Model of Barriers to Adherence as a Mediator between Family Support and Asthma Control and Quality of Life
Results
Relationship between Demographic Variables and Model Variables
Adolescents’ perception of family support did not significantly differ by gender or race. However, family support was positively associated with age (r=.19, p<.05) and family income (r=.19, p<.05). Older participants were more likely to perceive greater family support, and those from the families of higher family income reported greater family support.
Regarding asthma outcomes, males reported higher levels of asthma control (t=2.17, df=124, p=.03), QOL symptoms (t=2.00, df=124, p=.05) and QOL activity (t=3.05, df=124, p≤.001) than females. Whites teens reported higher levels of asthma control (t=2.74, df=124, p≤.001), QOL symptoms (t=3.10, df=124, p.≤.001) and QOL emotion (t=2.79, df=124, p≤.001) than their nonwhite counterparts. Age had negative association with QOL activity (r=−.30, p≤.001). The older one’s age, the lower the activity levels were. Given the significant influence of the demographic variables on outcomes measures, subsequent analyses were conducted controlling for these variables by including them as covariates of the outcome variables.
Direct Pathways between PFS and Asthma Outcomes
Regression analysis revealed a moderate positive association between family support and asthma control (β=.16, p=.05, r2=.16). Similarly, higher levels of family support were associated with greater levels of each domain of quality of life including symptom (β=.18, p=.02, r2=.18), emotion (β=.25, p=.002, r2=.17) and activity (β=.25, p=.002, r2=.19) domains.
Indirect Pathways between PFS and Asthma Outcomes via Barriers to Adherence
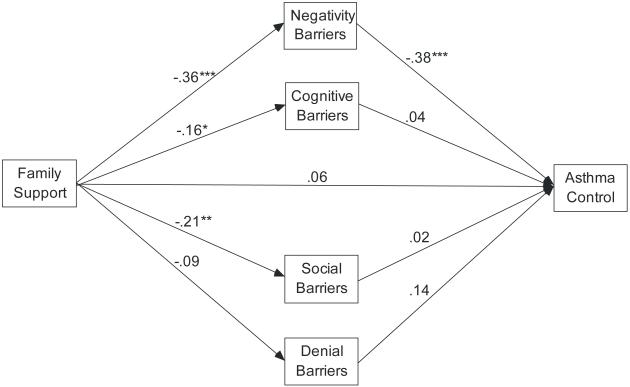
Correlations among the variables in the model can be seen in Table 1. Path analyses were performed to test the mediating effects of barriers to adherence in explaining the associations between family support and asthma outcomes. The indirect pathways between family support and asthma control are illustrated in Figure 2. This model fit the data well (χ2=25.87, df=19, p=.134, CFI=.928, TLI=.868, RMSEA=.05). Overall, the model explained 27% of the variance in asthma control (R2=.27). The direct pathway between family support and asthma control was no longer significant when the barriers were in the model as mediators. The Sobel test revealed a significant total indirect effect from family support to asthma control through barriers (β=.112, z=2.498, p=.012). Significant pathways were demonstrated between family support and the negativity barriers and between the negativity barriers and asthma control, indicating that higher levels of family support reduced negativity barriers which in turn improved asthma control. The path from family support to cognitive barriers and social barriers reached statistical significance, yet the path from these barriers to asthma control was not significant.
Table 1.
Correlations, Mean, and Standard Deviation of Variables in the Models (n=126)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Family Support | 1.00 | |||||||||
| 2. Barriers Total | -.34 ** | 1.00 | ||||||||
| 3. Barriers-Negativity | -.36 ** | .84 ** | 1.00 | |||||||
| 4. Barriers-Cognitive | -.17 | .72 ** | .38 ** | 1.00 | ||||||
| 5. Barriers-Social | -.21 * | .67 ** | .39 ** | .33 ** | 1.00 | |||||
| 6. Barriers-Denial | -.09 | .36 ** | .19 * | .12 | .13 | 1.00 | ||||
| 7. Asthma Control | .21 * | -.27 ** | -.36 ** | -.14 | -.12 | .11 | 1.00 | |||
| 8. QOL-Symptoms | .20 * | -.33 ** | -.36 ** | -.22 * | -.18 * | .05 | .72 ** | 1.00 | ||
| 9. QOL-Emotional | .25 ** | -.45 ** | -.42 ** | -.39 ** | -.28 ** | .08 | .59 ** | .83 ** | 1.00 | |
| 10. QOL-Activity | .20 * | -.35 ** | -.41 ** | -.25 ** | -.19 * | .13 | .57 ** | .69 ** | .72 ** | 1.00 |
| Mean | 13.93 | 2.26 | 2.07 | 2.67 | 2.12 | 2.32 | 12.85 | 51.56 | 45.69 | 26.29 |
| Standard Deviation | 4.61 | .53 | .63 | .83 | 0.83 | .90 | 2.44 | 15.05 | 11.25 | 6.56 |
p <.05
p < .01
Figure 2.
Model of Family Support, Barriers to Adherence, and Asthma Control (control variables of age, gender, race, and income are not pictured).
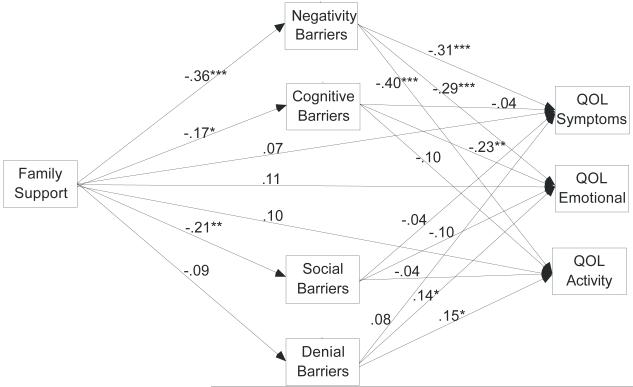
Figure 3 depicts indirect pathways between family support and the three domains of quality of life via the four subscales of barriers. Similarly, the direct pathways from family support to each domain of quality of life disappeared with the inclusion of barrier subscales in the model. This model also showed good fit with the data (χ2=25.87, df=19, p=.134, CFI=.980, TLI =.947, RMSEA=.05). Overall, the model explained about 28% of the variance in QOL symptom (R2=.28), 36% of the variance in QOL emotion (R2=.36), and 41% of the variance in QOL activity domain (R2=.41). The Sobel tests supported significant total indirect effects of family support to QOL symptoms (β=.121, z=2.793,p=.005), QOL emotional function (β=.149, z=2.901,p=.004), and QOL activity (β=.157, z=3.147,p=.002) through barriers to adherence. Of the four barrier subscales, negativity was the most prominent barrier that mediated the relationships between family support and all three domains of quality of life. The findings indicated that family support reduced the negative perceptions about medication and the providers, which in turn improved the quality of life. Cognitive barriers appeared to be mediating between family support and QOL emotion because of the significant paths. However, the Sobel test was borderline significant (β=.039, z=1.628, p=.104), most likely due to the small sample size.
Figure 3.
Model of Family Support, Barriers to Adherence, and Quality of Life (control variables of age, gender, race, and income are not pictured).
Discussion
Overall, higher levels of family support were associated with greater asthma control and quality of life in adolescents. This finding mirrors previous studies in which children of families with favorable psychosocial attributes were more likely to manifest well controlled asthma [21], while family conflicts increased risk for asthma morbidity in children [13]. Many studies have espoused that family’s overall psychosocial functioning (e.g., family relationships, conflict, expressiveness and cohesion) have a profound influence on psychological adaptation in children with asthma [3, 4, 22]. Therefore, it is no surprise that the current study revealed family support as a precursor to the quality of life in adolescents with asthma.
This study expanded existing knowledge by attempting to catch a glimpse of the mechanism that may explain the relationship between family factors and asthma outcomes. Our data suggest that family support could alter the negative effects of barriers to adherence, ultimately improving asthma outcomes in adolescents. This finding is in line with studies suggesting adverse family circumstances, such as inadequate family support, as roadblocks to adolescents’ adequate adherence to asthma management [1]. Unsupportive family atmosphere may make it difficult for adolescents to adhere to daily asthma management that often relies on effective communication, supervision and division of responsibilities among family members [23].
We found that adolescents’ negative perceptions about treatment and the providers were the most pertinent factor in understanding the relationship between family support and asthma outcomes. The finding suggests that families with high levels of support can ameliorate the negative perceptions through effective negotiation and communication that fosters positive views in its adolescents about asthma treatment. Furthermore, the supportive parents can take more active roles in bridging communication gaps between adolescents and their providers, thus abating the adolescents’ mistrust or dissatisfaction with their providers. Our findings also suggest that the family could counterbalance adolescents’ cognitive challenges (e.g., forgetfulness or difficulties in understanding treatment regimens) by providing structured and predictive family routines and environment that are conducive to adherence. Of the three domains of quality of life, cognitive barriers were associated only with emotional functioning. Lack of family support often coincides with relational or situational hardships in the family which increases the adolescents’ emotional vulnerability and amplifies negative emotional reactions to their asthma. Non significant mediating effects of social barriers and denial may have been the artifact of low reliability in these subscales primarily due to small number of items. Further study with more reliable measures of these constructs is warranted to determine the extent to which these types of barriers to adherence are associated with asthma outcomes.
Concurring with this study, several researchers viewed family support as a critical factor in promoting adherence in adolescents [24, 25]. Literature has consistently documented widespread issues involving adolescents’ inadequate adherence [26-30]. Parents’ attempts to ensure their adolescents’ adherence impinge upon adolescents’ desire for independence [8, 31, 32]. Moreover, family-based routines (e.g., regular mealtime or holiday rituals) which have been known to be linked to adherence and positive asthma outcomes wane as children transition to adolescents [33]. Nonetheless, family involvement and support continues to be beneficial and essential in achieving optimum asthma control in adolescents.
Family support enabling effective communication and nurturing relationships is pivotal in alleviating barriers to adherence, thus improving asthma outcomes in adolescents. Family interventions involving parents have been found effective in enhancing treatment adherence and promoting disease outcomes in youths with diabetes [34], cystic fibrosis [35] and HIV [36]. A family intervention for adolescents (11-14 years) with asthma and their parents has been pilot tested and demonstrated its feasibility and potential effectiveness in fostering youths’ asthma self-management [37]. Further research evaluating a similar program that promotes family support is warranted to affirm short- and long-term asthma outcomes in adolescents of a broader age range.
Caution should be exercised in interpreting the findings due to several limitations. First, the cross-sectional design prohibits making inference to causality among the study variables. A longitudinal study is warranted to inform temporal sequence between family support and adherence and asthma outcomes. The small convenient sample limits the generalizability of the findings. The majority were recruited from clinics, explaining the nearly 80% of the sample being on controller medication despite their low SES. Thus, caution is required in applying the findings to other low income families with limited access to health care. To gauge asthma control, instead of using an existing instrument (e.g., Asthma Control Test), we devised our own measure so that response options conformed to the specific criteria by the EPR3 guidelines. Given that our measure assessed the same types of symptom expressions as the existing instrument, we do not anticipate that our findings were affected by the use of the devised measure in a significant way. Finally, a broad age range (13-20 years) of the sample warrants caution in interpreting our findings. Our data suggests that the perception of family support differ by age, perhaps in part due to changes in parent-adolescent relationship dynamics from early to late adolescence. In early adolescence, the ongoing parent involvement can be perceived negatively by adolescents who are beginning to embrace emerging independence and identity, while in later adolescence family involvement diminishes and youths tend to achieve separation from the family, leading to more positive views on the family [38]. Moreover, age has an important implication for asthma self-management. Orrell-Valente [39] reported a strong linear relationship between age and adolescents responsibility for controller medication (by age 15, 75%; and by age 19, 100%). Given these important implications of age for family support and asthma management, our findings based on the broad age range should be interpreted with caution.
Overall, our study findings echo the imperative of family partnership in adequate asthma management purported by the national guidelines [10]. Prior to gauging the feasibility of family partnership, clinicians need to first assess family dynamics and relationships. In doing so, administering a brief questionnaire can be considered, such as the Parent-Adolescent Communication Scale (PACS) which is a valid instrument assessing parent-adolescent relationship reflected in communication quality and styles [40]. The scale has parent and adolescent forms and can be administered with ease at busy clinical settings. Information from such a measure will allow clinicians to identify risk factors within the family that impede proper asthma care, thus making a timely intervention possible. This study underscores the importance of the family in understanding adolescents’ adherence to asthma management and improving their asthma outcomes and calls for an intervention that strengthens the family support system.
Acknowledgments
This study was supported by a grant from the NIH/NINR: Contract number R21 NR009837.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaugars AS, Klinnert MD, Bender BG. Family influences on pediatric asthma. J Pediatr Psychol. 2004;29:475–491. doi: 10.1093/jpepsy/jsh051. [DOI] [PubMed] [Google Scholar]
- 2.Fiese B, Winter M, Anbar R, Howell K, Poltrock S. Family climate of routine asthma care: Associating perceived burden and mother-child interaction patterns to child well-being. Fam Process. 2008;47:63–79. doi: 10.1111/j.1545-5300.2008.00239.x. [DOI] [PubMed] [Google Scholar]
- 3.Bender BG, Annett RD, Ikle D, DuHamel TR, Rand C, Strunk RC, CAMP research group Relationship between disease and psychological adaptation in children in the childhood asthma management program and their families. Arch Pediatr Adolesc Med. 2000;154:706–13. doi: 10.1001/archpedi.154.7.706. [DOI] [PubMed] [Google Scholar]
- 4.Wood BL, Lim J, Miller BD, et al. Family emotional climate, depression, emotional triggering of asthma, and disease severity in pediatric asthma: Examination of pathways of effect. J Pediatr Psychol. 2007;32:542–551. doi: 10.1093/jpepsy/jsl044. [DOI] [PubMed] [Google Scholar]
- 5.Logan D, Zelikovsky N, Labay L, Spergel J. The illness management survey: Identifying adolescents’ perceptions of barriers to adherence. J Pediatr Psychol. 2003;28:383–392. doi: 10.1093/jpepsy/jsg028. [DOI] [PubMed] [Google Scholar]
- 6.Cohen R, Franco K, Motlow F, Reznik M, Ozuah PO. Perceptions and attitudes of adolescents with asthma. J Asthma. 2003;40:207–211. doi: 10.1081/jas-120017992. [DOI] [PubMed] [Google Scholar]
- 7.Jones IR, Ahmed N, Kelly M, et al. With an attack I associate it more with going into hospital: Understandings of asthma and psychosocial stressors; are they related to use of services? Soc Sci Med. 2008;66:765–775. doi: 10.1016/j.socscimed.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Penza-Clyve SM, Mansell CB, McQuaid EL. Why don’t children take their asthma medications? A qualitative analysis of children’s perspectives on adherence. J Asthma. 2004;41:189–197. doi: 10.1081/jas-120026076. [DOI] [PubMed] [Google Scholar]
- 9.Rhee H. Adolescents’ psychosocial experiences living with asthma: A focus group study. J Pediatr Health Care. 2007;21:99–107. doi: 10.1016/j.pedhc.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 10.National Heart, Lung, and Blood Institute . Expert panel report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: 2007. [Google Scholar]
- 11.Warschburger P, Busch S, Bauer CP, Kiosz D, Stachow R, Petermann F. Health-related quality of life in children and adolescents with asthma: Results from the ESTAR study. J Asthma. 2004;41:463–470. doi: 10.1081/jas-120033989. [DOI] [PubMed] [Google Scholar]
- 12.Juniper EF, Wisniewski ME, Cox FM, Emmett AH, Nielsen KE, O’Byrne PM. Relationship between quality of life and clinical status in asthma: A factor analysis. Eur Respir J. 2004;23:287–291. doi: 10.1183/09031936.04.00064204. [DOI] [PubMed] [Google Scholar]
- 13.Chen E, Bloomberg GR, Fisher E, Strunk RC. Predictors of repeat hospitalizations in children with asthma: The role of psychosocial and socioenvironmental factors. Health Psychol. 2003;22:12–18. doi: 10.1037//0278-6133.22.1.12. [DOI] [PubMed] [Google Scholar]
- 14.US Census Bureau [Accessed January 18, 2010];American community survey (ACS), income & wealth. Available at: http://www.census.gov/Press-Release/www/releases/archives/income_wealth/012528.html.
- 15.Procidano ME, Heller K. Measures of perceived social support from friends and from family: Three validation studies. Am J Community Psychol. 1983;11:1–24. doi: 10.1007/BF00898416. [DOI] [PubMed] [Google Scholar]
- 16.Rhee H, Belyea MJ, Cirzynski S, Brasch J. Barriers to asthma self-management in adolescents: Relationships to psychosocial factors. Pediatr Pulmonol. 2009;1:183–191. doi: 10.1002/ppul.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res. 1996;5:35–46. doi: 10.1007/BF00435967. [DOI] [PubMed] [Google Scholar]
- 18.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 19.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test the significance of the mediated effect. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 21.Meijer AM, Griffioen RW, van Nierop JC, Oppenheimer L. Intractable or uncontrolled asthma: Psychosocial factors. J Asthma. 1995;32:265–274. doi: 10.3109/02770909509044834. [DOI] [PubMed] [Google Scholar]
- 22.Klinnert MD, Kaugars AS, Strand M, Silveira L. Family psychological factors in relation to children’s asthma status and behavioral adjustment at age 4. Fam Proc. 2008;47:41–61. doi: 10.1111/j.1545-5300.2008.00238.x. [DOI] [PubMed] [Google Scholar]
- 23.Brenner M. Childhood asthma: A developmental and biopsychosocial model for treatment. J Asthma. 1991;28:401–403. doi: 10.3109/02770909109110621. [DOI] [PubMed] [Google Scholar]
- 24.La Greca AM. Peer influences in pediatric chronic illness: An update. J Pediatr Psychol. 1992;17:775–784. doi: 10.1093/jpepsy/17.6.775. [DOI] [PubMed] [Google Scholar]
- 25.Kyngas HA. Compliance of adolescents with asthma. Nurs Health Sci. 1999;1:195–202. doi: 10.1046/j.1442-2018.1999.00025.x. [DOI] [PubMed] [Google Scholar]
- 26.Bender B, Wamboldt FS, O’Connor SL, et al. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and doser CT. Ann Allergy Asthma Immunol. 2000;85:416–421. doi: 10.1016/s1081-1206(10)62557-4. [DOI] [PubMed] [Google Scholar]
- 27.Dinwiddie R, Muller WG. Adolescent treatment compliance in asthma. J R Soc Med. 2002;95:68–71. doi: 10.1258/jrsm.95.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price J, Kemp J. The problems of treating adolescent asthma: What are the alternatives to inhaled therapy? Respir Med. 1999;93:677–684. doi: 10.1016/s0954-6111(99)90033-1. [DOI] [PubMed] [Google Scholar]
- 29.Zebracki K, Drotar D. Outcome expectancy and self-efficacy in adolescent asthma self-management. Child Health Care. 2004;33:133–149. [Google Scholar]
- 30.McQuaid EL, Kopel SJ, Klein RB, Fritz GK. Medication adherence in pediatric asthma: Reasoning, responsibility, and behavior. J Pediatr Psychol. 2003;28:323–333. doi: 10.1093/jpepsy/jsg022. [DOI] [PubMed] [Google Scholar]
- 31.Rhee H, Wenzel J, Steeves RH. Adolescents’ psychosocial experiences living with asthma: A focus group study. J Pediatr Health Care. 2007;21:99–107. doi: 10.1016/j.pedhc.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Ayala GX, Miller D, Zagami E, Riddle C, Willis S, King D. Asthma in middle schools: What students have to say about their asthma. J Sch Health. 2006;76:208–214. doi: 10.1111/j.1746-1561.2006.00098.x. [DOI] [PubMed] [Google Scholar]
- 33.Fiese BH, Wamboldt FS, Anbar RD. Family asthma management routines: Connections to medical adherence and quality of life. J Pediatr. 2005;146:171–176. doi: 10.1016/j.jpeds.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 34.Wysocki T, Harris MA, Buckloh LM, et al. Effects of behavioral family systems therapy for diabetes on adolescents’ family relationships, treatment adherence, and metabolic control. J Pediatr Psychol. 2006;31:928–938. doi: 10.1093/jpepsy/jsj098. [DOI] [PubMed] [Google Scholar]
- 35.Stark LJ, Quittner AL, Powers SW, et al. Randomized clinical trial of behavioral intervention and nutrition education to improve caloric intake and weight in children with cystic fibrosis. Arch Pediatr Adolesc Med. 2009;163:915–921. doi: 10.1001/archpediatrics.2009.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellis DA, Naar-King S, Cunningham PB, Secord E. Use of multisystemic therapy to improve antiretroviral adherence and health outcomes in HIV-infected pediatric patients: Evaluation of a pilot program. AIDS Patient Care STDS. 2006;20:112–121. doi: 10.1089/apc.2006.20.112. [DOI] [PubMed] [Google Scholar]
- 37.Bruzzese JM, Unikel L, Gallagher R, Evans D, Colland V. Feasibility and impact of a school-based intervention for families of urban adolescents with asthma: Results from a randomized pilot trial. Fam Process. 2008;47:95–113. doi: 10.1111/j.1545-5300.2008.00241.x. [DOI] [PubMed] [Google Scholar]
- 38.Shulman S, Prechter E. Adolescent perception of family climate and adaptation to residential schooling. J Youth Adolesc. 1989;18:439–449. doi: 10.1007/BF02132779. [DOI] [PubMed] [Google Scholar]
- 39.Orrell-Valente JK, Jarlsberg LG, Hill LG, Cabana MD. At what age do children start taking daily asthma medicines on their own? Pediatrics. 2008;122:e1186–92. doi: 10.1542/peds.2008-0292. [DOI] [PubMed] [Google Scholar]
- 40.Barnes HL, Olson DH. Parent-adolescent communication and the circumplex model. Child Dev. 1985;56:438–447. [Google Scholar]