Abstract
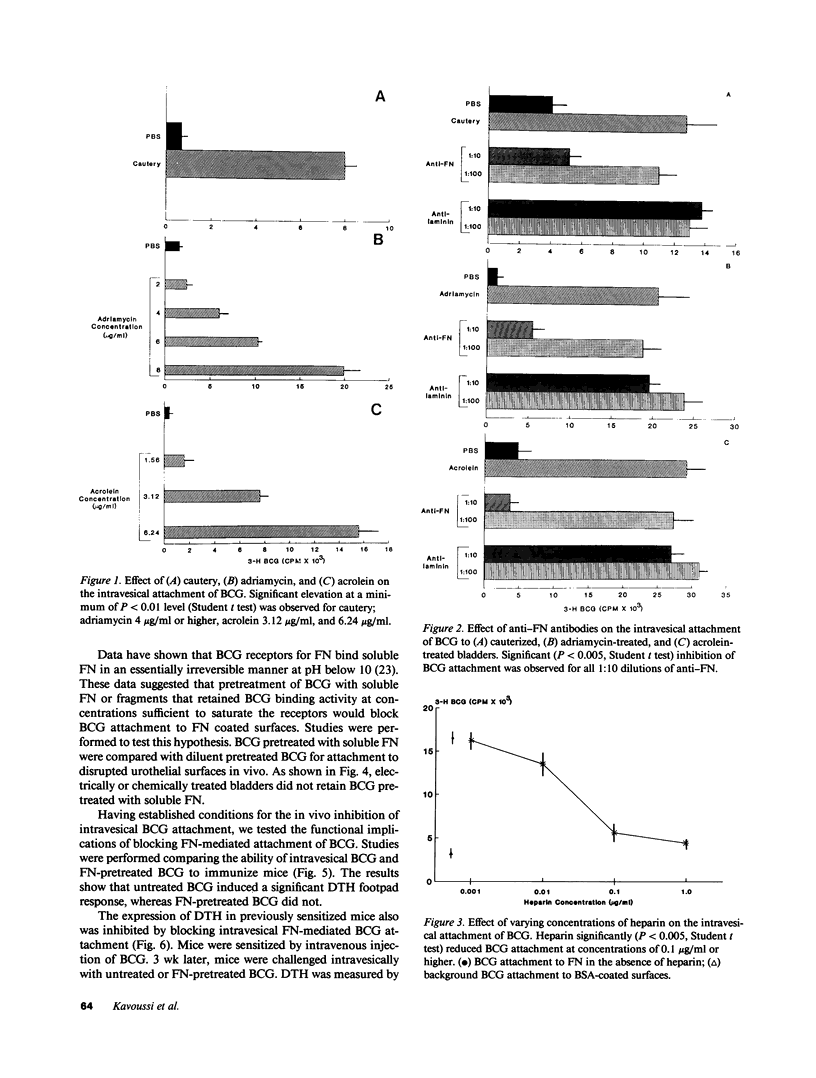
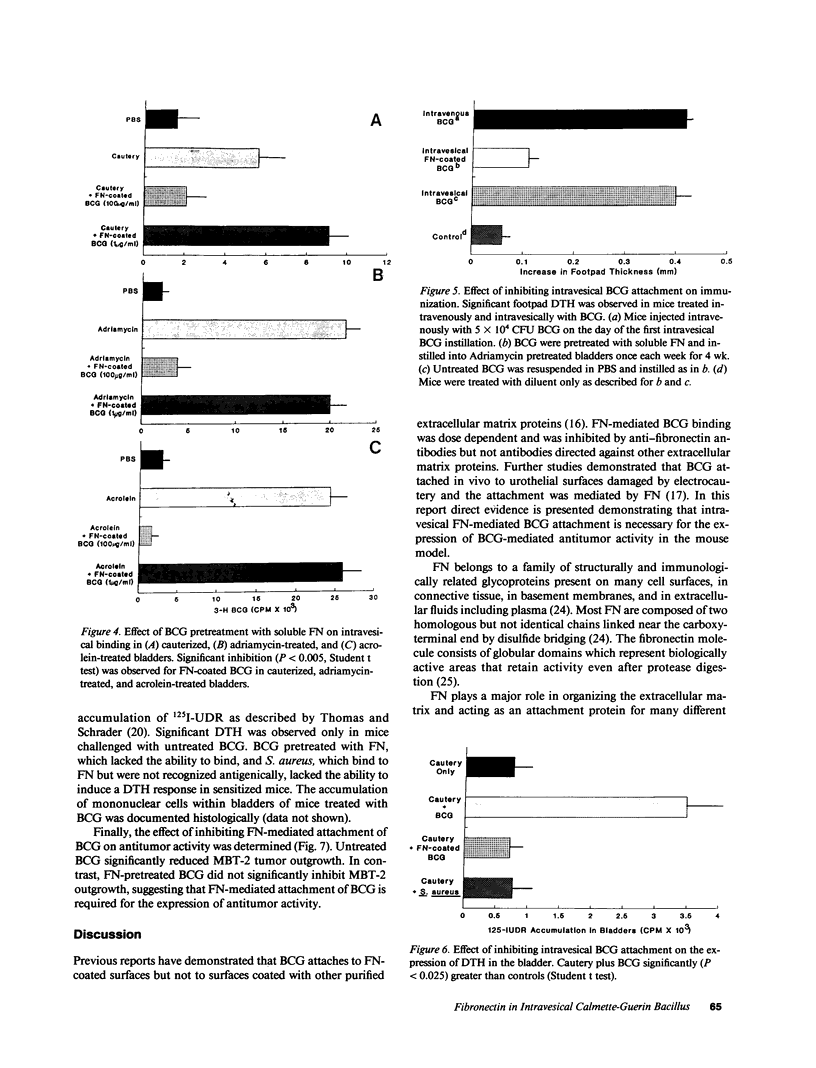
Adjuvant intravesical Calmette-Guerin bacillus (BCG) is an effective treatment for superficial bladder cancer. The mechanisms by which BCG mediates antitumor activity are not known. We investigated the initial interaction of BCG with the bladder mucosa to determine whether binding was essential for the development of antitumor activity. Herein, we show that bladder urothelial disruption induced by acrolein, adriamycin, or electrocautery resulted in BCG binding in areas of urothelial damage. Binding induced by each method was inhibited by anti-fibronectin (FN) antibodies but not by antibodies to the basement membrane component laminin. Intravesical BCG binding also was inhibited by pretreating BCG with soluble FN. Inhibition of intravesical FN-mediated BCG attachment prevented immunization via the intravesical route. Moreover, the expression of both delayed hypersensitivity in the bladder of BCG-immunized mice and antitumor activity was inhibited by blocking FN-mediated intravesical BCG attachment. These data suggest that intralumenal attachment of BCG appears to be mediated by FN. Moreover, these data suggest that intravesical FN mediated attachment of BCG is a requisite step in BCG-mediated antitumor activity in the murine bladder tumor model.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIOZZI G., BENACERRAF B., GRUMBACH F., HALPERN B. N., LEVADITI J., RIST N. Etude de l'activité granulopexique du système réticulo-endothélial au cours de l'infection tuberculeuse expérimentale de la souris. Ann Inst Pasteur (Paris) 1954 Sep;87(3):291–300. [PubMed] [Google Scholar]
- Bast R. C., Bast B. S. Critical review of previously reported animal studies of tumor immunotherapy with nonspecific immunostimulants. Ann N Y Acad Sci. 1976;277(00):60–93. doi: 10.1111/j.1749-6632.1976.tb41692.x. [DOI] [PubMed] [Google Scholar]
- Brosman S. A. Experience with bacillus Calmette-Guerin in patients with superficial bladder carcinoma. J Urol. 1982 Jul;128(1):27–30. doi: 10.1016/s0022-5347(17)52736-6. [DOI] [PubMed] [Google Scholar]
- Click E. M., Balian G. Domain structure of human plasma and cellular fibronectin. Use of a monoclonal antibody and heparin affinity to identify three different subunit chains. Biochemistry. 1985 Nov 5;24(23):6685–6696. doi: 10.1021/bi00344a058. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell Immunol. 1970 Sep;1(3):253–265. doi: 10.1016/0008-8749(70)90047-x. [DOI] [PubMed] [Google Scholar]
- Courtney H. S., Simpson W. A., Beachey E. H. Binding of streptococcal lipoteichoic acid to fatty acid-binding sites on human plasma fibronectin. J Bacteriol. 1983 Feb;153(2):763–770. doi: 10.1128/jb.153.2.763-770.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espersen F., Clemmensen I. Isolation of a fibronectin-binding protein from Staphylococcus aureus. Infect Immun. 1982 Aug;37(2):526–531. doi: 10.1128/iai.37.2.526-531.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUND J. The mode of action of immunologic adjuvants. Bibl Tuberc. 1956;(10):130–148. [PubMed] [Google Scholar]
- Fey F., Arnold W., Graffi A. Demonstration of the stimulation of the reticulohistiocytic system (RHS) of mice by treatment with BCG by means of biometric and histochemical techniques. Eur J Cancer. 1976 Aug;12(8):595–598. doi: 10.1016/0014-2964(76)90183-3. [DOI] [PubMed] [Google Scholar]
- Gardiner R. A., Seymour G. J., Lavin M. F., Strutton G. M., Gemmell E., Hazan G. Immunohistochemical analysis of the human bladder. Br J Urol. 1986 Feb;58(1):19–25. doi: 10.1111/j.1464-410x.1986.tb05420.x. [DOI] [PubMed] [Google Scholar]
- Haaff E. O., Catalona W. J., Ratliff T. L. Detection of interleukin 2 in the urine of patients with superficial bladder tumors after treatment with intravesical BCG. J Urol. 1986 Oct;136(4):970–974. doi: 10.1016/s0022-5347(17)45142-1. [DOI] [PubMed] [Google Scholar]
- Herr H. W., Pinsky C. M., Whitmore W. F., Jr, Sogani P. G., Oettgen H. F., Melamed M. R. Experience with intravesical bacillus Calmette-Guèrin therapy of superficial bladder tumors. Urology. 1985 Feb;25(2):119–123. doi: 10.1016/0090-4295(85)90525-4. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. R., Haaff E. O., Becich M., Lage J., Bauer W. C., Dresner S. M., Catalona W. J., Ratliff T. L. Prognostic value of purified protein derivative skin test and granuloma formation in patients treated with intravesical bacillus Calmette-Guerin. J Urol. 1986 Feb;135(2):268–271. doi: 10.1016/s0022-5347(17)45605-9. [DOI] [PubMed] [Google Scholar]
- Kuusela P., Vartio T., Vuento M., Myhre E. B. Attachment of staphylococci and streptococci on fibronectin, fibronectin fragments, and fibrinogen bound to a solid phase. Infect Immun. 1985 Oct;50(1):77–81. doi: 10.1128/iai.50.1.77-81.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm D. L., Thor D. E., Stogdill V. D., Radwin H. M. Bladder cancer immunotherapy. J Urol. 1982 Nov;128(5):931–935. doi: 10.1016/s0022-5347(17)53283-8. [DOI] [PubMed] [Google Scholar]
- Morales A., Eidinger D., Bruce A. W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976 Aug;116(2):180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- Mori K., Lamm D. L., Crawford E. D. A trial of bacillus Calmette-Guérin versus adriamycin in superficial bladder cancer: a South-West Oncology Group Study. Urol Int. 1986;41(4):254–259. doi: 10.1159/000281212. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Amrani D. L. The structure and biologic activities of plasma fibronectin. Blood. 1980 Aug;56(2):145–158. [PubMed] [Google Scholar]
- Mosher D. F. Physiology of fibronectin. Annu Rev Med. 1984;35:561–575. doi: 10.1146/annurev.me.35.020184.003021. [DOI] [PubMed] [Google Scholar]
- Pang A. S., Morales A. BCG induced murine peritoneal exudate cells: cytotoxic activity against a syngeneic bladder tumor cell line. J Urol. 1982 Jun;127(6):1225–1229. doi: 10.1016/s0022-5347(17)54303-7. [DOI] [PubMed] [Google Scholar]
- Pode D., Alon Y., Horowitz A. T., Vlodavsky I., Biran S. The mechanism of human bladder tumor implantation in an in vitro model. J Urol. 1986 Aug;136(2):482–486. doi: 10.1016/s0022-5347(17)44926-3. [DOI] [PubMed] [Google Scholar]
- Pommier C. G., Inada S., Fries L. F., Takahashi T., Frank M. M., Brown E. J. Plasma fibronectin enhances phagocytosis of opsonized particles by human peripheral blood monocytes. J Exp Med. 1983 Jun 1;157(6):1844–1854. doi: 10.1084/jem.157.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff T. L., Gillen D., Catalona W. J. Requirement of a thymus dependent immune response for BCG-mediated antitumor activity. J Urol. 1987 Jan;137(1):155–158. doi: 10.1016/s0022-5347(17)43909-7. [DOI] [PubMed] [Google Scholar]
- Ratliff T. L., Kavoussi L. R., Catalona W. J. Role of fibronectin in intravesical BCG therapy for superficial bladder cancer. J Urol. 1988 Feb;139(2):410–414. doi: 10.1016/s0022-5347(17)42445-1. [DOI] [PubMed] [Google Scholar]
- Ratliff T. L., Palmer J. O., McGarr J. A., Brown E. J. Intravesical Bacillus Calmette-Guérin therapy for murine bladder tumors: initiation of the response by fibronectin-mediated attachment of Bacillus Calmette-Guérin. Cancer Res. 1987 Apr 1;47(7):1762–1766. [PubMed] [Google Scholar]
- Reichert D. F., Lamm D. L. Long term protection in bladder cancer following intralesional immunotherapy. J Urol. 1984 Sep;132(3):570–573. doi: 10.1016/s0022-5347(17)49748-5. [DOI] [PubMed] [Google Scholar]
- See W. A., Chapman P. H. Heparin prevention of tumor cell adherence and implantation on injured urothelial surfaces. J Urol. 1987 Jul;138(1):182–186. doi: 10.1016/s0022-5347(17)43040-0. [DOI] [PubMed] [Google Scholar]
- Shapiro A., Ratliff T. L., Oakley D. M., Catalona W. J. Reduction of bladder tumor growth in mice treated with intravesical Bacillus Calmette-Guérin and its correlation with Bacillus Calmette-Guérin viability and natural killer cell activity. Cancer Res. 1983 Apr;43(4):1611–1615. [PubMed] [Google Scholar]
- Thomas W. R., Schrader J. W. Delayed hypersensitivity in mast-cell-deficient mice. J Immunol. 1983 Jun;130(6):2565–2567. [PubMed] [Google Scholar]
- Vaudaux P., Suzuki R., Waldvogel F. A., Morgenthaler J. J., Nydegger U. E. Foreign body infection: role of fibronectin as a ligand for the adherence of Staphylococcus aureus. J Infect Dis. 1984 Oct;150(4):546–553. doi: 10.1093/infdis/150.4.546. [DOI] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]